Introduction
Depression is a mental health disorder, which varies
from mild to severe changes in mood and affects physical, mental
and behavior health (1). According to
International Classification of Diseases-10 and Diagnostic and
Statistical Manual-IV, depression episodes are recognized as an
individual suffering from depressed or sad mood, showing a loss of
energy, diminished activity or loss of interest in activities,
which results in a significant reduction in productivity and has a
negative impact on overall health (1). The World Health Organization projects
unipolar major depression as a leading burden of disease worldwide
by 2030(2).
There are various factors that play a role in the
development of major depression, such as changes in environmental
factors, socioeconomic conditions, sedentary lifestyle, nutrition
and eating habits (1). These
conditions are linked to impacting stressful events in life.
Continuous exposure to stress can cause a compensatory increase in
synthesis and concentration of monoamines, specifically serotonin,
norepinephrine and dopamine in brain (3), which are responsible for regulating
appetite, the drive for sleep, emotions, stress and sexuality
(3). Despite the adaptive
consequence, changes in the nervous system can be excessive,
leading to an increase in vulnerability to the pathology of
depression (3). Chronic exposure to
stressful events not only affects neurotransmitter levels but also
induces neuroinflammation (4),
increase oxidative stress (5) and
affects the hypothalamic-pituitary-adrenal (HPA) axis (4). Changes in neurochemical levels can
result in dampening the psychological or physical impact of stress
and induce a response that impairs the ability in dealing with
stress (6). Current treatments mainly
target monoamines levels; however, this treatment is not efficient
for many patients (7). An alternative
approach is required to modulate neuroinflammation and resultant
oxidative stress.
Associations between oxidative stress and
inflammatory cytokines are well established in major depressive
disorders (5). Oxidative stress is an
outcome of imbalance between oxidant and antioxidant levels that
affects lipids and various other cellular biomolecules, and results
in the generation of various inflammatory cytokines (5). It was reported that oxidative stress is
elevated in major depressive syndrome and suppressed by standard
monoamine-targeting drugs, such as amitriptyline, fluoxetine of
imipramine (8). Thus, the present
study was designed to target oxidative stress that resulted from
chronic stress.
Diet is important in maintaining health. Green tea
is a popular beverage consumed daily by a wide population;
particularly in Japan and some parts of China, where ≤4 cups are
consumed daily (9). Its active
ingredients, including (+)-catechin, (+)-catechin gallate,
(-)-epicatechin, (-)-epicatechin gallate, (-)-epigallocatechin,
(-)-epigallocatechin gallate, (+)-gallocatechin and
(+)-gallocatechin gallate, have shown antioxidant,
anti-inflammatory and neuroprotective effects in various animal
models (10).
Epigallocatechin-3-gallate, a catechin found in green tea, has
shown catechol-o-methyltransferase inhibitory activity in
vitro (11). Another active
ingredient, (+)-catechin has shown anti-inflammatory effects in
lipopolysaccharide-induced neuroinflammation (10). (+)-Catechin is an active constituent
of the Tamarindus indica fruit pulp (12). Extract of it has shown aphrodisiac
effect in the previous study, which is suggesting that catechin
might alter monoamine levels in the brain (12). In chronic corticosterone-injected
rats, catechin administration showed a decrease in depression and
anxiety-like behaviors (13).
However, these described corticosterone injections may not reflect
stress-induced depression accurately. The chronic unpredictable
mild stress (CUMS) model in rodents mimics responses similar to
stressful life events in humans (14). Thus, the present study was designed to
assess the antidepressant activity of (+)-catechin in rats with
CUMS.
Materials and methods
Chemicals
Escitalopram oxalate (escitalopram; purity, >98%)
was provided by Micro Labs Ltd. and (+)-catechin hydrate, butylated
hydroxytoluene (BHT), thiobarbituric acid (TBA),
5,5-dithio-bis-(2-nitrobenzoic acid) (DTNB) and adrenaline
bitartrate were purchased from Sigma-Aldrich (Merck KGaA).
Carboxymethyl cellulose (CMC) was purchased from Loba Chemie Pvt.
Ltd., sodium chloride and trichloroacetic acid (TCA) were purchased
from SD Fine-Chem Ltd. and hydrogen peroxide, sodium bicarbonate,
potassium dihydrogen phosphate and disodium hydrogen phosphate were
purchased from HiMedia Laboratories Pvt. Ltd.
Animals
A total of 24 male Sprague Dawley rats (age, 12
weeks; weight, 180-200 g) were obtained from the Central Animal
Research Facility of the Manipal Academy of Higher Education.
Animals were acclimatized for 7 days before initiating the
experiment. The housing, breeding and storage conditions were the
same, with a controlled temperature (20±5˚C), humidity (55±5%) and
12-h light/dark cycles. Rats were kept in sterilized propylene
cages containing sterile husk and were provided with food and water
ad libitum. The study was approved by the Institutional
Animal Ethics Committee of Kasturba Medical College (Manipal,
India; no. IAEC/KMC/49/2016). All the experiments were performed
according to the Committee for the Purpose of Control and
Supervision of Experiments on Animals guidelines (15).
Experimental design of CUMS
Rats were randomly divided into four groups
(n=6/group): (i) Vehicle control, receiving 0.25% (w/v) CMC [dose
volume, 10 ml/kg per os (p.o.)]; (ii) CUMS control, receiving 0.25%
(w/v) CMC (dose volume, 10 ml/kg p.o.); (iii) escitalopram,
receiving escitalopram 10 mg/kg p.o. as suspension in 0.25% (w/v)
CMC (stock, 1 mg/ml; dose volume, 10 ml/kg) (14); and (iv) catechin, receiving
(+)-catechin hydrate at 50 mg/kg p.o. as suspension in 0.25% (w/v)
CMC (stock, 5 mg/ml; dose volume, 10 ml/kg) (16). All the rats were administered with
their treatments daily between 9-10 am and 1 h prior to daily
stress exposure for 8 weeks. All drug suspensions were prepared
fresh on a daily basis in 0.25% CMC (14).
The CUMS procedure was performed as previously
described (14). It involved the
daily application of various stressors for a period of 8 weeks. The
stressors included: (i) No food for 24 h, (ii) no water for 20 h,
(iii) tail pinch for 30-60 sec, (iv) electric shock at 0.5 mA for
10-15 sec, (v) cage tilt at 45˚ for 24 h, (vi) restraint in a rat
restrainer for 4-6 h, (vii) forced swimming for 15-20 min and
(viii) no food and water for 20-24 h. They were applied following
the schedule shown in Table I.
Vehicle control rats were not exposed to any stressors.
 | Table ISchedule for chronic unpredictable
mild stress in rats. |
Table I
Schedule for chronic unpredictable
mild stress in rats.
| Day | Week 1 | Week 2 | Week 3 | Week 4 | Week 5 | Week 6 | Week 7 | Week 8 |
|---|
| 1 | NF | NF, ES | ES | RS, TP | NW | ES | CT | RS |
| 2 | NW | NF, TP | RS, TP | ES | CT | NF, TP | ES | CT |
| 3 | CT | RS, TP | CT | NFW | RS | CT | RS | ES |
| 4 | TP | CT | NF, TP | ES | NF, TP | NW, TP | TP | NW, TP |
| 5 | RS | ES | NW, ES | CT | ES | RS | ES | NF |
| 6 | ES | NFW | RS, TP | FSa, NFW | RS, TP | FSa, NFW | NF | FSa, NFW |
| 7 | FS | RS, TP | FS | PT1 | ES | FST | NW | PT2 |
Sucrose preference test
The sucrose intake study was performed as described
previously (14) and involved an
adaptation phase, where rats were adapted to consume 1% (w/v)
sucrose before starting the CUMS experiment. In the next phase, the
test phase, sucrose compared with water consumption was measured.
During adaptation, rats were placed individually in a cage and
provided with two bottles for 3 days, one with water and one with
1% sucrose solution. Sucrose consumption was measured every 24 h
and a baseline value was obtained as the average consumed in 3
consecutive days. The tests were performed in weeks 4 and 8, and
food and water were removed for 24 h prior to the experiment. Then,
two bottles were placed in each cage, one with water and one with
100 ml 1% sucrose solution. After 24 h, the consumed volume was
measured in both bottles.
Forced swimming test (FST)
Rats were individually placed in a water tank made
of glass (height, 80 cm; diameter, 30 cm) at 23-25˚C. Experiments
were performed in two sessions. In the first session, animals were
trained to swim for 15 min. The second session (test session) was
conducted 24 h after the training session and 1 h post treatment.
In this test session, rats were studied for immobility time for 10
min. The study was conducted in weeks 4, 6 and 8. Immobility was
recorded as floating, with small movements or the head above the
surface of the water. The behavior was recorded using a video
camera and scored manually (14,17).
Evaluation of antioxidant
parameters
On completion of the study, rats were anaesthetized
with intraperitoneal injections of ketamine (87 mg/kg) and xylazine
(13 mg/kg). Once the animals reached the surgical plane of
anesthesia, an intracardiac perfusion was given using PBS, pH 7.4
until the liver was clear. The brain was isolated and homogenated
in PBS to make a 10% (w/v) homogenate. The homogenate was
centrifuged at 10,956 x g for 30 min at 4˚C and the supernatant was
used to determine endogenous antioxidant levels, including
catalase, reduced glutathione (GSH), superoxide dismutase (SOD) and
malondialdehyde (MDA) using a UV spectrophotometer (18). Total protein was determined in the
supernatant using Pierce™ BCA protein Assay kit (cat. no. 23225;
Thermo Fisher Scientific, Inc.) as per manufacturer
instruction.
Assay for antioxidant catalase
levels
A total of 50 µl tissue homogenate was added to 3 ml
of 50 mM potassium phosphate buffer (PPB; pH 7.0) containing
sufficient H2O2 (75 µl 35%
H2O2 added to 50 ml PPB) to establish an OD
between 3-5 at 240 nm. Changes in absorbance were recorded for 1
min. The activity of catalase was expressed as U/min/mg protein
(19).
Assay for GSH levels
Protein was precipitated from 750 µl tissue
homogenate by adding an equal volume of 5% (w/v) trichloroacetic
acid. The supernatant was collected after centrifugation (1,397 x
g, 10 min, 4˚C). A total of 500 µl supernatant was added to a
mixture of 3 ml PB (0.2 M; pH 8.0) and 500 µl DTNB (0.6 mM) and
incubated at room temperature for 10 min. Absorbance was recorded
at 412 nm. The amount of GSH was calculated by extrapolating the
absorbance using a standard curve of GSH and was expressed in µM
GSH/mg protein (19).
Assay of MDA levels
Tissue homogenate (0.5 ml) was heated in a mixture
of 2.5 ml TBA (0.375% w/v), TCA (15% w/v) and BHT (0.015% w/v)
solution at 90˚C for 10 min. The resulting mixture was centrifuged
at 1,397 x g for 10 min at 25˚C. The absorbance in the supernatant
was directly recorded at 525 nm. The absorbance was converted to
the amount of MDA formed using the molar extinction coefficient of
MDA and values were expressed in nM MDA/mg protein (19).
Assay for SOD levels
To 1.85 ml carbonate buffer (0.1 M; pH 10-11), 100
µl adrenaline (5 mM) and 50 µl of tissue homogenate were added.
Changes in the absorbance were recorded at 480 nm over 1 min at
room temperature. The change in absorbance was extrapolated to a
standard curve recorded for SOD activity. The activity was
expressed in U/mg protein (19).
Statistical analysis
Data were analyzed using GraphPad Prism 5.0
(GraphPad Software, Inc.). Statistical comparisons were performed
using one-way ANOVA followed by Tukey's multiple comparison tests.
Data are expressed as the mean ± standard error of the mean
representative of six experimental repeats. P<0.05 was
considered to indicate a statistically significant difference.
Results
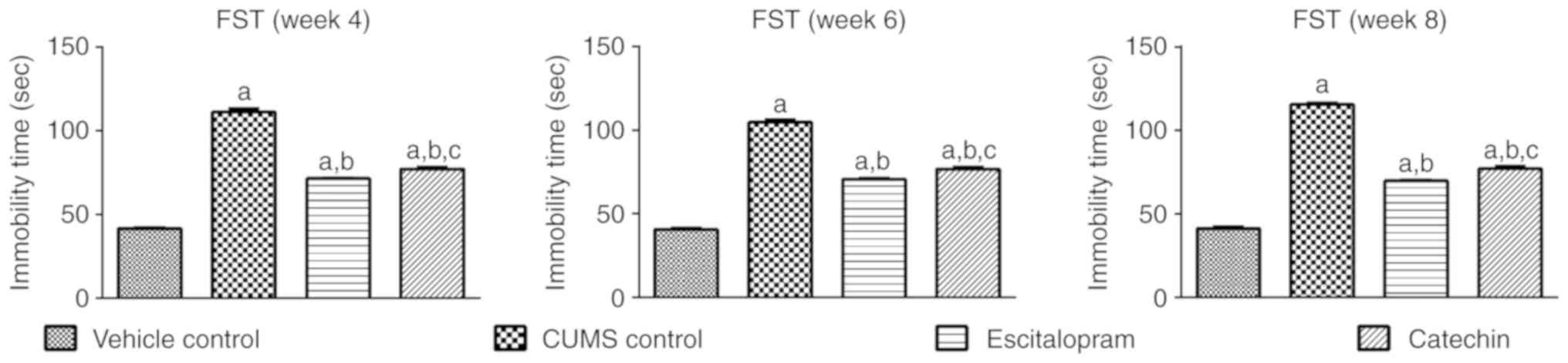
Catechin treatment affects FST in rats
subjected to CUMS
CUMS control rats showed a significant increase in
the immobility time in weeks 4, 6 and 8 compared with the vehicle
control group (111.30±2.11, 104.80±1.52 and 115.50±0.76 vs.
41.50±0.85, 40.67±0.72 and 41.33±1.12 sec, respectively; P<0.05;
Fig. 1). Escitalopram treated animals
subjected to CUMS displayed a significant reduction in immobility
during the 10 min FST in weeks 4, 6 and 8, with 71.50±0.50,
70.67±0.56 and 69.83±0.70 sec, respectively, compared with the CUMS
control group (P<0.05). This behavioral change indicated a
reduction in the degree of despair/helplessness. Catechin treated
animals subjected to CUMS also showed a significant reduction in
immobility time compared with the CUMS control at the end of week
4, 6 and 8, with 77.33±1.09, 76.83±0.95 and 77.00±1.51 sec,
respectively (P<0.05). The immobility time in the catechin
treated group was found to be significantly higher compared with
the escitalopram treated group in weeks 4, 6 and 8 (P<0.05).
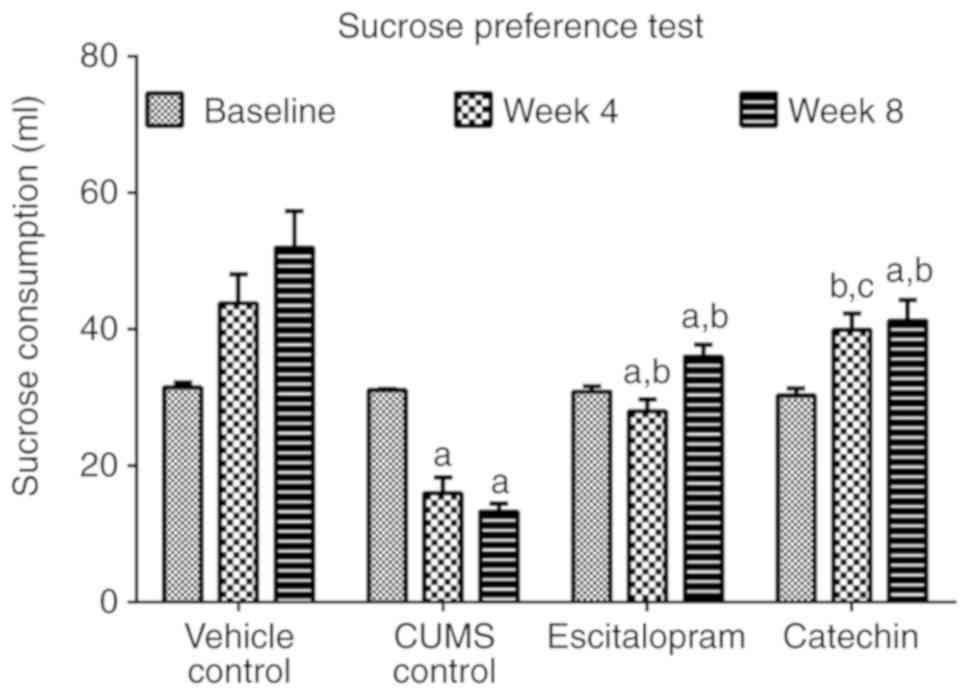
Catechin treatment affects the
consumption of sucrose water in rats subjected to CUMS
CUMS control animals showed a significant reduction
in sucrose consumption compared with the vehicle control group
observed at the end of week 4 and 8 (P<0.05). Additionally, in
comparison with the baseline values recorded for the CUMS control
group before subjecting the animals to CUMS, volumes of consumed
sucrose decreased over the course of the experiment and exposure to
CUMS, indicating anhedonia. Regular treatment of rats subjected to
CUMS with escitalopram and catechin significantly increased sucrose
consumption compared with the CUMS control group (P<0.05;
Fig. 2). In week 4, the sucrose
consumption in the catechin treated group was significantly
increased compared with the escitalopram treated animals
(P<0.05).
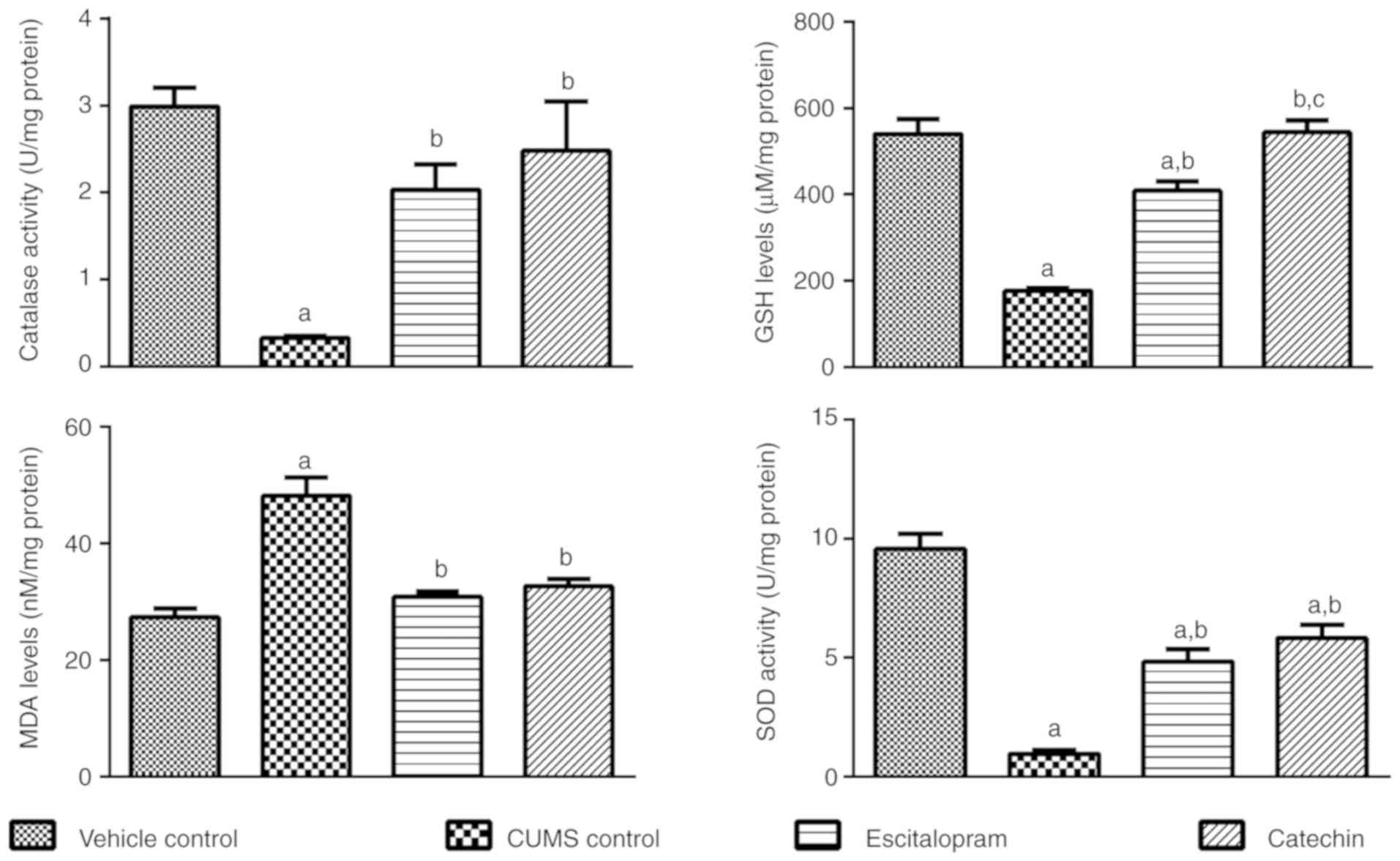
Catechin treatment affects the
endogenous antioxidant levels in the brain
The endogenous antioxidant activity, namely catalase
and SOD activity, and reduced GSH and MDA levels were evaluated in
brains of all animals after completion of the 8-week study
(Fig. 3).
Catalase activity is recovered in
catechin treated rats subjected to CUMS
In the CUMS control group a significant reduction in
catalase activity was observed in comparison with the vehicle
control group (P<0.05). Escitalopram and catechin treated
animals subjected to CUMS showed a significant increase in catalase
activity when compared with the CUMS control group (P<0.05).
Catechin affects SOD activity in rats
subjected to CUMS
A significant reduction in SOD activity in the CUMS
control group was observed in comparison with the vehicle control
group (P<0.05). Escitalopram and catechin treated animals
subjected to CUMS showed a significantly increased SOD activity in
comparison with the CUMS control group (P<0.05).
Reduced GSH levels are recovered in
catechin treated rats subjected to CUMS
A significant reduction in GSH levels in the CUMS
control group was observed when compared with the vehicle control
group (P<0.05). Regular treatment of animals subjected to CUMS
with escitalopram or catechin significantly increased the levels of
GSH compared with the CUMS control group (P<0.05). GSH activity
in the catechin treated animals was significantly increased
compared with the escitalopram treated animals (P<0.05).
Catechin treatment of rats subjected
to CUMS reverses changes in MDA levels
A significant increase in MDA levels in the CUMS
control group was observed when compared with the vehicle control
group (P<0.05). Escitalopram and catechin treated animals
subjected to CUMS showed significantly reduced levels of MDA
compared with the CUMS control (P<0.05).
Discussion
The CUMS induced depression model is a suitable
model for studying depression-like behavior in rodents. Chronic
exposure to stress leads to behavioral and pathological alterations
in rodents that are analogous to those observed in depressed
patients (20). Chronic exposure to
stress results in alterations in the central neurotransmitters,
including changes in levels and activities of noradrenaline,
serotonin and dopamine, in addition to the regulation of specific
receptors, disruption of the HPA axis, elevation of cortisol levels
leading to the activation of several proapoptotic factors, and the
generation of reactive oxygen species (ROS) causing
neurodegeneration and elevating the symptoms of depression
(4). The present study proposed to
assess the antidepressant activity of (+)-catechin in a rodent CUMS
model of depression.
FST is a reliable behavioral test to assess
depression in rodents (17). It helps
in determining the degree of despair/helplessness, which is
indicative of depression, and immobility observed in FST is
considered analogous to depression in humans (14). FST is used to evaluate the activity of
potential antidepressant drugs in rodent models of depression. The
forced immersion of rodents in water for an extended time period
leads to characteristic immobility. Antidepressant drugs decrease
this immobility time. In the current study, treatment with catechin
significantly reduced the FST immobility time at the end of week 4,
6 and 8 in animals subjected to CUMS compared with the CUMS control
group, thereby indicating an antidepressant effect of the
compound.
Anhedonia, or the lack of pleasure, is a major
depressive symptom in humans (14).
It can be assessed in rodents through a reduced preference for
sucrose (14). In the current study,
regular treatment of CUMS-subjected rats with catechin
significantly increased the consumption of sucrose water compared
with the CUMS control group after 4 and 8 weeks.
Oxidative stress is one of the main reasons for
neurodegeneration (21) and for the
further elevation of depression and its symptoms (22). Chronic exposure to stress results in
the activation of several proapoptotic factors, the generation of
excessive ROS and induces necrosis, thereby causing
neurodegeneration, which serves a key role in the pathology of
depression (5). The antioxidant
capacity of the brain homogenate is an indicator of oxidative
stress (5). Catalase is abundant in
peroxisomes. Metabolic activity in cells leads to hydrogen peroxide
production that can be toxic to the body causing the generation of
ROS and inducing cell damage and death. Catalases catalyze the
conversion of hydrogen peroxide to water and oxygen. Excessive
oxidative stress leads to decreased catalase activity resulting in
the accumulation of toxic hydrogen peroxide and ROS in the body
(22). An increase in oxidative
stress is reflected by a decrease in the levels of free radical
scavengers, such as catalase, SOD and GSH, and an increase in the
free radical generators, such as lipid peroxidation that is
quantified through MDA levels (22).
The present study showed that catechin significantly increased the
levels of endogenous antioxidants in the brain, including catalase,
SOD and GSH, and significantly decreased the levels of MDA, thereby
preventing lipid peroxidation in the brain.
In the current study, antidepressant effects of
catechin were compared with those of escitalopram, a known
antidepressant. In week 4, the immobility time in the escitalopram
group was significantly decreased compared with the catechin group,
while the sucrose consumption was significantly higher in the
catechin group compared with the escitalopram group. There was no
significant difference in oxidative stress indicators between the
catechin and escitalopram groups except for the GSH activity. These
results indicated that oxidative stress may not be the only target
of catechin. Thus, it was suggested that regular treatment with
catechin ameliorates the behavioral and neurochemical responses
involved in the pathophysiology of depression due to its
neuroprotective and antioxidant potential and the potential
modulation of neurotransmitter and HPA activity, thus exhibiting
antidepressant-like effects.
CUMS is associated with neuroinflammation and
correlates with the brains' oxidative status (23). This mechanistic aspect was not further
explored in the current study. The current study did not assess
monoamine levels or changes of expression of proteins in the brain;
these aspects may be explored in upcoming studies. Evaluating
further underlying mechanisms in addition to the antioxidant axis
may also be subject of future studies. Additionally, studies may
assess molecular mechanisms, cortisol levels and mRNA expression in
the brain and could help decoding the exact mechanism behind the
antidepressant activity of catechin.
The outcomes of the present study supported ideas to
use catechin as a nutritional supplement or as an adjuvant to the
conventional antidepressant therapy to augment the effect of drugs
(7). The antioxidant property
exhibited by catechin, as well as its ability to modulate the
levels of neurotransmitters may exert a long-term synergism with
first-line drugs. However, further research is required to assess
possible drug interactions in the co-administration with other
drugs.
In conclusion, catechin showed prevention of
anhedonia in Sprague Dawley rats subjected to CUMS that was
observed by improvements in the sucrose preference test and reduced
immobility times in FST. These observations were supported by a
determined reduction in oxidative stress. Thus, it was suggested
that catechin may be a novel therapeutic agent in treating
stress-associated disorders, such as depression.
Acknowledgements
The authors like to thank Ms Ashwathi R. Hegde and
Dr Muthukumar Amirthalingam (Department of Pharmaceutics, Manipal
College of Pharmaceutical Sciences, Manipal Academy of Higher
Education) for their support in this study in procuring chemicals.
The authors further thank Mr. Raju Krishna Ekden and Mr. N. B.
Shenoy from Micro Labs Ltd. for providing Escitalopram oxalate.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
AR, MG, RS, NaK, CMR and NiK designed the study,
analyzed the data and drafted the manuscript. AR, MG and MK
performed the in vivo behavioral study. SS, AR, MG and NiK
performed the antioxidant analysis. All the authors read and
approved the manuscript.
Ethics approval and consent to
participate
Institutional Animal Ethics Committee of Kasturba
Medical College approved the experimental procedures (approval no.
IAEC/KMC/49/2016).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Patel V: Cultural factors and
international epidemiology. Br Med Bull. 57:33–45. 2001.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Lépine JP and Briley M: The increasing
burden of depression. Neuropsychiatr Dis Treat. 7 (Suppl 1):S3–S7.
2011.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Wang F and Pereira A: Neuromodulation,
emotional feelings and affective disorders. Mens Sana Monogr.
14:5–29. 2016.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Slavich GM and Irwin MR: From stress to
inflammation and major depressive disorder: A social signal
transduction theory of depression. Psychol Bull. 140:774–815.
2014.PubMed/NCBI View
Article : Google Scholar
|
|
5
|
Lindqvist D, Dhabhar FS, James SJ, Hough
CM, Jain FA, Bersani FS, Reus VI, Verhoeven JE, Epel ES, Mahan L,
et al: Oxidative stress, inflammation and treatment response in
major depression. Psychoneuroendocrinology. 76:197–205.
2017.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Anisman H, Merali Z and Hayley S:
Neurotransmitter, peptide and cytokine processes in relation to
depressive disorder: Comorbidity between depression and
neurodegenerative disorders. Prog Neurobiol. 85:1–74.
2008.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Sarris J, Murphy J, Mischoulon D,
Papakostas GI, Fava M, Berk M and Ng CH: Adjunctive nutraceuticals
for depression: A systematic review and meta-analyses. Am J
Psychiatry. 173:575–587. 2016.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Behr GA, Moreira JC and Frey BN:
Preclinical and clinical evidence of antioxidant effects of
antidepressant agents: Implications for the pathophysiology of
major depressive disorder. Oxid Med Cell Longev.
2012(609421)2012.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Ni CX, Gong H, Liu Y, Qi Y, Jiang CL and
Zhang JP: Green tea consumption and the risk of liver cancer: A
meta-analysis. Nutr Cancer. 69:211–220. 2017.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Syed Hussein SS, Kamarudin MN and Kadir
HA: (+)-catechin attenuates NF-κB activation through regulation of
Akt, MAPK, and AMPK signaling pathways in LPS-induced BV-2
microglial cells. Am J Chin Med. 43:927–952. 2015.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Zhu BT, Shim JY, Nagai M and Bai HW:
Molecular modelling study of the mechanism of high-potency
inhibition of human catechol-O-methyltransferase by
(-)-epigallocatechin-3-O-gallate. Xenobiotica. 38:130–146.
2008.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Rai A, Das S, Chamallamudi MR, Nandakumar
K, Shetty R, Gill M, Sumalatha S, Devkar R, Gourishetti K and Kumar
N: Evaluation of the aphrodisiac potential of a chemically
characterized aqueous extract of Tamarindus indica pulp. J
Ethnopharmacol. 210:118–124. 2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Lee B, Sur B, Kwon S, Yeom M, Shim I, Lee
H and Hahm DH: Chronic administration of catechin decreases
depression and anxiety-like behaviors in a rat model using chronic
corticosterone injections. Biomol Ther (Seoul). 21:313–322.
2013.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Gill M, Kinra M, Rai A, Chamallamudi MR
and Kumar N: Evaluation of antidepressant activity of methanolic
extract of Saraca asoca bark in a chronic unpredictable mild stress
model. Neuroreport. 29:134–140. 2018.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Ministry of Environment and Forests and
Climate Change Government of India: Compendium of CPCSEA 2018.
Committee for the Purpose of Control and Supervision of Experiments
on Animals (CPCSEA), New Delhi, 2018. http://cpcsea.nic.in/WriteReadData/userfiles/file/Compendium%20of%20CPCSEA.pdf.
|
|
16
|
Zanwar AA, Badole SL, Shende PS, Hegde MV
and Bodhankar SL: Chapter 21 - Antioxidant role of catechin in
health and disease. In: Polyphenols in human health and disease.
Watson RR, Preedy VR and Zibadi S (eds.) Academic Press, San Diego,
CA. pp267–271. 2014. View Article : Google Scholar
|
|
17
|
Castagné V, Porsolt RD and Moser P: Use of
latency to immobility improves detection of antidepressant-like
activity in the behavioral despair test in the mouse. Eur J
Pharmacol. 616:128–133. 2009.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Kumar N, Choudhary A, Tiwari A, Rasal A,
Jetti R and Rao CM: Evaluation of hepatoprotective effect of
aqueous extract of Schrebera swietenioides Bark against
carbon tetrachloride-induced toxicity in wistar rats. Advanced Sci
Lett. 23:1917–1920. 2017. View Article : Google Scholar
|
|
19
|
Kumar N, Rai A, Reddy ND, Shenoy RR,
Mudgal J, Bansal P, Mudgal PP, Arumugam K, Udupa N, Sharma N and
Rao CM: Improved in vitro and in vivo hepatoprotective effects of
liposomal silymarin in alcohol-induced hepatotoxicity in Wistar
rats. Pharmacol Rep. (In Press). View Article : Google Scholar
|
|
20
|
Luo DD, An SC and Zhang X: Involvement of
hippocampal serotonin and neuropeptide Y in depression induced by
chronic unpredicted mild stress. Brain Res Bull. 77:8–12.
2008.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Cheruku S, Chamallamudi M, Ramalingayya G,
Biswas S, Gourishetti K, Nandakumar K, Devkar R, Mallik SB,
Nampoothiri M and Kumar N: Neuroprotective potential of methanolic
extract of Saraca asoca bark against doxorubicin-induced
neurotoxicity. Pharmacog Mag. 15:309–316. 2019. View Article : Google Scholar
|
|
22
|
Vaváková M, Ďuračková Z and Trebatická J:
Markers of oxidative stress and neuroprogression in depression
disorder. Oxid Med Cell Longev. 2015(898393)2015.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Thakare VN, Patil RR, Oswal RJ, Dhakane
VD, Aswar MK and Patel BM: Therapeutic potential of silymarin in
chronic unpredictable mild stress induced depressive-like behavior
in mice. J Psychopharmacol. 32:223–235. 2018.PubMed/NCBI View Article : Google Scholar
|