Introduction
Although the normal duration of pregnancy is 40
weeks, 5–10% (mean average 8%) of births take place preterm in
Europe, with prematurity constituting a main cause of perinatal
morbidity and mortality (1).
Pregnancy duration is primarily determined by a ‘placental clock’,
which is activated in early stage pregnancy (2) and involves the triggering of several
mechanisms including apoptotic events. Apoptosis occurs
physiologically in all regions of the human placenta throughout
pregnancy and, in its early stages, ensures successful placentation
and embryo development; at term increased apoptosis of all villous
components indicates initiation of delivery (3).
In response to hypoxia and oxidative stress, the
signalling pathways of hypoxia-induced apoptosis in human
trophoblasts are considered to involve activation and/or
upregulation of certain genes, including c-Jun N-terminal kinase
(JNK), a key apoptosis regulatory gene (4,5). JNK
serves as a mediator, bridging upstream hypoxia and oxidative
stress events and the downstream mammalian STE20-like protein
kinase 3 (MST3) gene (the human counterpart of the protein
serine/threonine kinase Ste20 in yeast), with subsequent caspase 3
(CASP3) activation (6). In human
placentas following at-term births and caesarean deliveries without
labour, Wu et al (4)
identified that MST3 served a crucial role in apoptosis and
detected significantly increased MST3 expression, trophoblast
apoptosis, oxidative stress and hypoxia, and reported that these
events were significantly correlated. In their study, trophoblast
apoptosis was induced by hypoxia via nitric oxide synthase
activation, which generated diverse components including
O2− that upregulated MST3 via JNK activation
(5). Other previous study has
investigated apoptotic genes in the placenta as contributors to
spontaneous preterm birth (sPTB). Dutta et al (7) screened a panel of phosphorylated
proteins to identify potential markers of activation and identified
higher JNK phosphorylation in sPTB patients than in patients with
premature rupture of membranes, suggesting specific pathways are
involved in sPTB.
As apoptosis triggered by oxidative stress may serve
a role in the pathophysiology of sPTB, we hypothesized that
susceptibility may be linked to genes encoding proteins that are
involved in apoptosis regulation. Consequently, single nucleotide
polymorphisms (SNPs) affecting expression levels of apoptotic
pathway genes (JNK, MST3 and CASP3) could plausibly contribute to
the risk of sPTB.
Evidence exists that inflammation serves a role in
the pathophysiology of sPTB (8–10). Given
that inflammation contributes to the initiation of sPTB, genes
encoding proteins involved in the regulation of inflammatory
mediators are plausible candidate genes, and genetic polymorphisms
affecting inflammatory-associated gene transcription and subsequent
expression levels may contribute to the development and progression
of inflammatory disorders (11). In
this regard, as apoptosis occurs in the inflammatory process, it
was hypothesized that mechanisms associated with apoptosis may be
involved.
Although previous data demonstrated that genetic
factors contributed to gestational length and risk of preterm birth
(12), non-genetic factors may also
influence oxidative stress and redox system imbalances in the
maternal-foetal intrauterine compartment (13). Maternal lifestyle (14) and health status, including diseases
with onset prior to or during pregnancy (15), may influence oxidative stress and
affect the apoptotic pathway leading to at-term birth or sPTB, both
of which also appear consequent to a mechanism involving genetic
and non-genetic factors, which may modulate the oxidative
stress-induced apoptosis.
For these reasons, the present study first evaluated
the role of certain SNPs (JNK: rs7560; MST3: rs9517320; CASP3:
rs1049216) in influencing expression of apoptotic pathway genes in
the placenta and maternal blood, and subsequently investigated
whether maternal lifestyle factors and health status may
potentially affect oxidative stress.
Materials and methods
Subjects
The present single-centre study enrolled 300
pregnant women at term (gestational age >37 weeks) as controls
and 43 preterm pregnant women (gestational age 22 weeks + 0 days to
36 weeks + 6 days) as sPTB cases on admission to the Department of
Obstetrics and Gynaecology of Hospital ‘S. Maria della
Misericordia’ (Perugia, Italy), for spontaneous delivery from
November 2013 to June 2015. Gestational age was determined by the
last menstrual period and confirmed by ultrasound dating before 20
weeks. Exclusion criteria were twin pregnancies, known genetic
foetal anomalies and indicated preterm delivery.
Each case and control completed a self-reported
questionnaire to record medical and obstetrical history, maternal
lifestyle factors and health status including the onset of diseases
prior to or during pregnancy, and smoking and eating habits.
The pregnant women were divided into 5 classes
according to the number of Mediterranean diet (MD) criteria
fulfilled as described by Meltzer et al (16). The present study used five MD criteria
as follows: Intake of ≥5 vegetables and fruit per day; ≥2 servings
of fish per week; use of olive oil for cooking and salad dressing;
≤2 servings of red meat per week; and ≤2 cups of coffee per
day.
The following pathologies with onset during
pregnancy were analysed: Genital and urinary tract infections,
current hypertensive diseases (high blood pressure, preeclampsia,
haemolysis/elevated liver enzymes/low platelet count syndrome) and
PROM or preterm PROM (P-PROM). sPTBs in previous pregnancies were
also considered.
Written informed consent was obtained from all
participants. The study protocol was approved by the Medical Ethics
Committee of the Region of Umbria (CEAS Umbria; approval no.
2157113). The experimental procedures adhered to the ethical
standards for human experimentation of the 1975 Declaration of
Helsinki (revised in 1983).
Selection of SNPs
To select SNPs a computational analysis was
performed using the polymiRTS database 3.0 (http://compbio.uthsc.edu/miRSNP/) to identify genetic
variants responsible for transcriptional variation (17) and the Preterm Birth database (dbPTB)
(http://ptbdb.cs.brown.edu/dbPTBv1.php) to identify
links with sPTB. Candidate SNPs were selected for analysis based on
biological plausibility [their position in micro (mi)RNA target
sites] and their potential role in sPTB.
DNA sampling and genotyping
Placenta tissue samples at time of delivery were
frozen in liquid nitrogen within 10 min of delivery and stored at
−80°C until further analysis. Following delivery, 10 ml venous
blood was drawn from each participant, collected in tubes
containing EDTA (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany),
immediately centrifuged at 1,600 × g per 10 min at room temperature
to separate the buffy coat from plasma and frozen at −80°C for
subsequent DNA extraction.
DNA was extracted from placenta tissue with a QiAmp
DNA Mini kit (Qiagen GmbH, Hilden, Germany) and from the buffy coat
with a NucleoSpin Blood kit (Macherey-Nagel GmbH & Co. KG,
Duren, Germany) according to the manufacturer's instructions.
SNP genotyping was performed by real-time polymerase
chain reaction (7300 Real Time PCR System; Applied Biosystems;
Thermo Fisher Scientific, Inc., Waltham, MA, USA) using TaqMan SNP
Genotyping Assays (Thermo Fisher Scientific, Inc.). The following
polymorphisms were analysed: JNK -rs7560 (T>G), MST3 -rs9517320
(A>C) and CASP3 -rs1049216 (A>G) with minor allele
frequencies (MAFs) of 0.31, 0.47 and 0.40, respectively. These
polymorphisms were selected based on MAF >0.05 to avoid rare
polymorphisms and enable identification in our population, and with
localization in the sites of interaction of miRNAs, suggestive of
an influence on expression by interfering with miRNA function.
The amplification reaction for all SNPs was
performed by Real-Time PCR 7300 detection system (Applied
Biosystems, Life Technologies, Carlsbad, CA, USA) in a total volume
of 20 µl containing 10 µl TaqMan Universal PCR Master Mix
[containing dNTPs, uracil-DNA glycosylase (UNG) and appropriate
buffer; Thermo Fisher Scientific, Inc.] 5 µl extracted DNA and 1 µl
TaqMan SNP Genotyping Assay (Thermo Fisher Scientific, Inc.)
specific for each SNP. The thermal profile consisted of a 2-min
cycle at 50°C for UNG treatment, a 10-min cycle at 95°C for DNA
denaturation and 50 cycles of 15 sec at 92°C and 1 min at 60°C for
DNA denaturation, primer hybridization and probe-DNA targeting and
amplification, respectively. In each experiment, unknown samples in
a single well and two wells for no template control (NTC) reactions
(reactants + sterile distilled water) were amplified for each SNP
that was analysed. Following PCR, fluorescence signals were
analysed by the Applied Biosystems 7300 Real Time-PCR System
(software 7300 System v1.4.0) using TaqMan SNP Genotyping assay to
determine the distribution of gene-specific polymorphisms in the
study population. For each sample, a unique pair of fluorescent dye
detectors is used: One fluorescent dye detector is a perfect match
to the wild-type (allele 1, VIC 551 nm) and the other fluorescent
dye detector is a perfect match to the mutation (allele 2, FAM 517
nm). Each pregnant woman was classified as homozygous when the
polymorphism was present in both alleles (homozygous for the mutant
allele) or in neither of the two alleles (homozygous for the
wild-type allele). Subjects were defined as heterozygous when the
polymorphism was present in only one allele.
Statistical analysis
Differences in allele/genotype frequencies and
categorical variables between cases and controls were analysed
using the χ2 test. The Mann-Whitney U test was used to
analyse differences in continuous variables. As variables were
distributed asymmetrically, the results were expressed as median,
minimum and maximum.
Multiple logistic regression analysis was used to
assess the association between the incidence of sPTB and
dichotomous variables (presence/absence of alleles), in which odds
ratios (ORs) and 95% confidence intervals (CIs) were calculated.
The logistic regression model predicts the probability of disease
according to the explanatory variables. Our multivariate analysis
selected variables (genetic factors, maternal lifestyle and health
status) with a statistical difference between sPTB cases and
controls reaching P<0.25 in a univariate analysis. Data analysis
was performed using IBM SPSS version 20.0 (IBM Corp., Armonk, NY,
USA).
Results
Demographic characteristics
The current study recruited a total of 343 pregnant
women: 300 who delivered at term (controls) and 43 who delivered
spontaneously preterm (sPTB). Table I
lists the demographic characteristics of these subjects. Age and
pre-pregnancy body mass index were similar between the groups.
Placenta and neonatal weights were significantly lower (P<0.001)
in the sPTB group, confirming premature births. The majority of
women in each group was Caucasian, with smaller populations of
subjects of African, Oriental, Hispanic and Indo-Asian origin. An
increased proportion of African women were included in the sPTB
group compared with in the control group (P=0.001).
 | Table I.Demographic characteristics of
enrolled subjects. |
Table I.
Demographic characteristics of
enrolled subjects.
| Characteristic | Controls | Cases | P-value |
|---|
| n | 300 | 43 |
|
| Age, years | 34 (19–46) | 32 (20–45) |
0.181 |
| Pregravid body mass
index, kg/m2 | 21.5 (15.8–42.2) | 22.4 (16.9–38.1) |
0.326 |
| Ethnicity (%) |
|
|
|
|
Caucasian | 285 (96.0) | 37 (86.0) |
|
|
African | 3 (1.0) | 3 (7.0) |
0.001 |
|
Oriental | 1 (0.3) | 2 (4.7) |
|
|
Hispanic | 7 (2.4) | 0 (0.0) |
|
|
Indo-Asian | 1 (0.3) | 1 (2.3) |
|
| Gestational age at
delivery, weeks | 39 (37–42) | 35 (23–36) | <0.001 |
| Placenta weight,
g | 575 (358–938) | 472 (200–830) | <0.001 |
| Neonatal weight,
g | 3.340
(1,128–4,570) | 2,163
(350–3,420) | <0.001 |
SNPs: Foetal and maternal genetic
aspects
Table II presents the
genotype frequencies of all SNPs analysed in placenta and maternal
blood samples from cases and controls. No significant differences
were identified in genotype distribution between the two groups.
The genotype results of paired combinations of the SNPs were
combined (JNK/CASP3; JNK/MST3; MST3/CASP3) yielding nine classes
for each combination in placenta and maternal blood, respectively.
No significant intergroup differences were identified in any of
these combinations (Tables III and
IV).
 | Table II.Genotype frequencies of analysed
single nucleotide polymorphisms in placenta and maternal blood
samples of cases and controls. |
Table II.
Genotype frequencies of analysed
single nucleotide polymorphisms in placenta and maternal blood
samples of cases and controls.
|
|
|
Genotype
frequencies |
|---|
|
|
|
|
|---|
|
|
| JNK |
| MST3 |
| CASP3 |
|
|---|
|
|
|
|
|
|
|
|
|
|---|
| Allele 1/allele
2 | G/T | G/G | T/T | P-value | A/C | A/A | C/C | P-value | G/A | G/G | A/A | P-value |
|---|
| Placenta |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Controls | n | 116 | 29 | 155 | 0.277 | 143 | 56 | 100 | 0.764 | 118 | 15 | 167 | 0.358 |
|
| % | 38.7 |
9.7 | 51.7 |
| 47.8 | 18.7 | 33.4 |
| 39.3 |
5.0 | 55.7 |
|
|
Cases | n | 22 | 4 | 17 |
| 18 | 9 | 16 |
| 19 | 4 | 20 |
|
|
| % | 51.2 |
9.3 | 39.5 |
| 41.9 | 20.9 | 37.2 |
| 44.2 |
9.3 | 46.5 |
|
| Maternal blood |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Controls | n | 108 | 39 | 153 | 0.965 | 139 | 55 | 106 | 0.414 | 108 | 18 | 174 | 0.409 |
|
| % | 36.0 | 13.0 | 51.0 |
| 46.3 | 18.3 | 35.3 |
| 36.0 |
6.0 | 58.0 |
|
|
Cases | n | 16 | 5 | 22 |
| 24 | 8 | 11 |
| 20 | 2 | 21 |
|
|
| % | 37.2 | 11.6 | 51.2 |
| 55.8 | 18.6 | 25.6 |
| 46.5 |
4.7 | 48.8 |
|
 | Table III.Genotype frequencies of paired single
nucleotide polymorphisms in placenta samples of cases and
controls. |
Table III.
Genotype frequencies of paired single
nucleotide polymorphisms in placenta samples of cases and
controls.
|
|
| JNK/CASP3 |
|
|---|
|
|
|
|
|
|---|
| Placenta |
| GT/GA | GG/GA | TT/GA | GT/AA | GG/AA | TT/AA | GT/GG | GG/GG | TT/GG | P-value |
|---|
| Controls | n | 49 | 9 | 52 | 9 | 3 | 3 | 69 | 17 | 89 | 0.145 |
|
| % | 16.3 | 3.0 | 17.3 | 3.0 | 1.0 | 1.0 | 23.0 | 5.7 | 29.7 |
|
| Cases | n | 10 | 2 | 7 | 1 | 0 | 3 | 11 | 2 | 7 |
|
|
| % | 23.3 | 4.7 | 16.3 | 2.3 | 0.0 | 7.0 | 25.6 | 4.7 | 16.3 |
|
|
|
|
|
JNK/MST3 |
|
|
|
|
|
|
|
Placenta |
| GT/AC | GT/AA | GT/CC | GG/AC | GG/AA | GG/CC | TT/AC | TT/AA | TT/CC | P-value |
|
| Controls | n | 53 | 12 | 72 | 14 | 9 | 32 | 54 | 8 | 45 | 0.459 |
|
| % | 17.7 |
4.0 | 24.1 |
4.7 | 3.0 | 10.7 | 18.1 | 2.7 | 15.1 |
|
| Cases | n | 12 | 1 | 5 | 4 | 1 | 4 | 6 | 2 | 8 |
|
|
| % | 27.9 |
2.3 | 11.6 |
9.3 | 2.3 |
9.3 | 14.0 | 4.7 | 18.6 |
|
|
|
|
|
MST3/CASP3 |
|
|
|
|
|
|
|
Placenta |
| AC/GA | AC/GG | AC/AA | AA/GA | AA/GG | AA/AA | CC/GA | CC/GG | CC/AA | P-value |
|
| Controls | n | 63 | 18 | 36 | 7 | 5 | 3 | 72 | 33 | 62 | 0.258 |
|
| % | 21.1 |
6.0 | 12.0 | 2.3 | 1.7 | 1.0 | 24.1 | 11.0 | 20.7 |
|
| Cases | n | 5 | 4 | 10 | 3 | 1 | 0 | 10 | 4 | 6 |
|
|
| % | 11.6 |
9.3 | 23.3 | 7.0 | 2.3 | 0.0 | 23.3 |
9.3 | 14.0 |
|
 | Table IV.Genotype frequencies of paired single
nucleotide polymorphisms in maternal blood samples of cases and
controls. |
Table IV.
Genotype frequencies of paired single
nucleotide polymorphisms in maternal blood samples of cases and
controls.
|
|
| JNK/CASP3 |
|
|---|
|
|
|
|
|
|---|
| Maternal blood | GT/GA | GG/GA | TT/GA | GT/AA | GG/AA | TT/AA | GT/GG | GG/GG | TT/GG | P-value |
|---|
| Controls | n | 38 | 17 | 53 | 4 | 3 | 11 | 66 | 19 | 89 | 0.194 |
|
| % | 12.7 |
5.7 | 17.7 | 1.3 | 1.0 |
3.7 | 22.0 |
6.3 | 29.7 |
|
| Cases | n | 3 | 3 | 14 | 0 | 0 | 2 | 13 | 2 | 6 |
|
|
| % |
7.0 |
7.0 | 32.6 | 0.0 | 0.0 |
4.7 | 30.2 |
4.7 | 14.0 |
|
|
|
|
|
JNK/MST3 |
|
|
|
|
|
|
| Maternal
blood | GT/AC | GT/AA | GT/CC | GG/AC | GG/AA | GG/CC | TT/AC | TT/AA | TT/CC | P-value |
|
| Controls | n | 43 | 23 | 72 | 18 | 7 | 30 | 47 | 9 | 51 | 0.379 |
|
| % | 14.3 |
7.7 | 24.0 |
6.0 | 2.3 | 10.0 | 15.7 | 3.0 | 17.0 |
|
| Cases | n | 11 | 1 | 12 | 1 | 2 | 5 | 4 | 2 | 5 |
|
|
| % | 25.6 |
2.3 | 27.9 |
2.3 | 4.7 | 11.6 |
9.3 | 4.7 | 11.6 |
|
|
|
|
|
MST3/CASP3 |
|
|
|
|
|
|
| Maternal
blood | AC/GA | AC/GG | AC/AA | AA/GA | AA/GG | AA/AA | CC/GA | CC/GG | CC/AA | P-value |
|
| Controls | n | 50 | 23 | 35 | 8 | 5 | 5 | 81 | 27 | 66 | 0.827 |
|
| % | 16.7 |
7.7 | 11.7 | 2.7 | 1.7 | 1.7 |
2.7 |
9.0 | 22.0 |
|
| Cases | n | 10 | 4 | 6 | 1 | 1 | 0 | 13 | 3 | 5 |
|
|
| % | 23.3 |
9.3 | 14.0 | 2.3 | 2.3 | 0.0 | 30.2 |
7.0 | 11.6 |
|
To determine whether foetal/maternal genotype
interactions impacted on the risk of sPTB, placenta (foetal) and
maternal blood (maternal) genotypes were combined for each SNP,
obtaining nine classes of foetal/maternal genotypes. The
foetal/maternal genotype frequencies for the SNPs of the JNK, MST3
and CASP3 genes did not differ significantly between the controls
and sPTB cases (Table V).
 | Table V.Genotype frequencies of
placenta/maternal blood combinations of analysed SNPs in controls
and cases. |
Table V.
Genotype frequencies of
placenta/maternal blood combinations of analysed SNPs in controls
and cases.
|
|
| JNK |
|
|---|
|
|
|
|
|
|---|
| Placenta/maternal
blood | GT/GT | GT/GG | GT/TT | GG/GT | GG/GG | GG/TT | TT/GT | TT/GG | TT/TT | P-value |
|---|
| Controls | n | 49 | 30 | 39 | 18 | 7 | 3 | 40 | 1 | 113 | 0.329 |
|
| % | 16.3 | 10.0 | 13.0 |
6.0 | 2.3 | 1.0 | 13.3 | 0.3 | 37.7 |
|
| Cases | n | 10 | 2 | 9 | 1 | 3 | 0 | 6 | 0 | 12 |
|
|
| % | 23.2 |
4.7 | 20.9 |
2.3 | 7.0 | 0.0 | 14.0 | 0.0 | 27.9 |
|
|
|
|
| MST3 |
|
|
|
|
|
|
|
Placenta/maternal blood | AC/AC | AC/AA | AC/CC | AA/AC | AA/AA | AA/CC | CC/AC | CC/CC | P-value |
|
| Controls | n | 66 | 34 | 42 | 35 | 20 | 1 | 36 | 65 | 0.312 |
|
| % |
22.1 |
11.3 |
14.0 |
11.7 |
6.7 |
0.3 |
12.0 |
21.7 |
|
| Cases | n | 11 | 4 | 3 | 5 | 4 | 0 | 6 | 9 |
|
|
| % |
25.6 |
9.3 |
7.0 |
11.6 |
9.3 |
0.0 |
14.0 |
20.9 |
|
|
|
|
| CASP3 |
|
|
|
|
|
|
|
Placenta/maternal blood | GA/GA | GA/GG | GA/AA | GG/GA | GG/GG | AA/GA | AA/AA | P-value |
|
| Controls | n | 61 | 10 | 47 | 7 | 8 | 40 | 127 | 0.505 |
|
| % | 20.3 |
3.3 |
15.7 |
2.3 |
2.7 |
2.3 |
42.3 |
|
| Cases | n | 10 | 2 | 6 | 3 | 1 | 8 | 13 |
|
|
| % | 23.3 |
4.7 |
14.0 |
7.0 |
2.3 |
18.6 |
30.2 |
|
Maternal lifestyle factors
With regard to maternal lifestyle factors, the
majority of controls and sPTB cases declared that they had never
smoked (cases, 79.1%; controls, 70.3%); a percentage of each group
stated they gave up smoking immediately following conception
(cases, 11.6%; controls, 20.0%) and smaller percentages of each
group had continued smoking during pregnancy (cases, 9.3%;
controls, 9.7%). No statistical significance was identified
regarding smoking status in the sPTB group versus the control group
(P=0.407). No pregnant woman fulfilled all of the MD criteria. The
majority of controls (58.0%) had a good MD (3 or 4 criteria
fulfilled) while the majority of sPTB cases (60.5%) had a mediocre
MD (1 or 2 criteria fulfilled). This difference tended towards
significance (P=0.069). A significant difference was identified in
the practice of physical activities. Unlike the majority of
controls (51.5% practising physical activity), the majority of sPTB
women (69.8%) did not practice any physical activities P=0.014).
Urinary and genital tract infections were similarly distributed
between the groups (cases, 7.0%; controls, 11.0%; P=0.596) while
hypertensive diseases and PROM/P-PROM were significantly more
frequent among sPTB women (9.3 and 32.6%, respectively; P=0.001;
P<0.001) than controls (0.3 and 2.0%, respectively). The
frequency of sPTB in previous pregnancies was similar in both
groups (cases, 4.7%; controls, 1.0%; Table VI).
 | Table VI.Maternal lifestyle factors and
maternal health status during and prior to pregnancy in the studied
groups. |
Table VI.
Maternal lifestyle factors and
maternal health status during and prior to pregnancy in the studied
groups.
| Maternal lifestyle
factor | Controls, n
(%) | Cases, n (%) | P-value |
|---|
| Smoking |
|
|
|
| Never
smoked | 211 (70.3) | 34 (79.1) | 0.407 |
| Active
smoker | 29 (9.7) | 4
(9.3) |
|
|
Ex-smoker | 60 (20.0) | 5 (11.6) |
|
| Eating habits
according to Mediterranean diet criteria |
|
|
|
| 1
criterion | 18 (6.0) | 6 (14.0) | 0.069 |
| 2
criteria | 108 (36.0) | 20 (46.5) |
|
| 3
criteria | 121 (40.3) | 13 (30.2) |
|
| 4
criteria | 53 (17.7) | 4 (9.3) |
|
| Physical
activity |
|
|
|
|
Absent | 145 (48.5) | 30 (69.8) |
|
|
Present | 154 (51.5) | 13 (30.2) | 0.014 |
|
| Maternal health
status | Controls, n
(%) | Cases, n
(%) | P-value |
|
| Current urinary and
genital tract infections |
|
|
|
|
Absent | 267 (89.0) | 40 (93.0) | 0.596 |
|
Present | 33 (11.0) | 3 (7.0) |
|
| Current
hypertensive diseases |
|
|
|
|
Absent | 299 (99.7) | 39 (90.7) | 0.001 |
|
Present | 1 (0.3) | 4 (9.3) |
|
| Current
PROM/preterm PROM |
|
|
|
|
Absent | 294 (98.0) | 29 (67.4) | <0.001 |
|
Present | 6 (2.0) | 14 (32.6) |
|
| Previous
spontaneous preterm birth |
|
|
|
|
Absent | 297 (99.0) | 41 (95.3) | 0.120 |
|
Present | 3 (1.0) | 2 (4.7) |
|
Combinations of genetic and lifestyle
factors
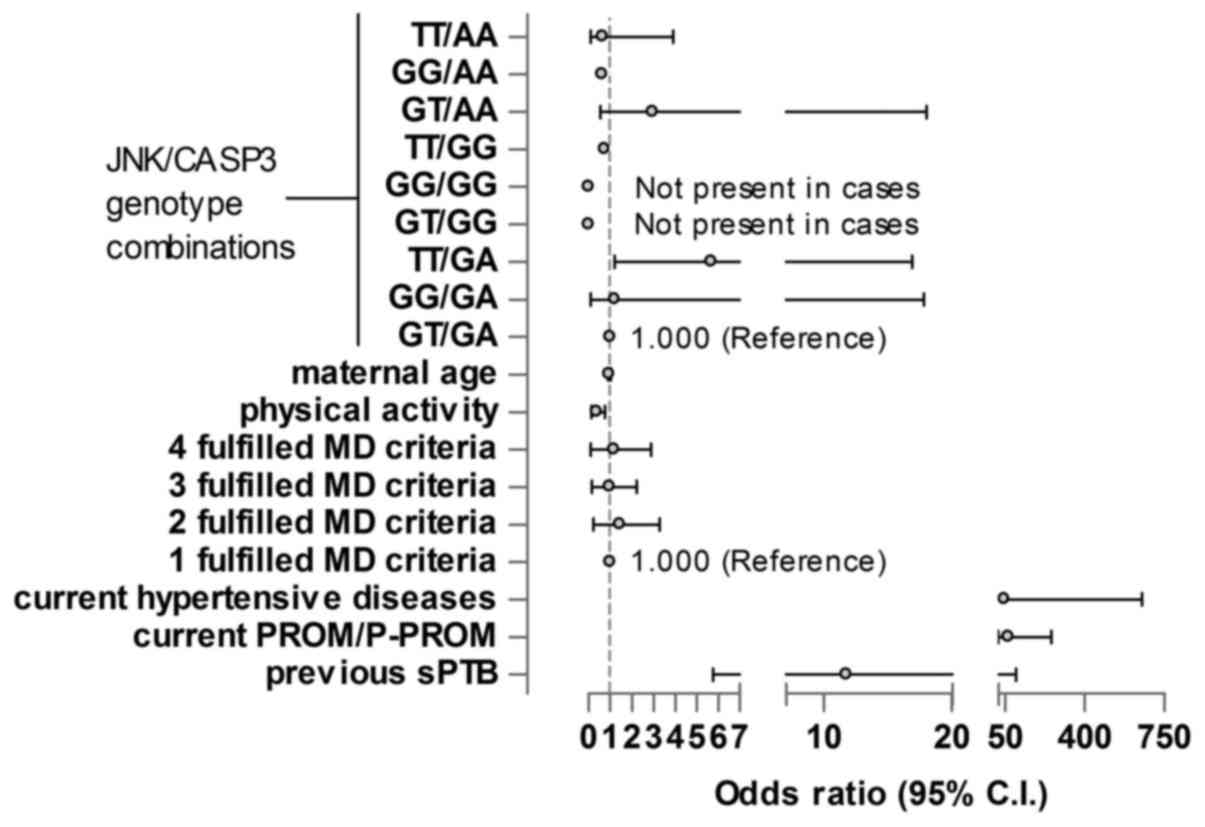
The JNK/CASP3 genotype combination in maternal
blood, maternal age, physical activity, diet, current hypertensive
diseases, current P-PROM/PROM and previous sPTB were the selected
variables to be analysed. The GT/GA genotype was selected as the
reference genotype. The TT/GA genotype of JNK/CASP3 was
significantly associated with sPTB; pregnant women carrying the
TT/GA genotype had higher risk of sPTB than those with the
reference genotype GT/GA (OR, 5.7; P=0.037) independently of all
other maternal factors. Physical activity reduced the risk of sPTB
(OR, 0.309; P=0.009). Current hypertensive diseases, PROM/P-PROM in
the current pregnancy and previous sPTB significantly increased the
risk of sPTB (ORs, 47.2, 65.6 and 11.7; P=0.004, <0.001 and
0.03, respectively; Fig. 1).
Discussion
In the current preliminary study investigating SNPs
in genes involved in placental apoptosis in at-term births and
sPTB, SNPs were selected by computational analysis using the
polymiRTS database 3.0 (17) and
dbPTB, and PubMed to search for literature supportive of the
database findings. To the best of our knowledge, the current study
has evaluated SNPs, namely rs7560, rs9517320 and rs1049216 SNPs of
the JNK, MST3 and CASP3 genes, respectively, that have not been
studied previously in the context of sPTB.
The JNK variant rs7560 is a G/T single-nucleotide
variation on human chromosome 14. The long arm of chromosome 14
contains protein-coding genes and two loci of key importance to the
immune system; more than 60 disease-associated genes have been
localized on several loci of chromosome 14 (18). The MST3 variant rs9517320 is an A/C
transversion substitution on human chromosome 13. It participates
in the mitogen-activated protein kinase cascade, and this variant
is considered as a longevity variant, based on its potential
association with the life-spans of individuals sharing similar
environmental and living conditions (19). The CASP3 variant rs1049216 is a G/A
transition substitution on human chromosome 4. It is located in the
3′UTR and, through ribosome binding, initiation or elongation, may
alter mRNA transcript stability (20). In putative microRNA target sites,
these SNPs may serve a biological role in regulating
post-transcriptional gene expression through mRNA destabilization
or translation repression, thus impacting on gene functions and
phenotypes that are involved in trophoblast apoptosis.
Consequently, they may underlie sPTB risk in pregnant women.
The present findings demonstrated that, in the
placenta and maternal blood, any of the three individual SNPs or
their paired combinations (JNK/CASP3; JNK/MST3; MST3/CASP3) in
placenta or maternal blood samples were not associated with sPTB
susceptibility. Furthermore, no significant associated was
identified when the placental genotype was combined with the
maternal for each SNP in order to assess the foetal-maternal
interaction.
Since sPTB involves complex interactions between
SNPs, maternal lifestyle and health status (21), the present study also investigated
non-genetic factors. Although sPTB did not associate with smoking
or diet, a significant association was identified with physical
activity, in concurrence with a previous report that physical
exercise could decrease the risk of sPTB (22). Multivariate analysis indicated that
the risk of sPTB was higher in pregnant women carrying the TT
genotype for JNK and the GA genotype for CASP3, and in pregnant
women with current hypertensive diseases, PROM/P-PROM or previous
sPTB. Current urinary and genital tract infections did not appear
to impact on sPTB risk.
In investigating the etiopathogenesis of sPTB,
studies have focused on genetically determined molecular mechanisms
in the inflammatory process (23,24),
identifying that cytokine gene polymorphisms were related to sPTB
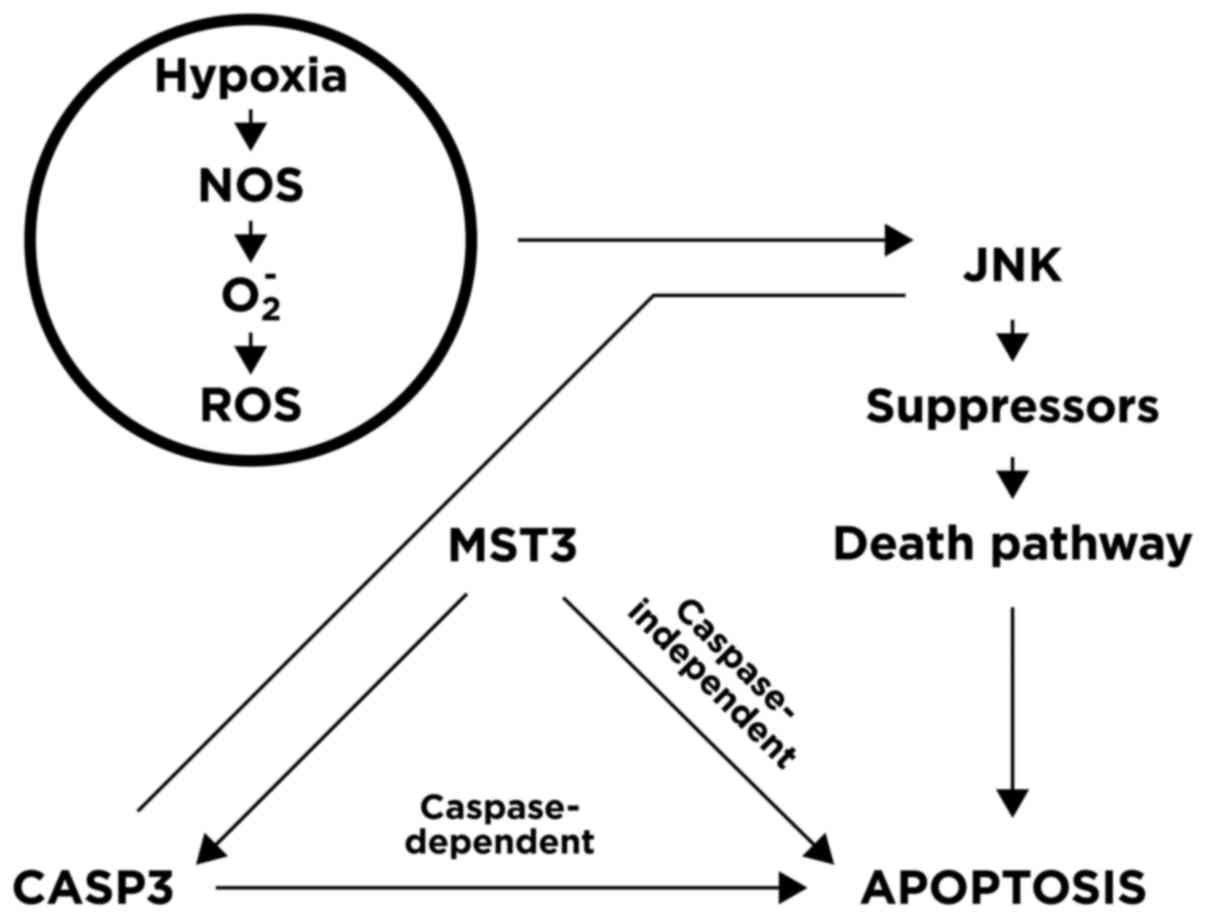
(24). Placental apoptosis may also
be involved: An imbalance between reactive oxygen species and
antioxidants may result in excessive oxidative stress that triggers
trophoblast apoptosis and initiates labour by upregulating
expression of the JNK, MST3 and CASP3 genes, as depicted in
Fig. 2.
Consequently, as in the inflammatory process, SNPs
were hypothesized to influence genes involved in placental
apoptosis in the current study. It was also speculated that sPTB
susceptibility may be increased by the combination of foetal and
maternal genotypes. Romero et al (25) observed that different combinations of
maternal and foetal genetic variants [including those of the genes
interleukin-6 receptor (IL6R1), tissue inhibitor of
metalloproteinases 2 (TIMP2), insulin like growth factor 2 (IGF2)
and collagen type iv alpha 3 chain (COL4A3)] involved in
inflammation and extracellular matrix metabolism, influenced sPTB
susceptibility differently. Notably, rs8192282 in foetal IL6R1
significantly doubled the risk of sPTB (OR=2.07, 95% CI=1.42–3-02;
P=0.000148). Furthermore, haplotypes for COL4A3 in the mother and
for IGF2 and IL2 in the foetus were associated with preterm
labour/delivery (25). Similar
findings were reported by Ota et al (26) and Roberts and Cooper (27), with these studies demonstrating a
foetal-maternal interaction in the aetiology of preeclampsia. In
pregnancy the interaction between maternal and foetal genetic
backgrounds may also influence a polymorphism-pathology association
and increase sPTB susceptibility independently or synergistically.
The significant association with combined JNK and CASP3 genotypes
suggested the apoptotic pathway was involved in the pathogenesis of
sPTB. Additionally, the identification that maternal lifestyle and
health status factors were involved in sPTB confirmed a
multifactorial mechanism involving genetic and non-genetic
factors.
The present study considered SNPs that, to the best
of our knowledge, have not been studied previously; all of which
have associations with the apoptotic pathway that triggered by
oxidative stress, with maternal lifestyle factors and with health
status. However, there were limitations to the current study.
Firstly, the cohort of women with sPTB was relatively small,
considering the frequency of preterm births and the MAF of each
SNP. The lack of significant association between any single SNP and
sPTB risk may have thus been due to the low number of women in the
sPTB group. However, even with the present sample size, subtle
difference in ethnicity was highlighted, which was analysed only in
terms of intergroup frequencies, but emerged as statistically
significant. Indeed, these preliminary results concur with those of
Dunlop et al (28), who
reported that the incidence of PTB was significantly higher among
African-American women. Since the present study detected some
significant associations and trends towards significance in other
parameters, confirmation of the findings and expanding the scope of
results by recruiting more pregnant women at risk of or having
experienced sPTB is warranted in the future. Secondly, as phenotype
is a function of a combination of alleles, some of which may
increase/decrease the risk of disease, multiple genetic
interactions in the process of apoptosis may serve a greater role
in disease susceptibility than any single variant. This hypothesis
is the basis of future planned investigation into the combination
of these SNPs in a consistent number of individuals to identify
predictive markers of sPTB.
In conclusion, considering that sPTB is
multifactorial, further studies are warranted to confirm the
present results in a larger cohort of women at risk. The ultimate
aim is to improve pregnancy care by introducing screening tests for
sPTB and drawing up guidelines on maternal lifestyle to reduce the
risk of sPTB.
Acknowledgements
The authors are thankful to Mrs. Nadia Belia for
performing blood sampling, and Dr Eleonora Giulietti, Dr Claudia
Giordano, Dr Giulia Babucci and Dr Francesca Pauselli for their
assistance with placenta sampling and patient management
(Department of Biomedical and Surgical Sciences, University of
Perugia, Perugia, Italy).
Funding
The current study was supported by the Italian
Ministry of Education University and Research-Relevant National
Interest Research Project (grant no. 20102CHST_003).
Availability of data and materials
The datasets used and analysed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
FT and EP contributed to conception and design of
the study, processed all placenta and blood samples, performed the
SNP genotyping and were primarily responsible for the writing of
the manuscript. VB performed the statistical analysis and
interpreted the results. GC enrolled the pregnant women and aided
in writing the manuscript. SM, CA and IG were responsible for
placenta and blood sampling and contributed to the interpretation
of data. MC performed SNP selection via the database searches and
contributed to the acquisition of patient demographic data. GCDR
contributed to conception and design of the study and revised the
manuscript critically for important intellectual content. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
All participants provided written informed consent
for participation in the study. The study protocol was approved by
the Ethics Committee of Region Umbria (approval no. 2157113). The
experimental procedures adhered to the ethical standards for human
experimentation of the 1975 Declaration of Helsinki (revised in
1983).
Consent for publication
Patients provided written informed consent for the
publication of associated data.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ferrero DM, Larson J, Jacobsson B, Di
Renzo GC, Norman JE, Martin JN Jr, D'Alton M, Castelazo E, Howson
CP, Sengpiel V, et al: Cross-country individual participant
analysis of 4.1 million singleton births in 5 countries with very
high human development index confirms known associations but
provides no biologic explanation for 2/3 of all preterm births.
PLoS One. 11:e01625062016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
McLean M, Bisits A, Davies J, Woods R,
Lowry P and Smith R: A placental clock controlling the length of
human pregnancy. Nat Med. 1:460–463. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Levy RR, Cordonier H, Czyba JC and Guerin
JF: Apoptosis in preimplantation mammalian embryo and genetics.
Ital J Anat Embryol. 106(Suppl 2): 101–108. 2001.PubMed/NCBI
|
|
4
|
Wu HY, Lin CY, Lin TY, Chen TC and Yuan
CJ: Mammalian Ste20-like protein kinase 3 mediates trophoblast
apoptosis in spontaneous delivery. Apoptosis. 13:283–294. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wu HY, Lin CY, Chen TC, Pan ST, Yuan CJ
and Wu HY1: Mammalian Ste20-like protein kinase 3 plays a role in
hypoxia-induced apoptosis of trophoblast cell line 3A-sub-E. Int J
Biochem Cell Biol. 43:742–750. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cohen M, Meisser A, Haenggeli L and
Bischof P: Involvement of MAPK pathway in TNF-alpha-induced MMP-9
expression in human trophoblastic cells. Mol Hum Reprod.
12:225–232. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Dutta EH, Behnia F, Boldogh I, Saade GR,
Taylor BD, Kacerovský M and Menon R: Oxidative stress
damage-associated molecular signaling pathways differentiate
spontaneous preterm birth and preterm premature rupture of the
membranes. Mol Hum Reprod. 22:143–157. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ruiz RJ, Jallo N, Murphey C, Marti CN,
Godbold E and Pickler RH: Second trimester maternal plasma levels
of cytokines IL-1Ra, Il-6 and IL-10 and preterm birth. J Perinatol.
32:483–490. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Nold C, Anton L, Brown A and Elovitz M:
Inflammation promotes a cytokine response and disrupts the cervical
epithelial barrier: A possible mechanism of premature cervical
remodeling and preterm birth. Am J Obstet Gynecol.
206:208.e201–e207. 2012. View Article : Google Scholar
|
|
10
|
Goepfert AR, Jeffcoat MK, Andrews WW,
Faye-Petersen O, Cliver SP, Goldenberg RL and Hauth JC: Periodontal
disease and upper genital tract inflammation in early spontaneous
preterm birth. Obstet Gynecol. 104:777–783. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang Y, Yang X, Zheng Y, Wu ZH, Zhang XA,
Li QP, He XY, Wang CZ and Feng ZC: The SEPS1 G-105A polymorphism is
associated with risk of spontaneous preterm birth in a Chinese
population. PLoS One. 8:e656572013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang G, Feenstra B, Bacelis J, Liu X,
Muglia LM, Juodakis J, Miller DE, Litterman N, Jiang PP, Russell L,
et al: Genetic associations with gestational length and spontaneous
preterm birth. N Engl J Med. 377:1156–1167. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Capra L, Tezza G, Mazzei F and Boner AL:
The origins of health and disease: The influence of maternal
diseases and lifestyle during gestation. Ital J Pediatr. 39:72013.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Smith LK, Draper ES, Evans TA, Field DJ,
Johnson SJ, Manktelow BN, Seaton SE, Marlow N, Petrou S and Boyle
EM: Associations between late and moderately preterm birth and
smoking, alcohol, drug use and diet: A population-based case-cohort
study. Arch Dis Child Foetal Neonatal Ed. 100:F486–491. 2015.
View Article : Google Scholar
|
|
15
|
Ehrenberg HM, Iams JD, Goldenberg RL,
Newman RB, Weiner SJ, Sibai BM, Caritis SN, Miodovnik M and
Dombrowski MP; Eunice Kennedy Shriver National Institute of Child
Health and Human Development (NICHD) Maternal-Fetal Medicine Units
Network (MFMU): Maternal obesity, uterine activity, and the risk of
spontaneous preterm birth. Obstet Gynecol. 113:48–52. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Meltzer HM, Brantsæter AL, Nilsen RM,
Magnus P, Alexander J and Haugen M: Effect of dietary factors in
pregnancy on risk of pregnancy complications: Results from the
Norwegian Mother and Child Cohort Study. Am J Clin Nutr.
94:(Suppl):. 1970S–1974S. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ziebarth JD, Bhattacharya A, Chen A and
Cui Y: PolymiRTS Database 2.0: Linking polymorphisms in microRNA
target sites with human diseases and complex traits. Nucleic Acids
Res. 40(D1): D216–D221. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Heilig R, Eckenberg R, Petit JL,
Fonknechten N, Da Silva C, Cattolico L, Levy M, Barbe V, de
Berardinis V, Ureta-Vidal A, et al: The DNA sequence and analysis
of human chromosome 14. Nature. 421:601–607. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yashin AI, Wu D, Arbeev KG and Ukraintseva
SV: Joint influence of small-effect genetic variants on human
longevity. Aging (Albany NY). 2:612–620. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kuersten S and Goodwin EB: The power of
the 3′ UTR: Translational control and development. Nat Rev Genet.
4:626–637. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Romero R, Dey SK and Fisher SJ: Preterm
labor: One syndrome, many causes. Science. 345:760–765. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Domingues MR, Matijasevich A and Barros
AJ: Physical activity and preterm birth: A literature review.
Sports Med. 39:961–975. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Srinivas SK, Ma Y, Sammel MD, Chou D,
McGrath C, Parry S and Elovitz MA: Placental inflammation and viral
infection are implicated in second trimester pregnancy loss. Am J
Obstet Gynecol. 195:797–802. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Harper M, Zheng SL, Thom E, Klebanoff MA,
Thorp J Jr, Sorokin Y, Varner MW, Iams JD, Dinsmoor M, Mercer BM,
et al: Eunice Kennedy Shriver National Institute of Child Health
and Human Development (NICHD) Maternal-Fetal Medicine Units Network
(MFMU): Cytokine gene polymorphisms and length of gestation. Obstet
Gynecol. 117:125–130. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Romero RI, Velez Edwards DR, Kusanovic JP,
Hassan SS, Mazaki-Tovi S, Vaisbuch E, Kim CJ, Chaiworapongsa T,
Pearce BD, Friel LA, Bartlett J, et al: Identification of fetal and
maternal single nucleotide polymorphisms in candidate genes that
predispose to spontaneous preterm labor with intact membranes. Am J
Obstet Gynecol. 202:431.e1–34. 2010. View Article : Google Scholar
|
|
26
|
Ota S, Miyamura H, Nishizawa H, Inagaki H,
Inagaki A, Inuzuka H, Suzuki M, Miyazaki J, Sekiya T, Udagawa Y, et
al: Contribution of fetal ANXA5 gene promoter polymorphisms to the
onset of pre-eclampsia. Placenta. 34:1202–1210. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Roberts JM and Cooper DW: Pathogenesis and
genetics of pre-eclampsia. Lancet. 357:53–56. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Dunlop AL, Kramer MR, Hogue CJ, Menon R
and Ramakrishan U: Racial disparities in preterm birth: An overview
of the potential role of nutrient deficiencies. Acta Obstet
Gyunecol Scand. 90:1332–1341. 2011. View Article : Google Scholar
|