Introduction
Actin filament-associated protein 1 (AFAP1)
encodes a motor fiber-associated protein, which organizes a network
for linking other proteins, including SRC proto-oncogene,
non-receptor tyrosine kinase (Src) and protein kinase C (1). This network alters the structure and
function of actin filaments, thus contributing to cytophagy, cell
motion, invasion and metastasis (2).
This gene has been demonstrated to be over-expressed in the human
breast cancer cell line MDA-MB-231 where its silencing resulted in
a deficiency in actin stress fiber cross-linking and diminished
linkage with fibronectin (3). The
contribution of AFAP1 in the carcinogenesis process has been
further emphasized by the observed over-expression of the encoded
protein in prostate carcinoma types despite the absence or low
level of expression of this gene in normal prostatic epithelium and
benign prostatic hyperplasia (4). The
effect of AFAP1 knockdown on the repression of cell
proliferation has been documented in vitro and in
vivo (4). One long non-coding RNA
(lncRNA), namely AFAP1-antisense RNA 1 (AS1) is
transcribed form the antisense strand of this gene and may
potentially regulate its expression (5). Numerous studies have reported the
dysregulation of this lncRNA in human malignancies in association
with numerous pathological tumor features (5-7). A
previous meta-analysis has demonstrated that AFAP1-AS1
overexpression is indicative of poor patient survival and the
malignant behavior of a tumor (8).
Previously, the expression of AFAP1 and AFAP1-AS1 was
evaluated in breast cancer samples and the upregulation of
AFAP1-AS1 in tumor tissues compared with adjacent
non-cancerous tissues (ANCTs) was detected (9). AFAP1-AS1 expression has also been
revealed to be elevated in gastric cancer tissues and cells
compared with noncancerous gastric tissues and control cells
(6,10). However, there is no data regarding the
expression of AFAP1 in gastric cancer. In the present study,
the expression levels of AFAP1 and AFAP1-AS1 were
assessed in gastric cancer samples and ANCTs to verify results of
previous studies on AFAP1-AS1 expression and to investigate
the function of AFAP1 in gastric cancer pathogenesis.
Materials and methods
Patients
The present study was performed on samples obtained
from 30 patients (23 males and 7 females) diagnosed with gastric
cancer mean ± standard deviation (range) age 42.53±10.1 years,
ranging from 14-55 years old. Patients were admitted in Imam
Khomeini hospital, Tehran during February 2016 to December 2017.
Tumoral and ANCTs were excised from the patients during surgical
removal of the gastric tumor. Patients had no former history of
chemo/radiotherapy; those with familial disease were also excluded.
Inclusion criteria were availability of clinical data and
appropriate tissue for RNA extraction. All tissue samples were
inspected by pathologists to evaluate the existence of tumoral
cells. Tumor-node-metastasis staging was performed based on the
American Joint Committee on Cancer (AJCC) system, 8th edition
(11). The cutoff values for the age
groups were determined based on recent study (12). The study protocol was ethically
approved by the ethical committee of Shahid Beheshti University of
Medical Sciences (Tehran, Iran). All patients signed written
informed consent forms.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from tumoral tissues and
ANCTs using TRIzol™ reagent (Invitrogen; Thermo Fisher Scientific,
Inc., Waltham, MA, USA) according to the manufacturer's protocol.
Approximately 75 ng RNA samples was converted to cDNA using Applied
Biosystems High-Capacity cDNA Reverse Transcription kit according
to manufacturer protocols (Applied Biosystems; Thermo Fisher
Scientific, Inc.). Relative expressions of AFAP1 and
AFAP1-AS1 were quantified in the Rotor Gene 6000 Real-Time
PCR Machine using TaqMan® Universal PCR Master Mix
(Applied Biosystems; Thermo Fisher Scientific, Inc.). Transcript
levels were normalized to those of hypoxanthine
phosphoribosyltransferase 1 (HPRT1). The sequences of
primers and probes are presented in Table I. The thermocycling
conditions included a primary denaturation step at 95˚C for 15 min,
40 cycles of 95˚C for 15 sec and 60˚C for 55 sec. qPCR analyses
were performed using the Pfaffl method (13).
Evaluation of Helicobacter pylori (H
pylori) presence in tissues
The presence of H. pylori in tissues were
assessed with RT-qPCR using primers against H. pylori 16s
rRNA (forward, 5'-AGCGTTACTCGGAATCACTG-3' and reverse,
5'-CACATACCTCTCACACACTC-3') at a final concentration of 0.2
pmol/µl. Reactions were performed on 100 ng synthesized cDNA as
described above. The thermocycling conditions were as follows: 95˚C
for 15 min and then 95˚C for 15 sec and 60˚C for 1 min for 40
cycles followed by melting curve analysis. TaqMan®
Universal PCR Master Mix (Applied Biosystems; Thermo Fisher
Scientific, Inc.) was used. qPCR analyses were performed using the
Pfaffl method (13).
Statistical analysis
Demographic and clinical data were presented as the
mean ± standard deviation or as percentages. Relative expression
levels of genes in tumoral tissues compared with ANCTs were
assessed using REST 2009 software (Qiagen GmbH, Hilden, Germany).
The difference in the expression levels of genes between paired
tumoral tissues and ANCTs were evaluated using a Student's paired
t-test. The association between clinical data and the relative
expression of genes was assessed using a χ2 or
Mann-Whitney test. The correlation between the relative expression
of AFAP1 and AFAP1-AS1 was assessed using a
regression model. P<0.05 was considered to indicate a
statistically significant difference. The diagnostic power of
transcript levels of genes in gastric cancer was assessed by
plotting a receiver operating characteristic (ROC) curve.
Results
General data of the patients
Thirty patients (23 males and 7 females) diagnosed
with gastric were enrolled in the study. The degree of
invasiveness, histological form and the presence othe H.
pylori infection were analyzed, as well as other features.
Demographic and clinical data of the patients with gastric cancer
are presented in Table II.
Relative expression of AFAP1 and
ASAP1-AS1 genes in tumor tissues compared with ANCTs
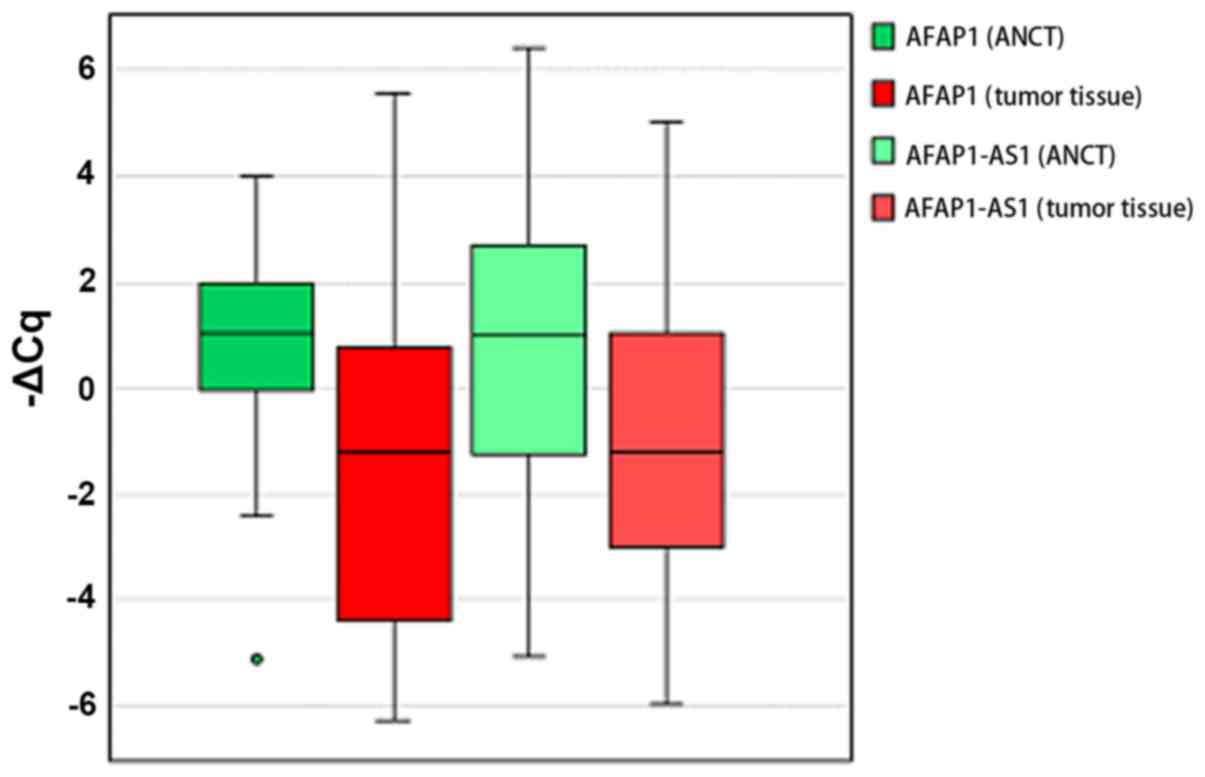
The two genes were significantly downregulated in
gastric tumor samples compared with ANCTs (expression ratios 0.26
and 0.36, P=0.001 and P=0.04 for AFAP1 and AFAP1-AS1,
respectively). Fig. 1 presents the
-ΔCq values (Cq HPRT1-Cq target gene) in tumoral tissues and
ANCTs.
Association between the expression of
levels of AFAP1 and ASAP1-AS1 and tumor characteristics
Relative expression levels of the two genes were
revealed to be associated with the location of the primary tumor,
in that AFAP1 and AFAP1-AS1 were significantly
downregulated in all cardia tumors compared with their paired ANCTs
(P=0.04 and P=0.001, respectively). There were indications of a
significant association between the expression levels of AFAP1 and
peritoneal invasion and smoking history (P=0.05). However, the
expression levels of neither gene were associated with the presence
of H. pylori in samples (P=0.65 and P=0.08, respectively).
Table III presents the results of the association analysis between
the relative expression levels of AFAP1 and AFAP1-AS1
in tumoral samples compared with ANCTs and tumor
characteristics.
The present study additionally compared the relative
expression levels of each gene between the distinct categories of
patients and identified a significantly lower expression of
AFAP1 in younger patients compared with older patients
(P=0.01) and in high grade (grade 3 and 4) tumor types compared
with a lower grade (grade type 2; P=0.04) and a significantly
higher expression of AFAP1-AS1 in patients with lymphatic or
vascular invasion compared with those who did not (P=0.01). Table
IV presents the results of the association analysis between the
transcript levels of genes in tumoral tissues and tumoral
features.
Correlation between the expression
levels of AFAP1 and AFAP1-AS1
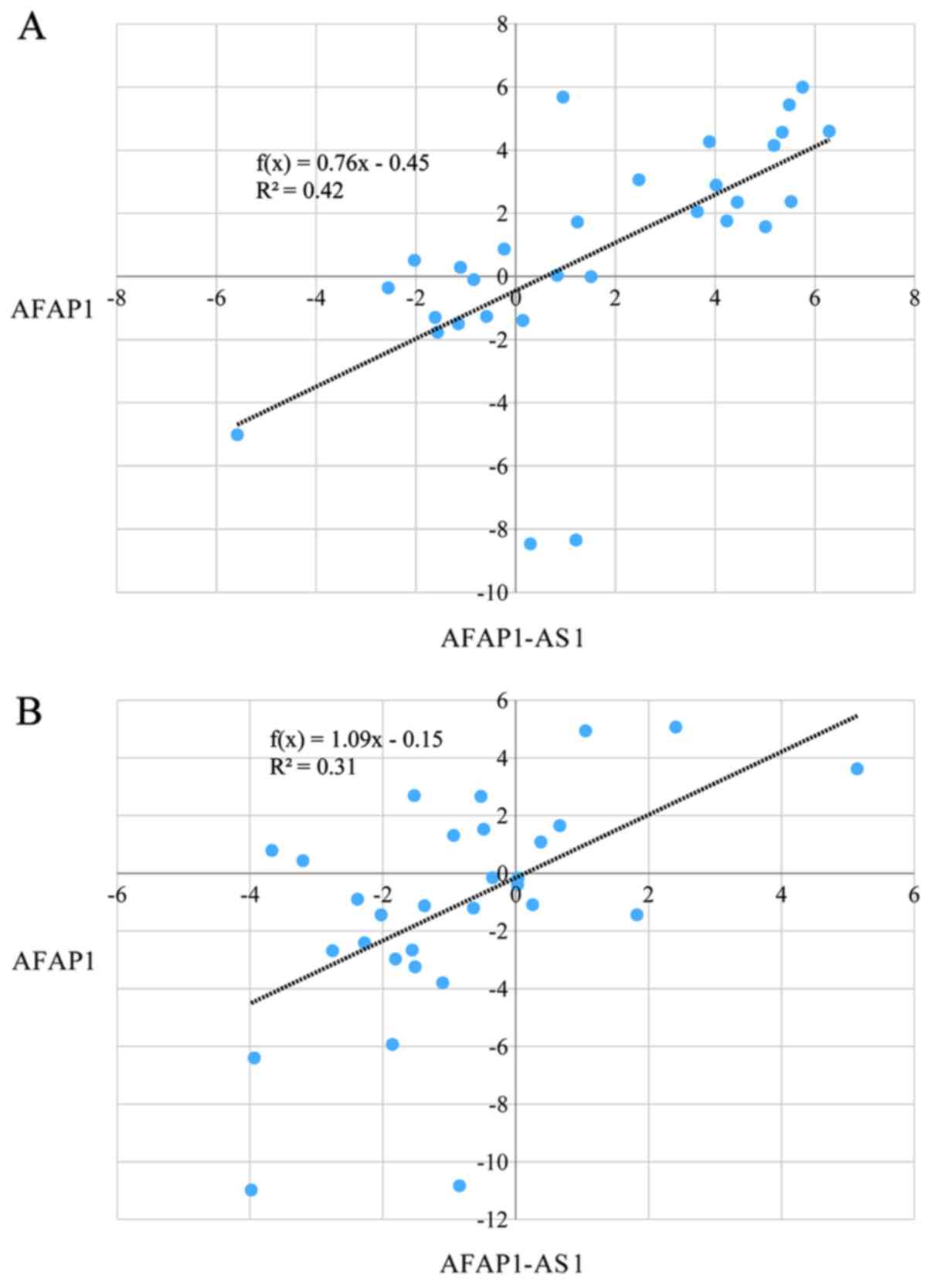
Significant pairwise correlations were identified
between transcript levels of AFAP1 and AFAP1-AS1 in
tumoral tissues and ANCTs (R2 =0.42 and 0.31, P<0.05;
Fig. 2A and B, respectively).
ROC curve analysis
The diagnostic power of AFAP1 and
AFAP1-AS1 in gastric cancer was calculated based on area
under curve (AUC) values of 0.75 and 0.67, respectively. The
combination of the transcript levels of these genes significantly
enhanced the diagnostic power when compared with the diagnostic
power of each gene (AUC, 0.76; P<0.001). Table V exhibits the
parameters of the ROC curve analysis.
Discussion
In the present study, the downregulation of
AFAP1 and AFAP1-AS1 in gastric cancer samples
compared with ANCTs was demonstrated. This observation is in
contrast with the previously reported expression pattern and
function of AFAP1-AS1 in gastric cancer (6,10). Such an
inconsistency may be due to the function of ethnicity-based and
environmental factors in the determination of lncRNAs expression.
The latter is supported by the fact that non-coding RNAs contribute
to the regulation of gene expression in response to environmental
signals (14). Future studies are
required to assess the function of putative environmental factors
on the expression of AFAP1-AS1 in association with gastric
cancer risk. The presence of certain genomic variants within the
promoter region of AFAP1-AS1 may additionally affect
expression of this lncRNA and may be responsible for the observed
downregulation of this lncRNA in Iranian patients. Downregulation
of these genes in cancerous tissues may be due to epigenetic
changes which have occurred during the process of tumorigenesis.
Alternatively, the differential expression of transcription factors
between tumoral and non-tumoral tissues may affect expression of
these genes in these two sets of samples.
Feng et al (10) reported positive associations between
the elevated expression levels of AFAP1-AS1 in gastric
cancer samples and lymph node metastasis, Tumor-Node-Metastasis
stage and poor patient outcome. In line with their results, the
present study demonstrated higher expression levels of
AFAP1-AS1 in patients with lymphatic/vascular invasion
compared with those who did not.
The present study additionally detected the
downregulation of AFAP1 in gastric cancer tissues compared
with ANCTs. AFAP1 has been recognized as an Src binding
partner. It may also modulate actin filament integrity in response
to cellular cues, and may link Src family members to actin
filaments (1). Based on the
previously reported upregulation of Src in gastric cancer samples
(15), it was hypothesized that the
observed downregulation AFAP1 in gastric cancer tissues may
be due to a compensatory mechanism to lessen the effects of Src on
cell proliferation. Alternatively, this observation implies the
existence of a negative regulatory feedback between Src and
AFAP1. Assessment of AFAP1 protein levels or function
is required for the verification of these hypotheses.
Notably, the present study detected significant
associations between the expression levels of the two genes and the
location of the primary tumor (P=0.04 and 0.001, respectively) in
that AFAP1 and AFAP1-AS1 were downregulated in all
cardia tumor types compared with their paired ANCTs. This
observation is consistent with the previously reported dissimilar
gene signature between cardia and non-cardia tumor types (16). Additionally, this expression pattern
signifies the function of these genes as diagnostic markers for
tumor types originating from the cardia region.
Furthermore, the lower expression levels of
AFAP1 were detected in younger patients compared with older
patients and in high grade tumor types compared with low grade
tumor types. Previous studies have revealed dissimilarities in the
clinicopathological traits between younger and older patients with
gastric cancer. These studies indicate a poor patient outcome in
younger patients as a result of late diagnosis and a more
aggressive tumor phenotype (17,18).
Altogether, it may be hypothesized that there exist associations
between AFAP1 low expression levels and determinants of an
aggressive tumor phenotype. Further evidence for this hypothesis
has been provided by the observed trends toward an association
between the expression level of AFAP1 and peritoneal
invasion. The association between smoking history and AFAP1
expression may indicate the impact of the interaction between
smoking, altered actin filament structure and cancer development. A
previous study has reported the function of cigarette smoke extract
in the induction of actin filament reconstitution (19). Future studies are required to assess
the interactive and combinational functions of these factors in
gastric cancer evolution.
The present study additionally detected significant
pairwise correlations between the transcript levels of these genes
in tumoral tissues and ANCTs. Future functional studies are
necessary to investigate whether AFAP1-AS1 may affect the
expression of AFAP1 at genomic, transcriptomic or protein
levels.
Finally, the diagnostic power of AFAP1 and
AFAP1-AS1 in gastric cancer were determined to be AUC=0.75
and 0.67, respectively. The combination of the transcript levels of
these genes marginally enhanced the diagnostic power. Therefore,
the present study indicates the potential application of these
transcripts as diagnostic biomarkers in gastric cancer. However,
this hypothesis should be verified in a larger cohort of
patients.
The present study has a number of limitations.
First, the present study did not implement mechanistic experiments
to unravel the cause of the differential expression of the genes
between the two sets of samples. Second, the study power was
limited by the relative small size of studied samples. Taken
together, the results of the present study indicates the role of
AFAP1 and AFAP1-AS1 in gastric cancer and their
application as biomarkers.
Acknowledgements
Not applicable.
Funding
The present study was supported by Shahid Beheshti
University of Medical Sciences (grant no. 16204).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
FE and MT performed the experiments. MT designed the
study. AN collected the data and participated in the experiments.
VKO analyzed the dat. SGF wrote the manuscript and supervised the
study. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The study protocol was approved by ethical committee
of Shahid Beheshti University of Medical Sciences. All patients
provided written consent for participation in the study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Baisden JM, Qian Y, Zot HM and Flynn DC:
The actin filament-associated protein AFAP-110 is an adaptor
protein that modulates changes in actin filament integrity.
Oncogene. 20:6435–6447. 2001.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Liu FT, Xue QZ, Zhu PQ, Luo HL, Zhang Y
and Hao T: Long noncoding RNA AFAP1-AS1, a potential novel
biomarker to predict the clinical outcome of cancer patients: A
meta-analysis. OncoTargets Ther. 9:4247–4254. 2016.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Dorfleutner A, Stehlik C, Zhang J, Gallick
GE and Flynn DC: AFAP-110 is required for actin stress fiber
formation and cell adhesion in MDA-MB-231 breast cancer cells. J
Cell Physiol. 213:740–749. 2007.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Zhang J, Park SI, Artime MC, Summy JM,
Shah AN, Bomser JA, Dorfleutner A, Flynn DC and Gallick GE:
AFAP-110 is overexpressed in prostate cancer and contributes to
tumorigenic growth by regulating focal contacts. J Clin Invest.
117:2962–2973. 2007.PubMed/NCBI View
Article : Google Scholar
|
|
5
|
Zhao H, Zhang K, Wang T, Cui J, Xi H, Wang
Y, Song Y, Zhao X, Wei B and Chen L: Long non-coding RNA
AFAP1-antisense RNA 1 promotes the proliferation, migration and
invasion of gastric cancer cells and is associated with poor
patient survival. Oncol Lett. 15:8620–8626. 2018.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Ye F, Gong Y, Chen XH, Yu MY, Zuo ZK, Pei
DN, Liu W, Wang Q, Zhou J, Duan L, et al: Long noncoding
AFAP1-antisense RNA 1 is upregulated and promotes tumorigenesis in
gastric cancer. Oncol Lett. 15:7523–7530. 2018.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Bo H, Gong ZJ, Zhang WL, Li XY, Zeng Y,
Liao QJ, Chen P, Shi L, Lian Y, Jing Y, et al: Upregulated long
non-coding RNA AFAP1-AS1 expression is associated with progression
and poor prognosis of nasopharyngeal carcinoma. Oncotarget.
6:20404–20418. 2015.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Liu RH, Wang MY, Chen LY, Yin ZJ, Ji QK,
Wang YY and Jin BZ: Meta-analysis of the prognostic value of long
non-coding RNA AFAP1-AS1 for cancer patients in China. Oncotarget.
9:8100–8110. 2017.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Dianatpour A, Faramarzi S, Geranpayeh L,
Mirfakhraie R, Motevaseli E and Ghafouri-Fard S: Expression
analysis of AFAP1-AS1 and AFAP1 in breast cancer. Cancer Biomark.
22:49–54. 2018.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Feng Y, Zhang Q, Wang J and Liu P:
Increased lncRNA AFAP1-AS1 expression predicts poor prognosis and
promotes malignant phenotypes in gastric cancer. Eur Rev Med
Pharmacol Sci. 21:3842–3849. 2017.PubMed/NCBI
|
|
11
|
Amin MB, Edge SB, Greene F, Byrd DR,
Brookland RK, Washington MK, Gershenwald JE, Compton CC, Hess KR,
Sullivan DC, et al: (eds). AJCC Cancer Staging Manual. 8th edition.
Springer International Publishing, New York, NY. 2017.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Esfandi F, Ghafouri-Fard S, Oskooei VK and
Taheri M: β-Secretase 1 and its Naturally Occurring Anti-Sense RNA
are Down-Regulated in Gastric Cancer. Pathol Oncol Res. Feb 25.
2019.(Epub ahead of print). PubMed/NCBI View Article : Google Scholar
|
|
13
|
Pfaffl MW: A new mathematical model for
relative quantification in real-time RT-PCR. Nucleic Acids Res.
29(e45)2001.PubMed/NCBI
|
|
14
|
Sun YZ, Zhang DH, Ming Z, Li JQ and Chen
X: DLREFD: a database providing associations of long non-coding
RNAs, environmental factors and phenotypes. Database (Oxford) 2017.
bax084. 2017.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Okamoto W, Okamoto I, Yoshida T, Okamoto
K, Takezawa K, Hatashita E, Yamada Y, Kuwata K, Arao T, Yanagihara
K, et al: Identification of c-Src as a potential therapeutic target
for gastric cancer and of MET activation as a cause of resistance
to c-Src inhibition. Mol Cancer Ther. 9:1188–1197. 2010.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Wang G, Hu N, Yang HH, Wang L, Su H, Wang
C, Clifford R, Dawsey EM, Li JM, Ding T, et al: Comparison of
global gene expression of gastric cardia and noncardia cancers from
a high-risk population in china. PLoS One. 8(e63826)2013.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Kim JH, Boo YJ, Park JM, Park SS, Kim SJ,
Kim CS and Mok YJ: Incidence and long-term outcome of young
patients with gastric carcinoma according to sex: does hormonal
status affect prognosis? Arch Surg. 143:1062–1067; discussion 1067.
2008.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Llanos O, Butte JM, Crovari F, Duarte I
and Guzmán S: Survival of young patients after gastrectomy for
gastric cancer. World J Surg. 30:17–20. 2006.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Chen HW, Lii CK, Ku HJ and Wang TS:
Cigarette smoke extract induces expression of cell adhesion
molecules in HUVEC via actin filament reorganization. Environ Mol
Mutagen. 50:96–104. 2009.PubMed/NCBI View
Article : Google Scholar
|