Introduction
Pentraxin-3 (PTX3) is a long pentraxin identified in
early 1990s that differs from short pentraxins, such as C reactive
protein (CRP) and serum amyloid A, in gene organization, ligand
binding abilities and the inducing stimuli (1). Similar to the other pentraxins, PTX3 is
a member of the humoral innate immune system and serves a role in
protection against infections (1,2),
inflammation control and matrix deposition (3).
PTX3 and CRP are considered to be evolutionarily
related antibodies (1). However,
unlike CRP, which is induced by IL-6 and produced in the liver,
PTX3 is formed locally by several cells and tissues following
ligand binding to Toll-like receptors (TLR) and in response to
proinflammatory signals, such as IL-1β and TNF-α (2).
There are conflicting results regarding the
association between PTX3 atherogenic factors and obesity. Adipose
cells produce PTX3 when stimulated by TNF-α, although the exact
function it serves in this scenario is unknown (2). Zanetti et al (4) found that PTX3 serum levels are higher in
individuals with metabolic syndrome and are negatively associated
with HDL cholesterol and positively associated with plasma
triglyceride levels. They also found an association between PTX3
concentrations and carotid intima thickness. Lee et al
(5) described an inverse association
between PTX3 levels and metabolic syndrome, being overweight/obese
and parameters of dyslipidemia, suggesting a potential
cardioprotective role of this marker. However, animal studies
showed that PTX3 deficiency reduces metabolic inflammation and
prevents weight gain in mice fed with a high fat diet (6).
Although PTX3 has been largely studied in
association with obesity and dyslipidemia, few studies have
examined changes in PTX3 levels following bariatric surgery.
Santilli et al (7) studied
PTX3 levels in 12 obese patients following gastric banding, and
found that PTX3 levels were low in the preoperative period and they
increased following the procedure, and PTX3 levels were inversely
associated with platelet activation markers. Barazzoni et al
(8) found that severely obese
individuals had higher than normal PTX3 levels and following
Roux-en-Y gastric bypass, the levels increased further.
In the present study, PTX3 levels in a sample of
obese patients who underwent bariatric surgery were measured to
analyze the variability in its levels in association with weight
loss and changes in patients' metabolic profile.
Patients and methods
Patients
The present study was a prospective study and was
approved by the Committee of Ethics in Research, Sociedade
Evangélica Beneficente de Curitiba (Curitiba, Brazil; approval no.
2.325.452). All participants provided signed informed consent for
participation. A total of 84 obese patients undergoing bariatric
surgery and 94 non-obese controls without any known disease, all
aged >18 years, were recruited for the present study. Patients
were classed as obese if they had a body mass index (BMI) ≥30
kg/m2. This cohort was a convenience sample which
includes all the patients who underwent bariatric surgery in a 1
year period, in two university hospitals from same geographic
region and that agreed to participate in the study. In this sample
72/84 (85.7%) were females and 12/84 (14.2%) were males with a
median age of 36 years (range, 19-63 years). Patients with chronic
inflammatory diseases, a history of cancer, altered renal function
and obesity secondary to endocrinopathies (such as Cushing's
syndrome or hypothyroidism) were excluded. All patients underwent
Roux-en-Y gastric bypass, and this was performed by the same
surgical team following a multidisciplinary pre-operative
evaluation by qualified clinicians in the fields of nutrition,
endocrinology, cardiology and psychology. All patients underwent
preoperative upper gastrointestinal endoscopy and abdominal
ultrasound.
Data collection
Patients who underwent bariatric surgery were
monitored for 60 days prior to the surgery and followed up in the
post-operative period for 360 days. Epidemiological data regarding
BMI, abdominal circumference, blood pressure, lipid profile (total
cholesterol, HDL and LDL cholesterol, and triglycerides), fasting
glycemia, hemoglobin A1c, basal insulin levels, uric acid,
creatinine, albumin, blood cell count and transaminases were
obtained at the same time as PTX3 measurements in the pre- and
post-operative period.
Measurement of PTX3 levels
PTX3 levels were measured in plasma samples using a
commercially available ELISA kit (XpressBio; cat. no. XPEH0263)
with a detection range of 0.31-20 ng/ml and an inter-assay
precision coefficient variation of <10%.
Statistical analysis
All statistical analyses were performed in GraphPad
Prism version 6.01 (GraphPad Software, Inc.). Results were gathered
in frequency and contingency tables. A Shapiro Wilk test was used
to analyze data distribution. Comparison of nominal data was
performed using a χ2 test, comparisons of numerical data
were performed using a Wilcoxon matched-pairs signed rank test or a
Student's t-test based on the distribution of the data. Comparison
of PTX3 levels between patients (pre and post-operative) and
controls was performed using a Kruskal-Wallis test followed by a
post-hoc Dunn's test. Correlation analyses of PTX3 with the other
numerical variables were performed using Spearman's Rank
Correlation tests. P<0.05 was considered to indicate a
statistically significant difference.
Results
Characteristics of the recruited
cohort
In the pre-operative period, 32/84 patients (38.1%)
were diagnosed with dyslipidemia; 58/81 patients (71.6%) with
hepatic steatosis; 41/84 patients (48.8%) with arterial
hypertension; and 13/84 patients (15.5%) with diabetes mellitus.
The post-operative evaluation was performed for between 106-375
days following surgery (mean ± standard deviation, 217±56.7 days)
and the weight loss ranged from 14-72 kg with a median weight loss
of 33.1 kg. The characteristics of the patients in the pre- and
post-operative period are presented in Table I.
 | Table IPrimary characteristics of the
patients who underwent bariatric surgery. |
Table I
Primary characteristics of the
patients who underwent bariatric surgery.
| Variables | Before surgery | After surgery | P-value |
|---|
| BMI
(kg/m2) | | |
<0.0001d |
|
Range | 35.1-64.8 | 22.4-44.5 | |
|
Median
(IQR) | 44.6 (41.4-48.1) | 31.0 (28.4-34.3) | |
| Systolic BP, mm
Hg | | |
<0.0001d |
|
Range | 90-200 | 90-170 | |
|
Median
(IQR) | 125 (110-140) | 120 (110-120) | |
| Diastolic BP, mm
Hg | | |
<0.0001d |
|
Range | 60-110 | 50-100 | |
|
Median
(IQR) | 80 (70-90) | 70 (70-80) | |
| Abdominal
circumference, cm | | |
<0.0001d |
|
Range | 102-176 | 73-141 | |
|
Median
(IQR) | 126 (118-137) | 100 (93-110) | |
| Total cholesterol,
mg/dl | | |
<0.0001d |
|
Range | 120.0-291.0 | 116.0-234.0 | |
|
Median
(IQR) | 191.7
(167.4-222.0) | 167 (139-189) | |
| LDL cholesterol,
mg/dl | | |
<0.0001d |
|
Range | 60-184 | 44-146 | |
|
Mean ±
SD | 118.6±29.2 | 99.9±24.1 | |
| HDL cholesterol,
mg/dl | | | 0.96 |
|
Range | 29.2-81.0 | 28.7-79.0 | |
|
Median
(IQR) | 45.3 (36.0-53.0) | 46.0 (39.0-52.0) | |
| Triglycerides,
mg/dl | | |
<0.0001d |
|
Range | 68-462 | 38-343 | |
|
Median
(IQR) | 135.3
(101.8-178.8) | 94 (72.5-134.0) | |
| Fasting glucose,
mg/dl | | | 0.0002c |
|
Range | 66-408 | 60-137 | |
|
Median
(IQR) | 91 (82-103) | 87.5 (79.2-92.0) | |
| Hemoglobin A1c,
% | | |
<0.0001d |
|
Range | 4.7-9.9 | 4.0-8.1 | |
|
Median
(IQR) | 5.4 (5.2-5.9) | 5.2 (5.0-5.6) | |
| Basal insulin,
mIU/l | | |
<0.0001d |
|
Range | 3.0-168.7 | 1.6-42.4 | |
|
Median
(IQR) | 15.6 (8.3-27.8) | 6.8 (4.5-12.1) | |
| Uric acid,
mg/dl | | |
<0.0001d |
|
Range | 2.0-9.3 | 1.9-7.2 | |
|
Mean ±
SD | 4.8±1.45 | 4.3±1.20 | |
| Ferritin,
ng/ml | | | 0.10 |
|
Range | 7-1042 | 9.8-697.0 | |
|
Median
(IQR) | 165.0
(55.3-259.5) | 128.0
(73.0-243.0) | |
| Albumin, g/l | | | 0.02a |
|
Range | 3.4-4.8 | 3.1-5.3 | |
|
Median
(IQR) | 4.0 (3. 8-4.3) | 4.1 (4.0-5.3) | |
| Oxalacetic
transaminase, U/l | | | 0.58 |
|
Range | 9-90 | 9-52 | |
|
Median
(IQR) | 22.5
(18.0-33.0) | 25 (18-31) | |
| Pyruvate
transaminase, U/l | | | 0.06 |
|
Range | 6-145 | 9-79 | |
|
Median
(IQR) | 26.0
(17.0-39.2) | 24 (16-37) | |
| Creatinine,
mg/dl | | | 0.20 |
|
Range | 0.5-1.2 | 0.5-1.2 | |
|
Median
(IQR) | 0.8 (0.7-0.9) | 0.8 (0.7-0.9) | |
| Hemoglobin,
g/dl | | | 0.14 |
|
Range | 11.4-16.6 | 10.6-15.9 | |
|
Median
(IQR) | 13.1
(12.5-14.0) | 12.9
(12.5-13.6) | |
| Hematocrit, % | | | 0.059 |
|
Range | 31.5-49.7 | 32.5-47.6 | |
|
Mean ±
SD | 39.9±3.41 | 39.3±3.16 | |
| Platelets,
n/mm3 | | | 0.19 |
|
Range |
127,000-474,000 |
138,000-465,000 | |
|
Median
(IQR) | 241,500
(209,250-291,000) | 233,000
(195,200-288,250) | |
| Vitamin D,
ng/ml | | | 0.001b |
|
Range | 6.8-47 | 9.7-52.0 | |
|
Median
(IQR) | 25.5
(18.6-29.9) | 28.5
(24.2-33.7) | |
PTX3 levels in the pre- and
post-operative period
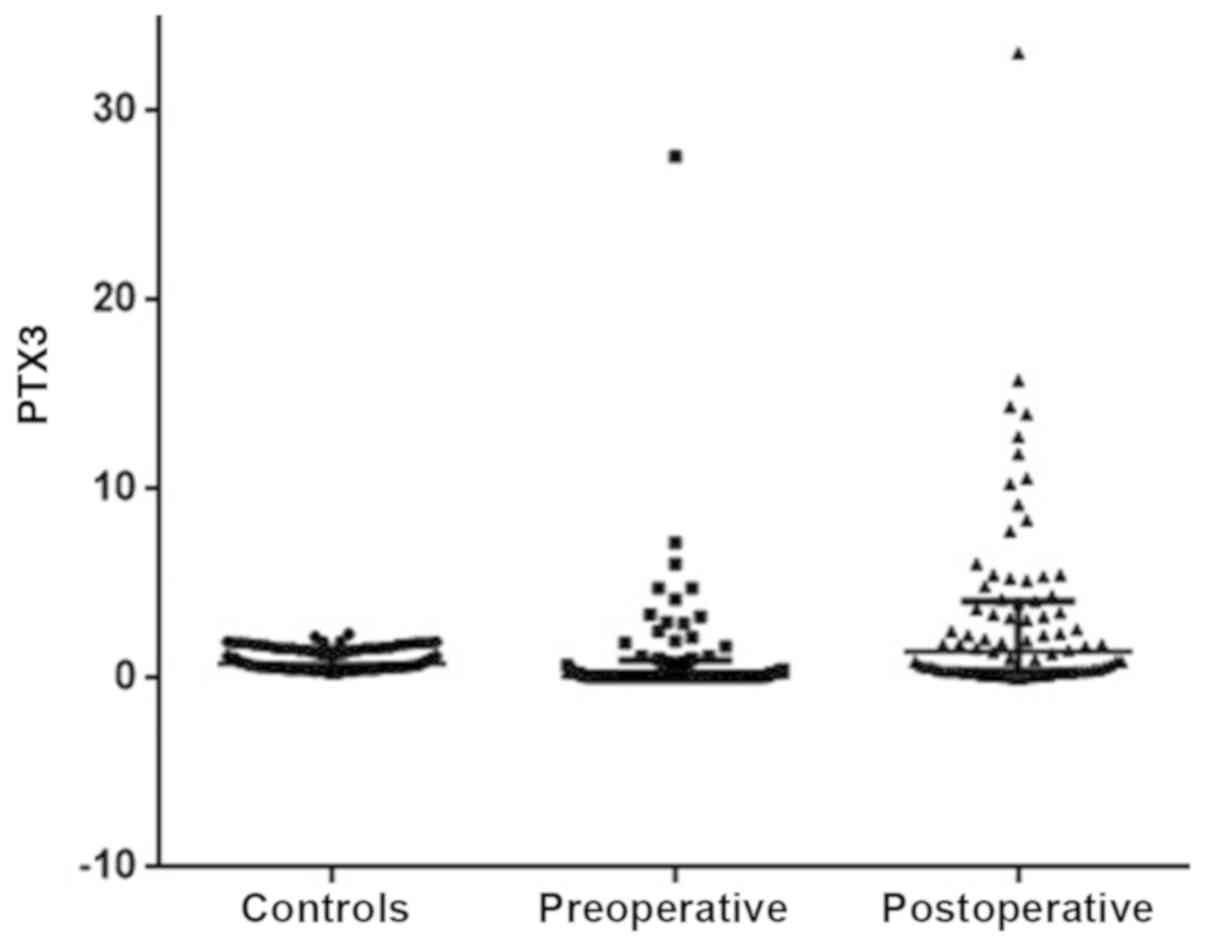
PTX3 levels in the control group and in the obese
patients pre- and post-surgery are presented in Fig. 1. Comparison of PTX3 levels in pre- and
post-operative patients and controls showed there was as
significant difference (P<0.0001). The post-hoc Dunn's test
showed that the PTX3 levels were significantly lower in the
controls compared with the levels in the pre-operative patients,
and the levels of PTX3 in the pre-operative patients were
significantly lower compared with the levels in the post-operative
patients (both P<0.05).
The variation in PTX3 values from pre- and
post-surgery had a median value of 0.55 ng/ml; (inter-quartile
range =0.12-2.97 ng/ml) and were not correlated with variations in
BMI (ρ=-0.01; P=0.91) or the number of days following surgery
(ρ=0.09; P=0.38).
No differences in PTX-3 serum concentrations were
observed between males and females. In the pre-operative period,
male patients had a median PTX3 level of 0.20 ng/ml (range,
0.10-1.77 ng/ml) and females had a median value of 0.10 ng/ml
(range, 0.10-0.90 ng/ml) (P=0.45). In the post-operative period,
the median value in males was 0.45 ng/ml (range, 0.20-3.50) and in
females it was 1.55 ng/ml (range, 0.40-4.15) (P=0.18).
Correlation analysis of the PTX3 levels prior to
surgery with clinical and metabolic variables did not show any
statistical significance. Correlation analysis of the PTX3 levels
following surgery with clinical and metabolic variables showed that
there was significant positive correlation with triglyceride levels
(although this correlation was modest; ρ=0.26; 95% CI, 0.03-0.46;
P=0.01). Detailed results of the correlation analyses between pre-
and post-surgery PTX3 levels with other variables are presented in
Table II.
 | Table IICorrelation analysis of variability
of values prior to and following bariatric surgery with the
variability in pentraxin-3 levels. |
Table II
Correlation analysis of variability
of values prior to and following bariatric surgery with the
variability in pentraxin-3 levels.
| Variables | Spearman ρ | 95% confidence
interval | P-value |
|---|
| Δ Body mass
index | -0.04 | -0.26-0.17 | 0.68 |
| Δ Total
cholesterol | -0.04 | -0.26-0.18 | 0.70 |
| Δ HDL
cholesterol | 0.08 | -0.14-0.30 | 0.47 |
| Δ
Triglycerides | -0.06 | -0.29-0.16 | 0.55 |
| Δ Basal
insulin | 0.07 | -0.20-0.34 | 0.59 |
| Δ Fasting
glycemia | 0.008 | -0.21-0.23 | 0.53 |
| Δ Hemoglobin
A1c | 0.007 | -0.20-0.34 | 0.59 |
| Δ Uric acid | -0.18 | -0.39-0.05 | 0.12 |
Discussion
The results of the present study showed that obese
patients have lower levels of PTX3 compared with non-obese patients
and that bariatric surgery results in restoration of physiological
levels. These results are in agreement with previous studies from
Ogawa et al (9) and
Osorio-Conles et al (10), who
both found an inverse association between the serum blood levels of
PTX3 and BMI. In addition, PTX3 serum levels are increased in obese
patients who underwent dietary intervention (11) and gastric banding (7).
There was no association between PTX3 levels and the
lipid profile or the glycemic controls; however, the elevation of
this biomarker itself is of interest for patients undergoing
bariatric surgery as it has been shown that PTX3 may exert a
protective role on the cardiovasculature (12). Animal studies of PTX3 null mice
demonstrated that they develop severe inflammatory changes in the
vascular walls, with an increased number of macrophages in
atherosclerotic plaques, suggesting that PTX3 may exert a
regulatory function in vascular associated inflammation (12). In an experimental ischemia-reperfusion
model of myocardial infarction, PTX3 deficient animals showed
significantly larger infarcts compared with the wild-type controls,
suggesting that PTX3 serves a role in myocardial damage and repair
(13). PTX3 deficient animals had
higher deposition of C3 (complement system) in the damaged tissue,
thus it is possible that the protective function of PTX3 may
transpire through prevention of tissue damage by excessive
complement deposition (13). PTX3
inhibits activation of the classic complement pathway by preventing
an interaction between immunoglobulins and C1q (14), and inhibits the alternate pathway by
controlling the deposition of H factor in apoptotic cells (15).
Norata et al (16) showed that HDL-cholesterol is capable
of inducing the expression of PTX3 mRNA in vascular endothelial
cells. They suggested that the protective effect of HDL on
atherosclerosis may partly be due to its modulation of PTX3
secretion.
Obesity is a low-grade inflammatory disease and a
significant risk factor of cardiovascular morbidity (17). A study on PTX3 gene expression in
cultured adipocytes found that PTX3 is upregulated locally, and
that the production of this biomarker is higher in visceral adipose
tissue compared with subcutaneous fat (10). However, in the same study, the blood
levels of PTX3 were inversely associated with obesity and thus the
authors hypothesized that local production of PXT3 regulates the
equilibrium between an inflammatory and anti-inflammatory response
(10). Therefore, if PTX3 exerts a
protective effect against metabolic syndrome, lowering blood PTX3
levels in obese patients may lead to a reduction in the
inflammatory process resulting from increased fat mass (11).
There was no association between PTX3 levels and
insulin levels, glycemic controls or any of the lipid profile
fractions assessed except for triglycerides following surgery.
Contrasting results regarding the association between PTX3 levels
and triglycerides have been reported. Zanetti et al
(4) found a positive correlation
between plasma triglyceride and PTX3 levels, whereas a negative
association was observed by Lee et al (5) and Yamasaki et al (18). A possible explanation for the
discrepant results may be due to small sizes in each study and the
use of patients with a specific disease (11). In the present study, a modest but
significant positive correlation was observed between PTX3 and
triglycerides levels in the post-operative period.
The present study has some limitations: The PTX3
measurements were taken at different intervals following surgery
and there was a relatively lower number of males in the
experimental cohort (14.3%). Additional studies, with a higher
proportion of male patients and fixed intervals of PTX3 measurement
will increase the reliability of the results and allow for
stratification by sex to remove this as a potential confounding
factor, thus clarifying the results further. Nevertheless, the
present study does show that bariatric surgery restores PTX3 levels
to physiological levels, demonstrating the beneficial effect of
this type of surgery on cardiovascular risk.
In conclusion, the study showed that bariatric
surgery restores PTX3 levels to physiological levels. Additional
studies are required to understand the value increased PTX3 levels
in obese patients on the prevention of cardiovascular.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
AFT, RN, PANN and TS designed and performed the
experiments. SIM, AGC and JSCG collected the patients' data and
serum samples. AFT, RN and TS organized and analyzed data. All
authors were involved in writing the paper and approved the final
version of the manuscript.
Ethics approval and consent to
participate
The present study was a prospective study and was
approved by the Committee of Ethics in Research, Sociedade
Evangélica Beneficente de Curitiba (Curitiba, Brazil; approval no.
2.325.452). All participants provided signed informed consent for
participation.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ristagno G, Fumagalli F, Bottazzi B,
Mantovani A, Olivari D, Novelli D and Latini R: Pentraxin 3 in
cardiovascular disease. Front Immunol. 10(823)2019.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Balhara J, Koussih L, Zhang J and Gounni
AS: Pentraxin 3: An immuno-regulator in the lungs. Front Immunol.
4(127)2013.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Jaillon S, Bonavita E, Gentile S, Rubino
M, Laface I, Garlanda C and Mantovani A: The long pentraxin PTX3 as
a key component of humoral innate immunity and a candidate
diagnostic for inflammatory diseases. Int Arch Allergy Immunol.
165:165–178. 2014.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Zanetti M, Bosutti A, Ferreira C, Vinci P,
Biolo G, Fonda M, Valente M, Cattin L, Guarnieri G and Barazzoni R:
Circulating pentraxin 3 levels are higher in metabolic syndrome
with subclinical atherosclerosis: Evidence for association with
atherogenic lipid profile. Clin Exp Med. 9:243–248. 2009.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Lee R, Ahn HR, Shin MH, Kim HN, Lee YH,
Choi SW and Kweon SS: Association of plasma pentraxin-3 level with
lipid levels and cardiovascular risk factors in people with no
history of lipid-lowering medication: The Dong-gu Study. J
Atheroscler Thromb. 26:738–745. 2019.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Bonacina F, Moregola A, Porte R, Baragetti
A, Bonavita E, Salatin A, Grigore L, Pellegatta F, Molgora M,
Sironi M, et al: Pentraxin 3 deficiency protects from the metabolic
inflammation associated to diet-induced obesity. Cardiovasc Res.
115:1861–1872. 2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Santilli F, Guagnano MT, Innocenti P,
Aceto L, Vazzana N, Lattanzio S, Liani R, Tripaldi R, Creato V,
Romano M, et al: Pentraxin 3 and platelet activation in obese
patients after gastric banding. Circ J. 80:502–511. 2016.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Barazzoni R, Palmisano S, Gortan
Cappellari G, Giuricin M, Moretti E, Vinci P, Semolic A, Guarnieri
G, Zanetti M and Manzini N: Gastric bypass-induced weight loss
alters obesity-associated patterns of plasma pentraxin-3 and
systemic inflammatory markers. Surg Obes Relat Dis. 12:23–32.
2016.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Ogawa T, Kawano Y, Imamura T, Kawakita K,
Sagara M, Matsuo T, Kakitsubata Y, Ishikawa T, Kitamura K,
Hatakeyama K, et al: Reciprocal contribution of pentraxin 3 and
C-reactive protein to obesity and metabolic syndrome. Obesity
(Silver Spring). 18:1871–1874. 2010.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Osorio-Conles O, Guitart M, Chacón MR,
Maymo-Masip E, Moreno-Navarrete JM, Montori-Grau M, Näf S,
Fernandez-Real JM, Vendrell J and Gómez-Foix AM: Plasma PTX3
protein levels inversely correlate with insulin secretion and
obesity, whereas visceral adipose tissue PTX3 gene expression is
increased in obesity. Am J Physiol Endocrinol Metab.
301:E1254–E1261. 2011.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Witasp A, Carrero JJ, Michaëlsson K,
Ahlström H, Kullberg J, Adamsson V, Risérus U, Larsson A,
Helmersson-Karlqvist J, Lind L, et al: Inflammatory biomarker
pentraxin 3 (PTX3) in relation to obesity, body fat depots and
weight loss. Obesity (Silver Spring). 22:1373–1379. 2014.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Norata GD, Marchesi P, Pulakazhi Venu VK,
Pasqualini F, Anselmo A, Moalli F, Pizzitola I, Garlanda C,
Mantovani A and Catapano AL: Deficiency of the long pentraxin PTX3
promotes vascular inflammation and atherosclerosis. Circulation.
120:699–708. 2009.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Salio M, Chimenti S, De Angelis N, Molla
F, Maina V, Nebuloni M, Pasqualini F, Latini R, Garlanda C and
Mantovani A: Cardioprotective function of the long pentraxin PTX3
in acute myocardial infarction. Circulation. 117:1055–1064.
2008.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Nauta AJ, Bottazzi B, Mantovani A,
Salvatori G, Kishore U, Schwaeble WJ, Gingras AR, Tzima S, Vivanco
F, Egido J, et al: Biochemical and functional characterization of
the interaction between pentraxin 3 and C1q. Eur J Immunol.
33:465–473. 2003.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Erreni M, Manfredi AA, Garlanda C,
Mantovani A and Rovere-Querini P: The long pentraxin PTX3: A
prototypical sensor of tissue injury and a regulator of
homeostasis. Immunol Rev. 280:112–125. 2017.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Norata GD, Marchesi P, Pirillo A, Uboldi
P, Chiesa G, Maina V, Garlanda C, Mantovani A and Catapano AL: Long
pentraxin 3, a key component of innate immunity, is modulated by
high-density lipoproteins in endothelial cells. Arterioscler Thromb
Vasc Biol. 28:925–931. 2008.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Garlanda C, Bottazzi B, Moalli F, Deban L,
Molla F, Latini R and Mantovani A: Pentraxins and atherosclerosis:
The role of PTX3. Curr Pharm Des. 17:38–46. 2011.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Yamasaki K, Kurimura M, Kasai T, Sagara M,
Kodama T and Inoue K: Determination of physiological plasma
pentraxin 3 (PTX3) levels in healthy populations. Clin Chem Lab
Med. 47:471–477. 2009.PubMed/NCBI View Article : Google Scholar
|