Introduction
Gastric cancer has the third highest mortality and
fourth highest morbidity rates of all cancers worldwide (1). In 2012, GloboCan statistics reported
almost 1 million new cases of gastric cancer, and more than 700,000
mortalities caused by gastric cancer (1). Gastric cancer is a multifactorial
disorder, in which genetic and environmental interactions serve an
important role in development and progression (2). Increasing age, gender, lifestyle,
dietary regime, environmental factors and Helicobacter
pylori infections are among the known risk factors for stomach
cancer (3,4). While dietary regime and lifestyle are
the most recognized factors, more effective identification of the
genetic risk factors is expected to improve understanding of the
basic molecular events involved in tumorigenesis (5). Various genetic and epigenetic changes
that have the potential to convert normal epithelial cells in the
stomach into malignant neoplasms may be responsible for the
development of both familial and sporadic gastric cancer (6,7). Studies
performed recently have demonstrated that a high number of genes
and various environmental factors are the causal agents of gastric
cancer, and the presence of different forms of alleles in genes
(polymorphisms) may promote the development of cancers; in this
regard, the P53 gene has been a research focus due to its
role as a major tumor suppressor gene (8,9). The
P53 gene is located on the short arm of chromosome 17 and
includes 11 exons (10). The
P53 codon 72 (Arg72Pro) polymorphism, located in exon 4, has
been investigated in numerous types of cancer (11). Substitution of a guanine base for
cytosine in this codon leads to the replacement of an arginine
amino acid to proline, which influences the activity of the
resulting protein (12). The proline
variant is effective in the repair of DNA damage, while the
arginine variant leads to a strong induction of apoptosis (13). To date, studies have been performed on
the P53 codon 72 polymorphism in breast, colorectal, skin
and stomach cancers; however, no comprehensive result has been
obtained (13–16). Considering the controversial results
regarding the role of P53 gene polymorphism in gastric
cancer, dependence of variants on geographical conditions, racial
differences and genetic differences probably exists in different
communities. This is indicated when considering the increase in
incidence of stomach cancer and associated mortalities in Iran,
particularly in northern parts of the country (17–20).
Therefore, the aim of the present study was to determine the
association of P53 gene polymorphism with gastric cancer in
Northern Iran as a high-risk region.
Materials and methods
Study population
This was a case-controlled study intending to
determine the association between P53 gene polymorphism and
gastric cancer in patients referred to the Tuba Clinic (Academic
Referral Center for the Mazandaran Province) from October 2016 to
April 2017 in Sari, Iran, compared with non-cancer patients. The
patients with gastric cancer (n=59) were diagnosed by oncologists
and confirmed by pathological examination, while the non-cancer or
control subjects (n=59) were healthy patients referred to the Tuba
Clinic for routine laboratory tests. The exclusion criteria were
the following: Presence of a tumor in a site other than the stomach
and a non-adenocarcinoma type of gastric cancer in the case group,
and a history of cancer and/or pregnancy in the control group.
Study and control subjects were compared regarding age and gender,
and study subjects were compared regarding age (≤55 vs. >55
years), gender and tumor differentiation (well vs. moderate vs.
poor), stage (I/II vs. III/IV) and location (proximal vs. distal)
(21). All data for the study
population, including age, gender, clinical and laboratory
diagnosis, were collected based on related checklists of the above
variables. The study received approval from the Ethics Committee of
Mazandaran University of Medical Sciences (Sari, Iran) and written
informed consent was obtained from all subjects following full
disclosure of the study objectives and procedures.
DNA extraction
Samples of 5–10 ml fasting venous blood were
obtained from the subjects and transferred to two tubes: A
serum-separating tube and a tube containing the anticoagulant EDTA.
Subsequently, the samples were transferred to 15 ml Falcon tubes
and brought to a total volume of 15 ml with lysis buffer I
(DynaBio™ Blood/Tissue DNA Extraction Mini kit, cat. no. KI0015;
Unilabs, Geneva, Switzerland). The tubes were agitated, incubated
for 5 min at room temperature and centrifuged (4,000 × g for 10 min
at 4°C). The upper layer of the solution was removed, the tubes
were re-filled with 15 ml of buffer I and the steps listed above
were repeated three times. A total of 2 ml buffer II was added to
the tubes, which were then incubated for 30 min in a 45°C water
bath. During incubation, the tubes were agitated periodically to
ensure that the sediment dissolved. A total of 0.5 ml 5 M sodium
perchlorate was added to the tubes and allowed to combine for 2–3
min. Subsequently, 2 ml cold chloroform was added to each tube
under a hood and the tubes were centrifuged at 4,000 × g for 5–7
min at 4°C. A total of 3 ml cold ethanol (99% v/v) were added to
the upper layer of the solution containing the DNA, and
precipitated DNA was observed and allowed to settle. Finally, DNA
was removed from the solution using a Pastor pipet and dried in the
open air to allow the ethanol to evaporate. DNA was eluted in
100–200 ml sterilized distilled water.
Amplification of the P53 region
The region of the P53 gene containing the
codon 72 (Arg72Pro) polymorphism on exon 4 was amplified by
polymerase chain reaction (PCR) using the following specific
primers: Forward, 5′-TTGCCGTCCCAAGCAATGGATGA-3′ and reverse,
5′-TCTGGGAAGGGACAGAAGATGAC-3′. The PCR reaction mixture contained
10 pmol of each primer, 200 ng genomic DNA, 1 U Taq DNA polymerase
(Denazist Asia Co., Mashhad, Iran), 1.5 µmol MgCl2, 200
mM of each dNTP and nuclease-free water to a final volume of 25 ml.
The reaction mixtures were preincubated for 10 min at 94°C. The PCR
conditions were 94°C for 30 sec and 55°C for 1 min, followed by
72°C for 1 min for 40 rounds. After confirmation of an amplified
fragment of the expected size (199 bp) (20) on a 1.5% agarose gel with ethidium
bromide staining, the PCR products were digested with 0.1 µl (10
U/µl) BstUI restriction enzyme (Fermentas, Vilnius,
Lithuania) at 60°C for 16 h. The DNA fragments were electrophoresed
through a 2% agarose gel containing gel stain (DNA Green Viewer™;
Parstous Biotechnology, Mashhad, Iran). The Pro allele is not
cleaved by BstUI at codon 72 and has a single band with
length of 199 bp. The Arg allele is cleaved by BstUI and
digested products are separated as two fragments of 113 and 86 bp
in length. The heterozygote genotype has 3 bands of 199, 113 and 86
bp in length (22).
Statistical analysis
Based on the results of a recent study (23) which estimated the frequencies of the
proportions of a polymorphism in case and control groups (P1 and
P2, respectively) with 95% confidence (α=0.05) and 80% test power
(β=0.20), the sample size of the present study for the gastric
cancer and non-cancer groups was at least 100 subjects (gastric
cancer, n=59 and non-cancer, n=59). Continuous variables were
expressed as the mean ± standard deviation, and categorical values
were expressed as frequencies. Statistical analyses by unpaired
Student's t-test and one-way analysis of variance were performed
using SPSS software version 19 (IBM Corp., Armonk, NY, USA). The
correlation of gastric cancer with P53 gene codon 72
polymorphism was assessed by logistic regression analysis.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Patient characteristics
In the present study, 59 patients with gastric
cancer and 59 healthy controls from Northern Iran were assessed for
P53 codon 72 genotype. The demographic characteristics of
all subjects are listed in Table I.
The sex distribution was not significantly different between the
two groups (P=0.056); however, the mean age of patients with cancer
was higher than that of the controls (P<0.001).
 | Table I.Demographic characteristics of
subjects in the patient and control groups. |
Table I.
Demographic characteristics of
subjects in the patient and control groups.
|
| Group |
|
|---|
|
|
|
|
|---|
| Variable | Gastric cancer | Control | P-value |
|---|
| Sex, n (%) |
|
|
|
|
Male | 36 (61.0) | 45 (76.3) | 0.056 |
|
Female | 23 (39.0) | 14 (23.7) |
|
| Age, mean ± SD | 62.9±11.8 | 29.8±13.2 | <0.001 |
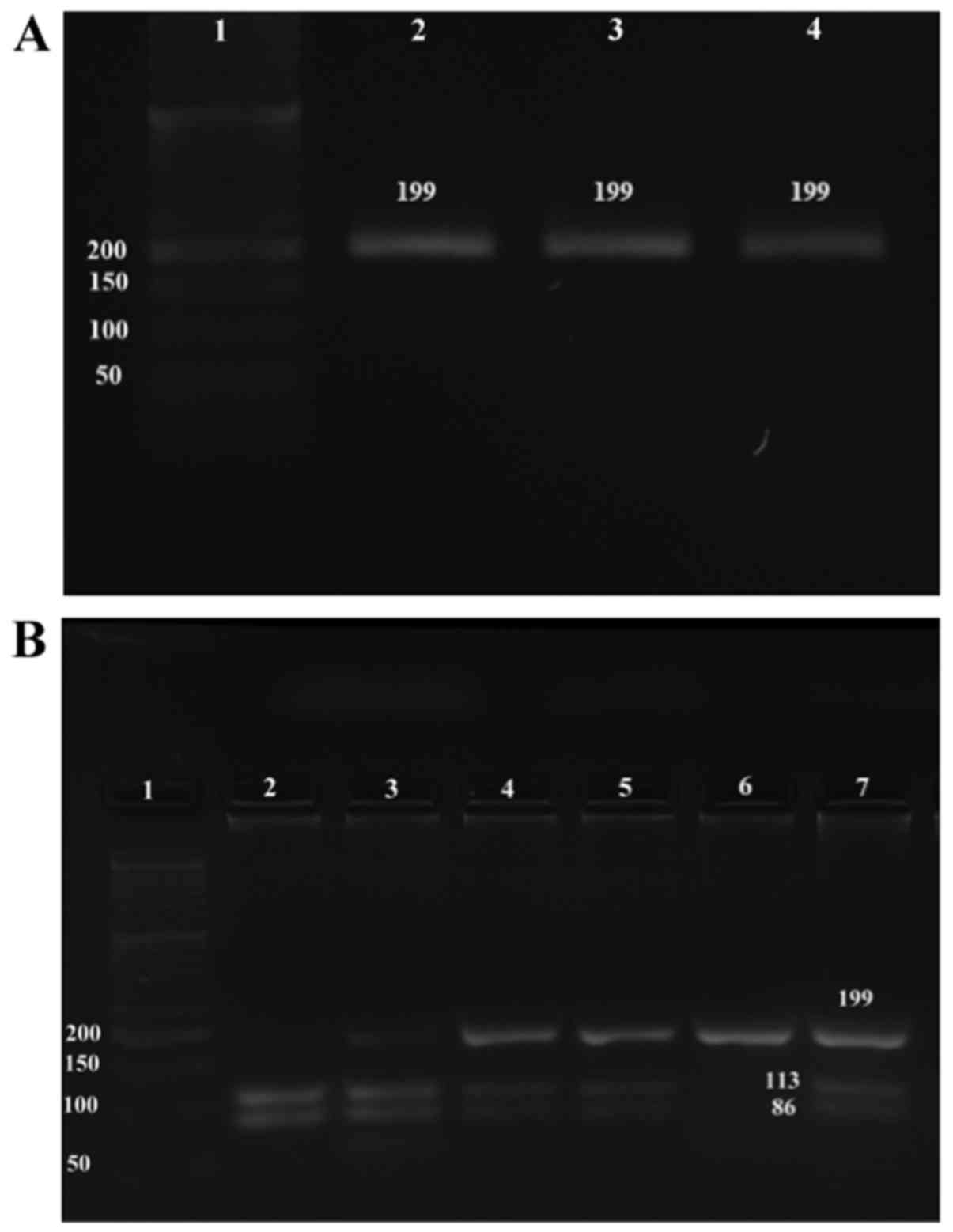
PCR-RFLP products of P53 codon 72
variants
Band visualization confirmed restriction digestion
of the P53 gene. The electrophoretic pattern of the
P53 gene segments were obtained following BstUI
digestion and 2% agarose separation (Fig.
1). Electrophoresis of the undigested amplified P53
product detected the expected 199 bp fragment (Fig. 1A). In the presence of proline, the
P53 allele remains unchanged on cleavage of the PCR product
with 10 U/µl BstUI, and the allele is detected as undigested
product. Thus, the homozygote genotype (Pro/Pro) yielded a single
199 bp band (Fig. 1B, lane 6). By
contrast, cleavage of the arginine homozygote genotype (Arg/Arg)
generated two bands at 113 and 86 bp (Fig. 1B, lane 2). The Arg/Pro heterozygote
genotype yielded all three fragments (Fig. 1B, lanes 3–5 and 7).
Distribution of P53 codon 72
polymorphism variants
The genotype frequencies of the P53 codon 72
polymorphism in Iranian gastric cancer cases and controls are
summarized in Table II. Genotype
frequencies did not differ significantly between the patients and
controls (P=0.4); the frequencies of the three genotypes Arg/Arg,
Arg/Pro and Pro/Pro in gastric cancer patients were 28.8, 49.2 and
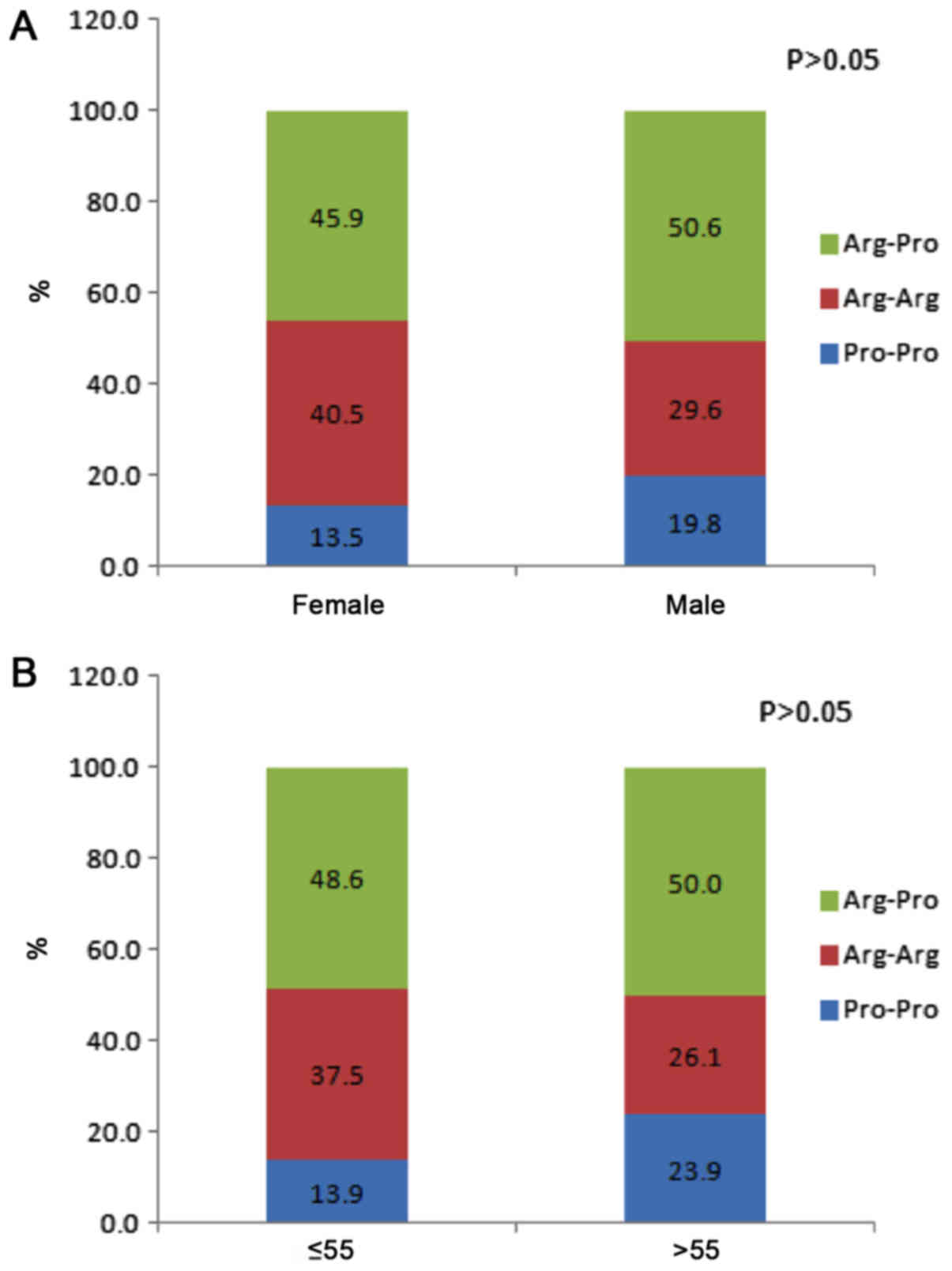
22.0%, and in controls were 37.3, 49.2 and 13.6%. When the patients
with gastric cancer were classified by sex and age group (≤55 or
>55 years), there were no significant differences in the
genotype distributions between males and females or patient age
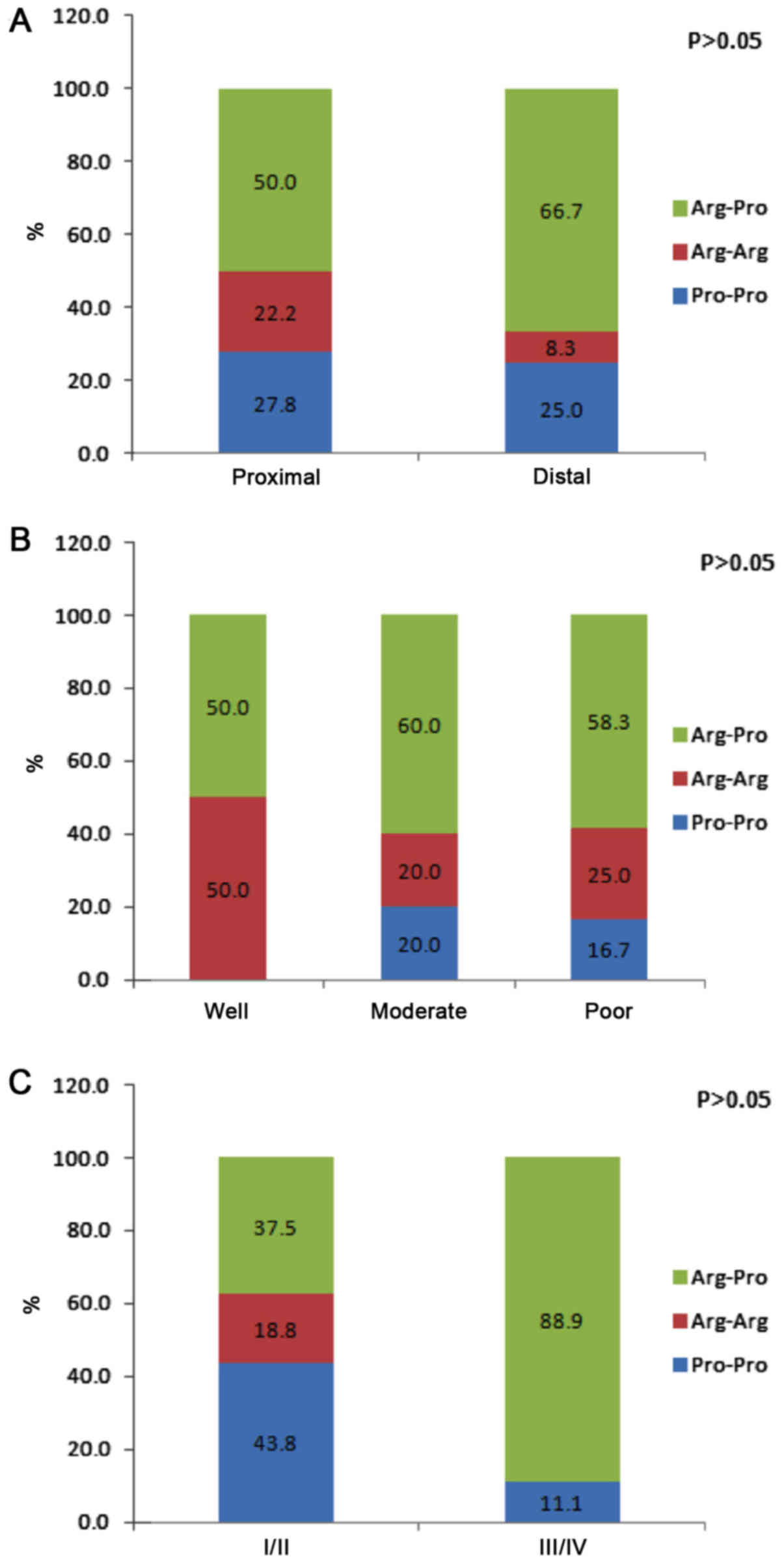
groups (Fig. 2). Fig. 3 presents genotype frequency based on
tumor location, histological differentiation and tumor stage. The
location of the tumor in 60% of patients was proximal and in 40%
was distal. Histological differentiation of the tumor samples was
defined as well, moderate and poor in 8.3, 41.7 and 50.0% of the
gastric cancer cases, respectively. There were no significant
differences in genotype frequencies based on tumor location,
histological differentiation or tumor stage.
 | Table II.Frequency of P53 codon 72
polymorphism genotypes in the patient and control groups. |
Table II.
Frequency of P53 codon 72
polymorphism genotypes in the patient and control groups.
|
| Group frequency, n
(%) |
|
|---|
|
|
|
|
|---|
| Genotype | Gastric cancer | Control | P-value |
|---|
|
Proline-Proline | 13 (22.0) | 8 (13.6) |
|
|
Arginine-Arginine | 17 (28.8) | 22 (37.3) | 0.4 |
|
Arginine-Proline | 29 (49.2) | 29 (49.2) |
|
| Total | 59
(100.0) | 59
(100.0) |
|
Discussion
In the present study, the genotype frequencies of a
P53 polymorphism in Iranian patients with gastric cancer did
not differ significantly to those in healthy controls; the
frequencies of the three genotypes, Pro/Pro, Arg/Arg and Arg/Pro
were 22, 28.8 and 49.2% in the case group and 13.6, 37.3 and 49.2%
in the control group. Similar to the present study, Chung et
al (24) reported that there was
no specific genotype of P53 polymorphism in a gastric cancer
cohort compared with other groups with or without H.
pylori-associated chronic gastritis in a Korean population,
though another study by Liu et al identified a significant
difference between P53 expression in primary tumor and
non-tumor tissue in gastric cancer patients (25). In the gastric cancer group in the
current study, the male to female ratio was 1.56, and there was no
significant difference in sex distribution between the case and
control groups. However, the mean age of patients in the case group
was significantly (P<0.0001) higher compared with that of the
control group. Furthermore, the location of the tumor in 60% of
patients was proximal (cardia, body, fundus and curve of the
stomach) and in 40% was distal (antrum of the stomach).
Histological differentiation of the tumor samples was well,
moderate and poor in 8.3, 41.7 and 50% of gastric cancer patients.
These results are in agreement with the study by Chung et al
(24). Additionally, 64% percent of
the gastric cancer cases were stage I/II and 36% were stage III/IV.
However, the study failed to identify significant differences in
P53 polymorphism variants between different tumor locations,
histological differentiations or tumor stages in Iranian gastric
cancer cases.
Zhou et al (26) analyzed the association between P53
codon 72 polymorphism and gastric cancer among a Chinese
population. High frequencies of Pro/Pro in cardia gastric cancer
patients and Arg/Arg in advanced gastric cancer patients suggested
that this polymorphism was associated with the location and stage
of gastric cancer. Shen et al (27) identified that the P53 Arg allele in
homozygote and heterozygote genotypes was associated with increased
risk of gastric cancer. Furthermore, a meta-analysis including
1,665 gastric cancer patients and 2,358 controls revealed high
frequencies of the Arg/Arg allele in advanced gastric cancer
patients, while the Pro/Pro allele was significantly higher in
patients with cardia gastric cancer compared with healthy controls.
They came to the conclusion that P53 codon 72 polymorphism is
likely associated with gastric cancer among Asian populations
(28). The same results were reported
in a meta-analysis by Liu et al (29); they also demonstrated an association
between polymorphism in P53 at codon 72 and gastric cancer among
Asian populations.
The association of polymorphism at codon 72 of P53
is not restricted to gastric cancer, and has been discussed in
various types of carcinoma. Buyru et al (9) reported that the Arg/Arg genotype was
notably correlated with breast cancer. In contrast, a Chinese
population contradicted any possible association of P53 codon 72
Pro/Arg polymorphism with ovarian cancer (30). Tang et al (31) conducted a meta-analysis on the role of
P53 codon 72 polymorphism in colorectal cancer. They determined no
association between the Pro/Arg variant and the risk of colorectal
cancer in their study population. Meanwhile, a different systematic
analysis identified high frequencies of the Pro/Pro allele in
colorectal cancer patients (32). The
risk of oral cancer with P53 codon 72 polymorphism was investigated
in a study by Jing et al (33). They reported that the Arg genotype was
associated with a reduced risk of oral cancer, and a high frequency
of the Pro/Pro allele in oral cancer patients. Furthermore, the
Arg/Arg genotype and reduced cancer risk has also been reported
(34). A study performed in a
Japanese population revealed that the Pro/Pro genotype at codon 72
was associated with increased risk of prostate cancer and its
progression (35). Two separate
meta-analyses by Jia et al (36) and Lao et al (37) suggested that the homozygote and
heterozygote genotypes of Pro participated in the development of
endometriosis in Asian and Caucasian populations. Analysis of this
variant among an Iranian population revealed that the Arg genotype
increased the risk of breast cancer while Pro served as a
protective factor (38).
As study limitations, the two population groups were
not matched based on age or gender, and there was no evaluation of
H. pylori infections, lifestyle or dietary regime. Other
limitations included the small sample size and sampling of
individuals of the same geographical region and race. Therefore, to
confirm the results, further studies considering different
geographical locations and races and a larger number of
participants are necessary. Nonetheless, the present findings
failed to indicate an association between P53 codon 72 polymorphism
and gastric cancer risk.
In conclusion, the present study identified no
significant association between Arg72Pro at codon 72 of P53 and
gastric cancer risk in North Iranian patients. Additionally, there
were no differences in genotype frequencies based on tumor
location, histological differentiation or tumor stage.
Acknowledgements
Not applicable.
Funding
The current study was supported by Mazandaran
University of Medical Sciences (grant no. MAZUMS.2649).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
AH and RA were responsible for study conception and
design. GJ, VO, YH, OA and MT were responsible for acquisition of
data. RA performed analysis of the data. AH and RA were responsible
for drafting of the manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Mazandaran University of Medical Sciences, Sari, Iran
(approval no. IR.MAZUMS.IMAMHOSPITAL.REC.1396.2649). Written
informed consent was obtained from all subjects following full
disclosure of the study objectives and procedures.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Wang C, Zhang J, Cai M, Zhu Z, Gu W, Yu Y
and Zhang X: DBGC: A Database of Human Gastric Cancer. PLoS One.
10:e01425912015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
El-Omar EM, Carrington M, Chow WH, McColl
KE, Bream JH, Young HA, Herrera J, Lissowska J, Yuan CC, Rothman N,
et al: Interleukin-1 polymorphisms associated with increased risk
of gastric cancer. Nature. 404:398–402. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ang TL and Fock KM: Clinical epidemiology
of gastric cancer. Singapore Med J. 55:621–628. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Eybpoosh S, Talebkhan Y, Saberi S,
Esmaeili M, Oghalaie A, Ebrahimzadeh F, Karimi T, Abdirad A,
Nahvijou A, Mohagheghi MA, et al: Mohammadi M. Age-specific gastric
cancer risk indicated by the combination of Helicobacter pylori
sero-status and serum pepsinogen levels. Iran Biomed J. 19:133–142.
2015.PubMed/NCBI
|
|
5
|
Zali H, Rezaei-Tavirani M and Azodi M:
Gastric cancer: Prevention, risk factors and treatment.
Gastroenterol Hepatol Bed Bench. 4:175–185. 2011.PubMed/NCBI
|
|
6
|
Yasui W, Sentani K, Sakamoto N, Anami K,
Naito Y and Oue N: Molecular pathology of gastric cancer: Research
and practice. Pathol Res Pract. 207:608–612. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Arjmand Kolukhi Z, Salehi Z, Mashayekhi F,
Najafi B and Mirpoor S: Analysis of Glu298Asp eNOS Gene
polymorphism in patients with Gastric Cancer in the Guilan
population. Analysis. 17:53–62. 2014.
|
|
8
|
Hossein Pour Feizi MA, Ravanbakhsh Gavgani
R, Pourahmad R, Pouladi N, Azarfam P and Montazeri V: Association
of p53 Arg/Pro polymorphism at codon 72 with risk of breast cancer
in east azerbaijani women. J Babol Univ Med Sci. 14:31–38.
2012.
|
|
9
|
Buyru N, Tigli H and Dalay N: P53 codon 72
polymorphism in breast cancer. Oncol Rep. 10:711–714.
2003.PubMed/NCBI
|
|
10
|
Gloria-Bottini F, Banci M, Saccucci P,
Neri A, Bottini E and Magrini A: p53 codon 72 polymorphism and
coronary artery disease: Evidence of interaction with ACP1. Med Sci
Monit. 18:CR712–CR715. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lin HY, Huang CH, Wu WJ, Chang LC and Lung
FW: TP53 codon 72 gene polymorphism paradox in associated with
various carcinoma incidences, invasiveness and chemotherapy
responses. Int J Biomed Sci. 4:248–254. 2008.PubMed/NCBI
|
|
12
|
Kalemi TG, Lambropoulos AF, Gueorguiev M,
Chrisafi S, Papazisis KT and Kotsis A: The association of p53
mutations and p53 codon 72, Her 2 codon 655 and MTHFR C677T
polymorphisms with breast cancer in Northern Greece. Cancer Lett.
222:57–65. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Weng Y, Lu L, Yuan G, Guo J, Zhang Z, Xie
X, Chen G and Zhang J: p53 codon 72 polymorphism and hematological
cancer risk: An update meta-analysis. PLoS One. 7:e458202012.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yu KD, Di GH, Yuan WT, Fan L, Wu J, Hu Z,
Shen ZZ, Zheng Y, Huang W and Shao ZM: Functional polymorphisms,
altered gene expression and genetic association link NRH:quinone
oxidoreductase 2 to breast cancer with wild-type p53. Hum Mol
Genet. 18:2502–2517. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liu Y, Zhang X, Han C, Wan G, Huang X,
Ivan C, Jiang D, Rodriguez-Aguayo C, Lopez-Berestein G, Rao PH, et
al: TP53 loss creates therapeutic vulnerability in colorectal
cancer. Nature. 520:697–701. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Stacey SN, Sulem P, Jonasdottir A, Masson
G, Gudmundsson J, Gudbjartsson DF, Magnusson OT, Gudjonsson SA,
Sigurgeirsson B, Thorisdottir K, et al Swedish Low-risk Colorectal
Cancer Study Group, : A germline variant in the TP53
polyadenylation signal confers cancer susceptibility. Nat Genet.
43:1098–1103. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Khatami F, Noorinayer B, Ghiasi S, Mohebi
R, Hashemi M and Zali MR: Single nucleotide polymorphisms of DNA
methyltransferase 1 gene and gastric cancer in iranian patients: a
case control study. Asian Pac J Cancer Prev. 10:1177–1182.
2009.PubMed/NCBI
|
|
18
|
Amoori N, Mahdavi S and Enayatrad M:
Epidemiology and trend of stomach cancer mortality in Iran. Int J
Epidemiol Res. 3:268–275. 2016.
|
|
19
|
Hedayatizadeh-Omran A, Rafiei A,
Alizadeh-Navaei R, Tehrani M, Valadan R, Moradzadeh K, Panbechi M
and Taghavi SM: Role of HER2 in brain metastasis of breast cancer:
A systematic review and meta-analysis. Asian Pac J Cancer Prev.
16:1431–1434. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hedayatizadeh-Omran A, Rafiei A, Khajavi
R, Alizadeh-Navaei R, Mokhberi V and Moradzadeh K: Association
between ghrelin gene (Leu72Met) polymorphism and ghrelin serum
level with coronary artery diseases. DNA Cell Biol. 33:95–101.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Uchino S, Noguchi M, Ochiai A, Saito T,
Kobayashi M and Hirohashi S: p53 mutation in gastric cancer: A
genetic model for carcinogenesis is common to gastric and
colorectal cancer. Int J Cancer. 54:759–764. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Matei MC, Negură L, Liliac L, Negură A and
Azoicăi D: Validation of PCR-RFLP techniques for the evaluation of
codon 72 of p53 and CYP1A1 gene's polymorphisms in relation with
ovarian cancer in a Romanian population. Rom J Morphol Embryol.
53:47–54. 2012.PubMed/NCBI
|
|
23
|
Pandey R, Misra V, Misra SP, Dwivedi M and
Misra A: Helicobacter pylori infection and a P53 codon 72 single
nucleotide polymorphism: A reason for an unexplained Asian enigma.
Asian Pac J Cancer Prev. 15:9171–9176. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chung WC, Lee KM, Lee BI, Chun JS, Lee SY,
Chang UI, Park SH, Yang JM, Choi KY and Chung IS: P53 genetic
polymorphism of gastric cancer in Korea. Korean J Intern Med.
21:28–32. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Liu X, Wang S, Xia X, Chen Y, Zhou Y, Wu
X, Zhang J, He S, Tan Y, Qiang F, et al: Synergistic role between
p53 and JWA: Prognostic and predictive biomarkers in gastric
cancer. PLoS One. 7:e523482012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhou Y, Li N, Zhuang W and Wu X: p53 Codon
72 polymorphism and gastric cancer risk in a Chinese Han
population. Genet Test Mol Biomarkers. 14:829–833. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Shen H, Solari A, Wang X, Zhang Z, Xu Y,
Wang L, Hu X, Guo J and Wei Q: P53 codon 72 polymorphism and risk
of gastric cancer in a Chinese population. Oncol Rep. 11:1115–1120.
2004.PubMed/NCBI
|
|
28
|
Zhou Y, Li N, Zhuang W, Liu GJ, Wu TX, Yao
X, Du L, Wei ML and Wu XT: P53 codon 72 polymorphism and gastric
cancer: A meta-analysis of the literature. Int J Cancer.
121:1481–1486. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Liu KJ, Qi HZ, Yao HL, Lei SL, Lei ZD, Li
TG and Zhao H: An updated meta-analysis of the p53 codon 72
polymorphism and gastric cancer risk. Mol Biol Rep. 39:8265–8275.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kang S, Duan LH, Zhang JH, Guo W, Wang N
and Li Y: Association of p53 gene polymorphism with susceptibility
to ovarian cancer. Zhonghua Fu Chan Ke Za Zhi. 39:754–758. 2004.(In
Chinese). PubMed/NCBI
|
|
31
|
Tang NP, Wu YM, Wang B and Ma J:
Systematic review and meta-analysis of the association between P53
codon 72 polymorphism and colorectal cancer. Eur J Surg Oncol.
36:431–438. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Liu Y, Qin H, Zhang Y, Shi T, Liu B, Sun Y
and Ma Y: P53 codon 72 polymorphism and colorectal cancer: A
meta-analysis of epidemiological studies. Hepatogastroenterology.
58:1926–1929. 2011.PubMed/NCBI
|
|
33
|
Jing G, Lv K and Jiao X: The p53 codon 72
polymorphism and the risk of oral cancer in a Chinese Han
population. Genet Test Mol Biomarkers. 16:1149–1152. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhang R, Chen W, Zhang W, Jiang Q, Liu C,
Lin Y, Hu Z, Yu S and Xu G: Genetic polymorphisms of p53 codon 72
and bladder cancer susceptibility: A hospital-based case-control
study. Genet Test Mol Biomarkers. 15:337–341. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Suzuki K, Matsui H, Ohtake N, Nakata S,
Takei T, Nakazato H, Okugi H, Koike H, Ono Y, Ito K, et al: A p53
codon 72 polymorphism associated with prostate cancer development
and progression in Japanese. J Biomed Sci. 10:430–435. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Jia S, Xu L, Chan Y, Wu X, Yang S, Yu H,
Yang H, Luo Y and Tang W: p53 codon 72 polymorphism and
endometriosis: A meta-analysis. Arch Gynecol Obstet. 285:1657–1661.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Lao X, Chen Z and Qin A: p53 Arg72Pro
polymorphism confers the susceptibility to endometriosis among
Asian and Caucasian populations. Arch Gynecol Obstet.
293:1023–1031. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Soleimani A, Rahmani Y, Farshchian N,
Delpisheh A, Khassi K, Shahmohammadi A and Amirifard N: The
evaluation of p53 polymorphism at codon 72 and association with
breast cancer in Iran: A systematic review and meta-analysis. J
Cancer Prev. 21:1–293. 2016. View Article : Google Scholar : PubMed/NCBI
|