Introduction
It is evident that mandibular prognathism (Online
Mendelian Inheritance in Man #176700) is a multifactorial phenotype
(1,2).
To identify the susceptibility loci of mandibular prognathism, the
first genome-wide association study (GWAS) was performed using
microsatellites in Japanese patients including 240 individuals with
mandibular prognathism and 360 individuals who were healthy
(3,4).
This previous GWAS (4) suggested that
six loci [1p22.3, 1q32.2 (Table I),
3q23, 6q23.2, 7q11.22 and 15q22.22] were identified as
susceptibility regions of mandibular prognathism. The mutations of
synovial sarcoma, X breakpoint 2 interacting protein, plexin A2
(PLXNA2) (Table II), Ras p21
protein activator 2, transcription factor 21, calneuron 1 and RAR
(retinoic acid receptor)-related orphan receptor α genes were
suggested as candidate genes, respectively.
 | Table I.Results of a previous genome-wide
association of mandibular prognathism using microsatellite
markers. |
Table I.
Results of a previous genome-wide
association of mandibular prognathism using microsatellite
markers.
|
|
|
|
| Allele frequency | Fishers exact test
P-value |
|
|---|
|
|
|
|
|
|
|
|
|---|
| Microsatellite | Cytobands | Alleles (n) | Significant
allelea | Cases | Controls | 2×2 | 2×m | Odds ratio (95%
confidence interval) |
|---|
| D1S1358i | 1q32.2 | 7 | 92 | 0.362 | 0.465 | 0.000422 | 0.007 | 0.65 (0.51–0.83) |
 | Table II.Information on nearest candidate gene
of susceptibility locus obtained from previous genome-wide
association study of mandibular prognathism using
microsatellites. |
Table II.
Information on nearest candidate gene
of susceptibility locus obtained from previous genome-wide
association study of mandibular prognathism using
microsatellites.
|
|
| Physical position of
amplicona |
|
|
|---|
| Microsatellite | Cytobands | From | To | Nearest gene | Gene
locationa |
|---|
| D1S1358i | 1q32.2 | 208365606 | 208365706 | PLXNA2 | (chr1: 208200588 -
208391267) |
PLXNA2 gene is located on chromosome 1q32.2 and
encodes plexin A2, a member of the plexin-A family of semaphorin
co-receptors (5). Semaphorins are a
large family of secreted or membrane-bound proteins that mediate
repulsive effects on axon path finding during nervous system
development (5). Semaphorin 3A
(sema3A) is a secreted protein and expressed by osteoblasts,
whereas sema3A is not detected in osteoclasts (6). Sema3A exerts an osteoprotective effect
by suppressing osteoclastic bone resorption and promoting
osteoblastic bone formation. Plexin A2 is expressed in osteoblasts,
and it is suggested that sema3A and plexin A2 binding stimulates
osteoblast differentiation (6).
Yoshida et al (7) reported
that sema3A may induce cell migration, proliferation and the
odontoblastic differentiation of human dental pulp stem cells.
However, human mandibular growth consists of a periosteal growth of
cortical bone and an endochondral growth of the mandibular condyle
(8). Active mandibular growth occurs
in an endochondral growth of the condyle (8). Gomez et al (9) reported that sema3A and plexin A2 mRNA
are expressed in mouse chondrogenic cell line MC 615. However, to
the best of our knowledge, there have been no previous studies on
the role of sema3A or plexin A2 in human chondrocytes.
The objectives of the present study were to examine
the function of sema3A and its receptor, plexin A2, in human
chondrocytes.
Materials and methods
Cell culture
Normal human chondrocytes (PromoCell GmbH,
Heidelberg, Germany) were seeded into a T25 culture vessel
(10,000–20,000 cells/cm2) and grown in commercial medium
(Chondrocyte Growth Medium; PromoCell GmbH) containing 10% fetal
calf serum (PromoCell GmbH). Cultures were maintained at 37°C in a
humidified atmosphere of 5% CO2 and 95% air, and the
medium was changed every 2–3 days. Subcultures were obtained by
removing the cells from the dish using 0.025% trypsin in
2-[4-(2-Hydroxyethyl)-1-piperazinyl] ethanesulfonic acid (HEPES)
buffered saline solution containing 0.01%
ethylenediaminetetraacetic acid (EDTA) (PromoCell GmbH).
Cells were seeded into 100 mm diameter culture
plates. After 1 day, they were exposed to new media with either: i)
A 100 ng/ml concentration, the concentration determined in a
previous study (7), of recombinant
human sema3A (R&D Systems, Inc., Minneapolis, MN, USA; high);
ii) A 1 ng/ml concentration of sema3A (low); or iii) without sema3A
(control). The effects of sema3A were analyzed at days 7 and 14 of
culturing, and representative data were obtained from five culture
plates of each group.
Protein assay and type II collagen and
human parathyroid hormone-related peptide (PTHrP) receptor 1
(PTH-R1) concentration determination
Cells and extracellular matrices were assayed for
concentrations of PTH-R1 on days 7 and 14 of culturing using an
Enzyme-linked Immunosorbent assay kit (cat no. SEC743Hu;
Cloud-Clone Corp., Houston, TX, USA) as previously described
(10). Cells and extracellular
matrices were detached with 0.025% trypsin in HEPES buffered saline
solution containing 0.01% EDTA, collected by centrifugation (220 ×
g for 3 min at 4°C), washed three times, resuspended in 1× PBS,
subject to ultrasonication, and centrifuged (1,500 × g for 10 min
at 4°C) to remove cellular debris in accordance with the
manufacturer's protocol.
The cells and extracellular matrices were also
assayed for concentrations of type II collagen using an enzyme
immunoassay kit (Type II Collagen Detection kit; Chondrex, Inc.,
Redmond, WA, USA) as previously described (11,12). In
accordance with the manufacturer's protocol, the cells and
extracellular matrices were solubilized by pepsin digestion (at 4°C
for 24 h) and elastase digestion (at 4°C for 24 h) prior to with
the assay. Culture media were also assayed for concentrations of
type II collagen. Protein content of the cells and extracellular
matrices were measured using a bicinchoninic acid assay reagent kit
(Thermo Fisher Scientific, Waltham, MA, USA) as previously
described (13,14). Bovine serum albumin was used as the
control. All assays were performed according to the manufacturer's
protocol.
Statistical methods
The data were represented as the mean ± standard
deviation. Representative data were obtained from five culture
plates of each group. Parametric tests were used in the present
study as the distribution of the concentrations of type II
collagen, PTH-R1 and the protein expression levels of control, low
and high sema3A groups were normally distributed. One-way analysis
of variance was used to compare the protein content and
concentrations of type II collagen and PTH-R1 among three groups.
Post hoc multiple comparisons were performed using Bonferroni's
test. Statistical analyses were performed using SPSS version 23.0
statistical package (IBM Corporation, Armonk, NY, USA). P<0.05
was considered to indicate a statistically significant
difference.
Results
Evaluation of human chondrocytes
response to sema3A treatment
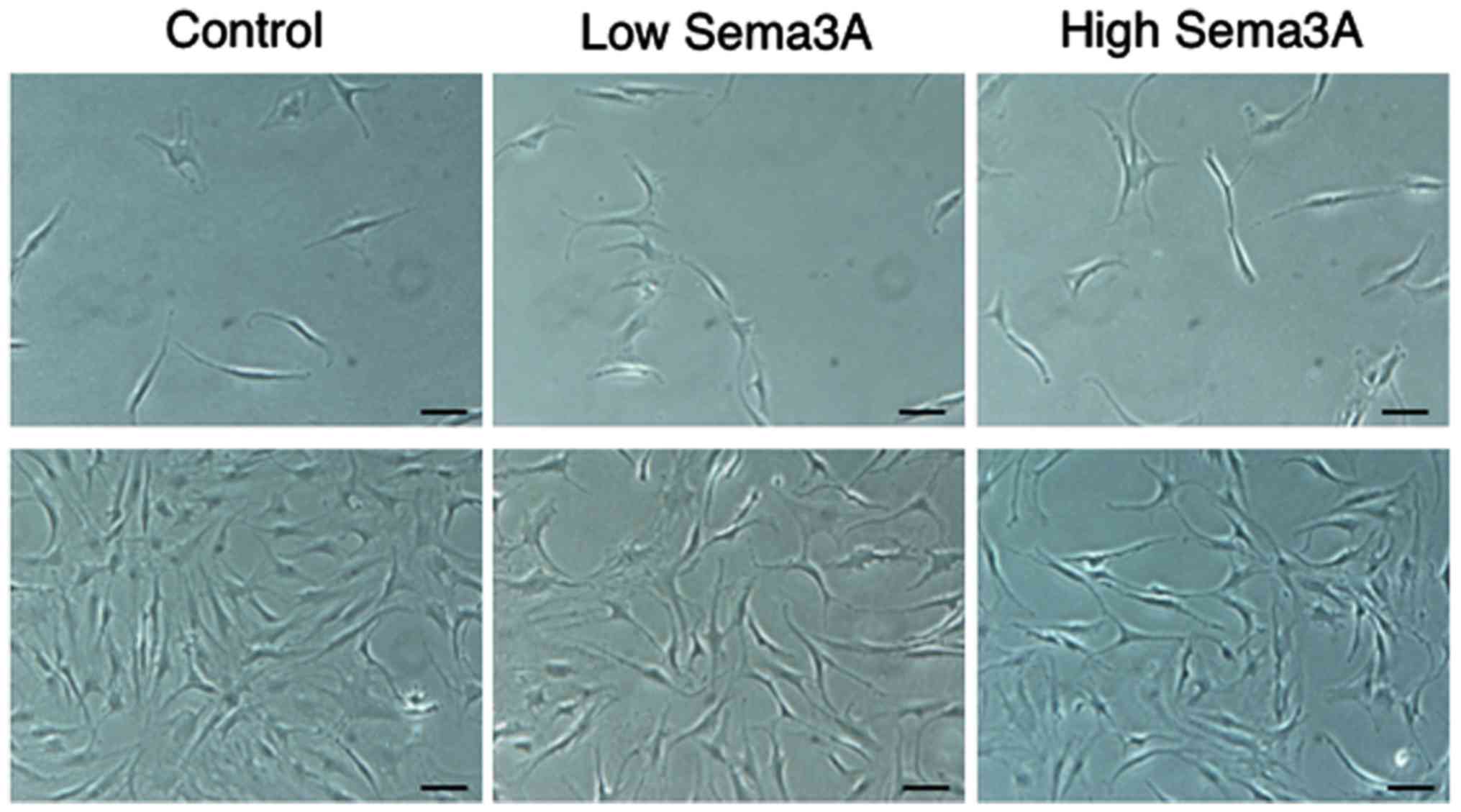
Fig. 1 reveals the
effect of recombinant human sema3A on the morphology of human
chondrocytes. At culture day 7, the cells were polygonal, and low
and high concentrations of exogenous sema3A appeared to promote the
growth of chondrocytes compared with the control. At culture day
14, the cells remained polygonal in the three groups.
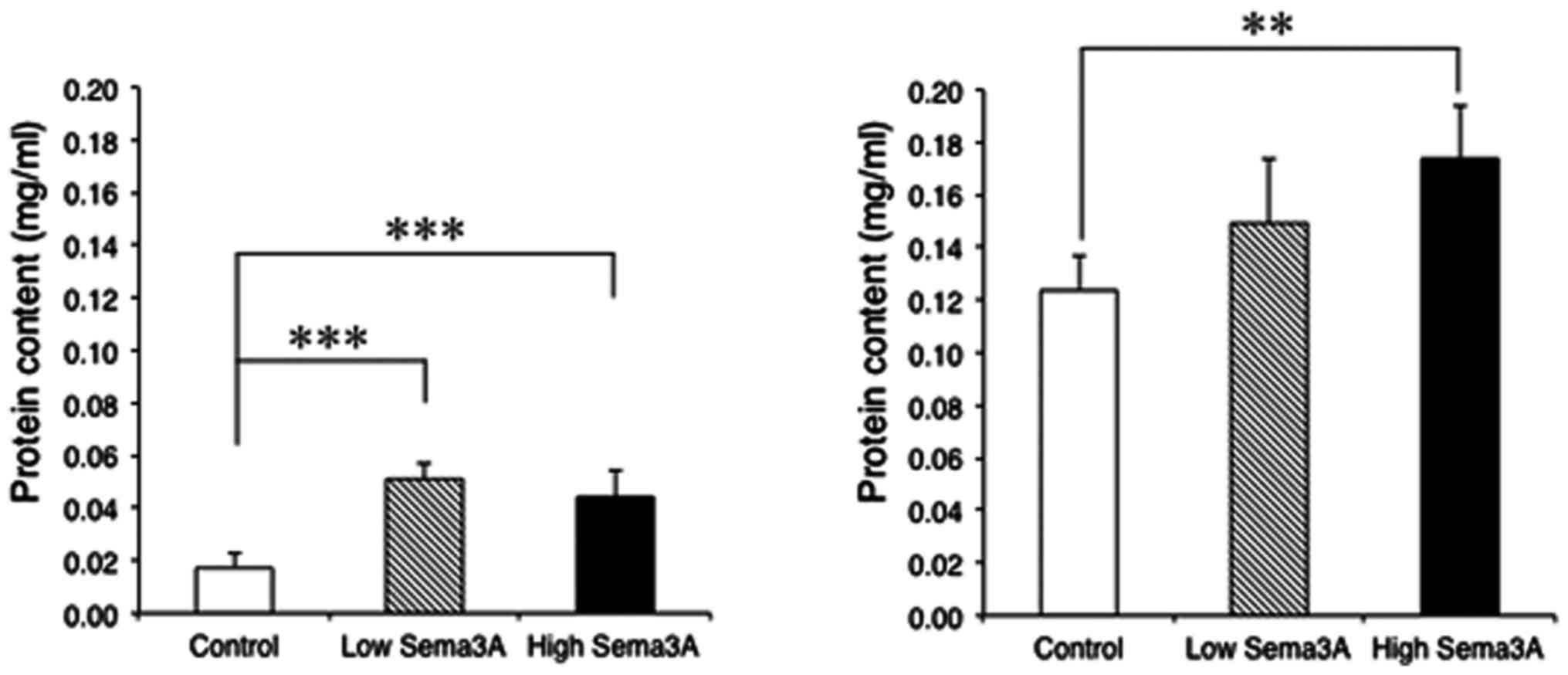
Fig. 2 demonstrates
the mean ± standard deviation of the protein content, in accordance
with cell growth and accumulation of extracellular matrices, of
human chondrocytes. At culture day 7, high and low concentrations
of exogenous sema3A significantly increased the protein content of
chondrocytes (0.044±0.010 and 0.051±0.006 mg/ml, respectively)
compared with the control (0.017±0.005 mg/ml) (P=0.0008 and
0.00002, respectively). At culture day 14, a high concentration of
sema3A significantly increased the protein content of chondrocytes
(0.174±0.020 mg/ml) compared with the control (0.124±0.014 mg/ml)
(P=0.002).
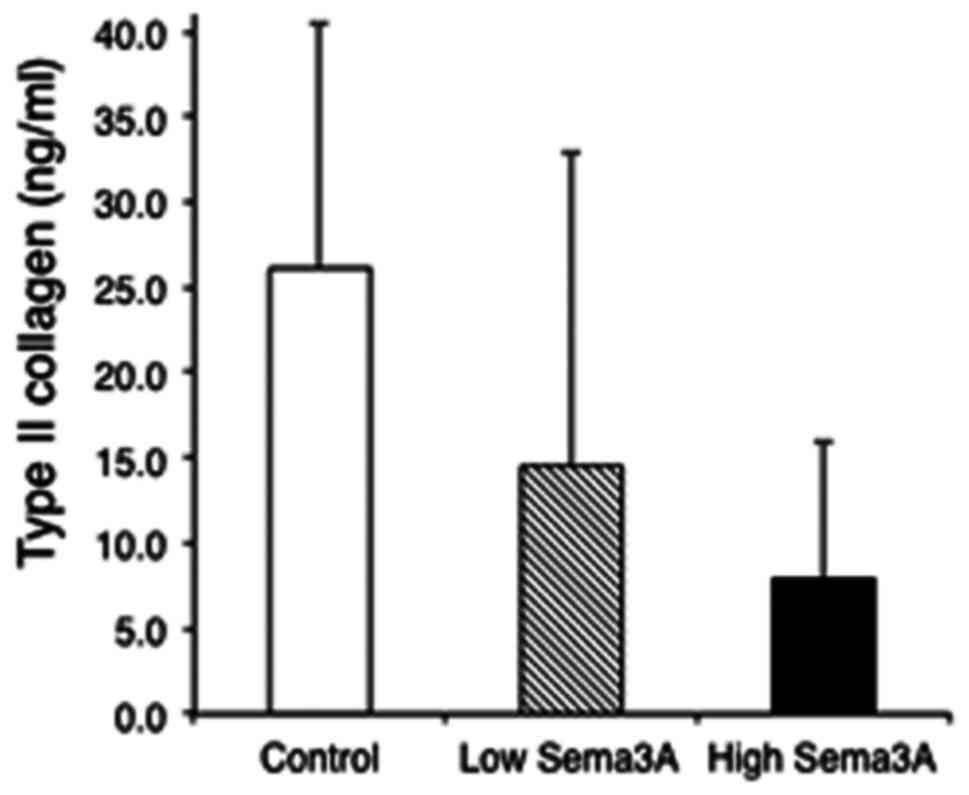
Effect of sema3A on type II collagen
expression in human chondrocytes
Fig. 3 presents the
mean ± standard deviation of concentrations of type II collagen in
the culture medium of human chondrocytes. At culture day 7,
exogenous sema3A dose-dependently decreased the concentrations of
type II collagen in the culture medium of chondrocytes, although
the differences were not statistically significant among the three
groups. At culture day 14, the concentration of type II collagen in
the culture medium of chondrocytes was not detected in the three
groups. Concentrations of type II collagen in cells and
extracellular matrices were not also detected in the three
groups.
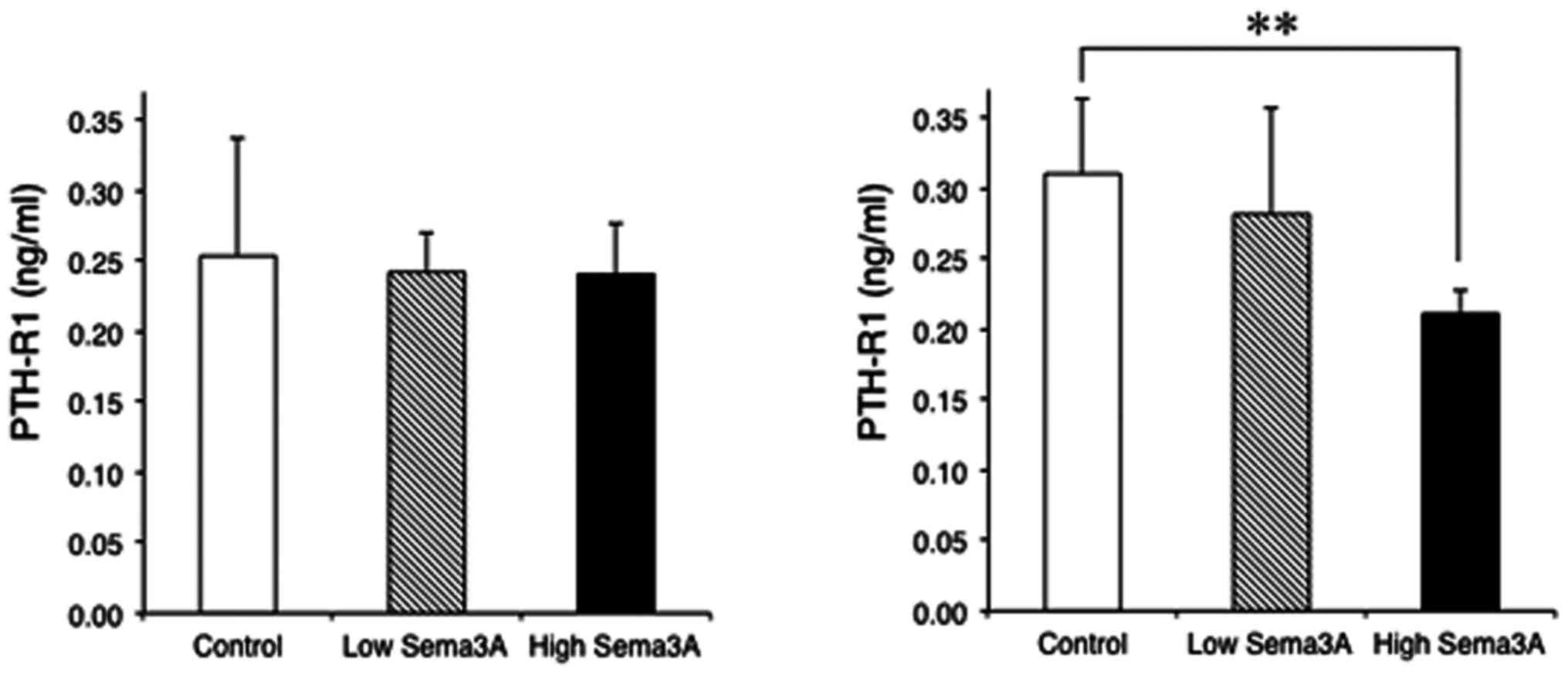
Effects of sema3A on PTH-R1 expression
in human chondrocytes
Fig. 4 presents the
mean ± standard deviation of concentrations of PTH-R1 in human
chondrocytes. At culture day 7, neither high nor low concentrations
of exogenous sema3A significantly influenced the concentration of
PTH-R1 of chondrocytes (0.241±0.036 and 0.242±0.029 ng/ml,
respectively) compared with the control (0.253±0.084 ng/ml). At
culture day 14, a high concentration of sema3A significantly
decreased the concentration of PTH-R1 in chondrocytes (0.211±0.017
ng/ml) when compared with the control (0.310±0.053 ng/ml)
(P=0.004).
Discussion
To investigate the effects of sema3A on the various
functions of human dental pulp stem cells, Yoshida et al
(7) added a 10 ng/ml concentration of
recombinant human sema3A in medium for human dental pulp stem
cells. To examine the function of sema3A in the regulation of
osteoclast differentiation, Hayashi et al (6) added a 500 ng/ml concentration of
recombinant sema3A in medium for osteoblasts. The concentrations of
recombinant human sema3A used in the present study were selected
according to these previous studies.
Type II collagen produced by cells is incorporated
into the extracellular matrix (11,12).
However, in the present study, type II collagen was not detected in
cells and matrix, nevertheless type II collagen was digested by
pepsin and elastase for solubilizing collagen. Instead of type II
collagen in extracellular matrix, concentrations of type II
collagen in culture medium were measured out, which was already
soluble.
Sema3A is expressed in osteoblasts (6). Plexin A2, which is a semaphorin
receptor, is also expressed in osteoblasts, and it has been
suggested that sema3A and plexin A2 binding stimulates osteoblast
differentiation (6). Gomez et
al (9) reported that sema3A and
plexin A2 mRNA were expressed in a mouse chondrogenic cell line. In
the present study, the addition of recombinant human sema3A
significantly (P<0.001) increased the protein content of human
chondrocytes (Fig. 2). Protein
content is thought to indicate the degree of cell growth and
accumulation of extracellular matrices (13,14). The
results of the present study and a previous study (9) therefore suggest that plexin A2 may be
expressed in not only mouse but also human chondrocytes, and that
exogenous sema3A may bind plexin A2 on proliferative human
chondrocytes and induce the acceleration of cell growth and/or
accumulation of extracellular matrices of chondrocytes, although
the present study did not assert whether the expression of plexin
A2 is constant or regulated by exogenous sema3A.
Active mandibular growth occurs in an endochondral
growth of the condyle of the temporomandibular joint (8). In the temporomandibular joint,
endogenous cytokines are reportedly produced by non-inflammatory
cells including osteoblasts and chondrocytes and interact with
their receptors (13). The
PTHrP gene is located on chromosome 12p12.1-p11.2 and
encodes a member of the parathyroid hormone family. The protein
PTHrP, via its receptor, PTH-R1, regulates endochondral bone
development. The PTHrP ligand is expressed in mouse
undifferentiated (proliferative) chondrocytes in the growth plate
of developing bones (15,16). PTH-R1 is expressed in mouse
chondrocytes located mainly in the proliferative zone and the
hypertrophic zone of the growth plate (15–17).
Expression of the PTH-R1 gene coincides with the type II
collagen gene (16). Activation of
PTH-R1 interferes with the early stages of chondrogenesis through
the cyclic AMP/protein kinase A signaling pathway (18). Namely, PTH-R1 functions to maintain
proliferative chondrocytes and delays the production of
hypertrophic chondrocytes in mice (18). Indian hedgehog is expressed in
pre-hypertrophic chondrocytes and stimulates the production of
PTHrP in mice (18,19). Experiments using chimaeric mice
(19) revealed that a negative
feedback loop functions in chondrogenesis. In the present study, to
examine whether sema3A is associated with the endochondral growth
of the condyle of the temporomandibular joint, effects of exogenous
sema3A on PTH-R1 in human chondrocytes were preliminarily
investigated.
In the present study, the addition of a high
concentration (100 ng/ml) of recombinant human sema3A significantly
(P=0.002) decreased the expression of PTH-R1 in human chondrocytes
at culture day 14 (Fig. 4). The novel
results of the present study suggested that exogenous sema3A may
function on plexin A2 in proliferative human chondrocytes and
suppresses PTH-R1 and/or PTHrP expression in proliferative human
chondrocytes, although the mechanism remains unknown. Suppression
of PTH-R1 and/or PTHrP expression potentially accelerates the
differentiation of hypertrophic chondrocytes and the subsequent
endochondral ossification at puberty (in other words, early
termination of condylar endochondral growth). The mutation of the
PLXNA2 gene may inhibit this action of sema3A on
chondrocytes and delay this early termination of condylar
endochondral growth. Therefore, the mutation of PLXNA2 gene
encoding plexin A2 may be a candidate gene of mandibular
prognathism, even though the results of the present study were
limited.
In the present study, there was no data of
expression of sema3A, plexin A2 and PTH-R1 when the calcium
concentration was changed. In general, PTH enhances the release of
calcium from the large reservoir contained in the bones. PTH and
PTH-R1 binding stimulates osteoblasts to increase their expression
of receptor activator of nuclear factor κ-B (RANKL). The binding of
RANKL to RANK stimulates these osteoclast precursors to fuse,
forming novel osteoclasts, which ultimately enhances bone
resorption (6). Interestingly, Ohta
et al (20) reported that
plexin A2 mediates cell-cell adhesion via a homophilic binding
mechanism under the presence of calcium ions. Therefore, the
association between the expression of plexin A2 and calcium
concentration will be analyzed in the future.
Another limitation in the present study lies in the
‘dedifferentiation’ of chondrocytes. Chondrocytes grow and express
the phenotypes of chondrocytes in monolayer cultures (21,22).
However, in monolayer cultures cultured over two weeks in a
previous study, the growing chondrocytes appeared to be
dedifferentiated (21,22). Chondrocytes dedifferentiated by
monolayer cultures transform into a fibroblast-like morphology and
express type I collagen accompanied with a fibroblast-like
phenotype (23,24), whereas proliferative chondrocytes are
polygonal and express type II collagen accompanied with a
chondrocyte phenotype. In the present study, the increased protein
content and the decreased type II collagen and PTH-R1 expression in
human chondrocytes at culture day 14 may result from the
dedifferentiation accelerated by exogenous sema3A.
To facilitate the ‘redifferentiation’ of the
dedifferentiated chondrocytes incubated by monolayer cultures over
two weeks, high cell density three-dimensional cultures performed
by assembling chondrocyte in alginate beads, synthetic polymer gels
or compressing into pellets (spheroid formation) have been
attempted (25,26). In future studies, three-dimensional
cultures for human chondrocytes will be utilized.
In conclusion, a previous GWAS had revealed
PLXNA2 encoding a member of the plexin-A family of
semaphorin co-receptors as one of the candidate genes for
mandibular prognathism. The present preliminary study proposed that
sema3A functions on not only murine but also human chondrocytes and
revealed the novel result that exogenous sema3A suppresses the
expression of PTH-R1 in human proliferative chondrocytes. Exogenous
sema3A may function on human chondrocytes by binding plexin A2.
Suppression of PTH-R1 and/or PTHrP expression potentially
accelerates the early termination of condylar growth. The mutation
of the PLXNA2 gene may delay the early termination of the
condylar growth. Therefore, the mutation of the PLXNA2 gene
encoding plexin A2 may be a candidate gene of mandibular
prognathism.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Japanese
Society for the Promotion of Science KAKENHI (Grants-in-Aid for
Scientific Research; grant no. JP16K20630) and National Institutes
of Health/National Institute if Dental and Craniofacial Research
(grant no. R01DE023538).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
TK conceived and designed the study, acquired the
data, analyzed and interpreted the data and drafted the article. AO
conceived and designed the study and revised the article for
important intellectual content. MH acquired the data, analyzed and
interpreted the data and revised the article for important
intellectual content. JYamaz analyzed and interpreted the data and
revised the article for important intellectual content. JYamas
analyzed and interpreted the data and revised the article for
important intellectual content. JI conceived and designed the study
and revised the article for important intellectual content.
Ethics approval and consent to
participate
Not applicable.
Patients consent for publication
Not applicable.
Competing interest
The authors declare that they have no competing
interests.
References
|
1
|
Uribe Moreno LM and Miller SF: Genetics of
the dentofacial variation in human malocclusion. Orthod Craniofac
Res. 18 Suppl 1:91–99. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kajii TS and Oka A: Candidate gene
analyses of mandibular prognathism. J Dent Oral Biol.
2:10682017.
|
|
3
|
Ikuno K, Kajii TS, Oka A, Inoko H,
Ishikawa H and Iida J: Microsatellite genome-wide association study
for mandibular prognathism. Am J Orthod Dentofacial Orthop.
145:757–762. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Saito F, Kajii TS, Oka A, Ikuno K and Iida
J: Genome-wide association study for mandibular prognathism using
microsatellite and pooled DNA method. Am J Orthod Dentofacial
Orthop. 152:382–388. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wray NR, James MR, Mah SP, Nelson M,
Andrews G, Sullivan PF, Montgomery GW, Birley AJ, Braun A and
Martin NG: Anxiety and comorbid measures associated with PLXNA2.
Arch Gen Psychiatry. 64:318–326. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hayashi M, Nakashima T, Taniguchi M,
Kodama T, Kumanogoh A and Takayanagi H: Osteoprotection by
semaphorin 3A. Nature. 485:69–74. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yoshida S, Wada N, Hasegawa D, Miyaji H,
Mitarai H, Tomokiyo A, Hamano S and Maeda H: Semaphorin 3A induces
odontoblastic phenotype in dental pulp stem cells. J Dent Res.
95:1282–1290. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Björk A: Prediction of mandibular growth
rotation. Am J Orthod. 55:585–599. 1969. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gomez C, Burt-Pichat B, Mallein-Gerin F,
Merle B, Delmas PD, Skerry TM, Vico L, Malaval L and Chenu C:
Expression of Semaphorin-3A and its receptors in endochondral
ossification: Potential role in skeletal development and
innervation. Dev Dyn. 234:393–403. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chang WM, Lin YF, Su CY, Peng HY, Chang
YC, Hsiao JR, Chen CL, Chang JY, Shieh YS, Hsiao M, et al:
Parathyroid hormone-like hormone is a poor prognosis marker of head
and neck cancer and promotes cell growth via RUNX2 regulation. Sci
Rep. 7:411312017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gründer T, Gaissmaier C, Fritz J, Stoop R,
Hortschansky P, Mollenhauer J and Aicher WK: Bone morphogenetic
protein (BMP)-2 enhances the expression of type II collagen and
aggrecan in chondrocytes embedded in alginate beads. Osteoarthritis
Cartilage. 12:559–567. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tekari A, Luginbuehl R, Hofstetter W and
Egli RJ: Chondrocytes expressing intracellular collagen type II
enter the cell cycle and co-express collagen type I in monolayer
culture. J Orthop Res. 32:1503–1511. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kajii TS, Okamoto T, Yura S, Mabuchi A and
Iida J: Elevated levels of beta-endorphin in temporomandibular
joint synovial lavage fluid of patients with closed lock. J Orofac
Pain. 19:41–46. 2005.PubMed/NCBI
|
|
14
|
Luppanapornlarp S, Kajii TS, Surarit R and
Iida J: Interleukin-1beta levels, pain intensity, and tooth
movement using two different magnitudes of continuous orthodontic
force. Eur J Orthod. 32:596–601. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Karaplis AC, Luz A, Glowacki J, Bronson
RT, Tybulewicz VL, Kronenberg HM and Mulligan RC: Lethal skeletal
dysplasia from targeted disruption of the parathyroid
hormone-related peptide gene. Genes Dev. 8:277–289. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Shukunami C, Shigeno C, Atsumi T, Ishizeki
K, Suzuki F and Hiraki Y: Chondrogenic differentiation of clonal
mouse embryonic cell line ATDC5 in vitro: Differentiation-dependent
gene expression of parathyroid hormone (PTH)/PTH-related peptide
receptor. J Cell Biol. 133:457–468. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Amizuka N, Kwan MY, Goltzman D, Ozawa H
and White JH: Vitamin D3 differentially regulates parathyroid
hormone/parathyroid hormone-related peptide receptor expression in
bone and cartilage. J Clin Invest. 103:373–381. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kronenberg HM: Developmental regulation of
the growth plate. Nature. 423:332–336. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chung UI, Schipani E, McMahon AP and
Kronenberg HM: Indian hedgehog couples chondrogenesis to
osteogenesis in endochondral bone development. J Clin Invest.
107:295–304. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ohta K, Mizutani A, Kawakami A, Murakami
Y, Kasuya Y, Takagi S, Tanaka H and Fujisawa H: Plexin: A novel
neuronal cell surface molecule that mediates cell adhesion via a
homophilic binding mechanism in the presence of calcium ions.
Neuron. 14:1189–1199. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Takigawa M, Shirai E, Fukuo K, Tajima K,
Mori Y and Suzuki F: Chondrocytes dedifferentiated by serial
monolayer culture form cartilage nodules in nude mice. Bone Miner.
2:449–462. 1987.PubMed/NCBI
|
|
22
|
Sandell LJ and Aigner T: Articular
cartilage and changes in arthritis. An introduction: Cell biology
of osteoarthritis. Arthritis Res. 3:107–113. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
von der Mark K, Gauss V, von der Mark H
and Müller P: Relationship between cell shape and type of collagen
synthesised as chondrocytes lose their cartilage phenotype in
culture. Nature. 267:531–532. 1977. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Benya PD, Padilla SR and Nimni ME:
Independent regulation of collagen types by chondrocytes during the
loss of differentiated function in culture. Cell. 15:1313–1321.
1978. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chen G, Sato T, Ushida T, Hirochika R and
Tateishi T: Redifferentiation of dedifferentiated bovine
chondrocytes when cultured in vitro in a PLGA-collagen hybrid mesh.
FEBS Lett. 542:95–99. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Caron MM, Emans PJ, Coolsen MME, Voss L,
Surtel DA, Cremers A, van Rhijn LW and Welting TJ:
Redifferentiation of dedifferentiated human articular chondrocytes:
Comparison of 2D and 3D cultures. Osteoarthritis Cartilage.
20:1170–1178. 2012. View Article : Google Scholar : PubMed/NCBI
|