Introduction
It has been established in previous research that
gastrointestinal (GI) motility function is affected by gender
(1). Healthy women have also been
identified to have slower gastric emptying of solids compared with
men (2). This may explain why
gastroparesis - a chronic stomach motility disorder in which there
is a delayed gastric emptying of food without mechanical
obstruction - is more common in women than men (3,4).
Furthermore, previous studies assessing colonic motility have
demonstrated faster colon transit (shorter transit time) in men
compared with women (5–7). In one study, women exhibited less
pressure activity in the colon, particularly in the
transverse/descending colon, than men (8). Sex-associated differences were also
evident in the anal sphincter contraction and anorectal motility
(9). For instance, Sun and Read
(10) identified that healthy men had
stronger anal sphincter pressures compared with women. In the
gallbladder, women have been observed to have a slower emptying
rate than men under normal conditions, and this may explain the
increased probability of gallstone development in women compared
with men (11). Sex-dependent
differences in esophageal motility in terms of duration and
velocity of esophageal contraction have also been also reported
(12).
In addition, sex-associated differences have been
observed in various functional GI disorders and colorectal
disturbances. For instance, inflammatory and irritable bowel
syndromes, chronic functional abdominal pain, pelvic floor
dysfunction, constipation, bloating, fecal incontinence, globus and
dysphagia are more prevalent in women compared with men (13,14).
Physiologically, phosphorylation of the 20-kDa
regulatory myosin light chain (MLC20) is considered an
essential step in GI smooth muscle contraction (15). This phosphorylation is initiated and
regulated by activation of the Ca2+/calmodulin-dependent
myosin light-chain kinase (MLCK), which transfers the phosphate
group from adenosine triphosphate (ATP) to the Ser19 hydroxyl group
of MLC20 (16). This
phosphorylation activates the actin-activated myosin ATPase and
actin-myosin interaction, thereby initiating smooth muscle
contraction (15,16).
Studies on sex differences in GI motility disorders
have generally focused on sex hormone/receptor-mediated effects on
tract function (3,17). However, these sex differences may also
be associated with alterations in the signaling mechanisms of
smooth muscle contractile machinery. For example, our group
previously demonstrated greater gastric smooth muscle contraction
in male rats compared with female (1). In association with this higher
contraction, there was greater activation of the small G protein
RhoA and its downstream effector, Rho-associated protein kinase
(ROCK), an important pathway in developing and maintaining smooth
muscle tone (18), in the male
stomach muscle cells compared with female (1). The effect of gender on the expression
and activity of other protein kinases and phosphatases that
regulate smooth muscle contraction is less clear.
The aim of the present study was to determine
whether the increased contractions of gastric muscle cells in males
compared with females are attributable in part to sex differences
in the phosphorylation of MLC20.
Materials and methods
Materials
A DC protein assay kit (500–0119) was obtained from
Bio-Rad Laboratories, Inc., Hercules, CA, USA. An MLCK ELISA kit
(CSB-EL015320RA) was purchased from Cusabio Technology LLC,
Baltimore, MD, USA. A phospho-MLC20 (pSer19 in rat)
cell-based ELISA kit (ABIN1380310) was purchased from
antibodies-online, Inc., Atlanta, GA, USA. An MLC20
ELISA kit (rat MLC polypeptide 9; MBS7201038) was purchased from
MyBioSource, San Diego, CA, USA. The MLCK inhibitor, ML-7
(ab120848) was purchased from Abcam, Cambridge, MA, USA. A 500-µm
Nitex mesh was purchased from Amazon, Seattle, WA, USA. All
remaining chemicals were obtained from Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany. Dimethylsulfoxide was used to prepare a stock
solution of ML-7.
Preparation of freshly dispersed
gastric smooth muscle cells (GSMCs)
Young mature male and female Sprague-Dawley rats
(~12 weeks of age, 250–300 g, n=37; 20 males and 17 females) were
provided by the animal house of the Jordan University of Science
and Technology, Irbid, Jordan. The animals were housed under
standardized conditions (temperature 20–22°C, humidity 50–60% and
12-h light/dark cycle) and allowed free access to food and tap
water throughout the experiments. Rats were euthanized by
inhalation of CO2 (4.5 l/min flow rate) in a
CO2 chamber (30 × 30 × 25 cm; 2 animals per exposure)
for at least 5 min. To further confirm euthanasia an incision was
made through the diaphragm with a scalpel blade. Following
euthanasia the stomach was immediately excised. The current study
protocols were approved and followed the guidelines of the Animal
Care and Use Committee at Jordan University of Science and
Technology.
GSMCs were isolated from the circular muscle layer
of the rat stomach by sequential enzymatic digestion, filtration
and centrifugation as described previously (19). Briefly, strips of circular muscle free
of mucosa from all regions of the stomach were dissected and
incubated at 31°C for 30 min in HEPES medium (pH was adjusted to
7.4) containing 120 mM NaCl, 4 mM KCl, 2.0 mM CaCl2, 2.6
mM KH2PO4, 0.6 mM MgCl2, 25 mM
HEPES, 14 mM glucose, 2.1% Eagle's essential amino acid mixture,
0.1% collagenase and 0.01% soybean trypsin inhibitor. The tissue
was continuously gassed with 100% oxygen throughout the isolation
procedure. Following two washes of the partially digested strips
with 50 ml of enzyme-free medium, the muscle cells were allowed to
disperse spontaneously for 30 min. The cells were harvested by
filtration through a 500-µm Nitex mesh and centrifuged twice at 350
× g for 10 min to eliminate broken cells and organelles. A dye
exclusion test was used following cell collection to determine the
number of viable cells present in the collected cell suspension.
Briefly, the cell suspension was mixed for less than 3 min at room
temperature with trypan blue dye and then visually examined with an
inverted Nikon TMS-F microscope (Nikon Corporation, Tokyo, Japan)
to determine whether cells took up or excluded the dye; live cells
possess intact cell membranes that exclude trypan blue whereas dead
cells do not. The cells were counted in a hemocytometer and it was
estimated that 95% of the cells excluded trypan blue. This cell
isolation procedure consistently yielded spindle-shaped and viable
GSMCs that exhibited notable contraction in response to contractile
stimuli. All the experiments were performed within 2–3 h of cell
dispersion.
Detection of MLCK and MLC20
protein levels by ELISA
A total of three repeated freeze-thaw cycles were
used to break up the membranes of the isolated GSMCs. Briefly, in
each cycle, cells were rapidly frozen on dry ice (−78.5°C) and left
for 3 min, then thawed immediately at room temperature for 30 min.
Following centrifugation of the lysates at 20,000 × g for 10 min at
4°C, the protein concentration of the supernatant was determined
with the DC protein assay kit from Bio-Rad Laboratories, Inc.
Samples of equal amounts of protein were quantitated for MLCK and
MLC20 by ELISA according to the manufacturers'
instructions.
MLC20 phosphorylation
assay
The phosphorylation level of MLC20
(pSer-19) was measured by using the phospho-MLC20 ELISA
kit. The assay was performed according to the manufacturer's
protocol, using 10 µl of protein lysate. The total starting protein
concentration for all samples was 1 mg/ml.
Measurement of contraction in
dispersed smooth muscle cells
Contraction in freshly dispersed GSMCs was
determined by scanning micrometry (20). Aliquots (0.4 ml) of cells containing
approximately 104 cells/ml were prepared and distributed
into either male or female groups. Cells were stimulated with
acetylcholine (ACh; 0.1 µM) for 1 min in the presence or absence of
the MLCK inhibitor, ML-7 (1 µM), and the reaction was terminated
with 1% acrolein at a final concentration of 0.1%. Acrolein kills
and fixes cells without affecting the cell length. The cells were
viewed using a 10× or 20× objective of the inverted Nikon TMS-F
microscope, and cell images were acquired using a Canon digital
camera (DS126291; Canon, Inc., Tokyo, Japan) and ImageJ acquisition
software v1.45 (National Institutes of Health, Bethesda, MD, USA).
The resting cell length was determined in control experiments in
which muscle cells were not treated with ACh. The mean length of at
least 50 muscle cells was measured with ImageJ from each group. The
contractile response to ACh was defined as the decrease in the mean
length of at least 50 cells and expressed as the percent of change
in length relative to mean resting length.
Statistical analysis
Data are expressed as the mean ± standard error of
the mean. Each experiment was performed on single gastric muscle
cells collected from at least 5–10 different rats of each sex.
Statistical analyses were performed using Prism 5.0 software
(GraphPad Software, Inc., La Jolla, CA, USA). Comparisons between
two groups were performed by unpaired Student's t-tests.
Comparisons between more than two groups were performed by one-way
analysis of variance followed by Fisher's post-hoc analysis.
P<0.05 was considered to indicate statistical significance.
Results
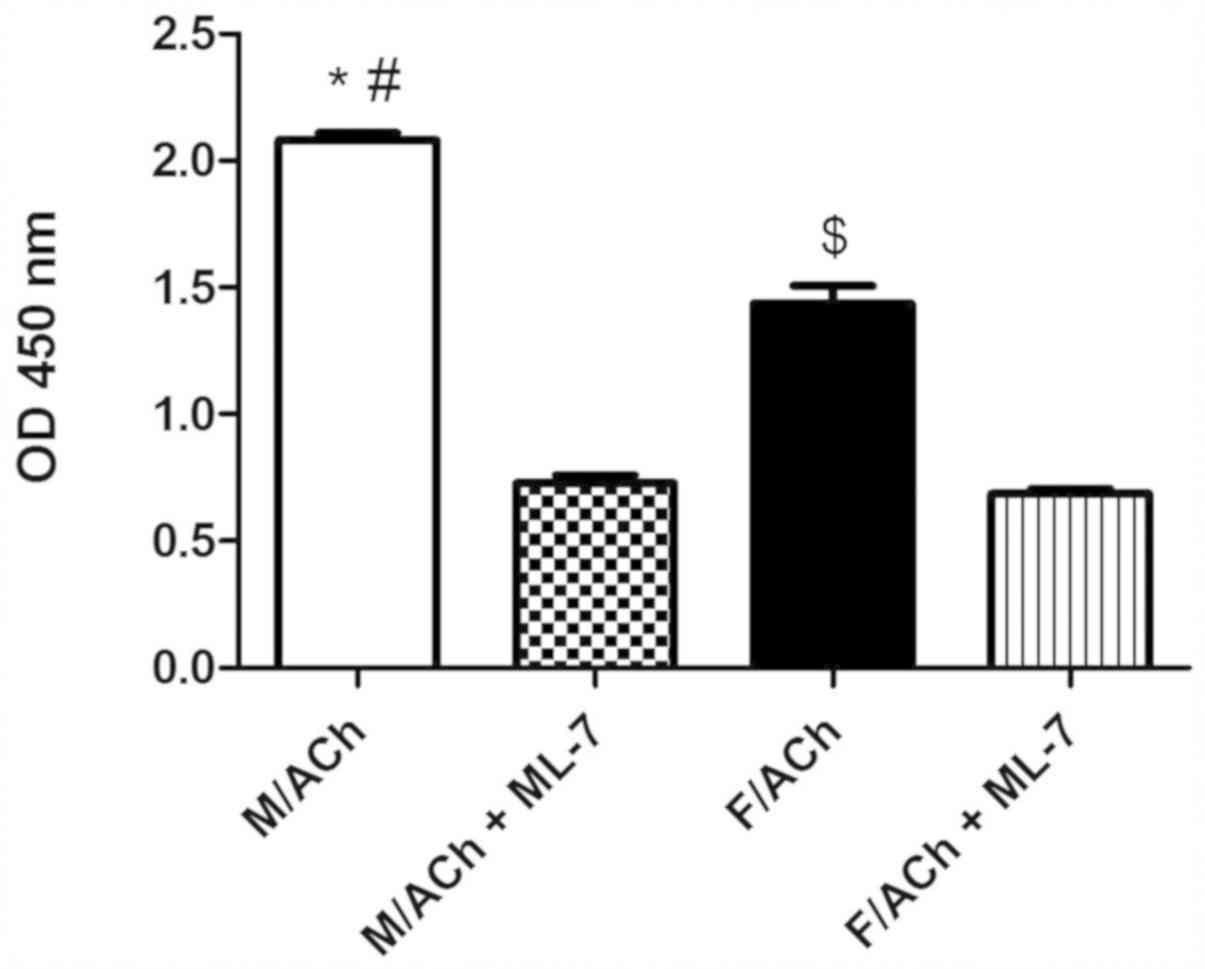
MLC20 phosphorylation
Treatment of freshly dispersed male or female
gastric muscle cells with ACh, a Gαq/13-coupled receptor agonist
(21), for 1 min significantly
increased MLC20 phosphorylation above the basal level
(P<0.05; data not shown). Notably, ACh-induced MLC20
phosphorylation was greater in male cells compared with female
cells (P<0.05; Fig. 1). The basal
MLC20 phosphorylation level was similar in the male and
female groups (data not shown). To determine whether the
sex-dependent phosphorylation of MLC20 was due to the
effect of MLCK activity, GSMCs were treated the MLCK inhibitor,
ML-7. ACh-induced MLC20 phosphorylation was
significantly inhibited by ML-7 in both sexes (P<0.05), and most
notably, sex differences were abolished (Fig. 1).
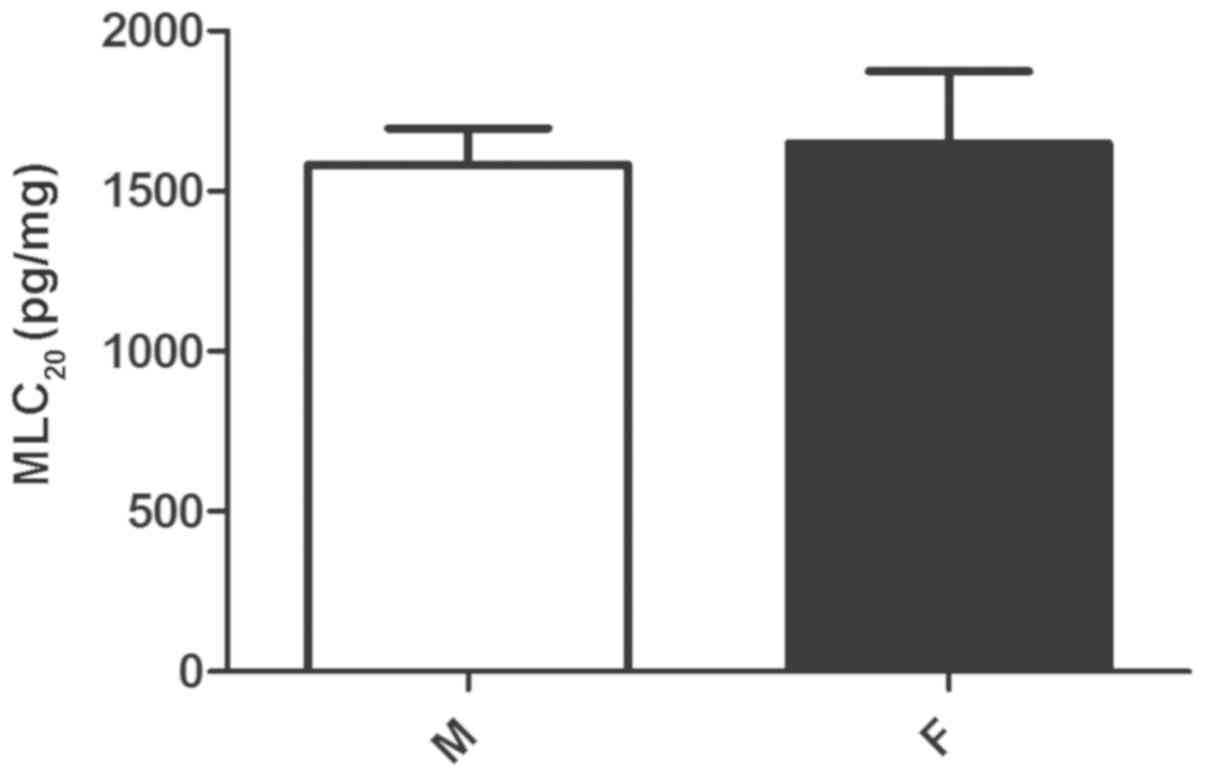
MLC20 expression
To determine whether the MLC20 protein
expression profile correlated with the phosphorylation differences
in MLC20, the protein level of MLC20 protein
level was compared between male and female gastric muscle cells by
ELISA. Despite the higher level of agonist-stimulated
MLC20 phosphorylation in male muscle cells compared with
female cells, the expression of MLC20 protein was
similar in both sexes (P>0.05; Fig.
2). This indicates the possibility that gender differences in
MLC20 phosphorylation may be due to an effect of other
upstream kinases, particularly of the key enzyme MLCK, on
MLC20 phosphorylation.
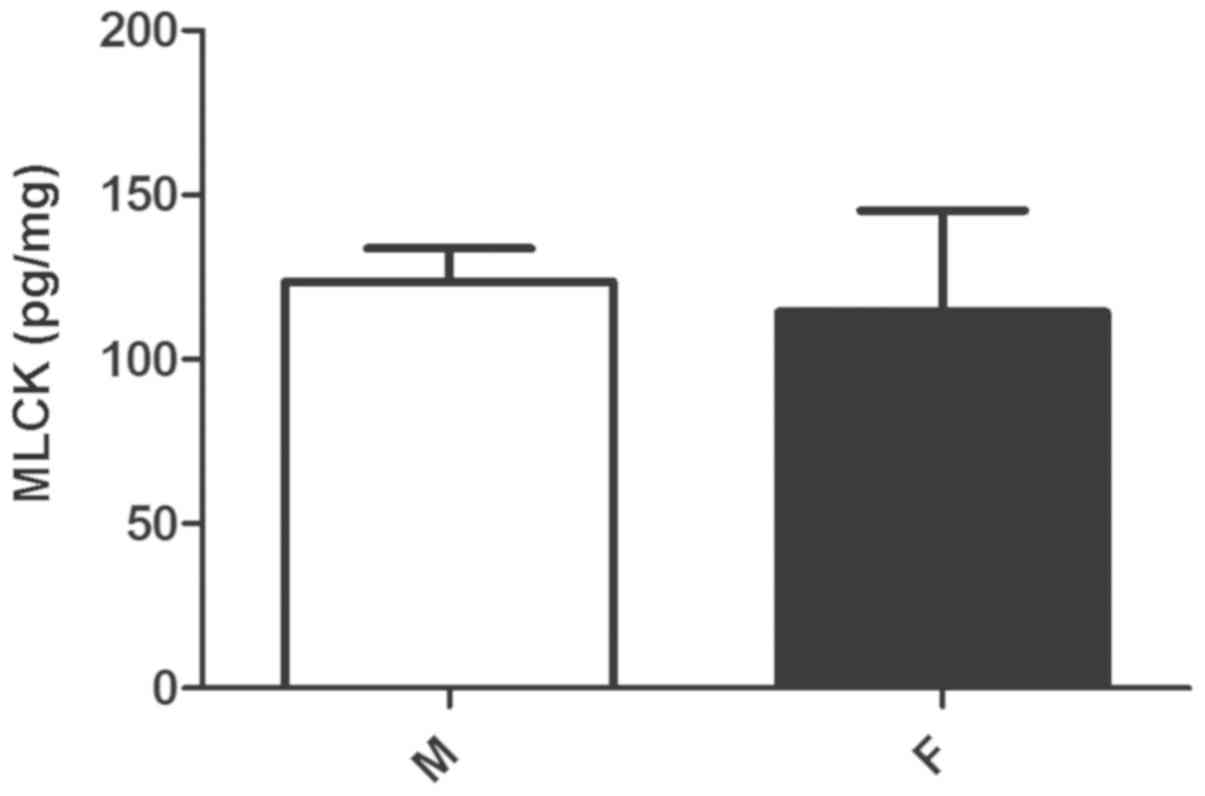
MLCK expression
Despite the greater effect of MLCK inhibition on
MLC20 phosphorylation in male compared with female
cells, the protein expression of MLCK did not differ significantly
between the groups of cells (P>0.05; Fig. 3).
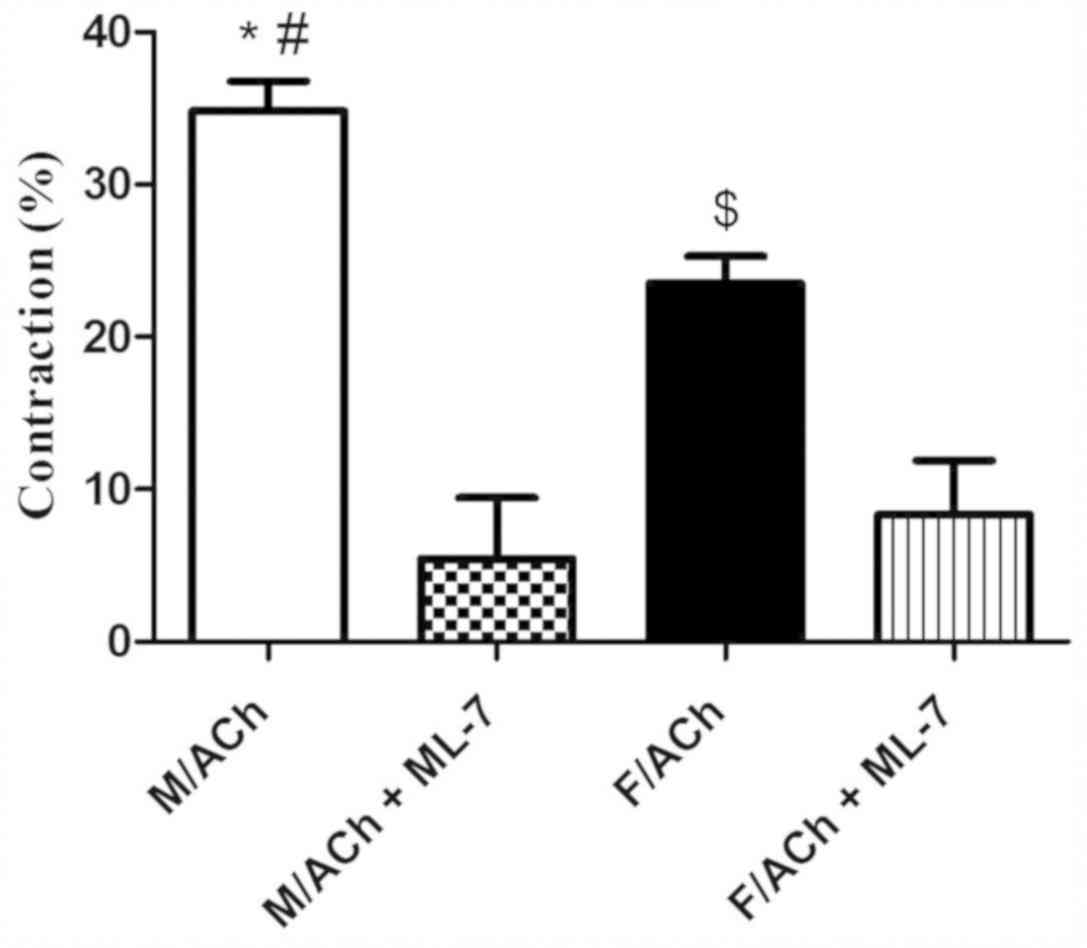
Smooth muscle contraction
The change in gastric muscle cell length in response
to ACh treatment was measured by scanning micrometry. Resting (not
treated with ACh) muscle cell lengths did not differ (P>0.05)
between the sexes (data not shown). ACh caused muscle cell
contraction in both the male and female groups. Notably,
contraction in response to ACh was significantly greater in male
cells compared with female cells (P<0.05; Fig. 4). To assess the effect of MLCK on the
sex-dependent muscle cell contraction, GSMCs were treated with
ML-7. Preincubation of cells with ML-7 significantly reduced
ACh-induced contraction in the male and female cells (P<0.05),
and this inhibition was to a greater extent in male compared with
female cells (Fig. 4). Most notably,
ML-7 abolished the sex differences in cell contraction (Fig. 4).
Discussion
In the present research, elevated MLC20
phosphorylation and contraction in response to ACh were identified
in GSMCs from male rats compared with those from females. However,
the protein levels of MLC20 were did not differ between
males and females. Inhibition of MLCK reduced MLC20
phosphorylation and ACh-induced contraction in both sexes and
abolished sex-dependent differences. These findings suggest that
phosphorylation of MLC20 by MLCK is regulated
differently in GSMCs from males versus females.
Sex differences in smooth muscle function have been
reported in various tissue organs and in different species. For
example, greater myogenic tone has been identified in the arteries
of male rats over that in females (22,23).
Furthermore, women have increased asthma prevalence with changes in
pulmonary smooth muscle contractile behavior compared with men
(24). Most notably, our group
recently reported greater muscle contraction in male stomach muscle
cells compared with the female counterparts (1).
Indeed, gender differences in smooth muscle
reactivity may be related to differential expression and/or
activity of sex hormone receptors (25), effects of sex hormones on the gene
expression of the specific receptors of contractile agonists
(26), or sex-related differences in
the signaling mechanisms of smooth muscle contraction downstream
from receptor activation (1). It is
established that phosphorylation of Ser19 on the regulatory light
chain of myosin II by Ca2+/calmodulin-dependent MLCK is
essential for the initiation of smooth muscle contraction (27). MLC20 phosphorylation may
also be increased through inhibition of MLC phosphatase by Rho
kinase and other important kinases, which sustains smooth muscle
contraction without a change in intracellular Ca2+ level
(28).
Research on gender-related differences in the
signaling mechanisms of smooth muscle contraction in the GI tract
is limited. In the vasculature, studies have demonstrated a smaller
basal intracellular Ca2+ level in female rats compared
with males, suggesting gender differences in
Ca2+-handling mechanisms (29,30). In
addition, stronger vasodilator response to the Rho kinase
inhibitor, Y27632, has been observed in the cerebral circulation of
male rats compared with females, although the measured protein
levels of RhoA and Rho kinase did not differ (31). Other studies have demonstrated that
gender differences in vascular reactivity reflect differences in
the expression and activity of PKC isoforms in vascular smooth
muscle (32–34). Whether these sex variations exist in
the GI tract muscle is currently unknown.
Parallel to previous findings outside the GI tract,
our group recently reported an increased contraction attributable
to greater RhoA/ROCK activation in the stomach smooth muscle cells
of male rats compared with females (1). This provided a basis for investigating
sex differences in other contraction signaling pathways in the GI
tract smooth muscle. To the best of our knowledge, the present
study is the first to indicate differential activation of the
MLCK/MLC20 pathway and its effect on contraction of
single GSMCs between the sexes in rats.
As sex-related differences in gastric contraction
may be due to differences in various types of stomach cells,
studying the muscle contraction and the regulation of the
MLCK/MLC20 pathway in a multicellular preparation may be
difficult and non-specific. For this reason, the present study was
performed on single smooth muscle cells freshly isolated from the
stomach of rat to avoid the contribution of other non-muscle cell
types.
Consistent with our previous study (1), treatment of muscle cells with ACh
induced muscle contraction and significantly enhanced
MLC20 phosphorylation level in male and female cells.
Despite the differences in MLC20 phosphorylation level,
the protein expression of MLC20 was indifferent between
the sexes. Thus, differences in the expression of MLC20
did not account for the difference in MLC20
phosphorylation between the sexes. These results indicate the
possibility that the sex-dependent elevation of MLC20
phosphorylation in males may be due to differences in the
activation of other upstream regulators of myosin, such as
MLCK.
To assess the role of MLCK in sex-dependent
differential MLC20 phosphorylation and contraction,
MLC20 phosphorylation and muscle contraction was
measured in the presence or absence of the MLCK inhibitor, ML-7.
ACh-induced muscle cell contraction was greater in male gastric
cells compared with female cells. The MLCK inhibitor ML-7 inhibited
both the phosphorylation of MLC20 and muscle
contraction. Most notably, inhibition of MLCK by ML-7 abolished the
observed sex differences and normalized contractions in response to
ACh between male and female cells. This suggests that enhanced MLCK
activity in the stomach of males may mediate the sex difference.
Indeed, the binding affinity of ML-7 for smooth muscle MLCK is ~100
times higher than its affinity for other enzymes, and it is more
specific than its parent form, ML-9, in inhibiting MLCK (35,36).
However, its ability to inhibit other enzymes including cyclic
adenosine monophosphate-dependent protein kinases, protein kinase C
(PKC) and calcium phosphodiesterase can not be excluded (35). A recent and advanced method for
testing the function of a specific enzyme is to treat cultured
GSMCs with small interfering RNA (siRNA) to block expression of the
target gene. However, due to limited laboratory facilities, these
siRNA experiments could not be performed in the present study.
Thus, future studies using siRNA and more specific inhibitors of
MLCK should be performed.
Various receptor agonists generate both
initial/transient (<1 min) Ca2+-dependent and
sustained (>5 min) Ca2+-independent contraction in GI
smooth muscle cells (37–39). As the present study treated single
gastric muscle cells with agonist for 1 min, the phosphorylation
assay and contraction results mostly represent sex differences in
the initial phase of contraction. During this phase,
MLC20 phosphorylation is mainly regulated by MLCK
(38). Future measurement of MLCK
activity by specific kinase assay techniques may aid to verify the
present results.
When regarding the inhibitory effects of female
steroid hormones, namely estrogen and progesterone, on muscle
contraction (40), the present
results are consistent with previous studies on human myometrial
muscle, which demonstrated significant reduction in
MLC20 phosphorylation upon KCl treatment in tissues from
pregnant women compared with nonpregnant women (41). This KCl-mediated MLC20
phosphorylation depends on Ca2+ influx through
voltage-sensitive Ca2+ channels (42). Both estrogen and progesterone have
been demonstrated to inhibit Ca2+ influx in smooth
muscle (43,44). In addition, the current findings are
in parallel with other results on enhanced myosin phosphatase
target subunit 1 expression and thus MLC phosphatase activity in
pregnancy, which resulted in decreased basal levels of
MLC20 phosphorylation (45).
In conclusion, the present study demonstrated that
phosphorylation of MLC20 and thus smooth muscle
contraction in response to ACh was greater in stomach cells from
males compared with those from females. This sex-dependent
phosphorylation was most probably mediated by increased activation
of MLCK in males compared with females, as the sex differences were
eliminated by the MLCK inhibitor ML-7. The exact mechanisms by
which the MLCK/MLC20 pathway is differentially regulated
between the sexes should be investigated in future studies. Sex
differences are present across the GI system, in which the
MLCK/MLC20 pathway serves a crucial role in GI motility
function (46). Further understanding
of the role of the MLCK/MLC20 pathway in modulating the
normal physiological as well as the pathophysiological functions of
the GI tract may enable more effective and sex-appropriate
treatments for various GI motility disturbances.
Acknowledgements
Not applicable.
Funding
The present work was supported by Jordan University
of Science and Technology, Irbid, Jordan (grant no. 20150371).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
Conception and design of the study, acquisition of
data, and drafting of the manuscript were performed by OAA.
Analysis and interpretation of data and revising the manuscript
critically for intellectual content were performed by OAA, ANA, MAA
and AGM. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The current study protocols were approved and
followed the guidelines of the Animal Care and Use Committee at
Jordan University of Science and Technology, Irbid, Jordan.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
MLC20
|
20-kDa regulatory myosin light
chain
|
|
GI
|
gastrointestinal
|
|
MLCK
|
myosin light chain kinase
|
|
GSMC
|
gastric smooth muscle cell
|
|
ACh
|
acetylcholine
|
|
ML-7
|
myosin light chain kinase
inhibitor
|
|
ROCK
|
Rho-associated protein kinase
|
|
PKC
|
protein kinase C
|
|
siRNA
|
small interfering RNA
|
References
|
1
|
Al-Shboul O: The role of the RhoA/ROCK
pathway in gender-dependent differences in gastric smooth muscle
contraction. J Physiol Sci. 66:85–92. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bennink R, Peeters M, Van den Maegdenbergh
V, Geypens B, Rutgeerts P, De Roo M and Mortelmans L: Comparison of
total and compartmental gastric emptying and antral motility
between healthy men and women. Eur J Nucl Med. 25:1293–1299. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Rao JN: Estrogens and gastroparesis: A
clinical relevance. Dig Dis Sci. 58:1449–1451. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Oh JH and Pasricha PJ: Recent advances in
the pathophysiology and treatment of gastroparesis. J
Neurogastroenterol Motil. 19:18–24. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Teff KL, Alavi A, Chen J, Pourdehnad M and
Townsend RR: Muscarinic blockade inhibits gastric emptying of
mixed-nutrient meal: Effects of weight and gender. Am J Physiol.
276:R707–R714. 1999.PubMed/NCBI
|
|
6
|
Meier R, Beglinger C, Dederding JP,
Meyer-Wyss B, Fumagalli M, Rowedder A, Turberg Y and Brignoli R:
Influence of age, gender, hormonal status and smoking habits on
colonic transit time. Neurogastroenterol Motil. 7:235–238. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lampe JW, Fredstrom SB, Slavin JL and
Potter JD: Sex differences in colonic function: A randomised trial.
Gut. 34:531–536. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Rao SS, Sadeghi P, Beaty J, Kavlock R and
Ackerson K: Ambulatory 24-h colonic manometry in healthy humans. Am
J Physiol Gastrointest Liver Physiol. 280:G629–G639. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zakari M, Nee J, Hirsch W, Kuo B, Lembo A
and Staller K: Gender differences in chronic constipation on
anorectal motility. Neurogastroenterol Motil. 29:292017. View Article : Google Scholar
|
|
10
|
Sun WM and Read NW: Anorectal function in
normal human subjects: Effect of gender. Int J Colorectal Dis.
4:188–196. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chukwuka UA, Kalu AK and Erondu OF:
Variabilities of gallbladder contraction indices and a simple
regression model for gallbladder and gastric emptying ratio. Pan
Afr Med J. 9:112011.PubMed/NCBI
|
|
12
|
Dantas RO, Ferriolli E and Souza MA:
Gender effects on esophageal motility. Brazilian journal of medical
and biological research. Rev Bras Pesqui Med Biol. 31:539–544.
1998.
|
|
13
|
Chang L, Toner BB, Fukudo S, Guthrie E,
Locke GR, Norton NJ and Sperber AD: Gender, age, society, culture,
and the patient's perspective in the functional gastrointestinal
disorders. Gastroenterology. 130:1435–1446. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chial HJ and Camilleri M: Gender
differences in irritable bowel syndrome. The journal of gender
specific medicine. J Gend Specif Med. 5:37–45. 2002.PubMed/NCBI
|
|
15
|
Somlyo AP and Somlyo AV: Signal
transduction and regulation in smooth muscle. Nature. 372:231–236.
1994. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kitazawa T, Gaylinn BD, Denney GH and
Somlyo AP: G-protein-mediated Ca2+ sensitization of smooth muscle
contraction through myosin light chain phosphorylation. J Biol
Chem. 266:1708–1715. 1991.PubMed/NCBI
|
|
17
|
Yang X, Liu R and Dong Y: Regulative
effects of ovarian steroids on rat gastric motility and
sensitivity. Sheng li xue bao: Acta physiologica Sinica.
58:275–280. 2006.PubMed/NCBI
|
|
18
|
Fukata Y, Amano M and Kaibuchi K:
Rho-Rho-kinase pathway in smooth muscle contraction and
cytoskeletal reorganization of non-muscle cells. Trends Pharmacol
Sci. 22:32–39. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Murthy KS and Makhlouf GM: Interaction of
cA-kinase and cG-kinase in mediating relaxation of dispersed smooth
muscle cells. Am J Physiol. 268:C171–C180. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Huang J, Mahavadi S, Sriwai W, Grider JR
and Murthy KS: Cross-regulation of VPAC(2) receptor desensitization
by M(3) receptors via PKC-mediated phosphorylation of RKIP and
inhibition of GRK2. Am J Physiol Gastrointest Liver Physiol.
292:G867–G874. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Murthy KS and Makhlouf GM: Differential
coupling of muscarinic m2 and m3 receptors to adenylyl cyclases
V/VI in smooth muscle. Concurrent M2-mediated inhibition via
Galphai3 and m3-mediated stimulation via Gbetagammaq. J Biol Chem.
272:21317–21324. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wellman GC, Bonev AD, Nelson MT and
Brayden JE: Gender differences in coronary artery diameter involve
estrogen, nitric oxide, and Ca(2+)-dependent K+ channels. Circ Res.
79:1024–1030. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Huang A, Sun D, Koller A and Kaley G:
Gender difference in myogenic tone of rat arterioles is due to
estrogen-induced, enhanced release of NO. Am J Physiol.
272:H1804–H1809. 1997.PubMed/NCBI
|
|
24
|
Fuseini H and Newcomb DC: Mechanisms
Driving Gender Differences in Asthma. Curr Allergy Asthma Rep.
17:192017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Collins P, Rosano GM, Sarrel PM, Ulrich L,
Adamopoulos S, Beale CM, McNeill JG and Poole-Wilson PA: 17
beta-Estradiol attenuates acetylcholine-induced coronary arterial
constriction in women but not men with coronary heart disease.
Circulation. 92:24–30. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Nickenig G, Strehlow K, Wassmann S, Bäumer
AT, Albory K, Sauer H and Böhm M: Differential effects of estrogen
and progesterone on AT(1) receptor gene expression in vascular
smooth muscle cells. Circulation. 102:1828–1833. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Dabrowska R and Hartshorne DJ: A Ca2+-and
modulator-dependent myosin light chain kinase from non-muscle
cells. Biochem Biophys Res Commun. 85:1352–1359. 1978. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Somlyo AP and Somlyo AV: Signal
transduction through the RhoA/Rho-kinase pathway in smooth muscle.
J Muscle Res Cell Motil. 25:613–615. 2004.PubMed/NCBI
|
|
29
|
Murphy JG and Khalil RA: Gender-specific
reduction in contractility and [Ca(2+)](i) in vascular smooth
muscle cells of female rat. Am J Physiol Cell Physiol.
278:C834–C844. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Xia Y and Khalil RA: Sex-related decrease
in [Ca2+]i signaling and Ca2+-dependent contraction in inferior
vena cava of female rat. Am J Physiol Regul Integr Comp Physiol.
298:R15–R24. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chrissobolis S, Budzyn K, Marley PD and
Sobey CG: Evidence that estrogen suppresses rho-kinase function in
the cerebral circulation in vivo. Stroke. 35:2200–2205. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kanashiro CA and Khalil RA: Signal
transduction by protein kinase C in mammalian cells. Clin Exp
Pharmacol Physiol. 25:974–985. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Li T, Xiao X, Zhang J, Zhu Y, Hu Y, Zang
J, Lu K, Yang T, Ge H, Peng X, et al: Age and sex differences in
vascular responsiveness in healthy and trauma patients:
Contribution of estrogen receptor-mediated Rho kinase and PKC
pathways. Am J Physiol Heart Circ Physiol. 306:H1105–H1115. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kanashiro CA and Khalil RA: Gender-related
distinctions in protein kinase C activity in rat vascular smooth
muscle. Am J Physiol Cell Physiol. 280:C34–C45. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Saitoh M, Ishikawa T, Matsushima S, Naka M
and Hidaka H: Selective inhibition of catalytic activity of smooth
muscle myosin light chain kinase. J Biol Chem. 262:7796–7801.
1987.PubMed/NCBI
|
|
36
|
Bain J, McLauchlan H, Elliott M and Cohen
P: The specificities of protein kinase inhibitors: An update.
Biochem J. 371:199–204. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Gerthoffer WT: Signal-transduction
pathways that regulate visceral smooth muscle function. III.
Coupling of muscarinic receptors to signaling kinases and effector
proteins in gastrointestinal smooth muscles. Am J Physiol
Gastrointest Liver Physiol. 288:G849–G853. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Murthy KS: Signaling for contraction and
relaxation in smooth muscle of the gut. Annu Rev Physiol.
68:345–374. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Hu W, Mahavadi S, Li F and Murthy KS:
Upregulation of RGS4 and downregulation of CPI-17 mediate
inhibition of colonic muscle contraction by interleukin-1beta. Am J
Physiol Cell Physiol. 293:C1991–C2000. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Datta S, Hey VM and Pleuvry BJ: Effects of
pregnancy and associated hormones in mouse intestine, in vivo and
in vitro. Pflugers Arch. 346:87–95. 1974. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Word RA, Stull JT, Casey ML and Kamm KE:
Contractile elements and myosin light chain phosphorylation in
myometrial tissue from nonpregnant and pregnant women. J Clin
Invest. 92:29–37. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Himpens B, Matthijs G and Somlyo AP:
Desensitization to cytoplasmic Ca2+ and Ca2+ sensitivities of
guinea-pig ileum and rabbit pulmonary artery smooth muscle. J
Physiol. 413:489–503. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Fomin VP, Cox BE and Word RA: Effect of
progesterone on intracellular Ca2+ homeostasis in human myometrial
smooth muscle cells. Am J Physiol. 276:C379–C385. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Salom JB, Burguete MC, Pérez-Asensio FJ,
Torregrosa G and Alborch E: Relaxant effects of 17-beta-estradiol
in cerebral arteries through Ca(2+) entry inhibition. J Cereb Blood
Flow Metab. 21:422–429. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Lontay B, Bodoor K, Weitzel DH, Loiselle
D, Fortner C, Lengyel S, Zheng D, Devente J, Hickner R and Haystead
TA: Smoothelin-like 1 protein regulates myosin
phosphatase-targeting subunit 1 expression during sexual
development and pregnancy. J Biol Chem. 285:29357–29366. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
He WQ, Peng YJ, Zhang WC, Lv N, Tang J,
Chen C, Zhang CH, Gao S, Chen HQ, Zhi G, et al: Myosin light chain
kinase is central to smooth muscle contraction and required for
gastrointestinal motility in mice. Gastroenterology. 135:610–620.
2008. View Article : Google Scholar : PubMed/NCBI
|