Introduction
Diabetes mellitus is a major health concern, with an
estimated ~300 million patients diagnosed globally, and with type 2
diabetes mellitus (T2DM) accounting for >90% of all cases
(1). Glucagon like peptide-1 (GLP-1)
derivatives in combination with first-line therapeutics are the
preferred regimen for the treatment for patients with T2DM due to
the promotion of β-cell proliferation and the efficacy of
non-violent hypoglycemia (2). The
half-life of biological GLP-1 is <4 min, as it is degraded in
the plasma by dipeptidyl-peptidase-IV (DPP-IV) (3). Prolonging the half-life of GLP-1 or its
derivatives has attracted a notable amount of research attention,
particularly GLP-1 receptor agonists, due to their short half-life
and the functional properties of GLP-1 receptor activation,
including the inhibition of glucagon secretion and the stimulation
of insulin secretion, each in a glucose-dependent manner (3). The current GLP-1 derivative strategy
includes the modification of residues required for degradation by
DPP-IV and structural modifications (4,5). For
example, arginine (34th residue) in liraglutide is
replaced by lysine, and the addition of a 16-carbon fatty acid
chain on the 24th lysine residue may abrogate
DPP-IV-mediated degradation, thereby prolonging its half-life
(6).
GLP-1 is secreted in response to increasing blood
glucose levels and serves to reduce the glucose concentration
(7). At present, the majority of
studies on this have utilized a T2DM db/db or ob/ob model mice with
their leptin receptor gene or leptin gene knocked out, in order to
determine the efficacy of GLP-1-derived therapeutics in vivo
(8,9).
Knockout T2DM mice are costly, and the construction cycle of these
mice may be time-consuming and, thus, not suitable for
higher-throughput identification of potential therapeutic
methods.
The acute hyperglycemia mouse model refers to a
model of instant hyperglycemia incited by an intraperitoneal
injection of glucose (IPG) in fasting healthy mice. The phase of
instant hyperglycemia in the mouse occurs prior to the first phase
of insulin secretion, typically 30-60 min following IPG, which is
subsequently followed by the slower second phase of insulin
secretion when normoglycemia is attained (10). Prior to the first phase of insulin
secretion, normal mice injected intraperitoneally with glucose are
in a state of hyperglycemia; however, physiologically, the mice
will attempt to maintain a high blood glucose level until the
hyperglycemic blood is detected (10).
A GLP-1 receptor agonist drug, 6-KTP, was utilized
in the present study as described previously (11). The GLP-1 derivative 6-KTP consists of
an albumin-binding domain (ABD), a protease-cleavable linker (LK)
and native GLP-1 (7-37), and is formed by the covalent conjugation
of GLP-1 with albumin mediated by thrombin and DPP-IV proteins
present in the blood (11).
The reaction time for the first phase of insulin
secretion varies with different strains of mice (12). The period within which 6-KTP exerts
its hypoglycemic effect should be determined in the acute
hyperglycemic period prior to the first phase of insulin and GLP-1
secretion. Therefore, the best-suited mouse model of acute
hyperglycemia should be determined first.
The blood glucose levels in acute hyperglycemia mice
are relative to the glucose quantity administered. Acute blood
glucose levels may exceed the upper limit of a blood glucose meter
(33.2 mmol/l) if the glucose quantity administered is too high, and
too low a quantity of glucose may result in an insufficient or lack
of initiation of a hyperglycemic response in the mouse model
(13). Therefore, the determination
of an optimum glucose quantity for IPG is required.
The hypoglycemic effect of 6-KTP is exerted in a
glucose-dependent manner. 6-KTP does not initiate a
glucose-lowering mechanism if the blood glucose level lies within
the physiological range, particularly if the administration of
6-KTP occurs too soon following IPG (11). However, if 6-KTP release is overly
delayed, it may overlap with the first phase of insulin secretion.
Therefore, the optimum time point of 6-KTP activity must be
determined, and should hypothetically lie in the time frame prior
to the first phase of insulin secretion (11).
The present study aimed to determine the ideal mouse
model for acute hyperglycemia, the optimum glucose quantity and the
optimum time point for the injection of 6-KTP.
Materials and methods
Intraperitoneal glucose tolerance test
(IPGTT)
Male KM and male C57BL/6J mice (4-6 weeks old,
average of 20 g per mouse, a total of 24 mice) were purchased from
the Guangdong Medical Laboratory Animal Center (Guangzhou, China).
The mice were fed commercial mouse food (finely ground, autoclaved,
low-fat diet) and housed under controlled conditions (~22˚C and
45-55% humidity, with a 12/12-h light/dark cycle) for the entire
experiment duration. All mice ate and drank freely. All in vivo
experiments were ethically approved by The Jinan University Ethics
Committee of Animal Welfare (Guangdong, China).
Following an overnight fast (16 h), each animal was
injected intraperitoneally with glucose (50% glucose solution, 2
g/kg body weight). Blood samples were collected from the tail vein
following 0, 5, 10, 15, 20, 30, 60, 120 and 180 min (n=8 per mouse
model). Blood glucose concentrations were measured using OneTouch
Ultra (Johnson & Johnson, New Brunswick, NJ, USA).
6-KTP was obtained from The Laboratory of
Biochemistry and Molecular Biology, Jinan University (Guangdong,
China). 6-KTP consists of three functional domains, an ABD (amino
acid sequence, LPHSHRAHSLPP), a protease cleavable linker (LK;
amino acid sequence, FNPRKTP) and bioactive GLP-1.
Intraperitoneal glucose injection
IPGTT was performed on male C57BL/6J mice (n=8 per
glucose mouse model) with a glucose dose of 0, 0.5, 2.0 and 4.0
g/kg body weight. The group of 0 g/kg glucose dose was injected
with PBS. Blood samples for glucose testing were collected from the
tail vein.
Injection of 6-KTP
Acute hyperglycemic male C57BL/6J mice (n=8 per
6-KTP mouse model) were subcutaneously injected with 0.2, 0.3, 0.6,
0.9, 1.2, 1.5 and 1.8 mg/kg 6-KTP. Blood samples for measuring the
blood glucose concentration were collected from the tail vein at 0,
10 and 30 min.
Male C57BL/6J mice (n=8 per mouse model) were
subsequently injected with 6-KTP (1.8 mg/kg body weight) at 60, 30
and 0 min prior to IPG. Blood samples for measuring the blood
glucose concentration were collected from the tail vein at 0, 10
and 30 min.
Acute hyperglycemic male C57BL/6J mice (n=8 per
6-KTP mouse model) were subcutaneously injected with 0.25, 0.5,
0.75 and 1 mg/kg 6-KTP to determine the association between the
6-KTP dose and the functional effects on blood glucose
concentration. Blood samples for measuring the blood glucose
concentration were collected from the tail vein at 0, 10 and 30
min.
Definition of 6-KTP hypoglycemic
activity in the acute hyperglycemia mouse model
The 6-KTP hypoglycemic activity unit was defined as
follows: i) A unit of 6-KTP hypoglycemic activity (U), Δ area under
the curve (AUC)0-30 min for 6-KTP per 100 mmol/l
min-1 was defined as one unit in acute hyperglycemia
mice; ii) the specific activity of 6-KTP (U/µg), the number of
units of hypoglycemic activity per 1 µg 6-KTP in the acute
hyperglycemia mouse model.
The conditions for defining a 6-KTP hypoglycemic
activity unit were as follows: i) 4-6 week-old male C57BL/6J mice
following 16 h fasting; ii) subcutaneous injection with 100 µl
6-KTP or PBS and a simultaneous intraperitoneal injection of 100 µl
glucose solution at a concentration of 0.4 g/ml; iii) blood samples
for blood glucose detection were collected from the tail vein at 0,
10 and 30 min.
Statistical analysis
All data were expressed as the mean ± standard error
of the mean. Statistical significance was determined using a
Student's t-test for comparison between two groups, or a one- or
two-way analysis of variance for comparisons between multiple
groups, as appropriate, followed by a Student-Newman-Keuls post hoc
test. All statistical analyses were performed using GraphPad Prism
6 and SPSS (GraphPad Software, Inc., La Jolla, CA, USA). P<0.05
was considered to indicate a statistically significant difference.
AUC0-30 min was calculated using GraphPad Prism 6.
Results
C57BL/6J mice are the best suited
strain for constructing an acute hyperglycemia mouse model
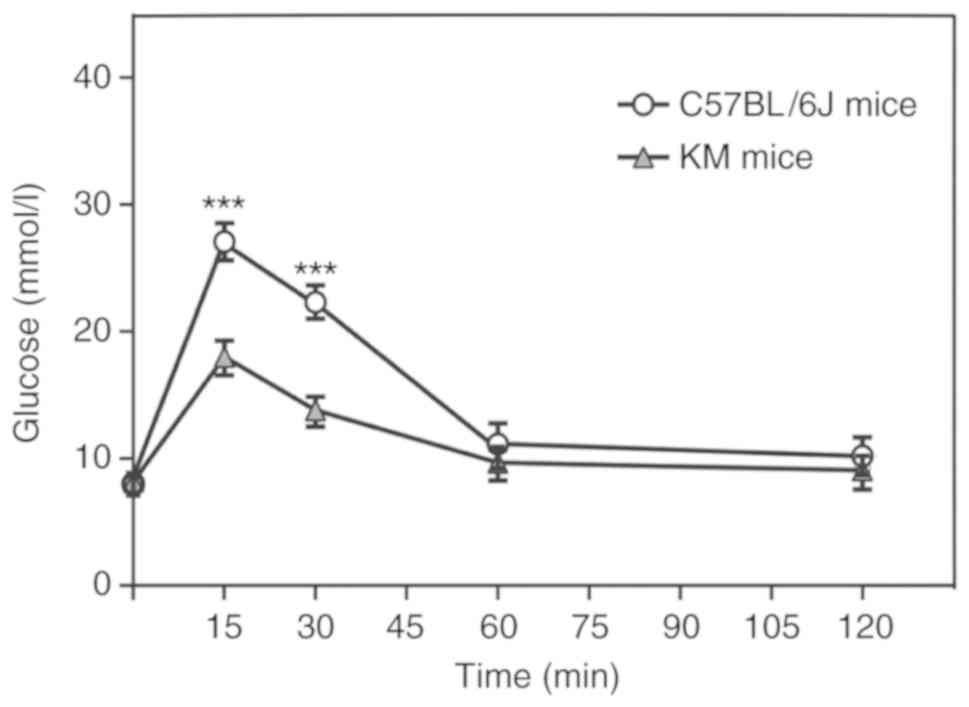
Acute hyperglycemia in mice was induced by an IPG
and induced insulin secretion within 30 min (Fig. 1). Different strains of mice exhibit
varying rates and time frames of increased insulin secretion
following IPG (9). The C57BL/6J and
KM mice, each of which are widely used in pharmacological research
due to the low cost of attaining and maintaining these strains
(14,15), were assessed for their suitability for
an acute hyperglycemia mouse model following IPG. There was no
significant difference between the blood glucose levels following
the first 15 and 30 min of IPG when compared with the same strain
at each time point (P<0.05; Table
I), although the blood glucose levels were significantly higher
in the C57BL/6J strain compared with KM mice following 15 and 30
min of IPG (P<0.001; Fig. 1).
Therefore, the C57BL/6J strain was selected for the subsequent
generation of an acute hyperglycemia mouse model.
 | Table IBlood glucose concentration of
C57BL/6J and KM mice at 15 and 30 min following an intraperitoneal
injection of glucose. |
Table I
Blood glucose concentration of
C57BL/6J and KM mice at 15 and 30 min following an intraperitoneal
injection of glucose.
| Mouse strain
(time) | Blood glucose
concentration, mM | SEM | P-value |
|---|
| C57BL/6J (15
min) | 26.97 | 0.61 | 0.183 |
| KM (15 min) | 17.8 | 0.74 | 0.173 |
| C57BL/6J (30
min) | 22.18 | 0.81 | 0.377 |
| KM (30 min) | 14.12 | 0.65 | 0.344 |
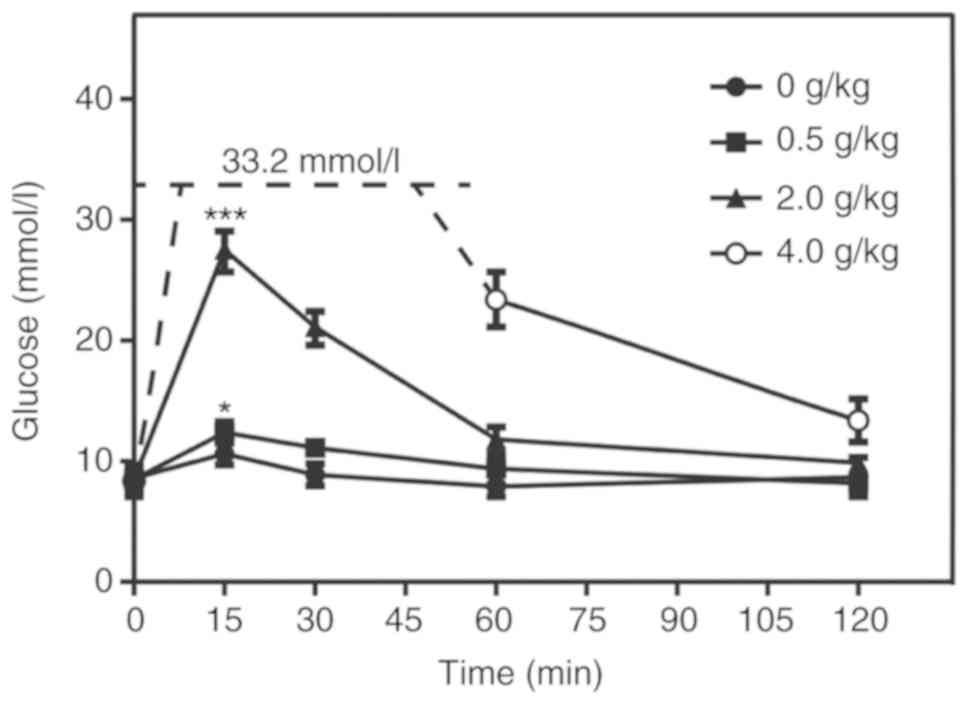
IPG with 2 g/kg glucose is the optimum
dose for an acute hyperglycemia mouse model
The optimum dose of glucose for the acute
hyperglycemia mouse model was determined by injecting mice with
four different doses of glucose. The optimum concentration should
ideally not exceed the limits of detection by conventional blood
glucose meters, but should increase the blood glucose concentration
sufficiently to induce a hyperglycemic response. In mice injected
with 0.5 g/kg glucose, the glucose level only differed
significantly compared with that in the control group subsequent to
15 min, and was not sustained (P<0.05; Fig. 2); thus, it was deemed unsuitable. In
mice treated with 4 g/kg glucose, the blood glucose level
approached (and possibly exceeded) the upper limit of the measuring
equipment (Fig. 2). The peak glucose
level in the group injected with 2 g/kg was sufficiently high
enough to induce a significant hyperglycemic response compared with
the control group, which was maintained for ≥30 min, whilst being
measurable using the available equipment (P<0.001; Fig. 2).
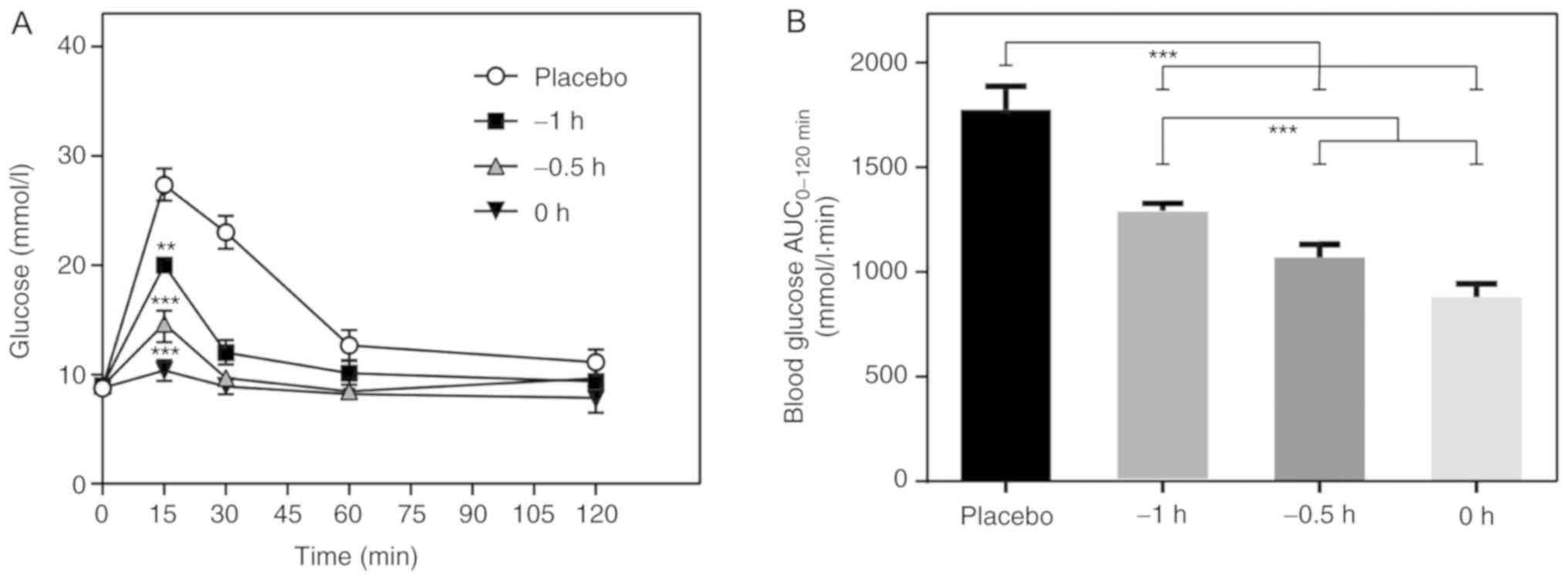
Injection of 6-KTP at 0 h is the
optimum time point for a maximal hypoglycemic effect
The timing of a subcutaneous injection of 6-KTP
should be such that a maximal hypoglycemic effect is initiated
prior to first-phase insulin secretion. Injection of 6-KTP at 0 h
was determined to be the optimum time point for 6-KTP to exert its
effects, with a significant difference in blood glucose compared
with the placebo group (P<0.001; Fig
3). All mice were injected with 2 g/kg glucose.
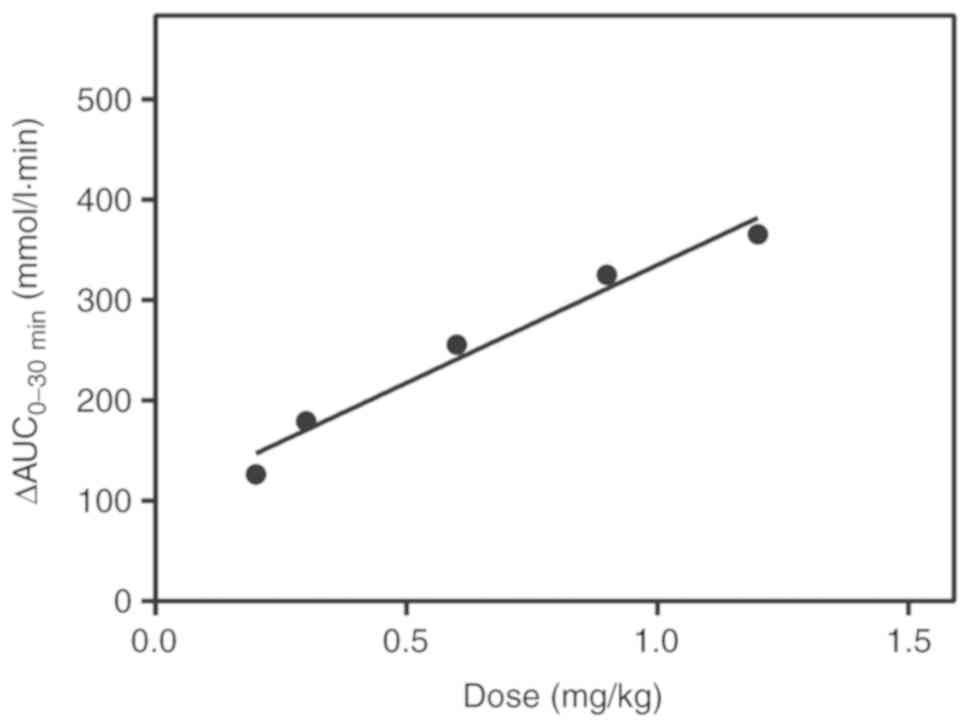
Dose-effect association between 6-KTP
and hypoglycemic activity
The AUC0-30 min revealed a linear
association between the 6-KTP dose and hypoglycemic activity
(Table II). Between 0.2 and 1.2
mg/kg 6-KTP, the linear regression equation was y=100.2+234.9x,
with an r2 value of 0.9705 (Fig. 4). The quantitative association between
6-KTP dose and the functional effect on blood glucose levels was
verified by using different 6-KTP doses. The recovery was
calculated as described previously (16) for the different doses of 6-KTP, and
was quantified by the equation ≤100±15% (between 0.2 and 1.2 mg/kg
6-KTP; Table III).
 | Table IIΔAUC0-30 min of different doses of
6-KTP in the acute hyperglycemia C57BL/6J mouse model. |
Table II
ΔAUC0-30 min of different doses of
6-KTP in the acute hyperglycemia C57BL/6J mouse model.
| | 6-KTP dose,
mg/kg |
|---|
| AUC (mmol/l
min-1) | PBS | 0.2 | 0.3 | 0.6 | 0.9 | 1.2 | 1.5 | 1.8 |
|---|
| aΔAUC0-30 min | 0 | 126.6±48.5 | 179.2±49.6 | 255.8±25.1 | 325.2±10.3 | 365.8±22.5 | 357.8±15.8 | 370.7±17.8 |
 | Table IIICalculated and experimental values of
ΔAUC0-30 min values of different 6-KTP doses. |
Table III
Calculated and experimental values of
ΔAUC0-30 min values of different 6-KTP doses.
| Dose of 6-KTP,
mg/kg | Experimental value,
mmol/l min-1 | Calculated value,
mmol/l min-1 | Recovery, % |
|---|
| 0.25 | 147.6 | 158.9 | 107.7 |
| 0.5 | 193.7 | 217.7 | 89 |
| 0.75 | 256.2 | 276.4 | 92.7 |
| 1 | 322.4 | 335.1 | 96.2 |
Discussion
In the present study, an acute hyperglycemic
C57BL/6J mouse model was developed. An intraperitoneal injection of
2 g/kg glucose alongside an injection of 6-KTP was used to
determine the blood glucose-lowering effect of 6-KTP at various
concentrations. The results demonstrated a linear association
between 6-KTP dose and hypoglycemic activity. An equation for
calculating the effect of each 6-KTP dose was developed based on
these results.
At present, potential therapeutic methods for T2DM
are primarily tested for biological activity using a mouse model of
T2DM, but this method has certain disadvantages. The low success
rate of constructing a mouse model of T2DM is likely to result in
the mice succumbing to mortality or low blood glucose in mice
(17,18). Meanwhile, ordering T2DM mice is
costly. A rapid detection method for measuring the activity of
GLP-1 derivatives over a period of 30 min was established in the
present study. The acute hyperglycemic mouse model was readily
inducible by an intraperitoneal injection of 2 g/kg glucose
solution into fasting C57BL/6J mice. The acute hyperglycemic mouse
model may be used for pharmacodynamic studies in the early stages
of drug screening, shortening the drug screening cycle. At present,
a 6-KTP-specific pharmaceutical formulation has been developed, and
the acute hyperglycemic mouse model may be used to verify whether
the formulation will affect the biological activity of 6-KTP by
this rapid testing method.
In the present study, 6-KTP was prepared strictly in
aseptic conditions. Subsequent to the completion of the test, the
mice were in good health and revealed no notable side effects
although no toxicology test was performed on the mice. Toxicology
tests examining the impact of 6-KTP in mice will be performed in
subsequent studies.
In conclusion, a rapid detection method for GLP-1
derivatives to lower blood glucose levels was established. This
method has the advantages of rapid detection and low cost, and may
serve as a template to develop mouse models for other similar
therapeutics.
Acknowledgements
Not applicable.
Funding
The present study was funded by The National Major
Scientific and Technological Special Project for ‘Significant New
Drugs Development’ during the Twelfth Five-year Plan Period (grant
no. 2013ZX09103003-018) and The National Natural Science Foundation
of China (grant no. 31500099).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
JH and XZ contributed to the design of the study and
to the acquisition and interpretation of the data. XY, HL and YR
drafted the manuscript and revised it for intellectual content. All
authors read and approved the final version of the manuscript for
publication.
Ethics approval and consent to
participate
All in vivo experiments performed in the present
study were ethically approved by The Jinan University Ethics
Committee of Animal Welfare (Guangdong, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Piya MK, Tahrani AA and Barnett AH:
Emerging treatment options for type 2 diabetes. Br J Clin
Pharmacol. 70:631–644. 2010.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Drucker DJ and Nauck MA: The incretin
system: Glucagon-like peptide-1 receptor agonists and dipeptidyl
peptidase-4 inhibitors in type 2 diabetes. Lancet. 368:1696–1705.
2006.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Bo A: GLP-1 for type 2 diabetes. Exp Cell
Res. 317:1239–1245. 2011.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Malik DK, Baboota S, Ahuja A, Hasan S and
Ali J: Recent advances in protein and peptide drug delivery
systems. Curr Drug Deliv. 4:141–151. 2007.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Pawar R, Ben-Ari A and Domb AJ: Protein
and peptide parenteral controlled delivery. Expert Opin Biol Ther.
4:1203–1212. 2004.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Perry CM: Liraglutide. Drugs.
71:2347–2373. 2011.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Dunning BE, Foley JE and Ahrén B: Alpha
cell function in health and disease: Influence of glucagon-like
peptide-1. Diabetologia. 48:1700–1713. 2005.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Wang Q and Brubaker PL: Glucagon-like
peptide-1 treatment delays the onset of diabetes in 8 week-old
db/db mice. Diabetologia. 45:1263–1273. 2002.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Ding X, Saxena NK, Lin S, Gupta NA and
Anania FA: Exendin-4, a glucagon-like protein-1 (GLP-1) receptor
agonist, reverses hepatic steatosis in ob/ob mice. Hepatology.
43:173–181. 2006.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Del Prato S and Tiengo A: The importance
of first-phase insulin secretion: Implications for the therapy of
type 2 diabetes mellitus. Diabetes Metab Res Rev. 17:164–174.
2001.PubMed/NCBI View
Article : Google Scholar
|
|
11
|
Li HJ, Ma Y, Chen Y, Sang Y, Zhou T, Qiu
M, Huang X, Zhou C and Su Z: A protease-based strategy for the
controlled release of therapeutic peptides. Angew Chem Int Ed Engl.
49:4930–4933. 2010.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Berglund O: Different dynamics of insulin
secretion in the perfused pancreas of mouse and rat. Acta
Endocrinol (Copenh). 93(54)1980.PubMed/NCBI
|
|
13
|
Voss EM, Bina DM, Mcneil LAD, Johnson ML
and Cembrowski GS: Determining acceptability of blood glucose
meters: Evaluating a blood glucose testing system. Lab Med.
27:679–682. 1996. View Article : Google Scholar
|
|
14
|
Collins S, Martin TL, Surwit RS and
Robidoux J: Genetic vulnerability to diet-induced obesity in the
C57BL/6J mouse: Physiological and molecular characteristics.
Physiol Behav. 81:243–248. 2004.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Pan Z: Study of the Biological
Characteristics of Sibp: KM mouse. Acta Laboratorium Animalisentia
Sinica. 1995.
|
|
16
|
Pharmacopoeia of the People's Republic of
China: Chemical Industry Press, 2015.
|
|
17
|
Lenzen S: The mechanisms of alloxan- and
streptozotocin-induced diabetes. Diabetologia. 51:216–226.
2008.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Ramanadham S, Doroudian A and McNeill JH:
Myocardial and metabolic abnormalities in streptozotocin-diabetic
Wistar and Wistar-Kyoto rats. Can J Cardiol. 6:75–82.
1990.PubMed/NCBI
|