Introduction
Olive oil is a key ingredient in the Mediterranean
diet, and a decreased incidence of cardiovascular disease and
several types of cancer in people living in the Mediterranean
region has been observed through epidemiological studies (1-3). The
regular intake of extra-virgin olive oil decreases the oxidant
status in humans, largely due to the anti-oxidant activities of
olive oil polyphenols (1-6).
Oleuropein and 3-hydroxytyrosol (3-HT) are natural
polyphenols present in olive oil, particularly in extra-virgin
olive oil. These polyphenols may be divided into simple phenols,
secoiridoids and lignans (2). 3-HT is
a simple phenol and formed from the hydrolysis of the secoiridoid
oleuropein (2). During the storage of
olive oil, hydrolysis of oleuropein results in the production of
3-HT (2). In vivo, oleuropein
is also time-dependently hydrolyzed into 3-HT in the stomach
following consumption (7). These
olive oil polyphenols are absorbed in the small intestine and
accumulate in the plasma, urine and liver (7). Oleuropein has demonstrated strong
anti-angiogenic properties, and it inhibits platelet aggregation
and macrophage-mediated low-density lipoproteins (LDL) oxidation
(3). 3-HT also decreases LDL
oxidation and stimulates mitochondrial biosynthesis to prevent
diabetes mellitus (2,3). In addition, these olive oil polyphenols
exert anti-cancer effects; oleuropein exhibits anti-cancer
activities in breast adenocarcinoma, melanoma, urinary bladder
carcinoma, colorectal adenocarcinoma, prostate cancer, lung
carcinoma, glioblastoma, renal cell adenocarcinoma and glioma
(3), while 3-HT significantly
inhibits cell proliferation of colon adenocarcinoma and exhibits
cytotoxicity in breast cancer cells (3). The anti-proliferative, pro-apoptotic,
anti-mutagenic, anti-inflammatory and anti-angiogenic effects of
olive oil polyphenols contribute to their anti-cancer activities
(1-3).
In the liver, olive oil polyphenols have been
demonstrated to inhibit inflammation by decreasing the production
of tumor necrosis factor-α, a proinflammatory cytokine, thereby
preventing the liver damage that leads to steatohepatitis and
hepatocellular carcinoma (HCC) (7).
Furthermore, 3-HT suppresses HCC cell proliferation and induces HCC
cell apoptosis by inhibiting the activation of NF-κΒ
(7).
Liver cancer is the second-most common cause of
cancer-associated mortalities in the world (8). Chronic hepatic inflammation and tissue
damage induce liver cancer (7,8). HCC
accounts for 85-90% of all cases of primary liver cancer (8). Frequent recurrence and metastasis in
patients with HCC have resulted in a relatively low survival rate
of patients with HCC (8), with
circulating HCC tumor cells considered to be the leading factor in
the metastatic process (9,10). A number of growth factor-growth factor
receptor signaling pathways are known to be involved in HCC
progression (11-13).
Transforming growth factor-α (TGF-α), a ligand for epidermal growth
factor receptor (EGFR), and EGFR signaling pathways, including
mitogen-activated protein kinases (MAPKs) and AKT pathways, are
also known to be involved in metastatic recurrence of patients with
HCC (11-14).
Polyphenols, including resveratrol and curcumin,
have been identified to suppress HCC invasion (15,16).
Although the anti-proliferative effects of olive oil polyphenols on
HCC cells have been demonstrated, their effects on the migration of
HCC cells remain unclear. The aim of the present study was to
clarify the effects of olive oil polyphenols on HCC cell migration.
It was demonstrated that oleuropein and 3-HT, olive oil
polyphenols, suppressed the TGF-α-induced migration of human
HCC-derived HuH7 cells.
Materials and methods
Antibodies and chemicals
Recombinant human TGF-α was obtained from R&D
Systems, Inc. Oleuropein and 3-HT were purchased from
Sigma-Aldrich; Merck KGaA. SB203580 was purchased from EMD
Millipore. PD98059 and Y27632 were purchased from Calbiochem; Merck
KGaA. Phospho-specific p38 MAPK antibodies (cat. no., 4511), p38
MAPK antibodies (cat. no., 9212), phospho-myosin phosphatase
targeting subunit 1 (MYPT-1) antibodies (cat. no., 4653), MYPT-1
antibodies (cat. no., 2634), phospho-specific AKT (T308) antibodies
(cat. no., 9275), AKT antibodies (cat. no., 9272),
phosphor-specific stress-activated protein kinase/c-Jun N-terminal
kinase (SAPK/JNK) antibodies (cat. nos., 4668 and 9252) were
purchased from Cell Signaling Technology, Inc. GAPDH antibodies
(cat. no., sc47724) were purchased from Santa Cruz Biotechnology,
Inc. An ECL Western blotting detection system was purchased from GE
Healthcare Life Sciences. Paraformaldehyde was obtained from Alfa
Aesar, Thermo Fisher Scientific, Inc. Other chemicals were
purchased from FUJIFILM Wako Pure Chemical Co. All other materials
were obtained from commercial sources. Oleuropein, 3-HT, PD98059,
SB203580 and Y27632 were dissolved in dimethyl sulfoxide (DMSO;
Sigma-Aldrich; Merck KGaA). The maximum concentration of DMSO was
0.1%, which did not affect cell viability and the phosphorylated
protein levels as determined by the cell migration assay and
western blot analyses, respectively.
Cell culture
Human HCC-derived HuH7 cells (17) were obtained from the Japanese
Collection of Research Bioresources Cell Bank (JCRB0403). The cells
were maintained in RPMI-1640 (Sigma-Aldrich; Merck KGaA) containing
10% fetal calf serum (FCS; Hyclone; GE Healthcare Life Sciences) at
37˚C in a humidified atmosphere of 5% CO2 and 95% air.
For western blot analyses, the cells were seeded into 100-mm
diameter dishes (7x105 cells/dish) in RPMI-1640 medium
containing 10% FCS. After 3 days, the medium was replaced with
serum-free RPMI-1640 medium. The cells were then used for
experiments after 24 h. For the cell migration assay, the cells
were seeded into 100-mm diameter dishes (4x105
cells/dish) in RPMI-1640 medium containing 10% FCS for 4 days and
then used for experiments.
Cell migration assay
A Transwell cell migration assay was performed using
a Boyden chamber (polycarbonate membrane with 8 µm pores,
Transwell; Costar; Corning, Inc.) as described previously (18,19).
Briefly, the cultured cells were seeded (1x105
cells/well) onto the upper chamber in serum-free RPMI-1640 medium.
When required, the cells were pretreated with oleuropein (0, 10, 30
or 100 µM), 3-HT (0, 1, 3 or 10 µM), PD98059 (0 or 50 µM), SB203580
(0 or 7 µM) or Y27632 (0 or 3 µM) in the upper chamber for 60 min
at 37˚C. To investigate whether or not 3-HT interacted physically
with TGF-α, the medium including 3-HT was removed from the Boyden
chamber following pretreatment. TGF-α was then added to the lower
chamber for 23 h at 37˚C. Subsequent to incubation, the cells on
the upperside of the membrane were mechanically removed. The
migrated cells adherent to the underside of the membrane were fixed
with 4% paraformaldehyde (Alfa Aesar, Thermo Fisher Scientific,
Inc.) for 20 min at room temperature, and stained with 1:500 DAPI
solution for 10 min at room temperature. Images of the migrated
cells were then captured and counted using fluorescence microscopy
at magnification, x20, by counting the stained cells in 3 randomly
chosen high power fields.
Western blot analysis
To examine the effect of time of TGF-α stimulation
on p38 MAPK and Rho kinase, the cultured cells were stimulated with
10 ng/ml TGF-α for the indicated periods (0, 1, 3, 5, 10, 20 and 30
min). To examine the effect of olive oil polyphenols on the
TGF-α-stimulated AKT, SAPK/JNK, p38 MAPK and Rho kinase pathways,
the cultured cells were pretreated with the indicated doses of 3-HT
(0, 1, 3 or 10 µM) or oleuropein (0, 10, 30 or 100 µM) for 60 min
and then stimulated with 10 ng/ml TGF-α or vehicle for 3 min for
AKT, 15 min for SAPK/JNK, 5 min for p38 MAPK and 1 min for MYPT-1
at 37˚C. The cells in each dish were washed twice with PBS and then
lysed and sonicated in 800 µl lysis buffer [62.5 mM Tris-HCl (pH
6.8), 2% SDS, 50 mM dithiothreitol and 10% glycerol]. Proteins were
separated via SDS-PAGE, as described by Laemmli (20). A total of 10 µl lysates were applied
per lane of all SDS-PAGE gels. Then, 10% SDS-PAGE gel was used for
analyses of AKT, SAPK/JNK, p38 MAPK and GAPDH, and 7.5% gel was
used to analyze MYPT-1. Proteins were then transferred to on
polyvinylidene difluoride membranes (Bio-Rad Laboratories, Inc.).
Blocking was performed prior to incubation with the primary
antibodies using 5% skim milk for 1 h at room temperature. Western
blot analyses were performed as described previously (18,19,21) using
phospho-specific AKT (T308) (1:1,000), AKT (1:10,000),
phospho-specific SAPK/JNK (1:1,000), SAPK/JNK (1:3,000),
phospho-specific p38 MAPK (1:20,000), p38 MAPK (1:20,000),
phospho-specific MYPT-1 (1:1,000), MYPT-1 (1:1,000) and GAPDH
(1:1,000) antibodies with horseradish peroxidase (HRP)-labeled
anti-rabbit IgG and anti-mouse IgG antibodies (1:1,000; cat. nos.,
7074 and 7076, respectively, Cell Signaling Technology, Inc.) as
secondary antibodies. Incubation time and temperature for primary
antibodies and secondary antibodies were 16-64 h at 4˚C and 1 h at
room temperature, respectively. The HRP activity was visualized on
an X-ray film using an ECL Western blotting detection system (GE
Healthcare Life Sciences.). Each protein was detected on different
gels. Densitometric analyses of the western blot analysis data were
performed using a scanner and an image analysis software program
(ImageJ v1.48; National Institutes of Health). The phosphorylated
protein levels were calculated as follows: The
background-subtracted signal intensity of each phosphorylation
signal was normalized to the respective intensities of total
protein and GAPDH, and then plotted as the fold increase in
comparison with that of the control cells without stimulation.
Statistical analyses
The data were analyzed by using SPSS software (v.
24.0, IBM Corp.). The data are expressed as the mean ± standard
deviation of data from 3 independent cell preparations performed in
triplicate. The statistical significance of the data from the cell
culture experiments was analyzed by two-way analysis of variance
followed by Tukey post-hoc test for multiple comparisons between
pairs. P<0.05 was considered to indicate a statistically
significant difference.
Results
Effects of oleuropein and 3-HT on the
TGF-α-induced migration of HuH7 cells
Whether or not olive oil polyphenols affected the
TGF-α-induced HuH7 cell migration was first examined. As
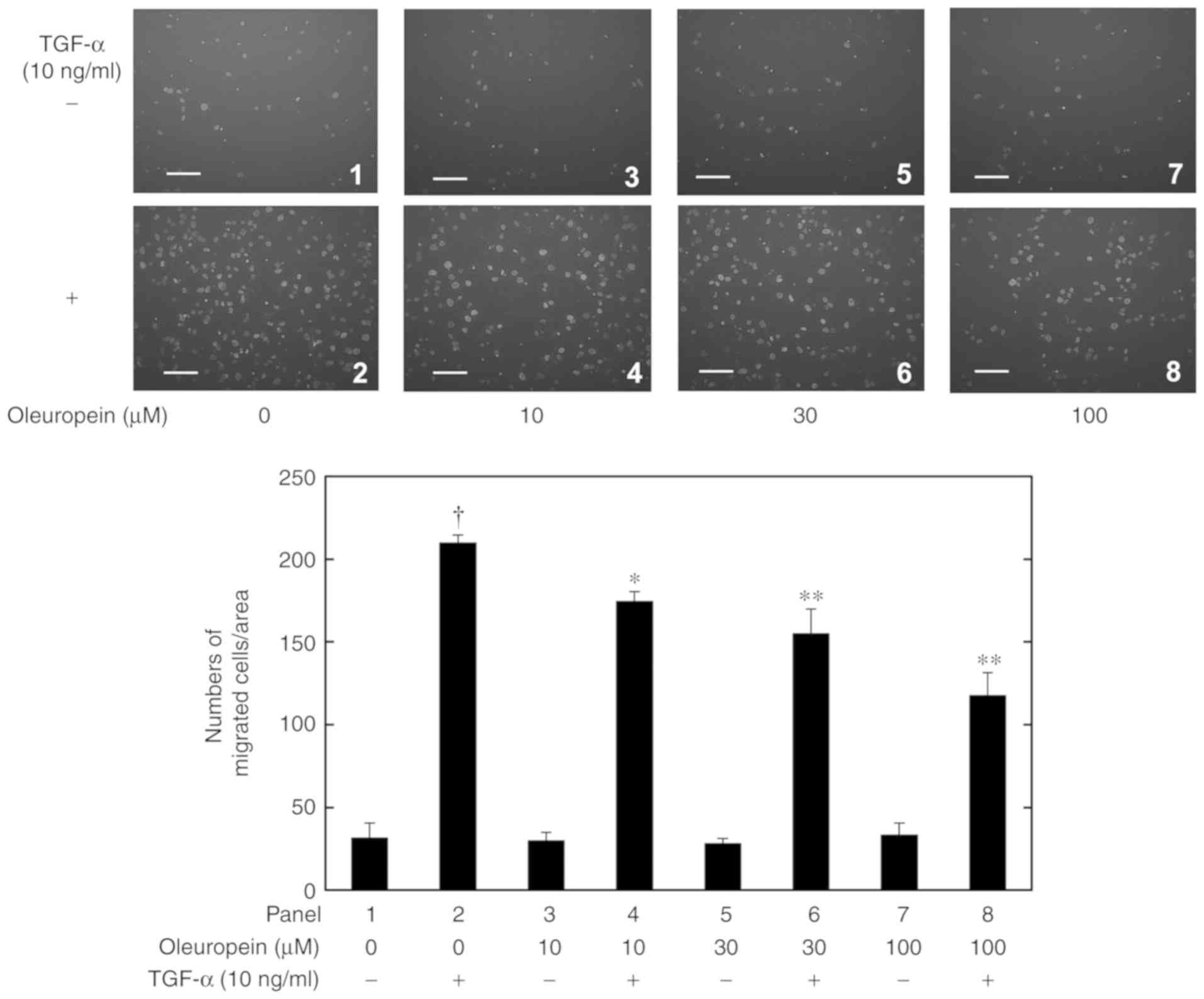
demonstrated in Fig. 1, oleuropein
significantly and dose-dependently suppressed the 10 ng/ml
TGF-α-induced migration of human HCC-derived HuH7 cells at
concentrations of 10-100 µM. Oleuropein alone did not affect the
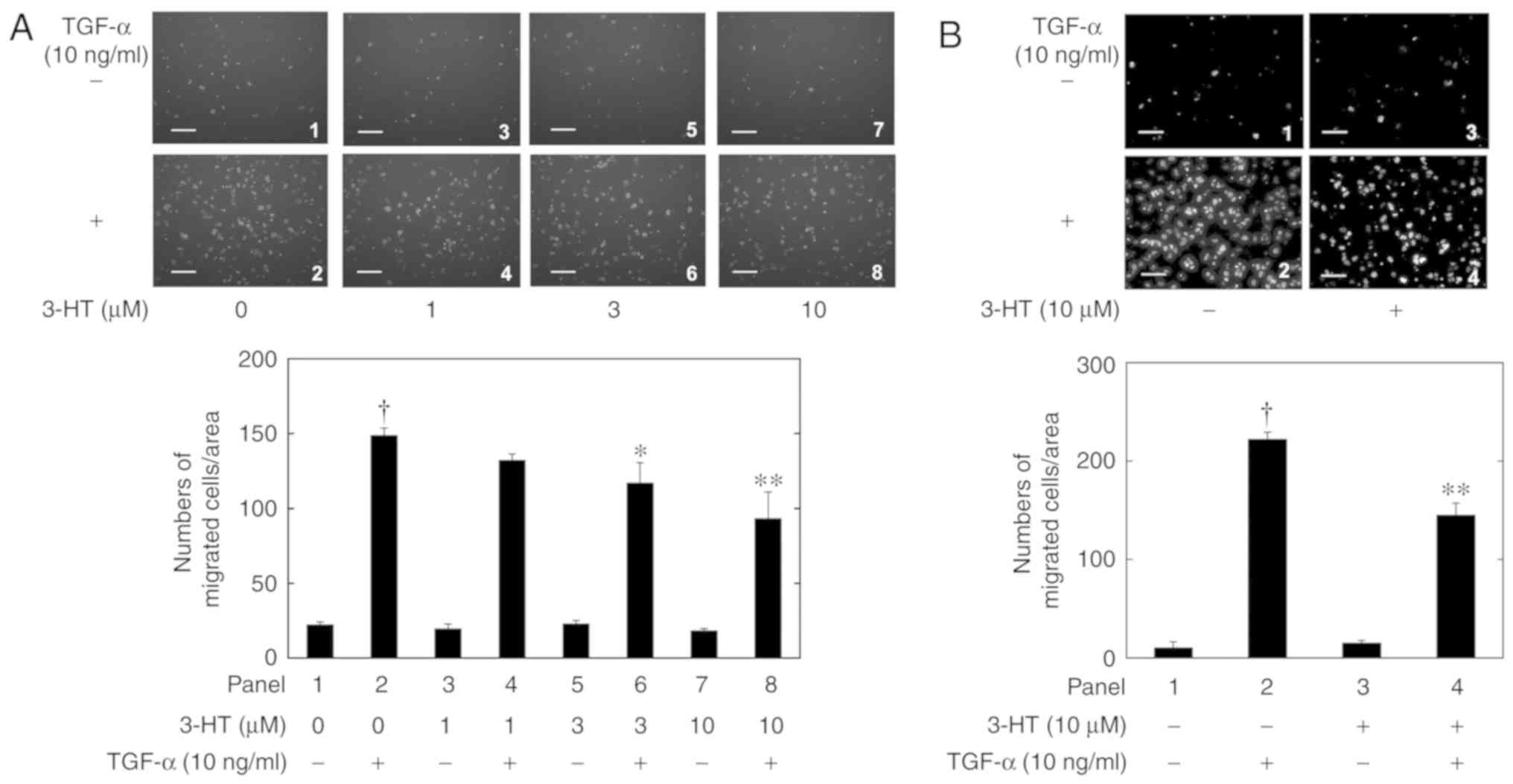
cell migration, even at 100 µM. Similarly, 3-HT, which also did not
affect the cell migration alone, significantly inhibited the 10
ng/ml TGF-α-induced HuH7 cell migration in a dose-dependent manner
at concentrations of 1-10 µM (Fig.
2A). In additon, whether or not 3-HT interacted physically with
TGF-α and inhibited its migratory activity was investigated.
Following pretreatment of HuH7 cells by 10 µM 3-HT for 60 min, the
medium including 3-HT was washed from the Boyden chamber, and then
the cells were stimulated with 10 ng/ml TGF-α. As indicated in
Fig. 2B, the TGF-α-stimulated
migration of HuH7 cells with 3-HT-pretreatment was significantly
decreased compared with the control cells.
Effects of PD98059, SB203580 or Y27632
on TGF-α-induced migration of HuH7 cells
Regarding the intracellular signaling system of
TGF-α, this study group demonstrated previously that TGF-α induced
the activation of the extracellular signal-regulated kinase (ERK),
SAPK/JNK and AKT pathways and promotes the cell proliferation of
human HCC-derived HuH7 cells (21).
In addition to these pathways, it is generally recognized that the
p38 MAPK (22) and Rho kinase
(23) pathways are also involved in
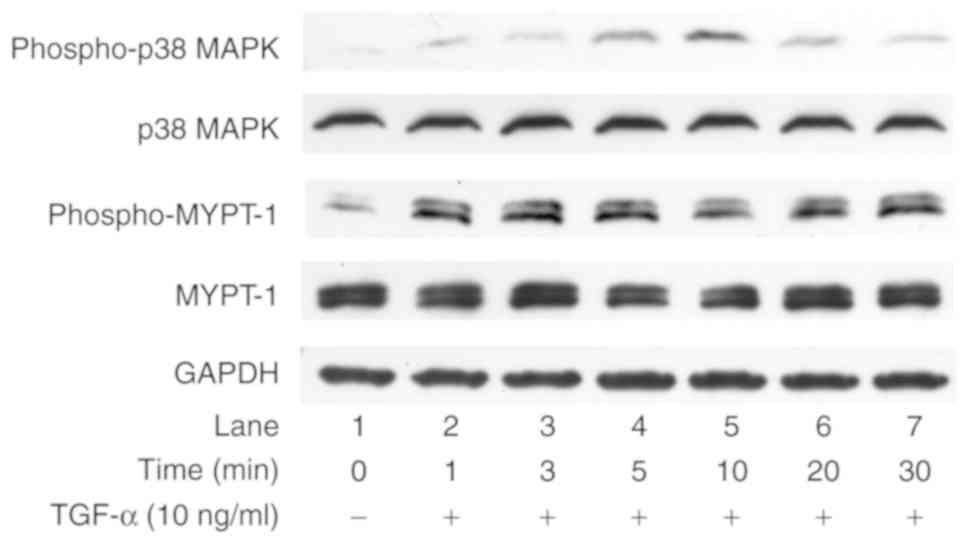
HCC cell functions. Therefore, the present study examined whether
or not TGF-α induced the phosphorylation of p38 MAPK and MYPT-1, a
downstream substrate of Rho kinase, in HuH7 cells. As revealed in
Fig. 3, TGF-α enhanced the
phosphorylation of p38 MAPK and MYPT-1 in HuH7 cells. These results
suggested that TGF-α activated the p38 MAPK and Rho kinase
pathways, in addition to the ERK, SAPK/JNK and AKT pathways, in
HuH7 cells.
This study group recently demonstrated that TGF-α
induced the migration of HuH7 cells via the SAPK/JNK (18) and AKT (19) pathways. Therefore, the present study
examined whether or not the ERK, p38 MAPK and Rho kinase pathways
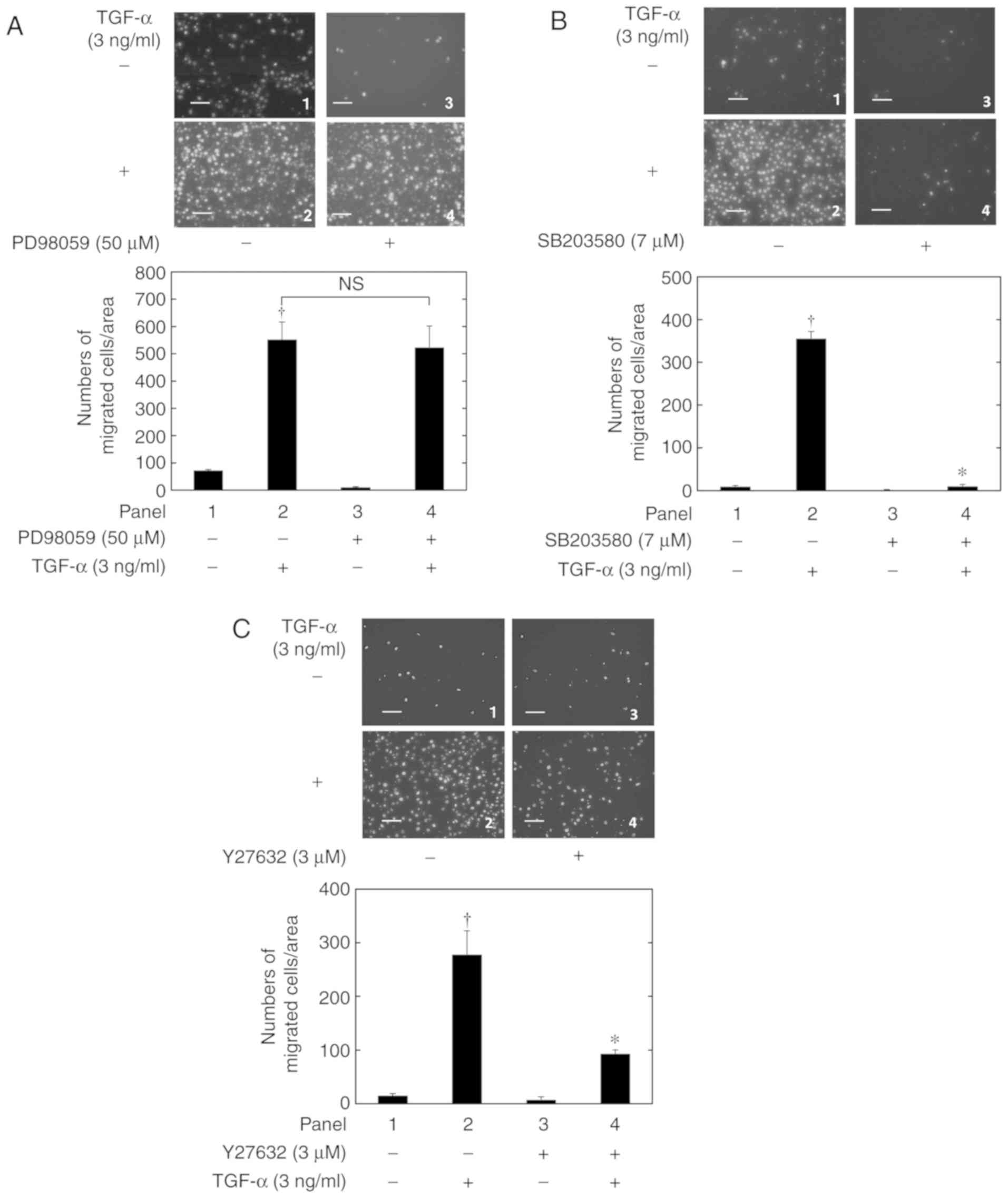
were also involved in the TGF-α-induced HuH7 cell migration.
PD98059, an inhibitor of the upstream kinase of ERK (24), did not exhibit a suppressive effect on
the TGF-α-induced HuH7 cell migration (Fig. 4A). By contrast, the p38 MAPK inhibitor
SB203580(25) (Fig. 4B) and the Rho kinase inhibitor
Y27632(26) (Fig. 4C) significantly suppressed the
TGF-α-induced HuH7 cell migration. This suggested that the p38 MAPK
and Rho kinase pathways, but not the ERK pathway, are involved in
the TGF-α-induced HuH7 cell migration.
Effects of olive oil polyphenols on
the TGF-α-stimulated phosphorylation of AKT, SAPK/JNK, p38 MAPK and
MYPT-1 in HuH7 cells
Whether or not 3-HT affected the TGF-α-activated
AKT, SAPK/JNK, p38 MAPK and Rho kinase pathways was examined. As
indicated in Fig. 5, the
TGF-α-stimulated phosphorylation of AKT, SAPK/JNK, p38 MAPK and
MYPT-1 was not suppressed by 3-HT concentrations ≤30 µM (Fig. 5A-D). In addition, 3-HT conversely
enhanced the TGF-α-induced activation of p38 MAPK at 20 and 30 µM
(Fig. 5C). Therefore, whether or not
oleuropein activated p38 MAPK activity as 3-HT in HuH7 cells was
investigated. As demonstrated in Fig.
5E, oleuropein did not affect the TGF-α-induced p38 MAPK
phosphorylation.
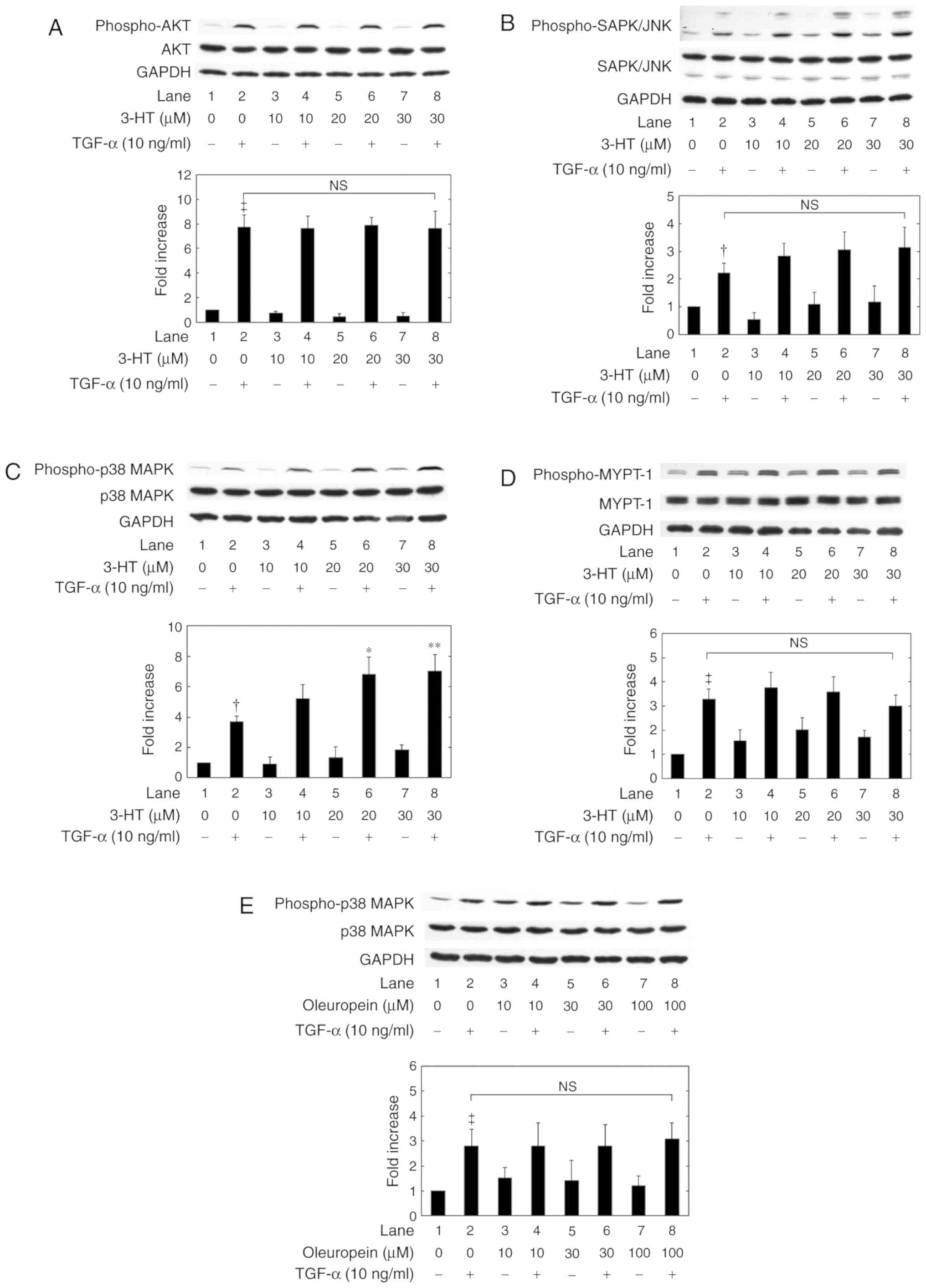 | Figure 5.Effects of 3-HT and oleuropein on
TGF-α-induced phosphorylation. The levels of (A) AKT, (B) SAPK/JNK,
(C) p38 MAPK and (D) MYPT-1 phosphorylation following 3-HT
treatment were measured using western blot analysis. (E) Effects of
oleuropein treatment on the TGF-α-induced phosphorylation of p38
MAPK in HuH7 cells. The cells were pretreated with the indicated
doses of 3-HT or oleuropein for 60 min and then stimulated by 10
ng/ml TGF-α or vehicle for (A) 3 min, (B) 15 min, (C) 5 min, (D) 1
min or (E) 5 min. The extracts of cells were then subjected to
SDS-PAGE with subsequent western blot analyses using antibodies
against phospho-specific AKT, AKT, phospho-specific SAPK/JNK,
SAPK/JNK, phospho-specific p38 MAPK, p38 MAPK, phospho-specific
MYPT-1, MYPT-1 or GAPDH. The histograms represent the
quantification of the levels of TGF-α-induced phosphorylation
obtained from laser densitometric analysis of 3 independent
experiments. The phosphorylation levels were corrected by the total
protein levels and the levels of GAPDH, and then expressed as the
fold increase compared with the basal levels presented in lane 1.
Each value represents the mean ± standard deviation of data from 3
independent cell preparations performed in triplicate.
†P<0.05 and ‡P<0.001 vs. control cells
without TGF-α. *P<0.05 and **P<0.01 vs.
cells with TGF-α alone. 3-HT, 3-hydroxytyrosol; TGF-α, transforming
growth factor-α; p38 MAPK, p38 mitogen-activated protein kinase;
MYPT-1, myosin phosphatase targeting subunit 1; SAPK/JNK,
stress-activated protein kinase/c-Jun N-terminal kinase; N.S., no
significant difference between the indicated pairs. |
Discussion
The beneficial effects of olive oil polyphenols on
human health, in particular their anti-oxidant effects, have been
evaluated by a number of studies (1-3). In
addition to their anti-oxidant activities, oleuropein and 3-HT are
also known to exhibit anti-cancer properties (1-3). For
example, 3-HT and other extra-virgin olive oil polyphenols were
demonstrated to induce apoptosis of HCC cells and inhibit their
proliferation (7,27-29).
However, while there are a number of previous studies concerning
the protective effects of olive oil polyphenols on the liver
(7), to the best of our knowledge,
none have described the effects of olive oil polyphenols on HCC
cell migration.
TGF-α, a ligand for EGFR, has demonstrated high
expression levels in human metastatic liver tumors (11). Furthermore, the activation of the EGFR
signaling pathway is known to enhance HCC cell movement (13), and this study group previously
demonstrated that TGF-α induced the migration of human HCC-derived
HuH7 cells (18,19). Therefore, in the present study,
whether or not olive oil polyphenols were involved in the
TGF-α-induced migration of HuH7 cells was examined.
It was demonstrated that the TGF-α-induced migration
of HuH7 cells was significantly and dose-dependently suppressed by
oleuropein and 3-HT. To the best of our knowledge, this is the
first study investigating the inhibitory effect of olive oil
polyphenols on HCC cell migration. The data suggested that
oleuropein and 3-HT at 10 and 1 µM, respectively, significantly
inhibited the TGF-α-induced migration of HuH7 cells, after 24 h of
treatment. Although oleuropein and 3-HT induced the apoptosis of
HCC cells, 24 h treatment with 20 µM oleuropein, and 48 h treatment
with 100 µM 3-HT did not decrease the viability of HuH7 cells
(27,28). The concentration and treatment period
of oleuropein and 3-HT required to suppress the TGF-α-induced
migration of HuH7 cells were much lower compared with those
required to induce apoptosis of HCC cells (27,28).
This study group previously demonstrated that TGF-α
induced the migration of HuH7 cells via the JNK (18) and AKT (19) pathways. In the present study, it was
revealed that the p38 MAPK and Rho kinase pathways were also
involved in the TGF-α-induced migration of HuH7 cells, while the
ERK pathway was not involved. Although the activation of the ERK
pathway is generally considered to induce HCC progression, it may
not be involved in TGF-α-induced HCC cell migration. Therefore, the
present study examined whether or not 3-HT decreased the
TGF-α-induced activation of the AKT, SAPK/JNK, p38 MAPK and Rho
kinase activities. Unexpectedly, the TGF-α-stimulated activations
of AKT, SAPK/JNK, P38 MAPK and Rho kinase were not suppressed by
3-HT up to 30 µM. Treatment with 100 µM 3-HT for 48 h was
demonstrated to suppress AKT activity in HuH7 cells (27). However, in the present study, 3-HT
exerted a suppressive effect on the TGF-α-induced migration of HuH7
cells, even at 1 µM for 24 h. Therefore, the suppressive effect on
AKT activity by the high doses and long treatment periods of 3-HT
may not be associated with the effect of 3-HT on the TGF-α-induced
migration of HuH7 cells.
Although both 3-HT and oleuropein suppressed the
TGF-α-stimulated migration of HuH7 cells, and p38 MAPK served as a
positive regulator in the cell migration, these olive oil
polyphenols did not inhibit TGF-α-induced p38 MAPK activation. On
the contrary, 3-HT activated p38 MAPK in HuH7 cells in the present
study. While, oleuropein failed to affect the TGF-α-induced p38
MAPK phosphorylation. These observations suggest that the
activation of p38 MAPK by 3-HT is not a common effect of olive oil
polyphenols. The activation of p38 MAPK by 3-HT may be independent
of the inhibitory effect on the migration. In addition, we proposed
that 3-HT decreased the level of TGF-α-stimulated migration of HuH7
cells, even when removed from the cell culture before TGF-α
stimulation. Thus, there was no plausible direct interaction
between 3-HT and TGF-α. Therefore, the suppressive effect of olive
oil polyphenols on the TGF-α-induced migration of HuH7 cells may be
mediated through a pathway other than the AKT, SAPK/JNK, p38 MAPK
and Rho kinase pathways, or it may occur at a point downstream of
these kinases. Additional studies are required to clarify the exact
roles of olive oil polyphenols in HCC.
In conclusion, the results from the present study
suggest that the olive oil polyphenols oleuropein and 3-HT
suppressed TGF-α-induced HCC cell migration.
Acknowledgements
The authors would like to thank Mrs. Yumiko Kurokawa
for her skillful technical assistance.
Funding
The present study was supported in part by JSPS
KAKENHI (grant nos. JP25460989 and JP17K094) from the Ministry of
Education, Culture, Sports, Science and Technology of Japan.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
NY, RMN and OK conceived and designed the
experiments. NY, RMN, AM and KT performed experiments. NY, RMN and
OK analyzed the data. NY, RMN and OK wrote the paper. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Vissers MN, Zock PL and Katan MB:
Bioavailability and antioxidant effects of olive oil phenols in
humans: A review. Eur J Clin Nutr. 58:955–965. 2004.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Echeverría F, Ortiz M, Valenzuela R and
Videla LA: Hydroxytyrosol and cytoprotection: A projection for
clinical interventions. Int J Mol Sci. 18(E930)2017.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Gorzynik-Debicka M, Przychozen P, Cappello
F, Kuban-Jankowska A, Marino Gammazza A, Knap N, Wozniak M and
Gorska-Ponikowska M: Potential health benefits of olive oil and
plant polyphenols. Int J Mol Sci. 19(E686)2018.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Salvini S, Sera F, Caruso D, Giovannelli
L, Visioli F, Saieva C, Masala G, Ceroti M, Giovacchini V, Pitozzi
V, et al: Daily consumption of a high-phenol extra-virgin olive oil
reduces oxidative DNA damage in postmenopausal women. Br J Nutr.
95:742–751. 2006.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Ruano J, López-Miranda J, de la Torre R,
Delgado-Lista J, Fernández J, Caballero J, Covas MI, Jiménez Y,
Pérez-Martínez P, Marín C, et al: Intake of phenol-rich virgin
olive oil improves the postprandial prothrombotic profile in
hypercholesterolemic patients. Am J Clin Nutr. 86:341–346.
2007.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Oliveras-López MJ, Molina JJ, Mir MV, Rey
EF, Martín F and de la Serrana HL: Extra virgin olive oil (EVOO)
consumption and antioxidant status in healthy institutionalized
elderly humans. Arch Gerontol Geriatr. 57:234–242. 2013.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Soto-Alarcon SA, Valenzuela R, Valenzuela
A and Videla LA: Liver protective effects of extra virgin olive
oil: Interaction between its chemical composition and the
cell-signaling pathways involved in protection. Endocr Metab Immune
Disord Drug Targets. 18:75–84. 2018.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Llovet JM, Zucman-Rossi J, Pikarsky E,
Sangro B, Schwartz M, Sherman M and Gores G: Hepatocellular
carcinoma. Nat Rev Dis Primers. 2(16018)2016.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Toso C, Mentha G and Manjo P: Liver
transplantation for hepatocellular carcinoma: Five steps to prevent
recurrence. Am J Transplant. 11:2031–2035. 2011.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Zhang Y, Shi ZL, Yang X and Yin ZF:
Targeting of circulating hepatocellular carcinoma cells to prevent
postoperative recurrence and metastasis. World J Gastroenterol.
20:142–147. 2014.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Jaskiewicz K and Chasen MR: Differential
expression of transforming growth factor alpha, adhesions molecules
and integrins in primary, metastatic liver tumors and in liver
cirrhosis. Anticancer Res. 15:559–562. 1995.PubMed/NCBI
|
|
12
|
Qin LX and Tang ZY: The prognostic
molecular markers in hepatocellular carcinoma. World J
Gastroenterol. 8:385–392. 2002.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Huang P, Xu X, Wang L, Zhu B, Wang X and
Xia J: The role of EGF-EGFR signaling pathway in hepatocellular
carcinoma inflammatory microenvironment. J Cell Mol Med.
18:218–230. 2014.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Muntané J, De la Rosa AJ, Docobo F,
García-Carbonero R and Padillo FJ: Targeting tyrosine kinase
receptors in hepatocellular carcinoma. Curr Cancer Drug Targets.
13:300–312. 2013.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Gao F, Deng G, Liu W, Zhou K and Li M:
Resveratrol suppresses human hepatocellular carcinoma via targeting
HGF-c-Met signaling pathway. Oncol Rep. 37:1203–1211.
2017.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Zhang HH, Zhang Y, Cheng YN, Gong FL, Cao
ZQ, Yu LG and Guo XL: Metformin incombination with curcumin
inhibits the growth, metastasis, and angiogenesis of hepatocellular
carcinoma in vitro and in vivo. Mol Carcinog. 57:44–56.
2018.PubMed/NCBI View
Article : Google Scholar
|
|
17
|
Nakabayashi H, Taketa K, Miyano K, Yamane
T and Sato J: Growth of human hepatoma cells lines with
differentiated functions in chemically defined medium. Cancer Res.
42:3858–3863. 1982.PubMed/NCBI
|
|
18
|
Matsushima-Nishiwaki R, Toyoda H, Nagasawa
T, Yasuda E, Chiba N, Okuda S, Maeda A, Kaneoka Y, Kumada T and
Kozawa O: Phosphorylated heat shock protein 20 (HSPB6) regulates
transforming growth factor-α-induced migration and invasion of
hepatocellular carcinoma cells. PLoS One.
11(e0151907)2016.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Matsushima-Nishiwaki R, Toyoda H,
Takamatsu R, Yasuda E, Okuda S, Maeda A, Kaneoka Y, Yoshimi N,
Kumada T and Kozawa O: Heat shock protein 22 (HSPB8) reduces the
migration of hepatocellular carcinoma cells through the suppression
of the phosphoinositide 3-kinase (PI3K)/AKT pathway. Biochim
Biophys Acta Mol Basis Dis. 1863:1629–1639. 2017.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Laemmli UK: Cleavage of structural
proteins during the assembly of the head of bacteriophage T4.
Nature. 227:680–685. 1970.PubMed/NCBI
|
|
21
|
Matsushima-Nishiwaki R, Adachi S, Yoshioka
T, Yasuda E, Yamagishi Y, Matsuura J, Muko M, Iwamura R, Noda T,
Toyoda H, et al: Suppression by heat shock protein 20 of
hepatocellular carcinoma cell proliferation via inhibition of the
mitogen-activated protein kinases and AKT pathways. J Cell Biochem.
112:3430–3439. 2011.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Min L, He B and Hui L: Mitogen-activated
protein kinases in hepatocellular carcinoma development. Semin
Cancer Biol. 21:10–20. 2011.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Wong CC, Wong CM, Au SL and Ng IO:
RhoGTPases and Rho-effectors in hepatocellular carcinoma
metastasis: ROCK N'Rho move it. Liver Int. 30:642–656.
2010.PubMed/NCBI
|
|
24
|
Alessi DR, Cuenda A, Cohen P, Dudley DT
and Saltiel AR: PD098059 is a specific inhibitor of the activation
of mitogen-activated protein kinase kinase in vitro and in vivo. J
Biol Chem. 270:27489–27494. 1995.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Cuenda A, Rouse J, Doza YN, Meier R, Cohen
P, Gallagher TF, Young PR and Lee JC: SB203580 is a specific
inhibitor of a MAP kinase homologue which is stimulated by cellular
stresses and interleukin-1. FEBS Lett. 364:229–233. 1995.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Shimokawa H and Rashid M: Development of
Rho-kinase inhibitors for cardiovascular medicine. Trends Pharmacol
Sci. 28:296–302. 2007.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Zhao B, Ma Y, Xu Z, Wang J, Wang F, Wang
D, Pan S, Wu Y, Pan H, Xu D, et al: Hydroxytyrosol, a natural
molecule from olive oil, suppresses the growth of human
hepatocellular carcinoma cells via inactivating AKT and nuclear
factor-kappa B pathways. Cancer Lett. 347:79–87. 2014.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Yan CM, Chai EQ, Cai HY, Miao GY and Ma W:
Oleuropein induces apoptosis via activation of caspases and
suppression of phosphatidylinositol 3-kinase/protein kinase B
pathway in HepG2 human hepatoma cell line. Mol Med Rep.
11:4617–4624. 2015.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Cusimano A, Balasus D, Azzolina A, Augello
G, Emma MR, Di Sano C, Gramignoli R, Strom SC, McCubrey JA,
Montalto G and Cervello M: Oleocanthal exerts antitumor effects on
human liver and colon cancer cells through ROS generation. Int J
Oncol. 51:533–544. 2017.PubMed/NCBI View Article : Google Scholar
|