Introduction
Gastroesophageal reflux disease (GERD) is commonly
treated by primary care physicians, and influences patients'
overall quality of life (QOL) (1).
Proton pump inhibitors (PPI) have been the mainstay of GERD
treatment for two decades, and are recommended in Japanese
guidelines (2). Despite evidence
confirming the efficacy of PPI treatment, a Japanese survey
reported over-the-counter medication use in patients who received
prescriptions for GERD (3). Some
patients with GERD are refractory to standard PPI treatment.
Japanese guidelines define PPI-resistant GERD as a condition in
which, i) an esophageal mucosal break did not heal and/or ii)
reflux symptoms considered to be due to GERD are not sufficiently
mitigated even after oral administration of PPI at a standard dose
for eight weeks (2). To overcome
treatment difficulties, double-dose PPI and/or additional
administration of a prokinetic drug and herbal medicine were
suggested. However, these unmet medical needs are not solved, and
many patients with PPI-resistant GERD continue to suffer with
impaired QOL (4).
Vonoprazan, a novel competitive acid blocker, became
a first-choice drug for GERD and Helicobactor pylori (H.
pylori) eradication therapy in Japan because of its strong acid
inhibition, superior to PPI (5,6). Hoshino
et al (7) reported the effect
of vonoprazan 20 mg on patients with PPI-resistant reflux
esophagitis with an 87.5% endoscopic healing rate and 76.2% of
these patients did not recur even with a decreased dose of
vonoprazan to 10 mg. These data encouraged us to evaluate the
effect of vonoprazan 10 mg on GERD symptoms. Despite interest in
the effect of vonoprazan in patients with PPI-resistant GERD, to
the best of our knowledge, few data have been reported on the
effect of vonoprazan on GERD symptoms. The effect of vonoprazan in
patients with PPI-resistant GERD without erosive changes of the
esophagus has not been well reported. The aim of the present study
was to determine the effect of vonoprazan 10 mg in patients with
PPI-resistant GERD.
Patients and methods
Twenty five patients with PPI-resistant GERD treated
with vonoprazan 10 mg daily from February 2016 to February 2017, at
Shinozaki Medical Clinic, were included in the present study. In
this study, PPI-resistant GERD was defined as GERD symptoms not
adequately improved even after a standard dose of PPI treatment for
more than eight weeks. The Izumo scale in clinical practice to
assess QOL of patients with gastrointestinal (GI) symptoms was
routinely used (8). The following data
were abstracted: Medical history, smoking habits, alcohol
consumption, Izumo scale score, kind of PPI prior to vonoprazan
treatment, acotiamide use, history of H. pylori eradication
and endoscopic findings. H. pylori infection was assessed
using serum anti-H. pylori antibody and/or the
13C-urea breath test. The degree of atrophy was
determined based on the findings of esophagogastroduodenoscopy
(EGD) using the Kimura-Takemoto classification, in which closed and
open types correspond to mild and severe atrophy, respectively
(9). Of 25 patients, one patient who
was not followed for more than one month was excluded from the
present study. Finally, 24 patients were included in the study
cohort. The Institutional Review Board approved this retrospective
study.
Izumo scale
The influence of GI symptoms on QOL of patients was
evaluated by the Izumo scale, a validated and widely used
questionnaire that assesses various abdominal symptoms (10–13). This
scale has good internal consistency with the Gastrointestinal
Symptom Rating Scale (12,14). It includes five domains with a total of
15 items: GERD (Q1-3), epigastric pain (Q4-6), postprandial
distress (Q7-9), constipation (Q10-12) and diarrhea (Q13-15). Each
item is scored 0 to 5 on a Likert scale based on the degree of
symptoms: 0 = not bothered, 1 = not so bothered, 2 = slightly
bothered, 3 = bothered, 4 = strongly bothered and 5 = intolerably
bothered. Each domain has three items and thus has a total score
from 0 to 15 points to assess the severity of each GI symptom
assessed. A higher score reflects more severe symptoms. In this
study, the score in the GERD domain of all patients were four or
more points. We used the Izumo scale before and four weeks after
starting vonoprazan. An ‘improvement of symptoms’ was defined as a
score that was reduced by 50% or more, ‘resolution of symptoms’ as
a score that was reduced to zero or one, and ‘aggravation of
symptoms’ as a score that increased by four or more points from the
initial score.
Statistical analysis
To compare scores of before and after the vonoprazan
therapy, The Wilcoxon rank sum test was used, and to compare scores
of groups, the Mann-Whitney U test was used. Data with a normal
distribution were analyzed with Student's t-test, and categorical
data were compared with the chi-squared test. Differences between
variables with P<0.05 were considered significant. Statistical
analysis was performed using BellCurve for Excel software (Social
Survey Research Information Co., Ltd. Tokyo, Japan).
Results
Baseline characteristics of 24
patients with PPI-resistant GERD
The 24 study patients included 79% (19/24) females
(Table I). All 24 patients underwent
EGD prior to vonoprazan therapy and none had a current H.
pylori infection. To evaluate the influence of erosions of the
esophagus despite prior PPI treatment, we divided patients into
erosive and non-erosive groups. An overall trend towards obesity
was recognized, especially in the erosive group. The degree of GERD
symptoms before starting vonoprazan were similar in both groups. Of
the 24 patients, 46% (11/24) had a history of H. pylori
eradication. Of the six patients in the erosive group, one, four
and one patient had Los Angeles (LA) grades A, B and C erosions,
respectively. The presence of hiatal hernia was significantly
higher in the erosive group than in the non-erosive group
(P=0.002). No patient had scleroderma.
 | Table I.Demographic data of patients with
proton pump inhibitor (PPI)-resistant gastroesophageal reflux
disease (GERD). |
Table I.
Demographic data of patients with
proton pump inhibitor (PPI)-resistant gastroesophageal reflux
disease (GERD).
| Variables | Total n = 24 | Erosive group n =
6 | Non-erosive group n =
18 | P-value |
|---|
| Age, years, mean ±
SD | 70.7 ± 9.6 | 67.3 ± 14.9 | 71.8 ± 6.6 | 0.347 |
| Sex, male, n (%) | 5 (21%) | 1 | 4 | 0.772 |
| Body mass index, mean
± SD | 24.5 ± 5.3 | 27.1 ± 6.5 | 23.9 ± 4.9 | 0.191 |
| Current smoker, n
(%) | 2
(8%) | 0 | 2 | 0.394 |
| Alcohol use,
>20g/day, n (%) | 3 (13%) | 0 | 3 | 0.285 |
| Severity of GERD,
Izumo scale score, mean ± SD | 5.8 ±
1.7 | 5.3 ± 1.5 | 5.9 ± 1.7 | 0.500 |
| PPI before starting
vonoprazan, n (EPZ 20 mg / LPZ 15 mg / OPZ 20 mg / RPZ 10 mg) | 13/4/2/5 | 3/1/0/2 | 10/3/2/3 |
| Prior use of
acotiamide, n | 5 (21%) | 1 | 4 | 0.772 |
| History of
Helicobactor pylori eradication, n (%) | 11 (46%) | 3 | 8 | 0.813 |
| Hiatal hernia, n
(%) | 9 (38%) | 6 | 3 | 0.002 |
| Grade of atrophy, n
(%) |
|
|
|
|
| None | 7 (29%) | 2 | 5 | 0.795 |
|
Closed-type | 9 (38%) | 3 | 6 | 0.465 |
|
Open-type | 8 (33%) | 1 | 7 | 0.317 |
Improvement and resolution rate of
GERD symptoms using vonoprazan 10 mg daily
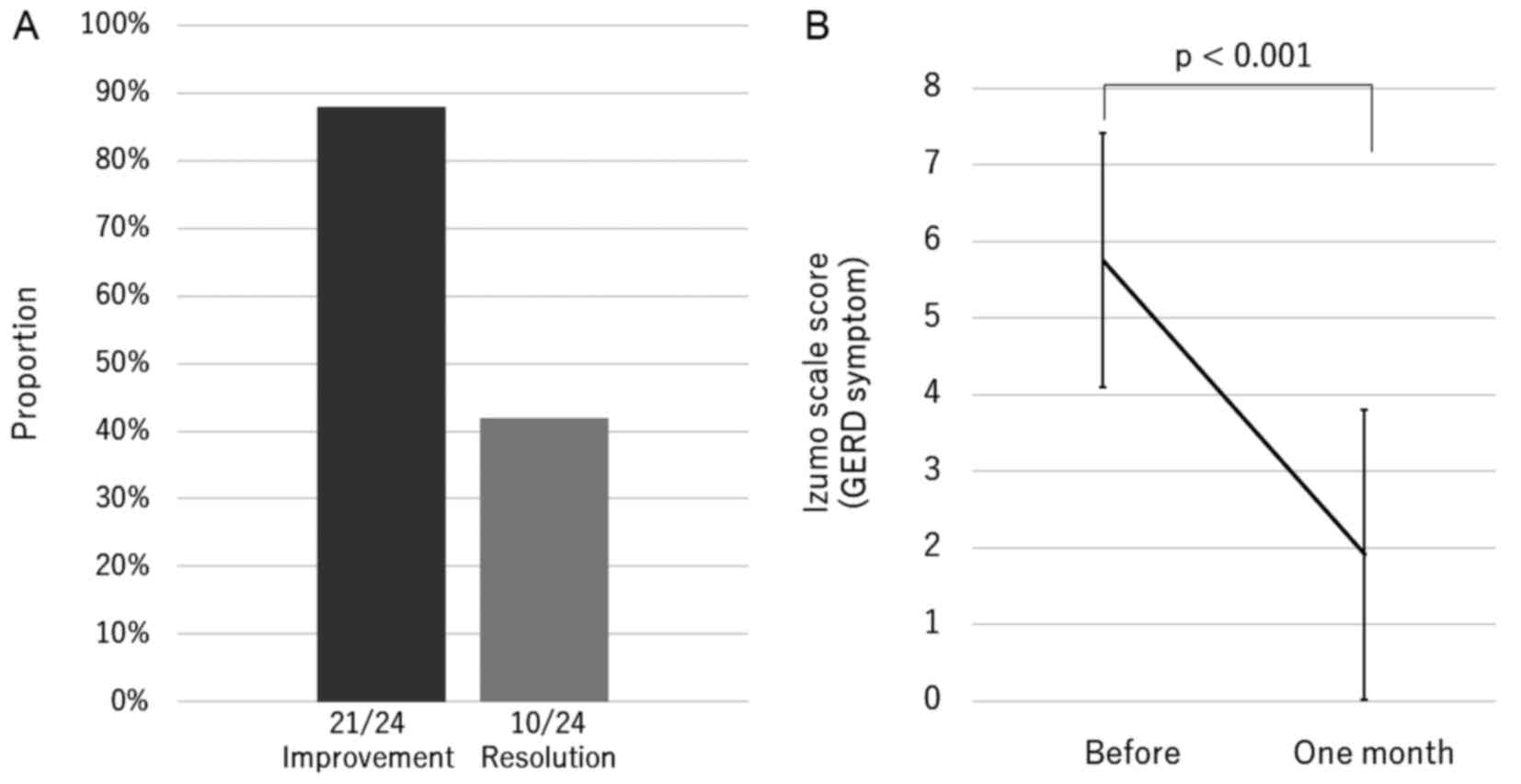
The overall rate of improvement and resolution of
GERD symptoms were 88 and 42%, respectively, and the score
significantly decreased to one-third of the pre-treatment value
(before 5.8±1.7 and one month 1.9±1.9, P<0.001) (Fig. 1). Vonoprazan 10 mg daily resulted in
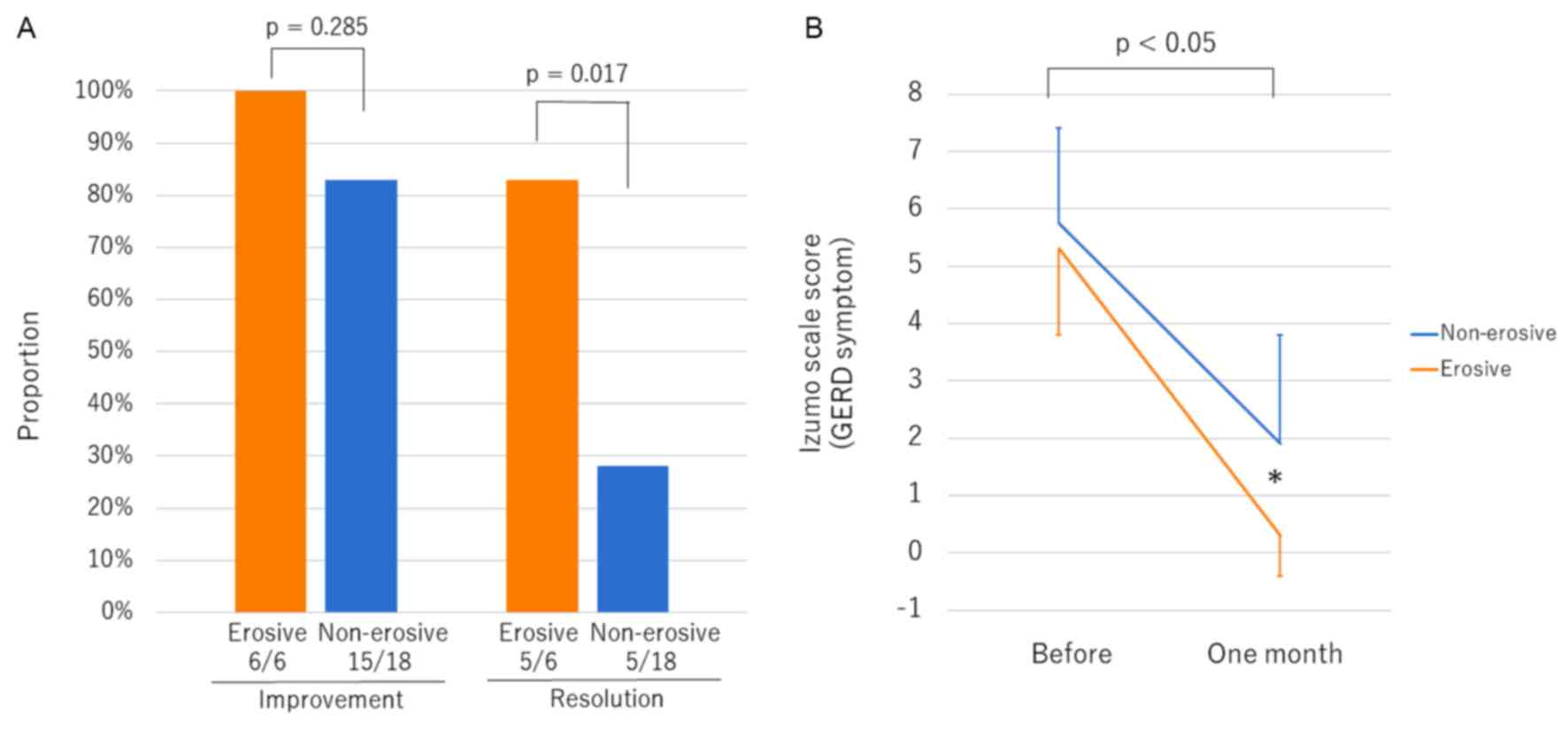
>80% improvement regardless of the degree of esophageal erosion
(Fig. 2A). Patients in the erosive
group showed significantly higher rates of resolution than those in
the non-erosive group (P=0.017). The score for GERD symptoms in the
erosive group after one month of vonoprazan therapy decreased to
almost zero, and the score was significantly lower than that of
patients in the non-erosive group (P=0.008) (Fig. 2B). No aggravation of symptoms or
adverse events were observed.
Vonoprazan effect on other GI symptoms
in patients with PPI-resistant GERD
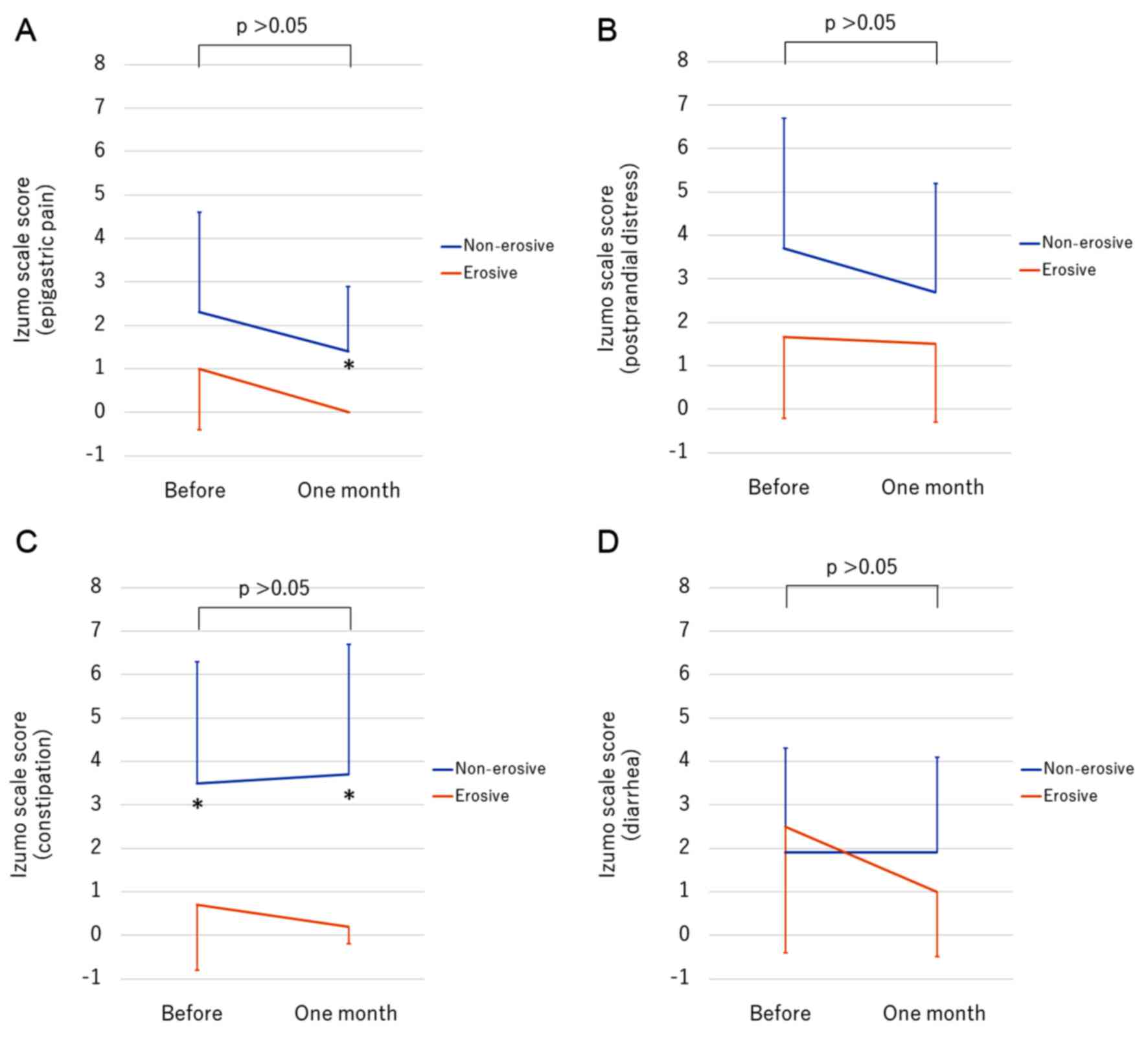
We evaluated other GI symptoms associated with GERD
before and after vonoprazan therapy. The scores for epigastric pain
comparing the erosive and the non-erosive groups were not
significantly different before therapy (P=0.191), but the scores
after treatment were significantly lower in the erosive group than
in the non-erosive group (P=0.025) (Fig.
3A). The scores for postprandial distress in the non-erosive
group showed a higher trend compared to the erosive group, without
a statistically significant difference (Fig. 3B). Although vonoprazan therapy did not
affect the score for constipation, the non-erosive group showed a
significantly higher score for constipation than the erosive group
both before and one month after starting vonoprazan (Fig. 3C). Vonoprazan therapy did not affect
the score for diarrhea (Fig. 3D).
Discussion
To the best of our knowledge, this is the first
report evaluating the efficacy of vonoprazan 10 mg in patients with
PPI-resistant GERD and other GI symptoms. Vonoprazan 10 mg resulted
in an 80% improvement regardless of the presence of erosions, and
had a significantly improved rate of resolution in patients in the
erosive group who had healed after adequate PPI treatment than the
non-erosive group.
Not limited to PPI-resistant GERD, patients with
erosions are more responsive to PPI therapy than those without
erosions in naïve GERD (15). The
presence of erosions despite standard PPI regimen suggests
insufficient gastric acid inhibition and thus stronger suppression
via an alternative mechanism is necessary. Unlike PPIs, acid
inhibition by vonoprazan is not influenced by the CYP2C19 genotype,
and its acid inhibition is superior to PPIs such as esomeprazole
(16). The present study shows that
vonoprazan 10 mg daily resulted in a 100% improvement and 83%
resolution of the GERD symptoms in patients with erosions. A recent
Japanese study reported an 88% (21/24) endoscopic healing rate by
vonoprazan 20 mg daily for four weeks in patients with
PPI-resistant reflux esophagitis (7).
The healing rate is similar to the resolution rate of GERD symptom
in the present study. Baseline LA classifications were mainly grade
B or greater (21/24, 88%) in their study (7) and 83% (5/6) in the present study. A
moderate or severe LA grade may exist frequently in patients with
PPI-resistant reflux esophagitis. Although the optimal dose of
vonoprazan for patients with erosions and PPI-resistant GERD
remains unclear, we believe that vonoprazan 10 mg daily is a viable
choice for patients with PPI-resistant GERD.
Few reports are available regarding the effect of
vonoprazan on patients with PPI-resistant GERD without erosions.
Despite an 83% improvement in the non-erosive group, the rate of
resolution (28%) is significantly lower than in the erosive group.
As a matter of course, non-erosive GERD is caused by heterogenous
conditions related not only to gastric acid but also GI motility
and sensitivity (15). The influence
of gastric acid is less in patients with non-erosive GERD than in
patients with erosive GERD, and strong acid inhibition by
vonoprazan may therefore be less effective in patients with
non-erosive GERD than in those with erosive GERD. Patients in the
non-erosive group in this study had a trend towards worse
epigastric pain, postprandial distress and constipation than those
in the erosive group. The presence of other GI symptoms may be
partially associated with the attenuated effect of vonoprazan in
patients without erosions. Additional use of prokinetic drugs or
cathartics may be suitable to resolve other symptoms related to
GERD in patients without erosions.
Japanese guidelines recommend a double-dose of PPI,
additional use of prokinetic drugs, histamine-2 receptor antagonist
(H2RA) or herbal medicine for the treatment of patients with
PPI-resistant GERD (2). A randomized
controlled trial reported that dose escalation of rabeprazole
improved endoscopic mucosal healing in patients with PPI-resistant
GERD with erosions (17). A
multicenter observational study reported that double-dose PPI
improved long-term QOL in patients with PPI-resistant GERD with
erosions (18). Therefore, strong acid
suppression is effective and justified for patients with
PPI-resistant GERD with erosions. Although the addition of bedtime
H2RA is effective for nocturnal acid breakthrough in the
short-term, its effect cannot be maintained because of H2RA
tolerance (19). Since vonoprazan
maintains the gastric pH level for 24 h, vonoprazan may be a
promising acid suppressor for the treatment of nocturnal acid
breakthrough (5). Vonoprazan directly
inhibits H+-K+ exchange on the gastric
luminal surface via an alternative mechanism, unlike PPIs, and its
effect is not influenced by the CYP2C19 genotype (16). Therefore, vonoprazan may be useful for
patients with PPI-resistant GERD, especially in patients who
rapidly metabolize these medications. Furthermore, vonoprazan may
decrease the need for anti-reflux surgery in patients with
PPI-resistant GERD.
There are some acknowledged limitations in the
present study. First, this is a single-center retrospective study
with a small number of patients. Second, patients and doctors were
not blinded to the therapy used. Third, the variety of PPIs used
before vonoprazan therapy were not homogeneous but a standard dose.
Fourth, the presence of the CYP2C19 genotype was not investigated.
A large prospective study is necessary to clarify the optimal dose
of vonoprazan for patients with erosive and non-erosive
PPI-resistant GERD.
In conclusion, this retrospective cohort study shows
satisfactory effectiveness of vonoprazan 10 mg daily in patients
with PPI-resistant GERD. Vonoprazan resolves GERD symptoms in
patients with erosions rather than those without erosions. Since
previous studies only used vonoprazan 20 mg daily, to the best of
our knowledge, this is the first study to describe the effect of
vonoprazan 10 mg in patients with PPI-resistant GERD. We believe
that vonoprazan 10 mg daily may become a first-line treatment for
patients with PPI-resistant GERD, but further study is
necessary.
Glossary
Abbreviations
Abbreviations:
|
GERD
|
gastroesophageal reflux disease
|
|
QOL
|
quality of life
|
|
PPI
|
proton pump inhibitor
|
|
GI
|
gastro-intestinal
|
|
H2RA
|
histamine-2 receptor antagonist
|
|
EGD
|
esophagogastro-duodenoscopy
|
|
LA
|
Los Angeles
|
References
|
1
|
Dimenäs E: Methodological aspects of
evaluation of Quality of Life in upper gastrointestinal diseases.
Scand J Gastroenterol Suppl. 199:18–21. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Iwakiri K, Kinoshita Y, Habu Y, Oshima T,
Manabe N, Fujiwara Y, Nagahara A, Kawamura O, Iwakiri R, Ozawa S,
et al: Evidence-based clinical practice guidelines for
gastroesophageal reflux disease 2015. J Gastroenterol. 51:751–767.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Suzuki H, Matsuzaki J, Okada S, Hirata K,
Fukuhara S and Hibi T: Validation of the GerdQ questionnaire for
the management of gastro-oesophageal reflux disease in Japan.
United European Gastroenterol J. 1:175–183. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Toghanian S, Johnson DA, Stålhammar NO and
Zerbib F: Burden of gastro-oesophageal reflux disease in patients
with persistent and intense symptoms despite proton pump inhibitor
therapy: A post hoc analysis of the 2007 national health and
wellness survey. Clin Drug Investig. 31:703–715. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sakurai Y, Mori Y, Okamoto H, Nishimura A,
Komura E, Araki T and Shiramoto M: Acid-inhibitory effects of
vonoprazan 20 mg compared with esomeprazole 20 mg or rabeprazole 10
mg in healthy adult male subjects-a randomised open-label
cross-over study. Aliment Pharmacol Ther. 42:719–730. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Shinozaki S, Nomoto H, Kondo Y, Sakamoto
H, Hayashi Y, Yamamoto H, Lefor AK and Osawa H: Comparison of
vonoprazan and proton pump inhibitors for eradication of
Helicobacter pylori. Kaohsiung J Med Sci. 32:255–260. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hoshino S, Kawami N, Takenouchi N, Umezawa
M, Hanada Y, Hoshikawa Y, Kawagoe T, Sano H, Hoshihara Y, Nomura T,
et al: Efficacy of vonoprazan for proton pump inhibitor-resistant
reflux esophagitis. Digestion. 95:156–161. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kakuta E, Yamashita N, Katsube T,
Kushiyama Y, Suetsugu H, Furuta K and Kinoshita Y: Abdominal
symptom-related QOL in individuals visiting an outpatient clinic
and those attending an annual health check. Intern Med.
50:1517–1522. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kimura K and Takemoto T: An endoscopic
recognition of the atrophic border and its significance in chronic
gastritis. Endoscopy. 1:87–97. 1969. View Article : Google Scholar
|
|
10
|
Shinozaki S, Osawa H, Sakamoto H, Hayashi
Y, Lefor A Kawarai and Yamamoto H: The effect of acotiamide on
epigastric pain syndrome and postprandial distress syndrome in
patients with functional dyspepsia. J Med Invest. 63:230–235. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yoshioka T, Okimoto N, Okamoto K and Sakai
A: A comparative study of the effects of daily minodronate and
weekly alendronate on upper gastrointestinal symptoms, bone
resorption, and back pain in postmenopausal osteoporosis patients.
J Bone Miner Metab. 31:153–160. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Okimoto E, Ishimura N, Morito Y, Mikami H,
Shimura S, Uno G, Tamagawa Y, Aimi M, Oshima N, Kawashima K, et al:
Prevalence of gastroesophageal reflux disease in children, adults,
and elderly in the same community. J Gastroenterol Hepatol.
30:1140–1146. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kinoshita Y and Chiba T: FUTURE study
group: Therapeutic effects of famotidine on chronic symptomatic
gastritis: Subgroup analysis from FUTURE study. J Gastroenterol.
47:377–386. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kinoshita Y and Chiba T: FUTURE study
group: Characteristics of Japanese patients with chronic gastritis
and comparison with functional dyspepsia defined by ROME III
criteria: Based on the large-scale survey, FUTURE study. Intern
Med. 50:2269–2276. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tack J and Fass R: Review article:
approaches to endoscopic-negative reflux disease: part of the GERD
spectrum or a unique acid-related disorder? Aliment Pharmacol Ther
19. Suppl 1:28–34. 2004. View Article : Google Scholar
|
|
16
|
Kagami T, Sahara S, Ichikawa H, Uotani T,
Yamade M, Sugimoto M, Hamaya Y, Iwaizumi M, Osawa S, Sugimoto K, et
al: Potent acid inhibition by vonoprazan in comparison with
esomeprazole, with reference to CYP2C19 genotype. Aliment Pharmacol
Ther. 43:1048–1059. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kinoshita Y and Hongo M: Japan TWICE study
group: Efficacy of twice-daily rabeprazole for reflux esophagitis
patients refractory to standard once-daily administration of PPI:
The Japan-based TWICE study. Am J Gastroenterol. 107:522–530. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kinoshita Y, Hongo M, Kusano M, Furuhata
Y, Miyagishi H and Ikeuchi S: RPZ Study Group: Therapeutic response
to twice-daily Rabeprazole on health-related quality of life and
symptoms in patients with refractory reflux esophagitis: A
multicenter observational study. Intern Med. 56:1131–1139. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Fackler WK, Ours TM, Vaezi MF and Richter
JE: Long-term effect of H2RA therapy on nocturnal gastric acid
breakthrough. Gastroenterology. 122:625–632. 2002. View Article : Google Scholar : PubMed/NCBI
|