Introduction
Dimethylsulphoxide (DMSO) is an organosulfur
compound, with the chemical formula C2H6OS,
which is widely used in the biology and medical fields (1–3). DMSO is
reported to possess anti-inflammatory and antioxidative capacities
(1,2). It
was shown that DMSO attenuated acute lung injury induced by
hemorrhagic shock (1). Moreover,
topical application of DMSO mitigated arthritis in animal models
from the reduction of pro-inflammatory cytokines in the joint
(2). Analgesic and local anesthetic
activity of DMSO has also been previously reported (3). DMSO is applied as a cryoprotective agent
to reduce ice crystal artifacts (4).
Most commonly, DMSO is widely used as a chemical solvent, and it is
known to be miscible in a wide range of organic solvents along with
water (5).
At present, stem cells are of great interest in
medicine, including their use in cell therapy and regeneration
fields (6). In a previous study, we
identified stem cells from human gingival (7). It was shown that stem cells derived from
gingiva had characteristics of stemness, including multipotency
with a high proliferation rate. More recently, our group fabricated
stem cell spheroids using the silicon elastomer-based concave
microwells (8). The effects of various
concentrations of DMSO on stem cells derived from intraoral area
are not known yet.
Thus, the present study evaluated the effects of
different concentrations of DMSO on the cell morphology, viability,
mRNA, and protein expression of stem cells derived from the
intraoral area, and found potential detrimental effects.
Materials and methods
Stem cells isolated from human
gingiva
The gingiva were obtained from healthy patients
visiting the Department of Periodontics, Seoul St. Mary's Hospital,
College of Medicine, The Catholic University of Korea (Seoul,
Korea). The Institutional Review Board reviewed and approved the
study (KC11SISI0348). All the procedures performed in studies
involving human participants were in accordance with the ethical
standards of the institutional and/or national research committee
and with the 1964 Helsinki Declaration and its later amendments or
comparable ethical standards. Informed consent was obtained from
the participants. All of the methods were performed in accordance
with the relevant guidelines and regulations. The gingiva were
placed in sterile phosphate-buffered saline (Welgene, Daegu, South
Korea) containing 100 U/ml penicillin and 100 µg/ml streptomycin
(Sigma-Aldrich, St. Louis, MO, USA). The epithelium of the obtained
tissue was removed, and the tissue was minced into 1–2 mm
fragments. The tissues were digested with media containing dispase
(1 mg/ml; Sigma-Aldrich) and collagenase IV (Sigma-Aldrich). The
cells were incubated in an environment with 5% CO2 and
95% O2 at 37°C in an incubator. Cells that were not
attached to the culture dish were removed, and the medium was
changed every 2–3 days.
Evaluation of cell morphology
The cells were plated in 96-well plates at a density
of 2.0×103 cells/well. The cells were incubated in
control medium (α-minimal essential medium (Gibco, Grand Island,
NY, USA) supplemented with 15% fetal bovine serum (Gibco), 200 mM
L-glutamine (Sigma-Aldrich), 10 mM of ascorbic acid 2-phosphate
(Sigma-Aldrich) in the presence of DMSO (Sigma-Aldrich) at final
concentrations of 0, 0.01, 0.1, 1, 3 and 10%. On days 1, 3, 5, 7
and 10, inverted microscopy (CKX41SF; Olympus Corp., Tokyo, Japan)
was used to evaluate the morphology of the tested stem cells.
Cell viability
Evaluation of the viability of the cells grown in
control medium was performed on days 1, 3, 5 and 7 with the Cell
Counting Kit-8 (CCK-8; Dojindo Molecular Technologies, Inc., Tokyo,
Japan) assay. Tetrazolium monosodium salt was added to the culture,
and the cells were incubated at 37°C for 2 h. A microplate reader
(BioTek Instruments Inc., Winooski, VT, USA) was used to find the
spectrophotometric absorbance at 450 nm. The tests were performed
in triplicate.
Immunofluorescence
An immunofluorescent assay was performed for
Runt-related transcription factor 2 (Runx2) (ab76956; Abcam,
Cambridge, UK) and collagen I (ab6308; Abcam) on days 1, 3, 5 and
7. The cells were fixed, permeabilized, blocked, and incubated with
primary antibodies. Mouse monoclonal Runx2 antibody was diluted at
1:50 and was incubated overnight incubation at 4°C. Mouse
monoclonal collagen I antibody was diluted at 1:67 and was
incubated overnight incubation at 4°C. The cultures were incubated
with fluorescein isothiocyanate-conjugated secondary antibody
(F2761; Thermo Fisher Scientific, Inc., Waltham, MA, USA) diluted
at 1:100 and incubated for 2 h at room temperature. The washed
cells were stained with 4′,6-diamidino-2-phenylindole (DAPI).
Analyses were performed using a fluorescence microscope (Axiovert
200; Zeiss GmbH, Jena, Germany).
Total RNA extraction and
quantification by quantitative polymerase chain reaction
(qPCR)
On day 11, isolation of total RNA was performed from
the cells grown in control medium on day 11 using a GeneJET RNA
Purification kit (Thermo Fisher Scientific, Inc., Waltham, MA,
USA). Quantities were determined using a spectrophotometer
(ND-2000; Thermo Fisher Scientific, Inc., Wilmington, DE, USA) with
ratios of absorbance at 260 and 280 nm.
The sense and antisense primers were designed based
on GenBank. The primer sequences used were: Collagen I, forward
5′-TCATGGCCCTCCAGCCCCCAT-3′; and reverse
5′-ATGCCTCTTGTCCTTGGGGTTC-3′; Runx2, forward
5′-AATGATGGTGTTGACGCTGA-3′; and reverse 5′-TTGATACGTGTGGGATGTGG-3′.
β-actin served as a housekeeping gene for normalization. mRNA
expression was detected by qPCR using SYBR-Green Real-Time PCR
Master Mixes (Enzynomics, Daejeon, South Korea) based on the
manufacturer's protocol. The experiments were performed in
triplicate.
Western blot analysis
On day 10, lysis buffer (Thermo Fisher Scientific,
Inc., Waltham, MA, USA) containing protease and phosphatase
inhibitor cocktail (Thermo Fisher Scientific, Inc.) as well as
phenylmethylsulfonyl fluoride (Sigma-Aldrich) was used as
solubilizing agent. The lysates were quantified using the BCA assay
(Thermo Fisher Scientific, Inc.). Protein samples were separated
and then transferred for immunoblotting. The membranes were
incubated with the primary antibodies overnight at 4°C, and then
with the secondary antibody for 1 h at room temperature. The
antibodies included those against collagen I, Runx2, and GAPDH, as
well as secondary antibodies linked with horseradish peroxidase.
The antibodies were purchased from Abcam and BD Bioscience (San
Jose, CA, USA).
Statistical analysis
Data were presented as the means ± standard
deviations of the experiments. The Shapiro-Wilk test was used to
test for normality. A one-way analysis of variance with a post-hoc
test was performed to determine the differences between the groups
using a commercially available program (SPSS 12 for Windows; SPSS,
Inc., Chicago, IL, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
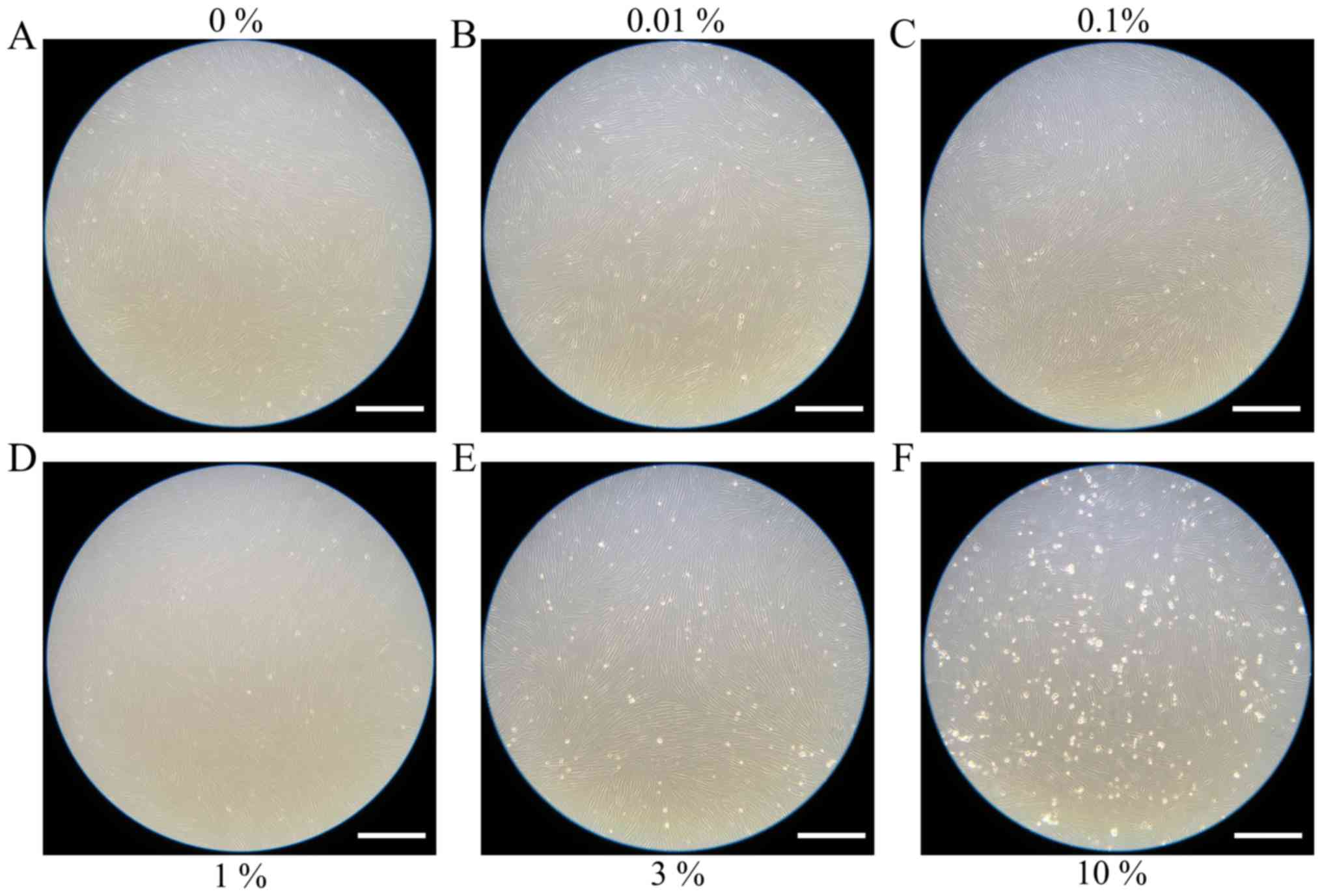
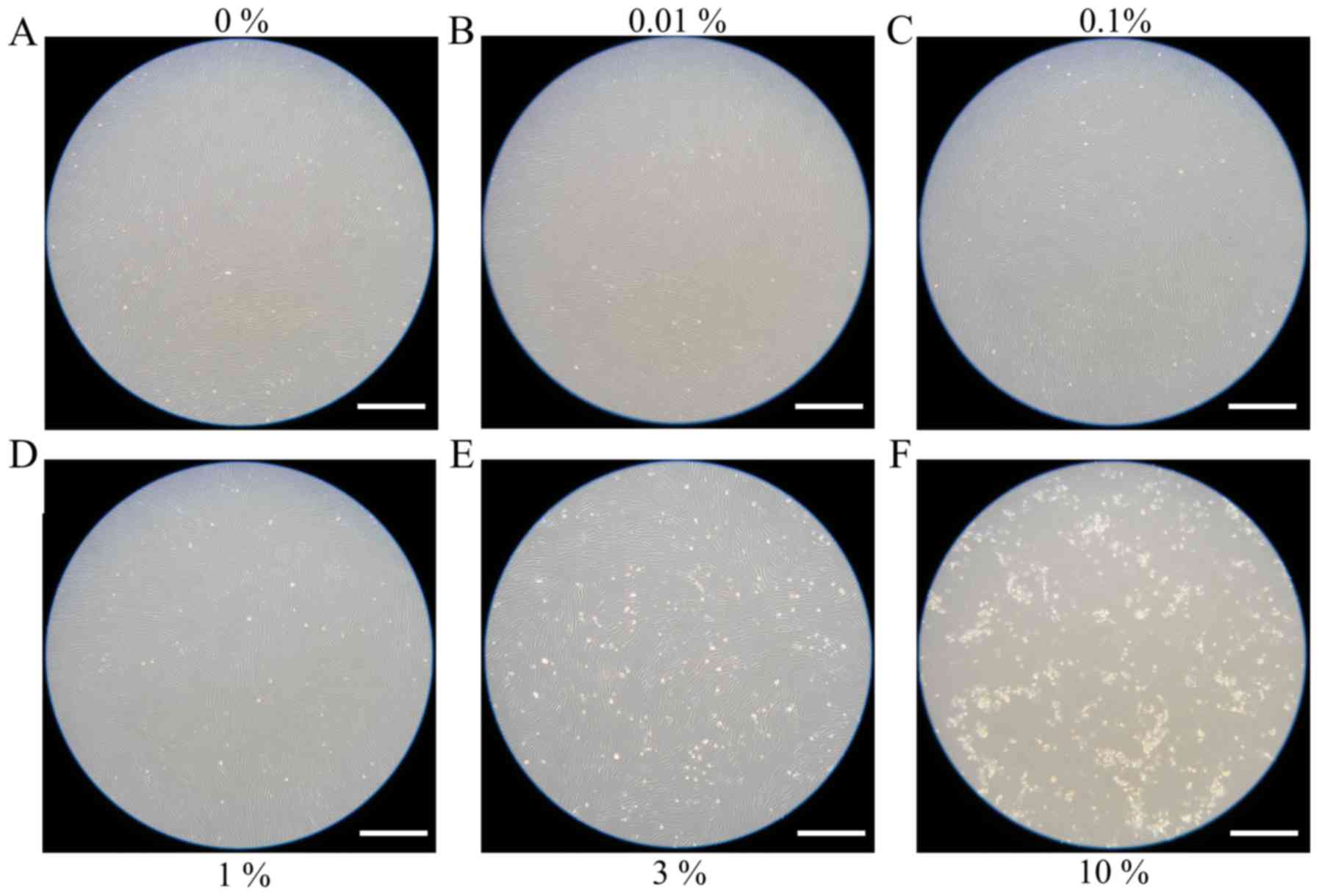
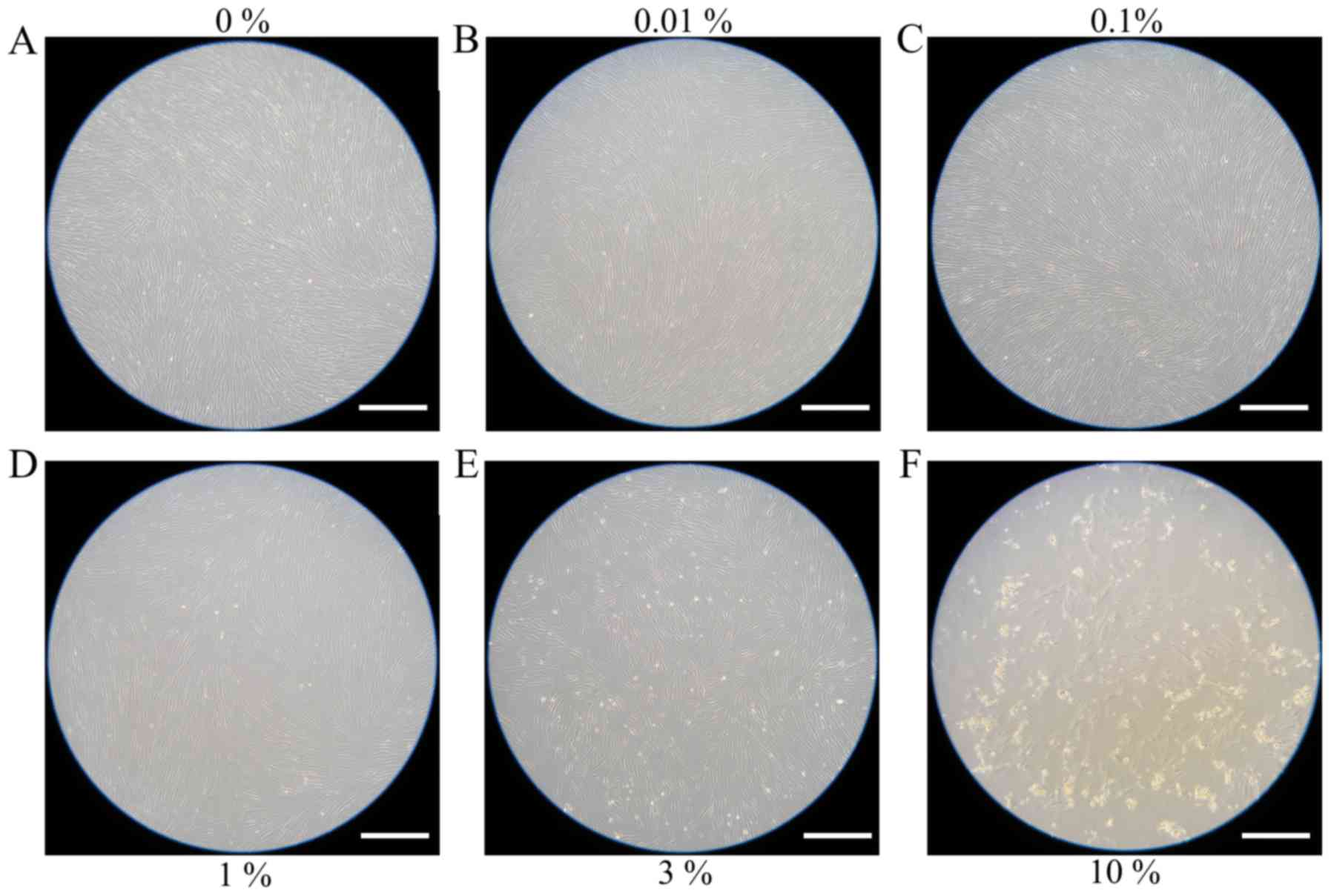
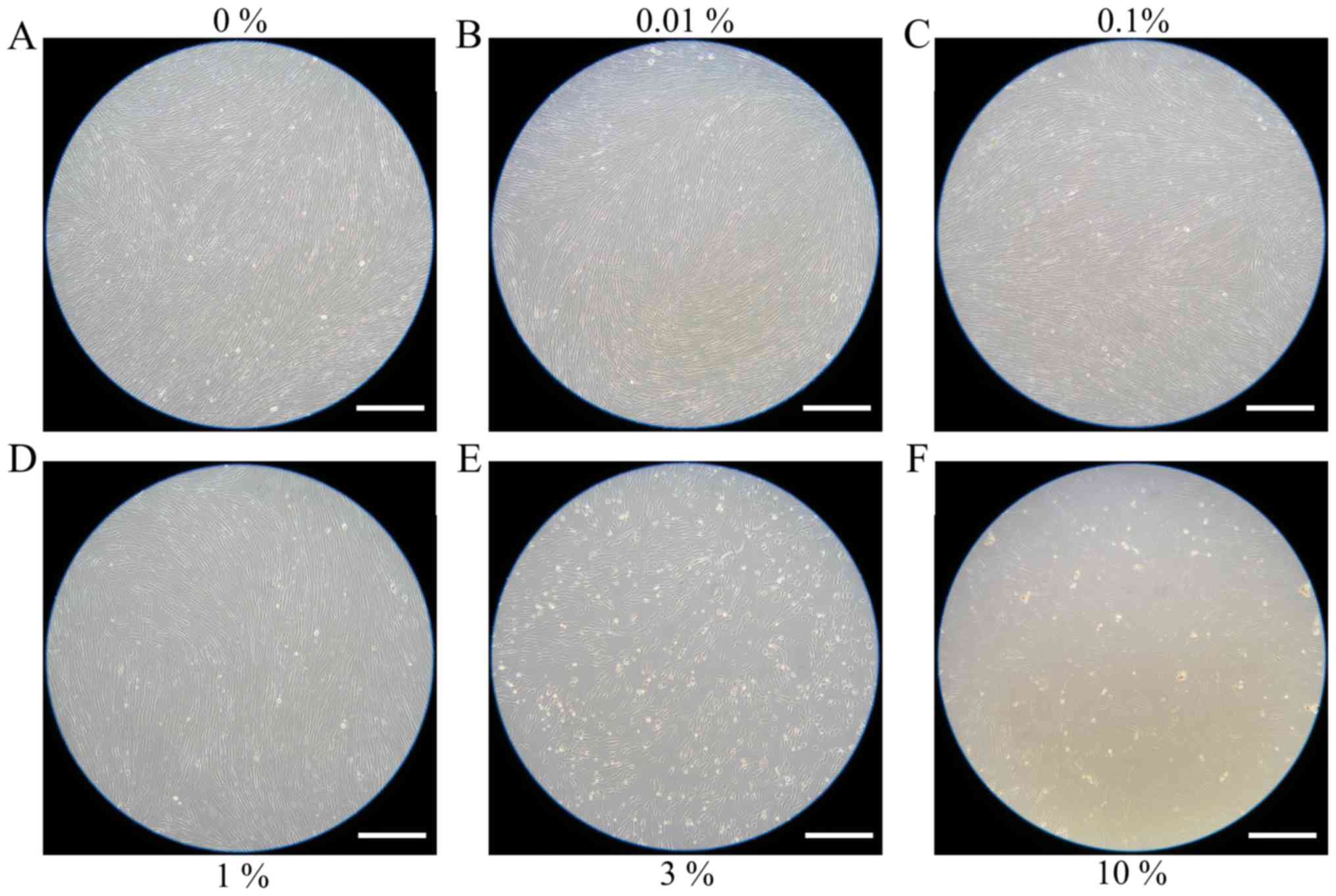
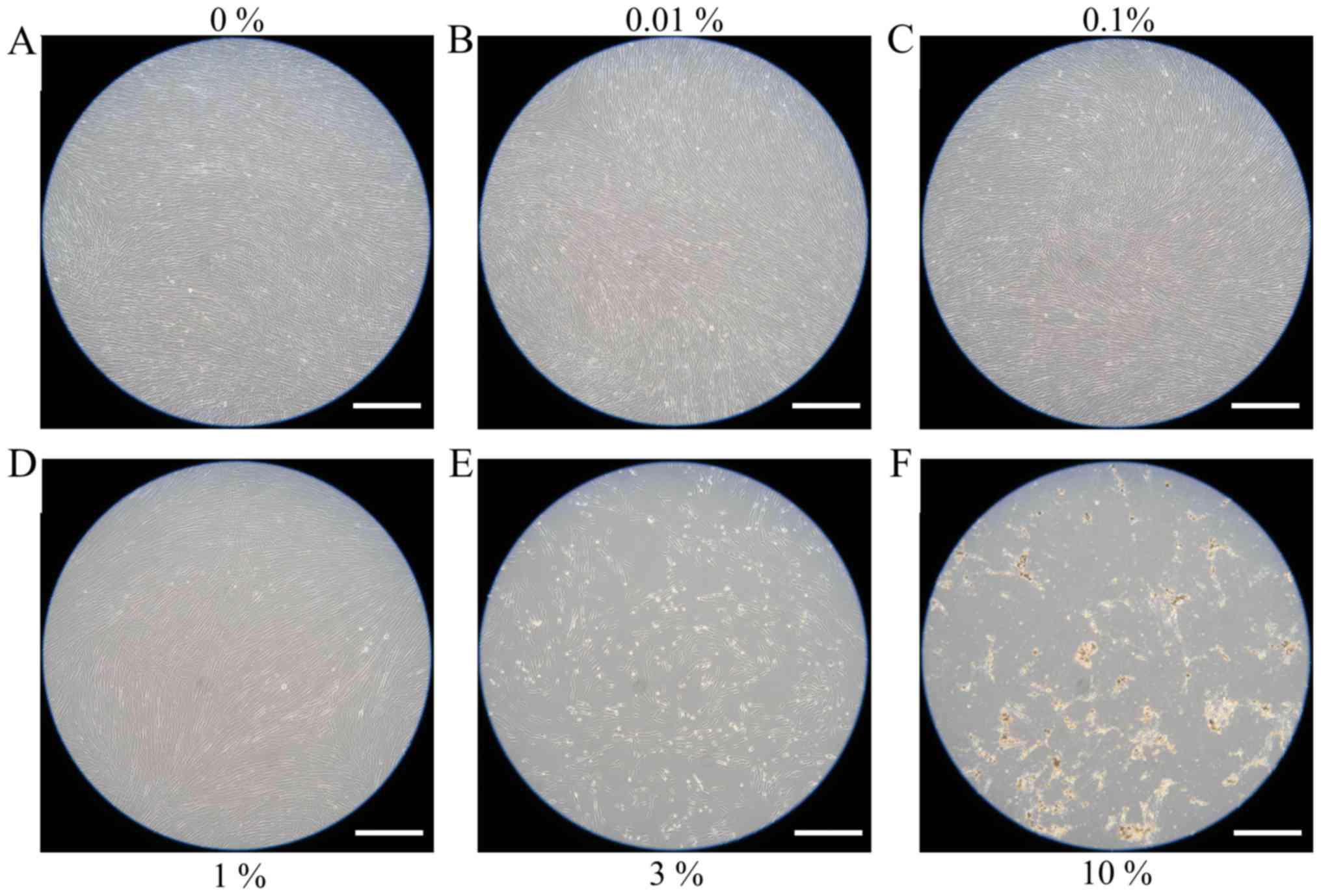
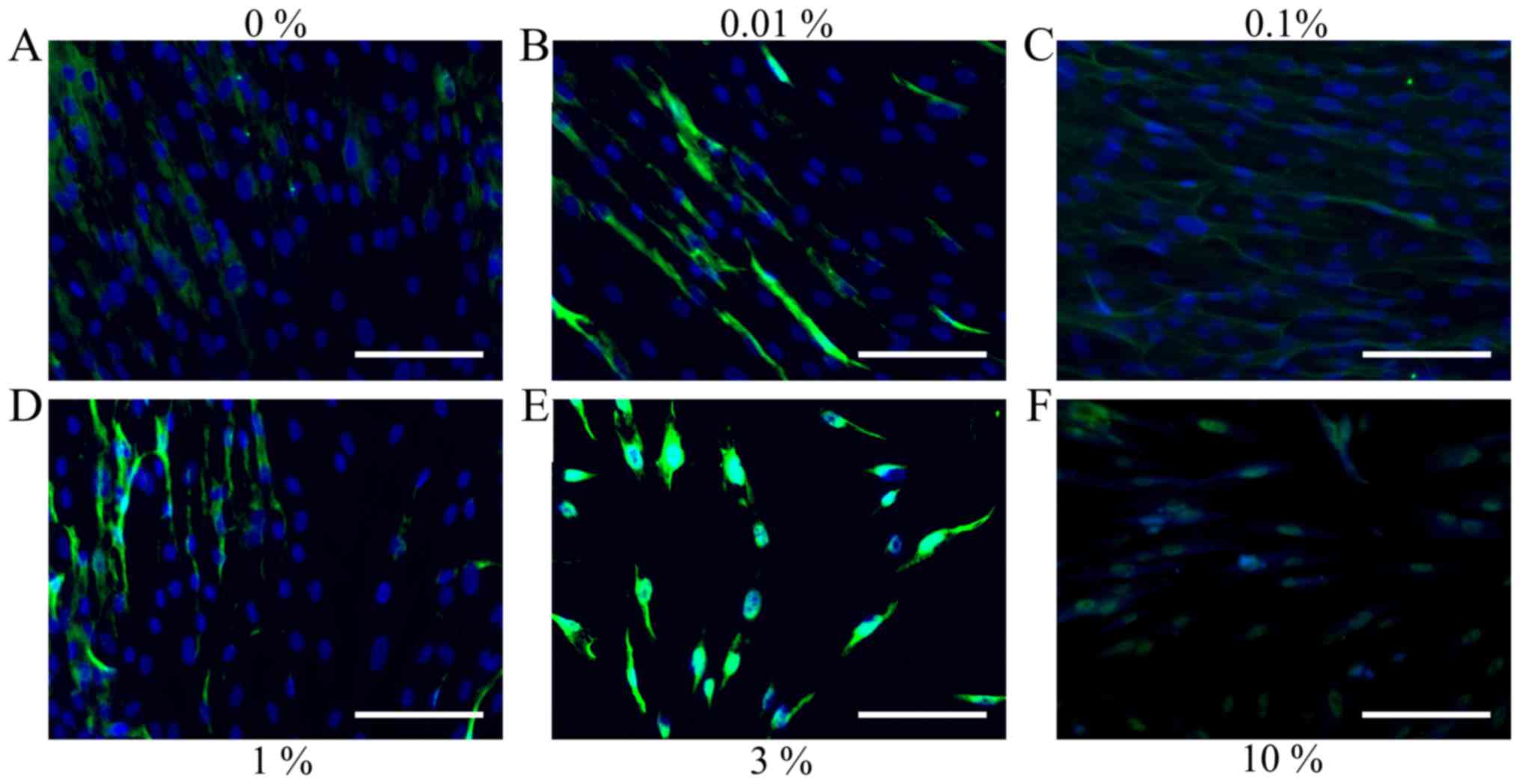
Evaluation of cell morphology
Cells in the control group showed normal fibroblast
morphology in growth media on day 1 (Fig.
1). The shape of cells for the 0.01, 0.1, 1, 3 and 10% groups
were similar to those in the control group. Cell morphology results
for days 3 and 5 are shown in Figs. 2
and 3, respectively. The shape of the
cells was similar to day 1. Cell morphology results for days 7 and
10 are shown in Figs. 4 and 5, respectively. The shape of the cells was
similar to day 1, however, cells from the 3 and 10% groups were
significantly different compared to the others. There were fewer
cells in the 3 and 10% groups and they were more round in
appearance.
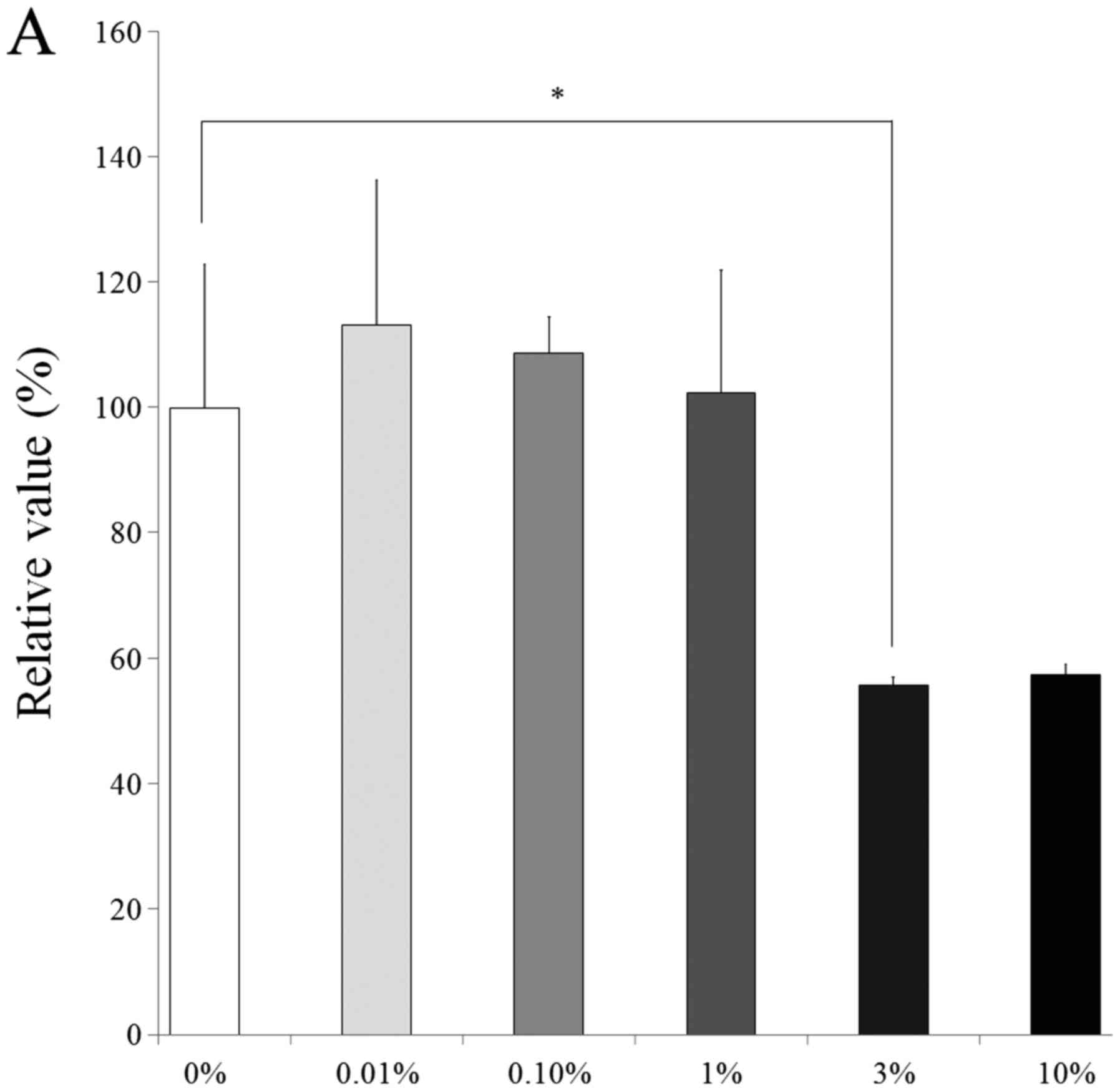
Cell viability
Results from the CCK-8 assay revealed cell viability
on days 1, 3, 5 and 7 (Fig. 6). The
relative values of CCK-8 at day 1 for 0.01, 0.1, 1, 3 and 10% were
113.2±23.2, 108.8±5.8, 102.4±19.6, 55.7±1.4 and 57.3±1.8%,
respectively, when the control (0% group) at day 1 was considered
100% (100.0±22.8%). The relative values of CCK-8 at day 7 for 0.01,
0.1, 1, 3 and 10% were 106.5±15.0, 109.1±19.9, 88.2±9.1, 67.1±4.7,
78.8±3.7, respectively, when the control (0% group) at day 7 is
considered 100% (100.0±1.2%).
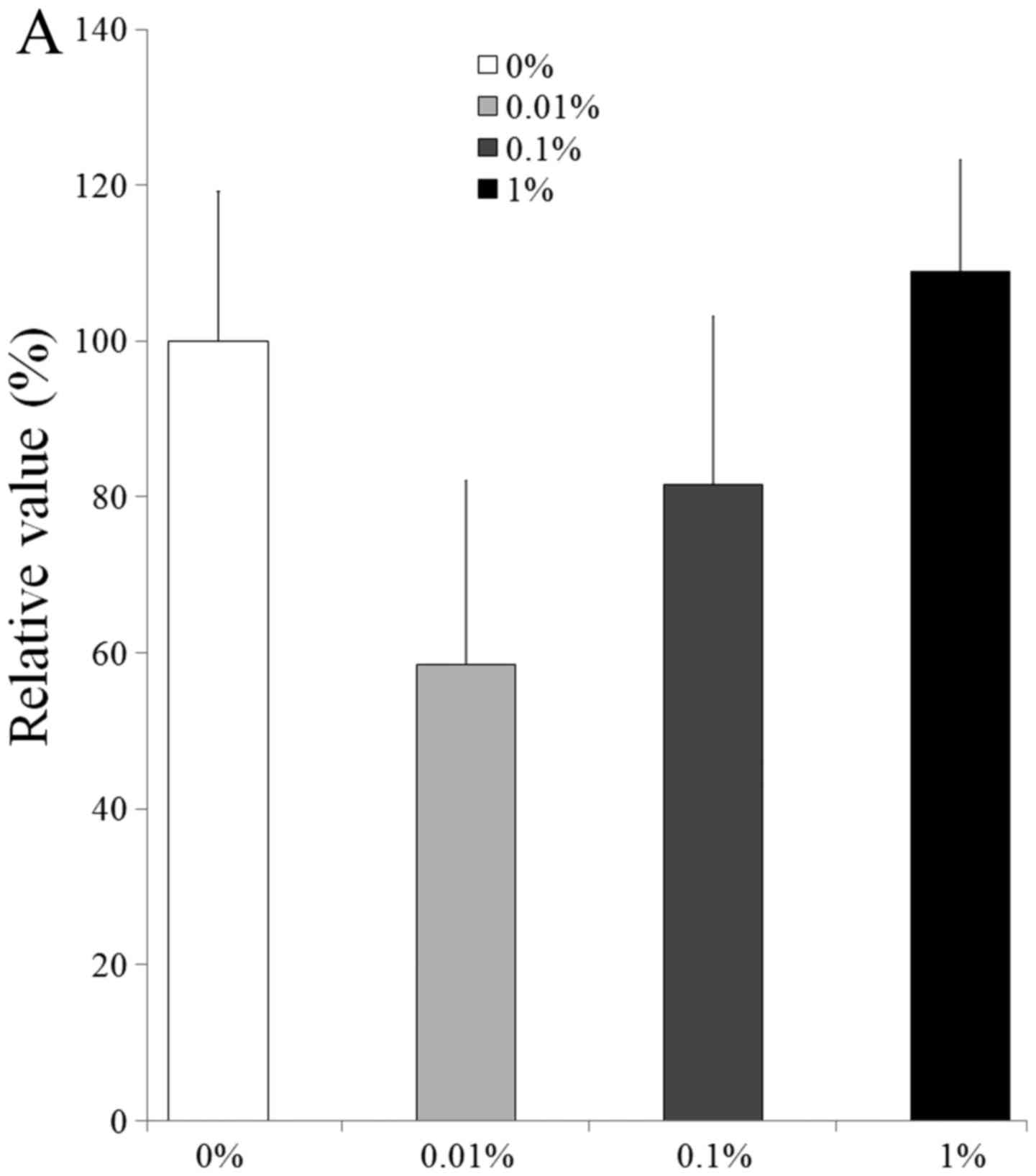
Validation of mRNA expression by
qPCR
The qPCR results for the mRNA levels of collagen I
and Runx2 are shown in Fig. 7. The
relative expression of collagen I in the control medium at day 11
for the 0, 0.01, 0.1 and 1% groups was 100.0±19.2, 58.4±23.6,
81.6±21.6 and 109.0±14.3%, respectively (Fig. 7A). The relative expression of Runx2 at
day 11 for the 0, 0.01, 0.1 and 1% groups was 100.0±14.4,
82.4±33.9, 121.2±6.6 and 174.1±39.7%, respectively (Fig. 7B).
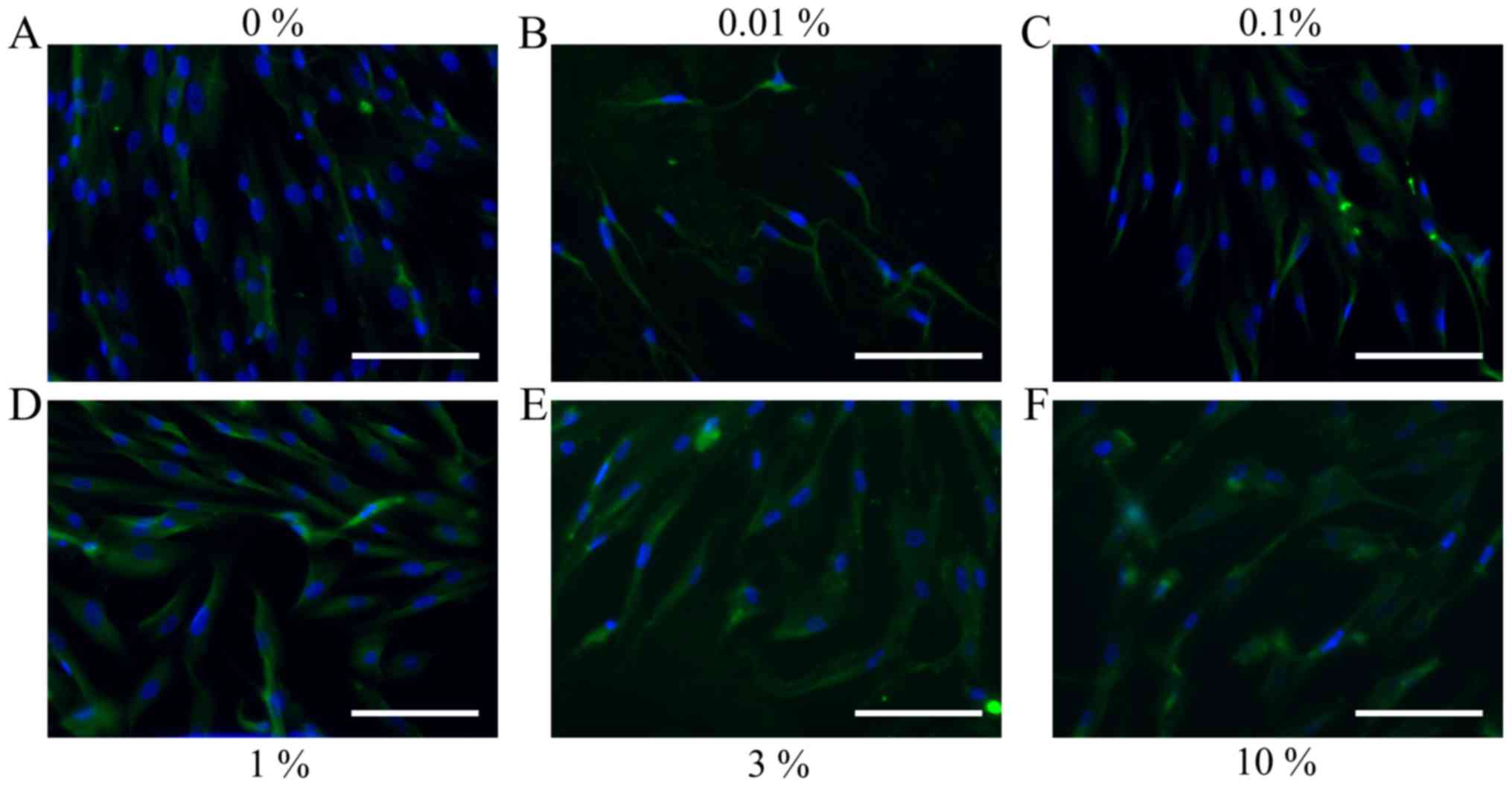
Immunofluorescence
The immunofluorescent assays for collagen I and
Runx2 for days 1, 3, 5 and 7 are shown in Figs. 8–15. A
significant change in collagen I expression was noted at a higher
concentration of the DMSO groups. The expression of Runx2 seemed to
show similar trends: there were notable changes with increasing
doses of DMSO.
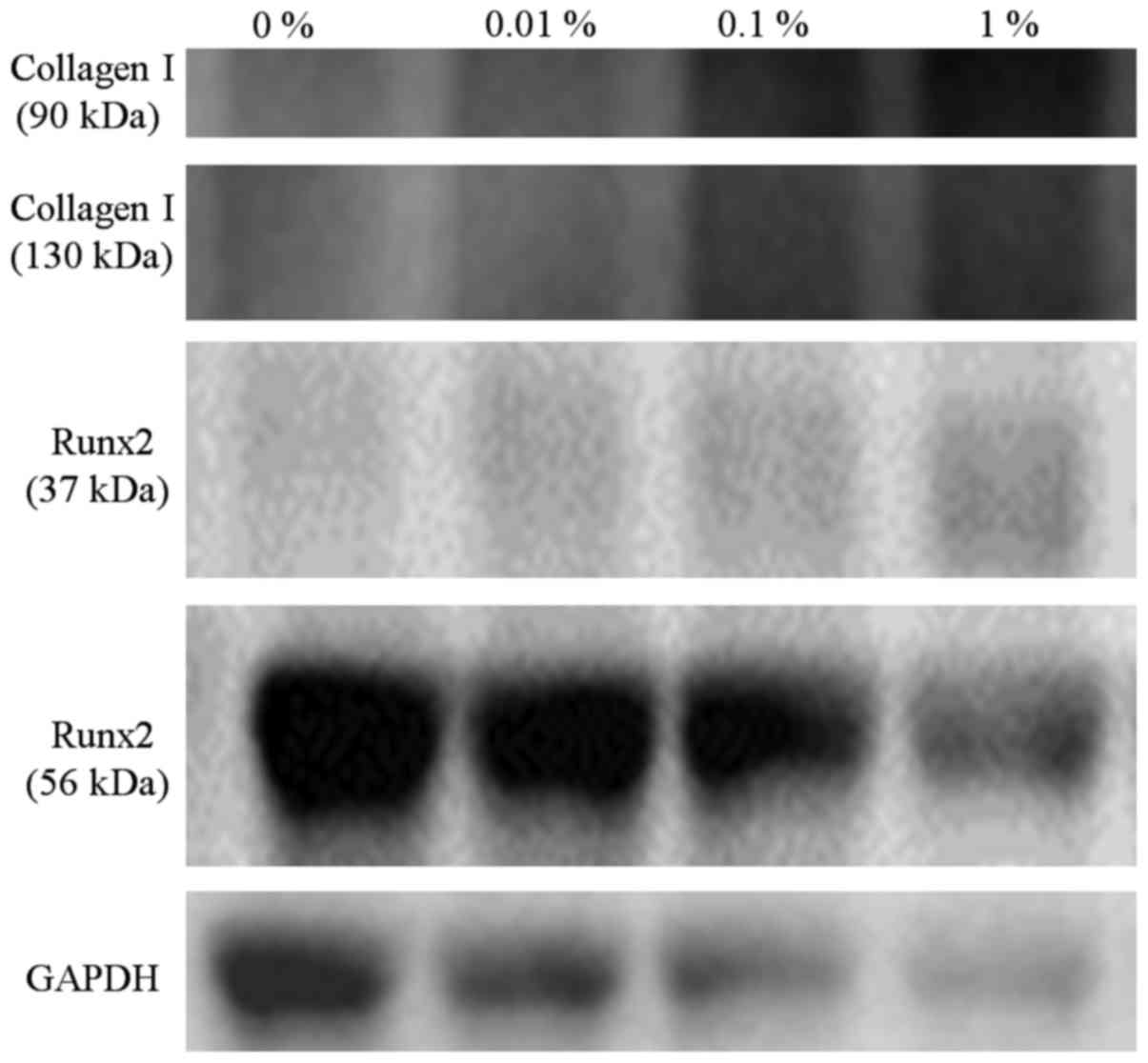
Western blot analysis
A western blot analysis was performed to detect the
protein expression of collagen I Runx2, and GAPDH at day 10
(Fig. 16). The relative expression of
collagen I (90 kDa) in growth media at day 10 for the 0, 0.01, 0.1
and 1% groups was 100.0, 136.0, 291.4 and 499.6%, respectively. The
relative expression of collagen I (130 kDa) in growth media at day
10 for the 0, 0.01, 0.1 and 1% groups was 100.0, 154.6, 468.0 and
769.2%, respectively.
The relative expression of Runx2 (37 kDa) in growth
media at day 10 for the 0, 0.01, 0.1 and 1% groups was 100.0,
137.2, 182.3 and 341.0%, respectively. The relative expression of
Runx2 (56 kDa) in growth media at day 10 for the 0, 0.01, 0.10 and
1% groups was 100.0, 91.5, 99.2 and 82.2%, respectively.
Discussion
This study tested the effects of the differential
concentration of DMSO on gingiva-derived stem cells and it was
shown that decreased cell viability along with the changes in
morphology were seen at higher concentrations. The change in mRNA
and protein expression of collagen I and Runx2 was also
identified.
Stem cells may be obtained from various tissues,
including bone marrow and fat (9).
Bone marrow-derived stem cells are widely used in medical fields in
applications including the regeneration of cartilage tissue
(9,10).
Adipose tissue is suggested to be an abundant and accessible source
of adult mesenchymal stem cells (11).
However, obtaining bone marrow-derived and adipose-derived stem
cells may produce issues regarding morbidity and pain (11,12).
However, obtaining stem cells from the intraoral area may be
attractive because the procedure can be performed under local
anesthesia (13). Gingival tissue can
be obtained during the daily dental practice and the gingival
tissue can be obtained several times with minimal morbidity
(14).
In the present study, we determined the effects of
DMSO on the morphology and viability of cells under predetermined
concentrations (0.01 to 10%). No significant changes of cell
morphology were noted in the low concentration. However, in the
higher concentrations of 3 and 10%, there were fewer cells with
rounder shapes. In the lower concentration of 0.01%, the cellular
viability was increased, but the treatment with higher
concentrations of DMSO of 3 and 5% resulted in a noticeable
decrease in cellular viability. Similarly, in the previous report,
low doses of DMSO showed the protective effects against
acetaminophen-induced liver injury (15). In the experimental design, DMSO is
widely used as a dissolving agent and an equal amount of DMSO is
applied to each sample to offset the effects of DMSO as a
dissolving agent (16). It should be
emphasized that the culture without a dissolving agent should be
used as a negative control, as the effect of DMSO can be falsely
attributed to the agents applied.
In this study, a CCK-8 assay, which is based on the
evaluation of mitochondrial activity, is used for the evaluation of
cellular viability (17). A qPCR and a
western blot analysis were performed to detect the mRNA and protein
expression of collagen I and Runx2 to achieve information on the
possible mechanisms. Collagen I is considered the most abundant
structural protein among the numerous types of collagen (18). Collagen I is shown to be involved in
biological events, including cell attachment, cell proliferation,
and remodeling (19).
Immunofluorescent data clearly showed that collagen I expression
was significantly reduced in the higher DMSO concentration.
Based on these findings, it was concluded that DMSO
could produce detrimental effects on the cellular morphology and
cellular viability of mesenchymal stem cells. Our results also
suggested that DMSO has toxic effects via reduced collagen I
expression.
Acknowledgements
This study was supported by Basic Science Research
Program through the National Research Foundation of Korea (NRF)
funded by the Ministry of Science, Information and Communication
Technology and Future Planning (NRF-2017R1A1A1A05001307).
References
|
1
|
Tsung YC, Chung CY, Wan HC, Chang YY, Shih
PC, Hsu HS, Kao MC and Huang CJ: Dimethyl sulfoxide attenuates
acute lung injury induced by hemorrhagic shock/resuscitation in
rats. Inflammation. 40:555–565. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Elisia I, Nakamura H, Lam V, Hofs E,
Cederberg R, Cait J, Hughes MR, Lee L, Jia W, Adomat HH, et al:
DMSO represses inflammatory cytokine production from human blood
cells and reduces autoimmune arthritis. PLoS One. 11:e01525382016.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Morris RW: Analgesic and local anesthetic
activity of dimethyl sulfoxide. J Pharm Sci. 55:438–440. 1966.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Farrant J, Walter CA, Lee H, Morris GJ and
Clarke KJ: Structural and functional aspects of biological freezing
techniques. J Microsc. 111:17–34. 1977. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Santos NC, Figueira-Coelho J,
Martins-Silva J and Saldanha C: Multidisciplinary utilization of
dimethyl sulfoxide: Pharmacological, cellular, and molecular
aspects. Biochem Pharmacol. 65:1035–1041. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jin SH, Kweon H, Park JB and Kim CH: The
effects of tetracycline-loaded silk fibroin membrane on
proliferation and osteogenic potential of mesenchymal stem cells. J
Surg Res. 192:e1–e9. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jin SH, Lee JE, Yun JH, Kim I, Ko Y and
Park JB: Isolation and characterization of human mesenchymal stem
cells from gingival connective tissue. J Periodontal Res.
50:461–467. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lee SI, Yeo SI, Kim BB, Ko Y and Park JB:
Formation of size-controllable spheroids using gingiva-derived stem
cells and concave microwells: Morphology and viability tests.
Biomed Rep. 4:97–101. 2016.PubMed/NCBI
|
|
9
|
Malgieri A, Kantzari E, Patrizi MP and
Gambardella S: Bone marrow and umbilical cord blood human
mesenchymal stem cells: State of the art. Int J Clin Exp Med.
3:248–269. 2010.PubMed/NCBI
|
|
10
|
Li Z, Ba R, Wang Z, Wei J, Zhao Y and Wu
W: Angiogenic potential of human bone marrow-derived mesenchymal
stem cells in chondrocyte brick-enriched constructs promoted stable
regeneration of craniofacial cartilage. Stem Cells Transl Med.
6:601–612. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zuk PA, Zhu M, Ashjian P, De Ugarte DA,
Huang JI, Mizuno H, Alfonso ZC, Fraser JK, Benhaim P and Hedrick
MH: Human adipose tissue is a source of multipotent stem cells. Mol
Biol Cell. 13:4279–4295. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Jeong SH, Lee JE, Kim BB, Ko Y and Park
JB: Evaluation of the effects of Cimicifugae Rhizoma on the
morphology and viability of mesenchymal stem cells. Exp Ther Med.
10:629–634. 2015.PubMed/NCBI
|
|
13
|
Park JB: Treatment of multiple gingival
recessions using subepithelial connective tissue grafting with a
single-incision technique. J Oral Sci. 51:317–321. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Park JB: Root coverage with 2 connective
tissue grafts obtained from the same location using a
single-incision technique. Quintessence Int. 40:371–376.
2009.PubMed/NCBI
|
|
15
|
Kelava T, Cavar I and Culo F: Influence of
small doses of various drug vehicles on acetaminophen-induced liver
injury. Can J Physiol Pharmacol. 88:960–967. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Park JB, Zhang H, Lin CY, Chung CP, Byun
Y, Park YS and Yang VC: Simvastatin maintains osteoblastic
viability while promoting differentiation by partially regulating
the expressions of estrogen receptors α. J Surg Res. 174:278–283.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ha DH, Yong CS, Kim JO, Jeong JH and Park
JB: Effects of tacrolimus on morphology, proliferation and
differentiation of mesenchymal stem cells derived from gingiva
tissue. Mol Med Rep. 14:69–76. 2016.PubMed/NCBI
|
|
18
|
Wong Po, Foo C and Kaplan DL: Genetic
engineering of fibrous proteins: Spider dragline silk and collagen.
Adv Drug Deliv Rev. 54:1131–1143. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ruggiero F, Exposito JY, Bournat P, Gruber
V, Perret S, Comte J, Olagnier B, Garrone R and Theisen M: Triple
helix assembly and processing of human collagen produced in
transgenic tobacco plants. FEBS Lett. 469:132–136. 2000. View Article : Google Scholar : PubMed/NCBI
|