Introduction
The establishment of an artificial airway, including
tracheal intubation or tracheotomy, is an important measure to
rescue and treat critically ill patients. When the artificial
airway is established, there is no upper respiratory tract warming
or humidification. As the warming and humidifying function of the
upper respiratory tract is lost, ciliary tracheal mucosal damage
occurs, the mucociliary transport function is lost, and secretion
is thickened and difficult to discharge. Humidification functions
in preventing hypothermia; warming and humidification are crucial
in preventing hypothermia, destruction of respiratory epithelial
tissue, bronchospasm, atelectasis and airway obstruction (1). The 2012 American College of Respiratory
Therapy Airway Wetness Guidelines (1)
propose that, in order to ensure the normal activity of the
mucociliary system, the gas inhaled through the artificial airway
must be at normal body temperature (37˚C) and relative humidity
(100%), which is absolute humidity (44 mg/l). The 2017 British
Thoracic Society guidelines for adult oxygen use suggest that
patients who require long-term oxygen therapy (>24 h) for
tracheotomy may be considered for the use of a large volume oxygen
humidification device, particularly for patients with poor
discharge of secretions (2). In
recent years, clinical researchers have used respiratory
humidification therapy devices with an active warming humidifier
(MR850, Fisher & Paykel Healthcare, Auckland, New Zealand),
heating pipelines and Venturi high-flow humidification oxygen
therapy equipment to treat patients with tracheotomy. Clinical
studies on the use of respiratory humidification therapies for
tracheotomy patients are limited. Corley et al (3) and others used high flow nasal cannula
(HFNC) high-flow oxygen therapy and low-flow oxygen therapy with
T-tubes in a tracheotomy. High-flow oxygen therapy improves the
patient's oxygen, and ivolves a different mechanism of action
between the transnasal and transtracheal incision modes of
delivery; thus, we maintained a certain positive ventilation
pressure. High-flow oxygen therapy in the tracheotomy patient
bypasses the larynx. The upper and lower respiratory tract may
affect the clinical effect of HFNC. The Venturi humidification
oxygen therapy system regulates the flow rate of gas, mainly by
adjusting the oxygen flow rate and the oxygen concentration
standard of the Venturi air oxygen mixer; however, the precise
mechanism of its operation remains controversial (4). In view of the above research status in
the actual application process, it is clear that there are
differences in the effects of humidification and ventilation
between the two oxygen therapy methods in patients with
tracheotomy. In order to further examine the effects, evaluation of
the two humidification oxygen therapy methods is required to
provide more choice.
The aim of the present study was to further examine
the effect of the two humidification oxygen therapy methods. This
involved use of a respiratory humidification therapy device and a
high-flow humidification oxygen therapy device to treat patients
with tracheotomy.
Materials and methods
Setting
The present study was conducted at the Department of
Critical Care, with ~39 beds in total, of Second People's Hospital
of Shenzhen (First Affiliated Hospital of Shenzhen University,
Shenzhen, China), Shenzhen is one of the largest cities and the
most populous city in South China. The study protocol was approved
by the Ethics Committee of the Department of Critical Care, Second
People's Hospital of Shenzhen (Shenzhen, China).
Patients
In total, 78 patients with tracheotomy in the
intensive care unit (ICU) were recruited to the study between July
2017 and December 2017, who were randomly divided into an
observation group (n=39) and a control group (n=39). The baseline
characteristics are shown in Table I.
All tracheotomy patients who met the inclusion criteria of the ICU
underwent respiratory humidification immediately, within 24 h of
being offline. Patients who had been taken offline in the ICU were
randomly assigned to the control group and the observation group
within 24 h. The inclusion criteria were as follows: i) 16-80 years
old; ii) tracheal incision patients evacuated from the ventilator;
iii) APACHE II score ≥15 points (5).
The exclusion criteria were as follows: i) Patients with a history
of chronic respiratory diseases, patients with chronic respiratory
diseases due to long-term chronic inflammation, repeated use of
antibiotics and patients in an immunosuppressive state. The use of
high-flow humidification oxygen therapy to improve airway
humidification cannot be used as an independent factor affecting
the lungs of patients with an infection, as the experimentally set
observation indicators are not specific to such patients; ii)
patents with a history of airway injury, lung trauma or lung
surgery; iii) patients with body temperature <35˚C, and those
with stage 4 heart failure patients requiring fluid restriction
(6); iv) pregnant woman. There were
no significant differences in gender, age, diagnosis or condition
between the two groups (P>0.05).
 | Table IComparison of sputum viscosity in the
control and experimental groups. |
Table I
Comparison of sputum viscosity in the
control and experimental groups.
| | 0 h | 48 h | 96 h | 7 days |
|---|
| Sputum viscosity | Control | Observation | Control | Observation | Control | Observation | Control | Observation |
|---|
| Grade I | 7 | 8 | 5 | 6 | 4 | 3 | 2 | 3 |
| Grade II | 26 | 25 | 18 | 28 | 19 | 30 | 18 | 31 |
| Grade III | 6 | 6 | 16 | 5 | 16 | 6 | 19 | 5 |
| Z-score | 0.086 | | 8.027 | | 6.457 | | 11.816 | |
| P-value | 0.958 | | 0.018 | | 0.040 | | 0.003 | |
Oxygen therapy
When the patients in the observation group had been
evacuated from the ventilator, the artificial airway high-flow
humidification oxygen therapy closed suction system was used for
oxygen therapy, and the gas flow rate was adjusted according to the
patient's requirements. The artificial airway high-flow
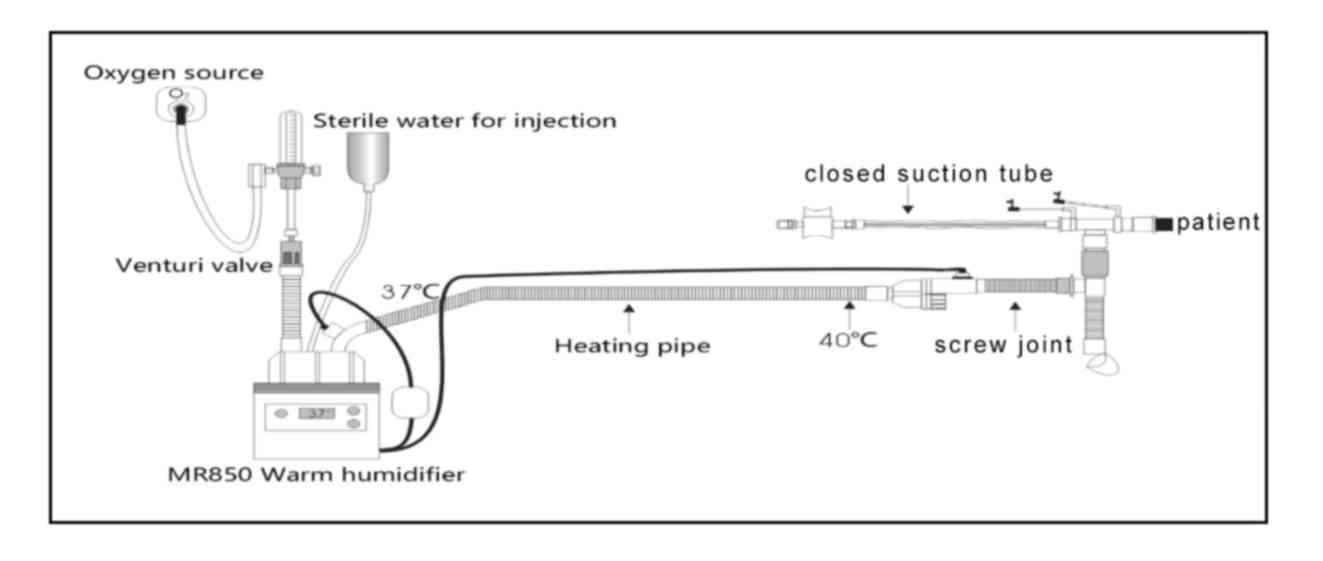
humidification oxygen therapy closed suction system is composed of
an automatic pressure regulating oxygen flow meter, a Fisher &
Paykel MR850 automatic temperature control heating system, a
Venturi air oxygen mixing valve (Fisher & Paykel Healthcare
Ltd.), oxygen connecting tube (Fisher & Paykel Healthcare
Ltd.), RT308 heating breathing tube (Fisher & Paykel Healthcare
Ltd.), screw joint (EM07-005, Excellentcare Medical Ltd.) and a
Closed Suction System (Tracheostomy; Pacific Hospital Supply Co.
Ltd.) (Fig. 1). The output gas flow
rate in the oxygen therapy system was adjusted to ≥40 l/min. The
humidifier was used in invasive mode to set up the output
temperature of the screw joint at 37˚C, and the relative humidity
at 100%.
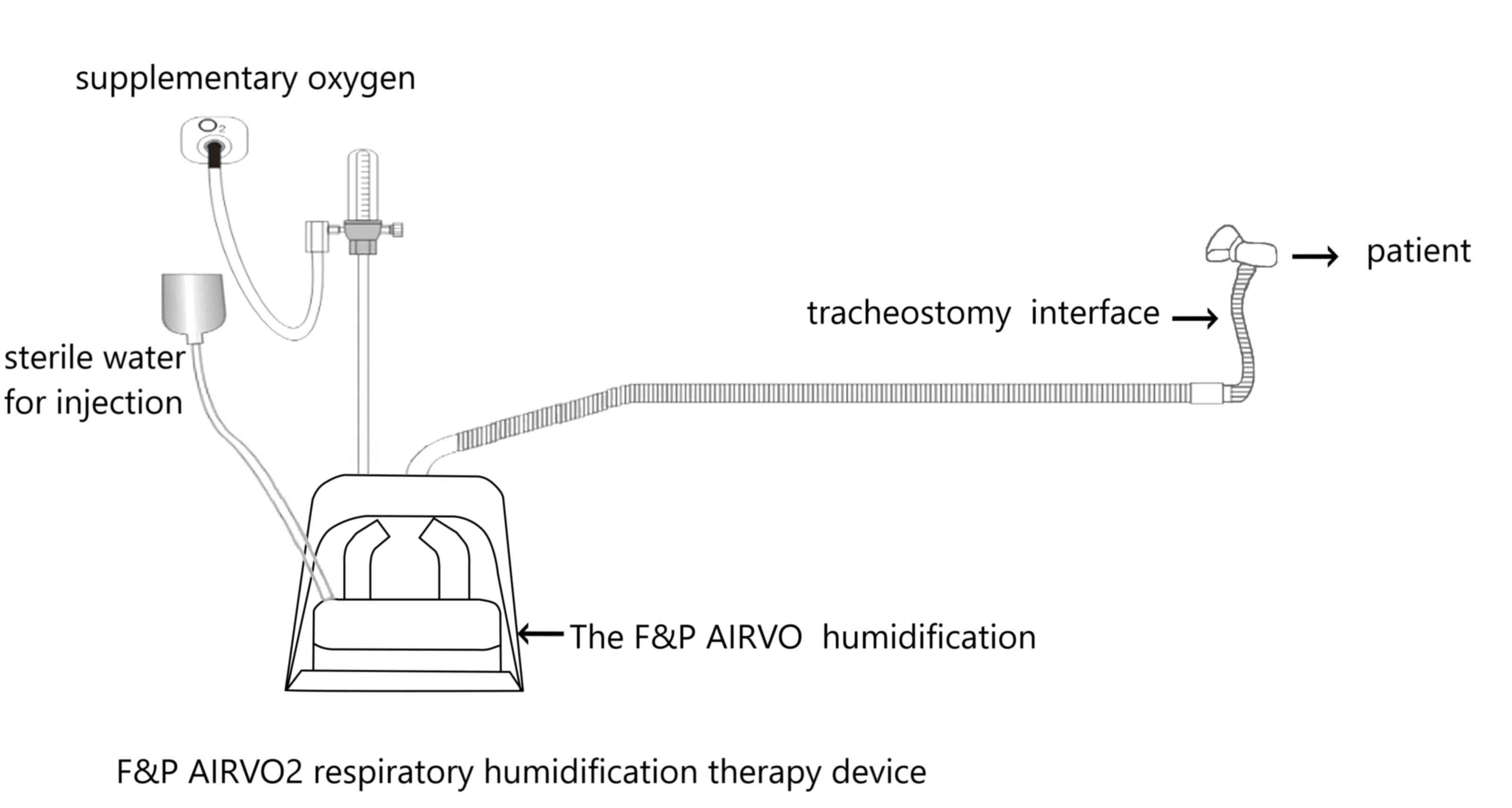
When patients in the control group had been
evacuated from the ventilator, the respiratory humidification
therapy device was used for oxygen therapy, and the gas flow rate
was adjusted according to the patient's requirements. A Fisher
& Paykel AIRVO2 (Fig. 2)
respiratory humidification therapy device and specific heating tube
were used to adjust the therapeutic instrument output gas flow rate
≥40 l/min and the output gas temperature at 37˚C.
Oxygen therapy equipment features
There was no difference in equipment quality as both
equipment and piping were obtained from Fisher & Paykel
Healthcare. Both types of oxygen therapy are high-flow oxygen
supply systems supplying gas at a flow rate of ≥40 l/min, which is
higher than patient inspiratory flow rate and can meet the demand
of a high-flow oxygen supply system (7). In addition, both contain an MR850
automatic temperature control heating system to control the
temperature at 37˚C. The differences between the systems are as
follows: The respiratory humidification therapy device can be used
for nasal and tracheal incision; the gas flow rate is accurate and
is not affected by the attached equipment; the pipeline and the
extension pipe joint material are provided with water-permeable and
airtight technology to effectively prevent condensed water inside
the pipe; the output gas temperature is maintained at 37˚C, thus no
external heating wire is required. The artificial airway
humidification oxygen therapy system is only used for tracheotomy
patients; the high-speed gas mainly depends on the automatic
pressure regulating oxygen flow meter and the Venturi effect,
whereas the gas flow rate is affected by the attached equipment;
the pipeline and the extension pipe joint material do not have
water-permeable and airtight technology, which produces a small
volume of condensed water; the output gas temperature is maintained
at 37˚C under the control of an external heating wire.
The humidification system used an MR850 heating
system, which comprises a humidification chamber and a heated-wire
circuit; the gas maintained the temperature of the water leaving
the chamber at 37˚C; the airway end temperature is automatically
controlled to 40˚C when breathing through the heated-wire circuit;
the temperature of the connecting tube through the closed suction
tube is 3˚C lower. Eventually, the temperature at which the
patient's airway enters the patient is exactly 37˚C and the
absolute humidity is 44 mg/l. These setting differences enabled a
higher inspiratory absolute humidity in the present study. For the
Airvo 2, only the chamber temperature was set, at 37˚C. The Airvo 2
servo-controls the proximal temperature at ~40˚C.
Compared parameters
Changes in sputum viscosity, arterial oxygen partial
pressure (PaO2), arterial blood carbon dioxide partial
pressure (PaCO2) and arterial oxygen saturation
(SaO2), in addition to the incidence of lung infection
were observed at 0, 48, 96 h and 7 days after oxygen therapy.
The arterial blood used for blood gas analysis was
usually drawn from the patient's wrist by a nurse in the ICU, who
was trained to do so 0, 2, 4 and 7 days after weaning from the
mechanical ventilation. Prior to the arterial blood gas test, the
nurse applied pressure to the arteries in the patient's wrist for
several seconds. The procedure, known as the modified Allen test,
assesses whether blood flow to the hand is normal. Once an artery
is located, a needle is inserted into the artery and blood is draw.
When the sample is obtained, care is taken to eliminate visible gas
bubbles, as these bubbles can dissolve into the sample and lead to
inaccurate results. The sealed syringe is taken to a blood gas
analyzer and the sample is analyzed within 30 min. The machine
aspirates this blood from the syringe and measures the pH and the
partial pressures of oxygen and carbon dioxide. The bicarbonate
concentration is also calculated. These results are usually
available for interpretation within 5 min.
Sputum viscosity grade
determination
As the artificial airway, the patients were randomly
assigned to receive either the high-flow humidification oxygen
therapy closed suction system or the AIRVO 2 humidifier (Fisher
& Paykel Healthcare) with the tracheostomy interface at the
onset of weaning from mechanical ventilation (time 0). Nurses in
the ICU were trained to perform tracheostomy management, including
suctioning and oral hygiene. The sputum samples were collected by
suction using a sterile technique 0, 2, 4 and 7 days after weaning
from mechanical ventilation. The suction procedure was based on
current knowledge. A sterile suction catheter was gently inserted
into the endotracheal tube for a maximum of 15 cm or until
resistance was detected; the duration of the suctioning procedure
was limited to <15 sec. The suctioning procedure was performed
according to clinical requirements, only when secretions were
present. Sputum viscosity was measured by the trained nurse, who
performed the first suctioning on each day. The sputum viscosity
was divided into three grades: Grade I: Diluted like rice soup or
foam, no sputum retention on the inner wall of the tube following
suction; grade II: Moderate adhesion, following suction, a small
quantity of sputum is retained in the inner wall of the tube, but
is easily removed by washing with water; grade III: Sticky and
yellow, a large amount of adhesive remains on the inner wall of the
suction tube, which is not easily removed by washing with water
(7).
Pulmonary infection determination
The secretions in the artificial airway were
collected in a blinded manner, and the sputum culture was detected
by quantitative analysis. Pathogens in the lower respiratory tract
secretions of >107 cfu/ml and reference to X-ray
chest imaging examination confirmed the occurrence of new pulmonary
infection (8). The detection time
should be between 48 h and 7 days following oxygen therapy
(9). In the present study, pulmonary
infection was defined as pneumonia occurring between 48 h and 7
days after oxygen therapy. Pneumonia was defined of the presence of
a new infiltrate (due to any pneumonia-causing pathogen) on chest
radiograph (CXR) and one or more clinical symptoms, including
fever, a new cough and dyspnea. The CXRs were interpreted by a
panel of radiologists trained in the standardized interpretation of
CXRs. The CXRs were classified as either demonstrating
consolidation, another infiltrate, both consolidation and another
infiltrate, or as being normal or uninterpretable. Standard culture
methods were used to detect putative pneumonia pathogens from
sputum samples for the purpose of ascribing etiology. A sterile
mucus extracting catheter attached to a suction device was gently
inserted into the endotracheal tube for a maximum of 15 cm or until
resistance was detected (the duration of the suctioning procedure
was limited to <15 sec) and sputum was collected into a sterile
trap. The suctioning procedure was performed according to clinical
requirements (only when secretions are present). The sputum
specimens were immediately sent to the laboratory and were washed
with saline. The presence of >25 white blood cells and <10
epithelial cells per low-power field of vision was regarded as
being indicative of a high-quality specimen. The sputum was
separated from the mucus by adding approximately five times the
volume of saline, following which the saline was removed by the
suction tube. Following the addition of the same volume of sputum
pretreatment solution, each specimen was diluted to 106
cfu/ml of the original concentration and inoculated into a blood
dish, a Chinese blue dish and a chocolate dish. Colony count and
bacterial type identification were performed using a Bact Alert 3D
full-automatic culture device (bioMérieux, Inc.) following
incubation for 18 h in the conditions of 5% CO2 and
37˚C.
Statistical analysis
Statistical analysis was performed using the SPSS 17
software package (SPSS, Inc.). All data are presented as the mean ±
standard deviation, and the difference between two groups were
compared with a t-test. The rank sum test was used for rank data
and the χ2 test was used for enumeration data. P<0.05
was considered to indicate a statistically significant
difference.
Results
Patients
A total of 78 patients were initially included in
the study, and all patients were successfully taken offline, in
which the patients were disconnected from the ventilator and
allowed to breath unassisted through the tracheostomy cannula.
Sputum viscosity in the control and
experimental groups
The sputum viscosity did not differ significantly
between the two groups on finishing oxygen therapy (P>0.05).
However, the number of patients with grade III sputum viscosity in
the control group was increased 48, 96 h and 7 days after oxygen
therapy, and the sputum viscosity grade between the two groups was
statistically significant (P<0.05) at the detected time
points.
Incidence of pulmonary infection in
the control and observation groups
Those patients who acquired a pulmonary infection in
the two groups 48 h and 7 days after oxygen therapy are shown in
Table III. The number of new
pulmonary infections in the control group significantly increased
within 7 days after taken offline compared with the observation
group (Table II). In the control
group, there were 13 cases of pulmonary infection. In the
observation group, there were five cases of pulmonary infection.
The incidence of pulmonary infection between the two groups was
statistically significant. Significant differences between the
control and observation groups 48, 96 h, and 7 days after oxygen
therapy were observed (P=0.018, 0.040 and 0.003, respectively;
Table I).
 | Table IIIBlood-gas indices in the control and
observation groups. |
Table III
Blood-gas indices in the control and
observation groups.
| Index | Group | 0 h | 48 h | 96 h | 7 days |
|---|
| PaO2
(mmHg) | Control | 91.56±6.07 | 87.08±6.74 | 86.20±5.69 | 86.84±6.50 |
| | Observation | 92.46±6.98 | 93.23±6.76 | 94.46±6.34 | 97.00±6.63 |
| | F-value | 0.606 | 4.025 | 6.054 | 7.373 |
| | P-value | 0.546 | <0.001 | <0.001 | <0.001 |
| PaCO2
(mmHg) | Control | 36.21±1.42 | 37.38±1.79 | 37.18±1.68 | 36.74±1.57 |
| | Observation | 36.95±2.22 | 36.97±1.55 | 37.00±1.56 | 36.89±1.59 |
| | F-value | 1.761 | 1.084 | 0.489 | 0.431 |
| | P-value | 0.082 | 0.282 | 0.626 | 0.668 |
| SaO2
(%) | Control | 96.94±1.56 | 96.25±1.14 | 95.72±1.48 | 94.85±1.41 |
| | Observation | 97.33±1.54 | 97.89±1.35 | 97.85±1.55 | 97.42±1.98 |
| | F-value | 1.096 | 5.791 | 6.218 | 6.593 |
| | P-value | 0.277 | <0.001 | <0.001 | <0.001 |
 | Table IIIncidence of pulmonary infection in
the control and observation groups. |
Table II
Incidence of pulmonary infection in
the control and observation groups.
| | Pulmonary
infection | | |
|---|
| Group | Positive, n (%) | Negative, n (%) | χ2
value | P-value |
|---|
| Control (n=39) | 13 (33.3) | 26 (66.7) | 4.622 | 0.032 |
| Observation
(n=39) | 5 (12.8) | 34 (87.2) | | |
Blood gas indices in the control and
observation groups
Blood gas analysis can provide a more comprehensive
understanding of the patient's ventilation function. Ventilation
function mainly depends on hypoxia and CO2 retention,
and the acid-base state and electrolyte imbalance in the body when
oxygen therapy finishes. No significant differences were observed
in the PaO2, PaCO2 or SaO2 between
the two groups (P>0.05). After 48, 96 h and 7 days, the
PaO2 gradually approached the normal range in the
control group, and the SaO2 also exhibited a downward
trend, whereas the PaCO2 did not change (P<0.05). The
PaO2 and SaO2, but not the PaCO2,
differed significantly between the two groups (P>0.05).
Discussion
Humidified effect of artificial airway
high-flow humidification oxygen therapy is superior to that of
respiratory humidification therapy in patients undergoing
tracheotomy
Although both oxygen therapy devices can be used for
patients with tracheotomy, the observation group and the control
group were respectively treated with different active warming and
humidifying oxygen therapy systems for oxygen therapy. The results
in Table I show that the sputum
viscosity was altered between 48 h and 7 days following oxygen
therapy. The sputum viscosity of the control group gradually
increased, the airway humidification rate decreased, and the sputum
viscosity grade different significantly compared with that in the
observation group (P<0.05). Warming and humidification can
improve the comfort of patients with non-invasive ventilation
(1). Patients undergoing nasal oxygen
therapy retain the warming and humidifying function in the upper
respiratory tract, however, the generation of condensed water in
the pipeline can reduce patient comfort and compliance, which means
respiratory humidification therapy devices are preferred in
patients who use nasal high-flow humidification oxygen therapy
(10-13)
and are mainly used in patients with acute heart failure and
hypoxemic respiratory failure (14,15). In
order to prevent the formation of condensed water, the pipeline and
the extension pipe joints use water-permeable and airtight
technology, which reduces the relative humidity of the delivered
gas but improves the comfort and compliance of patients with nasal
high flow oxygen therapy. However, when used for tracheotomy
patients, a decrease in relative humidity may result in the
thickening of endotracheal secretions, which in turn increases the
risk of catheter obstruction (1).
As patients undergoing tracheotomy lose the warming
and humidifying function of the upper respiratory tract, all
inhaled gas needs to be compensated for the heat and moisture lost
through the warming humidifier. An ideal high-flow humidification
oxygen therapy device can meet the demand of the patient's peak
flow rate, and ensures the generated air flow through the
humidifier reaches the temperature and humidity of the normal human
airway (44 mg/l, relative humidity 100%, gas temperature 37˚C)
(1,2,16). The
artificial airway high-flow humidification oxygen therapy system
used a Fisher & Paykel MR850 humidifier and a RT308 breathing
circuit equipped with a ventilator. This device is preferred for
use in artificial airway patients. When the invasive mode is
selected, the gas between the outlet of the humidifier and the
Y-type interface is heated to 40˚C, and is then attenuated to 37˚C
by exending the pipe joint. The temperature of the inhalation gas
of the patient is 37˚C, and the relative humidity reaches 100% to
ensure the effective discharge of secretions in the artificial
airway (1). Reports show that the
inhalation of humidified gas can affect the ciliary movement of
respiratory epithelial cells, and the incidence of lower
respiratory tract infection can increase with decreased airway
humidification (17-19).
As shown in Table II, the number of
new lung infections in the control group was increased 7 days after
oxygen therapy compared with the number in the observation group,
and this difference was statistically significant (P<0.05). The
differences in airway humidification between the two groups may
result in abnormality of the secreted discharge with consequent
pulmonary infection establishing. The artificial airway high-flow
humidification oxygen therapy system was superior to the
respiratory humidification therapy instrument in patients
undergoing tracheotomy.
Appropriate high-flow humidification
oxygen therapy equipment can be selected according to the time
requirement of the humidification oxygen therapy
Researchers have used T-tube and high-flow oxygen
therapy for patients with long-term tracheotomy following
cardiothoracic surgery, and showed that high-flow humidification
oxygen therapy increased oxygenation when long-term mechanical
ventilation intermittent training was used (3). As the results in Table III show, the difference in
PaO2 and SaO2 between the two groups was not
significant within 48 h of oxygen therapy (P>0.05), however, the
difference was significant between 48 h and 7 days after oxygen
therapy (P<0.05). As the control group patients had insufficient
airway humidification and poor drainage after 48 h, this may have
caused airway obstruction, atelectasis and pulmonary infection and
affected the patient's oxygenation index and ventilation effect,
eventually contributing to the poor prognosis of patients (20). The artificial airway humidification
oxygen therapy system supplied a satisfactory airway humidification
effect for a long duration in the observation group, and ensured
patient ventilation safety. It is clear that appropriate high-flow
humidification oxygen therapy equipment can be selected according
to the duration that the patient is treated with oxygen therapy. If
the tracheotomy patient can breathe without a ventilator within 48
h, either the artificial airway high-flow humidification oxygen
therapy system or the respiratory humidification therapy instrument
can be used for oxygen therapy; if the tracheotomy patients are
able to self-breathe for >48 h, it is recommended that an
artificial airway high-flow humidification oxygen therapy system is
used for oxygen therapy.
Constant-velocity high-flow gas is
essential for the safe use and humidification effect of the
artificial airway high-flow humidification oxygen therapy closed
suction system
Studies have suggested that the problem of an
additional ineffective cavity and airway resistance during the use
of an active warming humidification device should be considered, as
it may cause negative reactions including hypopnea and respiratory
muscle fatigue (21). Regarding the
different types of Venturi valves used in clinical practice, it is
not possible to monitor the gas flow rate of high-flow oxygen
therapy devices. In order to ensure that the patient's inspiratory
flow rate requirements are met and to avoid hypoventilation,
clinical researchers use Venturi valves for high-flow
humidification oxygen therapy and connect the tracheotomy mask or
T-tube connector at the suction end. When the output gas flow rate
of the oxygen therapy device is insufficient to meet the maximum
inspiratory flow rate of the patient, ambient air (oxygen
concentration 21%, temperature 24-26˚C, humidity 40-60%) can enter
from the expiratory hole of the mask or T-tube joint. However, this
increases the chance that the inhalation gas may be mixed with air
which has not been warmed and humidified, resulting in the actual
oxygen concentration and humidity being reduced. Therefore, the
present study used the automatic pressure regulating oxygen flow
meter to maintain the output gas flow rate ≥40 l/min, providing a
basis for the combined use of oxygen therapy equipment and a closed
suction system. The artificial high-flow humidification oxygen
therapy closed suction system can provide the same constant-speed
high-flow gas as the respiratory humidification therapy device, and
it reduces airway resistance and prevents side effects including
hypoventilation and respiratory muscle fatigue. Furthermore, it
ensures the gas inhaled by the patient is fully humidified by the
heating humidifier without reducing the oxygen concentration. As
shown in Table III, there was no
significant difference in PaCO2 between the two groups
(P>0.05).
However, the present study is not without
limitation. It did not consider several factors that have the
possibility to influence the results in the control and observation
groups, including the duration of incubation, the status of
nutrition (e.g., albumin), immunosuppressive status (e.g. diabetes
and immunosuppressive agents) and the administration of
antibiotics.
In conclusion, due to the clinical situation, a
large number of patients require long-term indwelling tracheotomy
catheters for oxygen therapy and expectoration, and the equipment
available to satisfy the long-term high-flow humidification oxygen
therapy requirements of these patients is limited. In the present
study, two types of active warming humidification oxygen therapy
equipment of equal quality, but with different design features,
were compared for their humidification and ventilation effects in
oxygen therapy for patients with tracheotomy. It was found that the
artificial airway high-flow humidification oxygen therapy closed
suction system was more suitable for the long-term oxygen therapy
of the patients with tracheotomy than the respiratory
humidification therapy instrument. With lower equipment and
material costs, it can improve airway humidification and oxygen
therapy effects and reduce lung infection.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Shenzhen's
Sanming Project Foundation of China (grant no. SZSM201612011).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
Performed the literature review: YM; conducted
research: YM, SY, PL and XXH; analyzed the data: YL, PL and YY. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Research
Ethics Boards and experimental procedures were approved by the
Ethics Committee of the Second People's Hospital of Shenzhen.
Written informed consent was provided by all participants.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
American Association for Respiratory Care
Restrepo RD Walsh BK: Humidification during invasive and
noninvasive mechanical ventilation: 2012. Respir Care 57: 782-788,
2012.
|
|
2
|
O'Driscoll BR, Howard LS, Earis J and Mak
V: British Thoracic Society Emergency Oxygen Guideline Group; BTS
Emergency Oxygen Guideline Development Group. BTS guideline for
oxygen use in adults in healthcare and emergency settings. Thorax.
72(Suppl 1):ii1–ii90. 2017.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Corley A, Edwards M, Spooner AJ, Dunster
KR, Anstey C and Fraser JF: High-flow oxygen via tracheostomy
improves oxygenation in patients weaning from mechanical
ventilation: A randomised crossover study. Intensive Care Med.
43:465–467. 2017.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Soto-Ruiz KM, Peacock WF and Varon J: The
men and history behind the Venturi mask. Resuscitation. 82:244–246.
2011.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Knaus WA, Draper EA, Wagner DP and
Zimmerman JE: APACHE II: A severity of disease classification
system. Crit Care Med. 13:818–829. 1985.PubMed/NCBI
|
|
6
|
Yancy CW, Jessup M, Bozkurt B, Butler J,
Casey DE Jr, Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi
JL, et al: ACCF/AHA guideline for the management of heart failure:
A report of the American College of Cardiology Foundation/American
Heart Association Task Force on Practice Guidelines. J Am Coll
Cardiol. 62:e147–e239. 2013.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Del Sorbo L, Vendramin A and Mehta S: High
flow oxygen therapy in adult critically ill patients. Minerva
Anestesiol. 83:402–411. 2017.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Lopez-Vidriero MT, Charman J, Keal E, De
Silva DJ and Reid L: Sputum viscosity: Correlation with chemical
and clinical features in chronic bronchitis. Thorax. 28:401–408.
1973.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Kalil AC, Metersky ML, Klompas M,
Muscedere J, Sweeney DA, Palmer LB, Napolitano LM, O'Grady NP,
Bartlett JG, Carratalà J, et al: Management of adults with
hospital-acquired and ventilator-associated pneumonia: 2016
clinical practice guidelines by the infectious diseases society of
america and the american thoracic society. Clin Infect Dis.
63:e61–e111. 2016.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Stéphan F, Barrucand B, Petit P,
Rézaiguia-Delclaux S, Médard A, Delannoy B, Cosserant B, Flicoteaux
G, Imbert A, Pilorge C, et al: High-flow nasal oxygen vs
noninvasive positive airway pressure in hypoxemic patients after
cardiothoracic surgery: A randomized clinical trial. JAMA.
313:2331–2339. 2015.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Miguel-Montanes R, Hajage D, Messika J,
Bertrand F, Gaudry S, Rafat C, Labbé V, Dufour N, Jean-Baptiste S,
Bedet A, et al: Use of high-flow nasal cannula oxygen therapy to
prevent desaturation during tracheal intubation of intensive care
patients with mild-to-moderate hypoxemia. Crit Care Med.
43:574–583. 2015.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Delorme M, Bouchard PA, Simon M, Simard S
and Lellouche F: Effects of high-flow nasal cannula on the work of
breathing in patients recovering from acute respiratory failure.
Crit Care Med. 45:1981–1988. 2017.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Papazian L, Corley A, Hess D, Fraser JF,
Frat JP, Guitton C, Jaber S, Maggiore SM, Nava S, Rello J, et al:
Use of high-flow nasal cannula oxygenation in ICU adults: A
narrative review. Intensive Care Med. 42:1336–1349. 2016.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Díaz-Lobato S, Folgado MA, Chapa A and
Mayoralas Alises S: Efficacy of high-flow oxygen by nasal cannula
with active humidification in a patient with acute respiratory
failure of neuromuscular origin. Respir Care. 58:e164–e167.
2013.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Hughes J and Doolabh A: Heated,
humidified, high-flow nasal oxygen usage in the adult Emergency
Department. Australas Emerg Nurs J. 19:173–178. 2016.PubMed/NCBI View Article : Google Scholar
|
|
16
|
McFadden ER Jr, Pichurko BM, Bowman HF,
Ingenito E, Burns S, Dowling N and Solway J: Thermal mapping of the
airways in humans. J Appl Physiol. 58:564–570. 1985.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Lacherade JC, Auburtin M, Cerf C, Van de
Louw A, Soufir L, Rebufat Y, Rezaiguia S, Ricard JD, Lellouche F,
Brun-Buisson C and Brochard L: Impact of humidification systems on
ventilator- associated pneumonia: A randomized multicenter trial.
Am J Respir Crit Care Med. 172:1276–1282. 2005.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Cinnella G, Giardina C, Fischetti A, Lecce
G, Fiore MG, Serio G Carravetta G, Dambrosio M and Fiore T: Airways
humidification during mechanical ventilation. Effects on
tracheobronchial ciliated cellsmorphology. Minerva Anestesiol.
71:585–593. 2005.(In English, Italian). PubMed/NCBI
|
|
19
|
Jiang M, Song JJ, Guo XL, Tang YL and Li
HB: Airway humidification reduces the inflammatory response during
mechanical ventilation. Respir Care. 60:1720–1728. 2015.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Roux NG, Plotnikow GA, Villalba DS,
Gogniat E, Feld V, Ribero Vairo N, Sartore M, Bosso M, Scapellato
JL, Intile D, et al: Evaluation of an active humidification system
for inspired gas. Clin Exp Otorhinolaryngol. 8:69–75.
2015.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Campbell RS, Davis K Jr, Johannigman JA
and Branson RD: The effects of passive humidifier dead space on
respiratory variables in paralyzed and spontaneously breathing
patients. Respir Care. 45:306–312. 2000.PubMed/NCBI
|