Introduction
Ulcerative colitis (UC) is a chronic inflammatory
disease of the colon characterized by recurrent periods of clinical
remission and disease relapse (1).
The incidence of colorectal cancer is increasing, and UC is a risk
factor of colorectal cancer (2,3). Despite
advances in therapeutic options, in a cohort study conducted in New
Zealand between 2005 and 2015, 2.7% of all patients with UC went
onto develop colorectal cancer (4).
UC has a marked negative impact on the quality of life of the
patients. The onset and reactivation of UC is associated with
genetic abnormalities that cause defects in the mucosal barrier and
alters the balance between the beneficial and pathogenic enteric
bacteria (5). In the colon, the mucus
layer is primarily composed of a large gel-forming mucin containing
the glycoprotein mucin2 (MUC2) (6).
MUC2 serves an important role in protecting the colon against
colitis, and this has been demonstrated by the fact that
MUC2-deficient mice develop spontaneous colitis (6,7).
Previously, Okumura et al (8) identified a novel protein present in the
inner mucosal layer called LY6/PLAUR domain containing 8 (LYPD8)
protein, which is selectively expressed in epithelial cells at the
uppermost layer of the large intestinal gland. The group
demonstrated that LYPD8 can bind to the flagellae (composed of
polymerized flagellin proteins) of live bacteria. LYPD8-/- mice
possess slightly increased numbers of Proteus species in the
luminal regions of the colon compared with wild-type mice (8). Proteus has been associated with
the pathogenesis of inflammatory bowel diseases in both mice and
humans (9,10) and LYPD8 promotes the segregation of
flagellated bacteria and colonic epithelia, thus reducing the risk
of intestinal inflammation (8-11).
As mentioned above, there are several reports on the
role of MUC2 and LYPD8 in UC; however, only two studies have
examined the role of MUC2 and LYPD8 in the context of severity of
inflammation and gene expression in UC (12,13).
Furthermore, to the best of our knowledge, there are no studies
comparing their gene expression in the lesioned and non-lesioned
regions of the colon in patients with UC. Therefore, the present
study aimed to investigate the association between the severity of
inflammation and MUC2 and LYPD8 expression levels in these
regions.
Patients and methods
Patients
Patients with UC who underwent treatment at Tottori
University Hospital (Tottori, Japan) and Nagasaki University
Hospital (Nagasaki, Japan) between August 2018 and July 2019 were
enrolled. Patients who disagreed to participation in the study were
excluded. A total of 18 patients with UC in the acute and remission
phases, including 6 females and 12 males, were examined. The mean
age ± standard deviation was 41.1±14.7 years (range, 18-74 years).
UC was diagnosed based on clinical symptoms, the results of
endoscopy, X-rays and histological findings. Patients with UC were
treated with 5-aminosalicylic acid, prednisolone (PSL), granulocyte
apheresis (G-CAP) and azathioprine (AZA). Biopsies of the lesioned
and non-lesioned areas of the colon were collected from the same
patient for 9-342 months following the initiation of treatment. The
distinction of normal or lesioned regions was based on endoscopy
images, and the expression levels of IL-8, MUC2 and LYPD8 were
compared between the lesioned and non-lesioned areas.
Samples were stratified into three groups based on
the Matts' histopathological grade (14); grade 1 (n=20), grade 2 (n=9) and grade
3 (n=7); for all regions, and the expression levels of IL-8, MUC2
and LYPD8 in the different grades were compared. All cases were
anonymized prior to analysis and written informed consent was
provided by all patients. The present study was approved by The
Institutional Review Board of Tottori University (Tottori, Japan)
and was performed in accordance with the Declaration of Helsinki
(15).
RNA extraction
The total RNA, including mRNA and miRNA, from the
tissues was extracted from biopsies using an miRNeasy Mini kit
(Qiagen China Co., Ltd.). The RNA was quantified using a BioSpec
Nano Spectrophotometer (Shimadzu Corporation) and the extracted RNA
samples were stored at -80˚C until further use.
Reverse transcription-quantitative
(RT-q)PCR
RNA was reverse transcribed into cDNA using a
High-Capacity cDNA Reverse Transcription kit (Thermo Fisher
Scientific, Inc.). The reverse transcription reactions were
performed in aliquots containing 1 µg total RNA, 1Χ RT buffer, 4 mM
dNTP mix, 1Χ RT random primer, 50 units Multiscribe reverse
transcriptase, 20 units RNase inhibitor and nuclease-free water
added to a final volume of 20 µl. The RT temperature protocol was:
25˚C for 10 min, 37˚C for 120 min and 85˚C for 5 min. The primer
sequences for qPCR were as follows: IL-8 forward,
5'-TTTTGCCAAGGAGTGCTAAAGA-3' and reverse:
5'-AACCCTCTGCACCCAGTTTTC-3'; MUC2 forward,
5'-ACAACTACTCCTCTACCTCCA-3' and reverse,
5'-GTTGATCTCGTAGTTGAGGCA-3'; LYPD8 forward,
5'-CTGAAGAACGTGTCCAGCAA-3' and reverse, 5'-CTGACAGGTGGCGTTACTGA-3';
and β-actin forward, 5'-GCATCCTCACCCTGAAGTA-3' and reverse,
5'-TGTGGTGCCAGATTTTCTCC-3'. qPCR was performed in 20 µl aliquots
containing 1 µl RT products using a 4 µl LightCycler® FastStart DNA
Master PLUS SYBR Green I (cat. no. 03515869001; Roche Diagnostics),
0.5 µM of each primer and 14.6 µl nuclease-free water on a Real
Time PCR LightCycler 1.5 Complete system (Roche Diagnostics). The
thermocycling conditions were as follows: Initial denaturation step
at 95˚C for 10 min, followed by 45 cycles of 95˚C for 10 sec, 60˚C
for 10 sec and 72˚C for 10 sec. The quantification cycle threshold
(Cq) was recorded for each mRNA using LightCycler version 3.5.28
(Roche Diagnostics) and β-actin was used as the endogenous control
for data normalization. The relative expression was calculated
using the following formula 2-ΔΔCq
= 2-(ΔCq, reagent
treatment-ΔCq, control) (16).
Statistical analysis
Differences in sex, presence/absence of recurrence,
presence/absence of treatments for each drug, and lesioned/normal
regions, were analyzed using a Student's t-test. Extent of disease,
disease type and the presence/absence of treatments for each drug
were analyzed using a one way ANOVA with a Tukey's post-hoc test.
The correlation between Matts' histopathological grade and gene
expression was analyzed using Kendall's Tau rank correlation
coefficient. Data are expressed as the mean ± standard deviation.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Patients
Of the 18 patients, 11 had pancolitis, 3 had distal
UC and 4 had left-sided UC. There were 8 cases of single attack
only, 6 cases of relapsing-remitting sub-type and 4 cases of
chronic continuous sub-type UC. Regarding treatment of these
patients: 10 patients were treated with 5-ASA; 3 patients were
treated with 5-ASA and PSL; 1 patient was treated with 5-ASA and
G-CAP; 1 patient was treated with 5-ASA and AZA; 1 patient was
treated with PSL and AZA; 1 patient was treated with 5-ASA, PSL and
AZA; and 1 patient was treated with 5-ASA, PSL, G-CAP and AZA
(Table I).
 | Table IClinical characteristics of 18
patients with ulcerative colitis. |
Table I
Clinical characteristics of 18
patients with ulcerative colitis.
| Characteristics | n |
|---|
| Age, years, mean
(SD) | 41.1 (14.7) |
| Sex |
|
Male | 12 |
|
Female | 6 |
| Location of disease,
n (%) |
|
Pancolitis | 11 (61.1) |
|
Left-side
UC | 4 (22.2) |
|
Distal
UC | 3 (16.7) |
| Disease type, n
(%) |
|
Single
attack only | 8 (44.4) |
|
Relapsing-remitting | 6 (33.3) |
|
Chronic
continuous | 4 (22.2) |
|
Acute
fulminant | 0 (0) |
| Treatment, n (%) |
|
5-ASA | 10 (55.5) |
|
5-ASA,
PSL | 3 (16.7) |
|
5-ASA,
G-CAP | 1 (5.6) |
|
5-ASA,
AZA | 1 (5.6) |
|
AZA,
PSL | 1 (5.6) |
|
5-ASA, PSL,
AZA | 1 (5.6) |
|
5-ASA, PSL,
G-CAP, AZA | 1 (5.6) |
Background
Men had significantly higher mRNA expression levels
of MUC2 expression compared with females (0.115±0.240 vs.
0.0653±0.0376, respectively; P<0.05; Table II). In addition, patients with the
relapsing-remitting sub-type displayed higher levels of MUC2
expression compared with patients with the single attack only
sub-type and the chronic continuous sub-type (0.355±0.266 vs.
0.0711±0.0612, respectively; P<0.01 and 0.355±0.266 vs.
0.0495±0.0320, respectively; both P<0.01; Table II). No association was observed
between IL-8, LYPD8 and MUC2 expression levels and the different
treatments (data not shown).
 | Table IIGene expression of IL-8, MUC2 and
LYPD8 classified by sex, location of disease, disease type and
presence of recurrence in the lesioned colon of patients with
ulcerative colitis. |
Table II
Gene expression of IL-8, MUC2 and
LYPD8 classified by sex, location of disease, disease type and
presence of recurrence in the lesioned colon of patients with
ulcerative colitis.
| Characteristics | IL-8,
x10-4 | MUC2,
10-2 | Lypd8,
x10-2 |
|---|
| Sex |
|
Male | 4.07±38.3 |
11.5±24.0a | 3.65±4.24 |
|
Female | 2.37±7.26 | 6.53±3.76 | 4.37±5.76 |
| Location |
|
Pancolitis | 3.05±21.0 | 8.99±23.7 | 4.23±4.51 |
|
Left | 16.2±58.5 | 5.92±17.8 | 3.18±6.54 |
|
Distal | 3.31±5.26 | 3.02±15.5 | 1.87±1.84 |
| Sub-type |
|
Single
attack only | 3.15±5.91 | 7.11±6.12 | 4.37±4.88 |
|
Relapsing | 11.2±28.5 |
35.5±26.6b,c | 3.47±2.75 |
|
Chronic | 4.83±60.5 | 4.95±3.20 | 5.26±6.49 |
| Recurrent | 16.5±62.7 | 21.6±20.4 | 1.60±4.86 |
| Non recurrent | 3.24±10.1 | 7.00±21.0 | 4.01±4.80 |
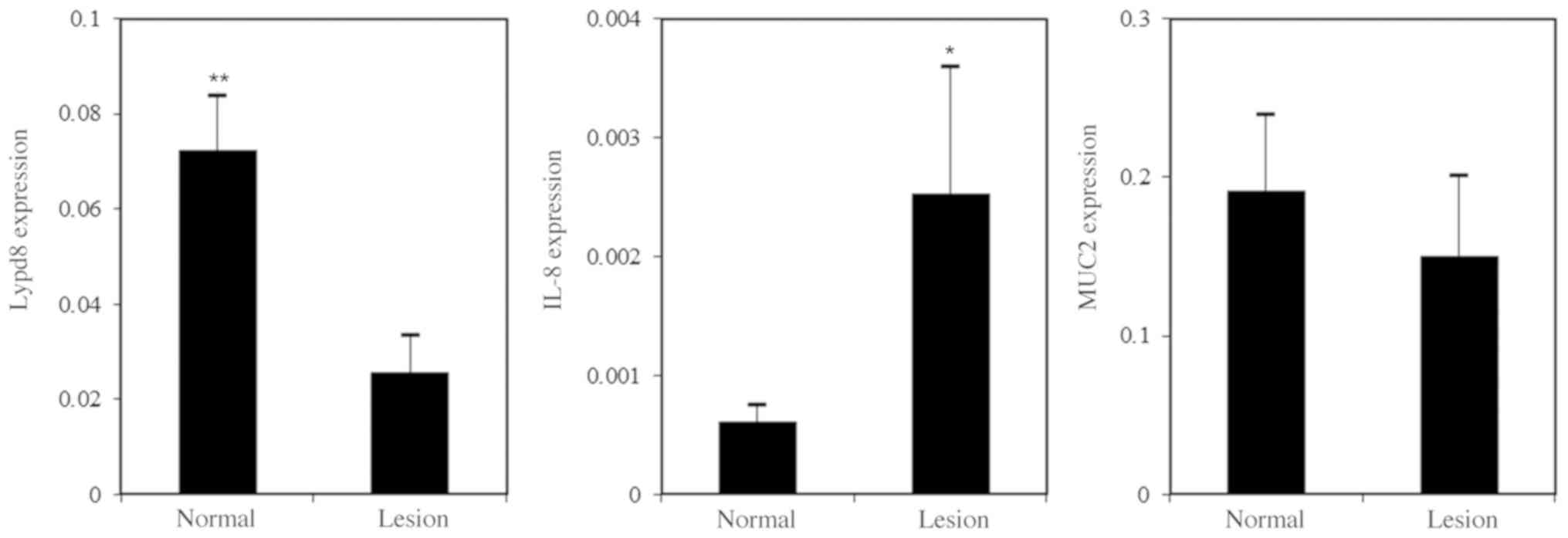
Normal vs. lesion
The expression levels of IL-8 were slightly higher
in the lesioned regions compared with the normal regions of the
colon (0.000431±0.00445 vs. 0.000327±0.000601, respectively;
P<0.05; Fig. 1). In contrast, the
decrease in LYPD8 expression levels in the lesioned regions
compared with normal regions was significant (0.0156±0.0335 vs.
0.0591±0.0485, respectively, P<0.001; Fig. 1).
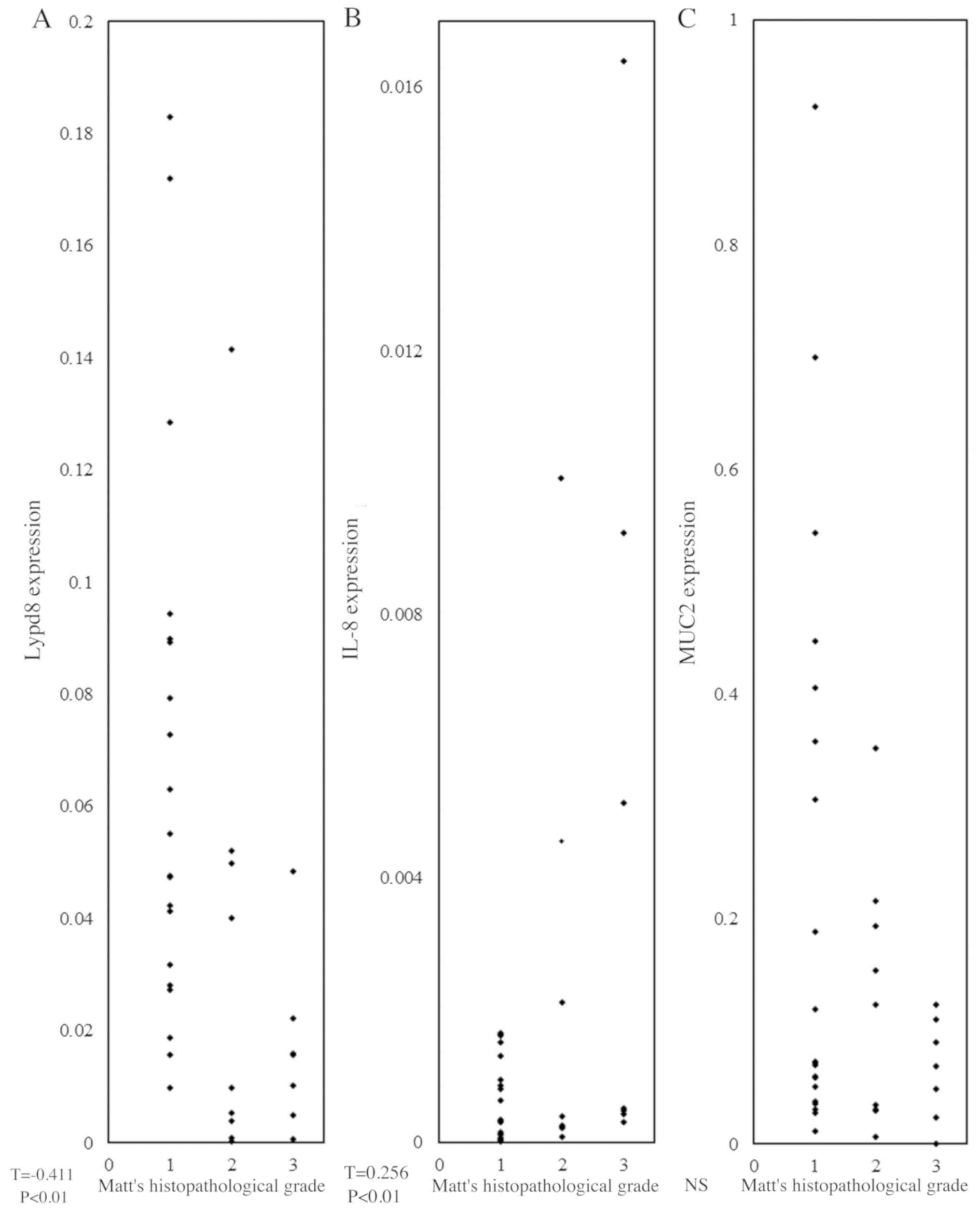
Matts' histopathological grade
There was a positive correlation between Matts'
histopathological grade and IL-8 expression levels (T=0.256;
P<0.01; Fig. 2), whereas a
negative correlation was observed between Matts' histopathological
grade and LYPD8 expression levels (T=-0.411; P<0.01; Fig. 2). There was no correlation between
Matts' histopathological grade and MUC2 expression levels (Fig. 2).
Discussion
While studying the relationship between LYPD8
expression levels and UC, Okumura et al (8) reported that LYPD8 expression levels were
decreased in patients with UC, indicating that LYPD8 may function
in the pathogenesis of inflammatory bowel diseases. The present
study showed that LYPD8 expression levels were lower in the
lesioned compared with matching non-lesioned regions of the colon
in patients with UC. This suggests that the expression levels of
LYPD8 are specifically downregulated in the inflamed mucosa of
patients with UC and that LYPD8 may be a novel inflammatory marker.
Furthermore, the present study showed that LYPD8 expression levels
and pathological mucosal inflammation were associated. LYPD8 is
expressed in the uppermost layer of the colonic gland, and is
secreted by the epithelial cells (8).
Destruction of the mucosal layer and depletion of mucin results in
the downregulation of LYPD8 expression levels in patients with UC
(8). The present study also showed
that decreased expression levels of LYPD8 were associated with the
severity of mucosal inflammation. Furthermore, LYPD8 expression was
not affected by sex, location of lesions, type of treatment,
combination of treatments, and presence/absence of recurrence;
therefore, it specifically reflected mucosal inflammation.
IL-8 expression levels are high in the inflamed
mucosa of patients with UC (17-19)
and Pearl et al (12) reported
that there was an association between the severity of inflammation
and the mucosal concentration of IL-8. The present study showed
that IL-8 expression levels were upregulated in the inflamed
regions of the colon in patients with UC, and this effect was
independent of patient background factors such as sex, location of
lesions, type of disease, combinations of treatment, and
presence/absence of recurrence. Mazzucchelli et al (20) reported that IL-8 expression is
restricted to histopathologically inflamed regions of the colon.
Therefore, IL-8 expression may not reflect patient background, but
instead only the severity of inflammation.
The intestinal tracts of mice infected with the
apicomplexan parasite Eimeria papillate showed greater MUC2
expression levels in females compared with males (21); however, to the best of our knowledge,
no previous studies have reported the association between MUC2
levels with sex differences in humans with UC. There may be
differences in the sensitivity of epithelial cells to UC due to
sex, resulting in differences in MUC2 expression. MUC2 expression
levels do not correlate with the severity of inflammation (6,22). The
present study showed that MUC2 expression levels were higher in
patients who had undergone a greater number of cycles of relapse
and remission, and this suggests that increased MUC2 expression
levels may reflect the number of courses of relapse and remission
of mucosal inflammation in UC. To the best of our knowledge, there
are no studies focused on MUC2 expression in patients examining the
location of the lesion, combinations of treatment, and
presence/absence of recurrence; thus, further studies are
required.
Previous studies have revealed that mucosal healing
is an important therapeutic goal for successfully treating patients
with UC as it may improve patient outcomes, potentially changing
the course of the disease by inducing sustained clinical remission
and reducing the need for hospitalization and surgery (23-25).
However, as there is no established definition of mucosal healing,
and it is difficult to assess the efficacy of various drugs
(24). One of the most commonly used
definitions of mucosal healing, as stated by the International
Organization of Inflammatory Bowel Disease, is the ‘absence of
friability, blood, erosions, and ulcers in all visualized segments’
corresponding to a Mayo Endoscopic sub score between 0 and
1(26). However, this definition does
not describe the histological healing of the mucosa (27). Additionally, although discrepancies
between histological and endoscopic data exist, these are rarely
assessed in therapeutic trials (28).
Mucosal healing may be multilaterally assessed by combining the
expression of mucosal markers such as LYPD8 with endoscopic and
histological indicators.
In conclusion, it was shown that IL-8 and LYPD8 were
specifically expressed in the inflamed mucosa compared with
non-inflamed mucosa of patients with UC, and that expression was
correlated with the severity of inflammation. Using IL-8 and LYPD8
expression as indicators, it may be possible to more accurately
determine the outcomes of therapeutic treatments in patients with
UC.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during the present
study are included in this article.
Authors' contributions
TO and TK made substantial contributions to the
acquisition, analysis and interpretation of the data, as well as in
drafting the manuscript. TK also established the LYPD8 primers. KH
and KN helped in acquiring the data and performed the biopsies. YI
and NU conceived and designed the study. HI assisted in the
conception and design of the study as well as in drafting the
manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by The Institutional
Review Board of Tottori University (Tottori, Japan) and was
performed in accordance with the Declaration of Helsinki. Written
informed consent was provided by all patients.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Tindall WN, Boltri JM and Wilhelm SM:
Mild-to-moderate ulcerative colitis: Your role in patient
compliance and health care costs. J Manag Care Pharm. 13((7 Suppl
A)): S2–S12; quiz S13-S14. 2007.PubMed/NCBI View Article : Google Scholar
|
|
2
|
International Agency for Research on
Cancer (IARC): Cancer Tomorrow. Estimated number of incident cases
from 2018 to. 2040, colon both sexes, all ages. IARC, Lyon, 2018.
http://gco.iarc.fr/tomorrow/graphic-line?type=0&population=900&mode=population&sex=0&cancer=39&age_group=value&apc_male=0&apc_female=0.
|
|
3
|
Farraye FA, Odze RD, Eaden J, Itzkowitz
SH, McCabe RP, Dassopoulos T, Lewis JD, Ullman TA, James T III,
McLeod R, et al: AGA medical position statement on the diagnosis
and management of colorectal neoplasia in inflammatory bowel
disease. Gastroenterology. 138:738–745. 2010.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Tranter-Entwistle I, Mullaney TG, Noah K,
Pearson J, Falvey J, Gearry R and Eglinton T: Long-term incidence
of dysplasia and colorectal cancer in an ulcerative colitis
population-based cohort. ANZ J Surg: Jan 23, 2020 (Epub ahead of
print).
|
|
5
|
Sartor RB: Mechanisms of disease:
Pathogenesis of Crohn's disease and ulcerative colitis. Nat Clin
Pract Gastroenterol Hepatol. 3:390–407. 2006.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Dorofeyev AE, Vasilenko IV, Rassokhina OA
and Kondratiuk RB: Mucosal barrier in ulcerative colitis and
Crohn's disease. Gastroenterol Res Pract.
2013(431231)2013.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Kawashima H: Roles of the gel-forming MUC2
mucin and its O-glycosylation in the protection against colitis and
colorectal cancer. Biol Pharm Bull. 35:1637–1641. 2012.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Okumura R, Kurakawa T, Nakano T, Kayama H,
Kinoshita M, Motooka D, Gotoh K, Kimura T, Kamiyama N, Kusu T, et
al: Lypd8 promotes the segregation of flagellated microbiota and
colonic epithelia. Nature. 532:117–121. 2016.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Garrett WS, Gallini CA, Yatsunenko T,
Michaud M, DuBois A, Delaney ML, Punit S, Karlsson M, Bry L,
Glickman JN, et al: Enterobacteriaceae act in concert with the gut
microbiota to induce spontaneous maternally transmitted colitis.
Cell Host Microbe. 8:292–300. 2010.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Mukhopadhya I, Hansen R, El-Omar EM and
Hold GL: IBD-what role do Proteobacteria play? Nat Rev
Gastroenterol Hepatol. 9:219–230. 2012.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Hansen R, Thomson JM, Fox JG, El-Omar EM
and Hold GL: Could Helicobacter organisms cause inflammatory bowel
disease? FEMS Immunol Med Microbiol. 61:1–14. 2011.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Pearl DS, Shah K, Whittaker MA,
Nitch-Smith H, Brown JF, Shute JK and Trebble TM: Cytokine mucosal
expression in ulcerative colitis, the relationship between cytokine
release and disease activity. J Crohns Colitis. 7:481–489.
2013.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Tytgat KM, van der Wal JW, Einerhand AW,
Büller HA and Dekker J: Quantitative analysis of MUC2 synthesis in
ulcerative colitis. Biochem Biophys Res Commun. 224:397–405.
1966.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Matts SG: The value of rectal biopsy in
the diagnosis of ulcerative colitis. Q J Med. 30:393–407.
1961.PubMed/NCBI
|
|
15
|
World Medical Association: World medical
association declaration of Helsinki: Ethical principles for medical
research involving human subjects. JAMA 310: 2191.2194, 2013.
|
|
16
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Bruno ME, Rogier EW, Arsenescu RI,
Flomenhoft DR, Kurkjian CJ, Ellis GI and Kaetzel CS: Correlation of
biomarker expression in colonic mucosa with disease phenotype in
Crohn's disease and ulcerative colitis. Dig Dis Sci. 60:2976–2984.
2015.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Lin X, Li J, Zhao Q, Feng JR, Gao Q and
Nie JY: WGCNA reveals key roles of IL8 and MMP-9 in progression of
involvement area in colon of patients with ulcerative colitis. Curr
Med Sci. 38:252–258. 2018.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Korolkova OY, Myers JN, Pellom ST, Wang L
and M'Koma AE: Characterization of serum cytokine profile in
predominantly colonic inflammatory bowel disease to delineate
ulcerative and Crohn's colitides. Clin Med Insights Gastroenterol.
8:29–44. 2015.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Mazzucchelli L, Hauser C, Zgraggen K,
Wagner H, Hess M, Laissue JA and Mueller C: Expression of
interleukin-8 gene in inflammatory bowel disease is related to the
histological grade of active inflammation. Am J Pathol.
144:997–1007. 1994.PubMed/NCBI
|
|
21
|
Dkhil MA: Sex-determined susceptibility
and differential MUC2 mRNA expression during the course of murine
intestinal eimeriosis. Parasitol Res. 114:283–288. 2015.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Niv Y: Mucin gene expression in the
intestine of ulcerative colitis patients: A systematic review and
meta-analysis. Eur J Gastroenterol Hepatol. 28:1241–1245.
2016.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Neurath MF and Travis SP: Mucosal healing
in inflammatory bowel diseases: A systematic review. Gut.
61:1619–1635. 2012.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Papi C and Aratari A: Mucosal healing as a
treatment for IBD? Expert Rev Gastroenterol Hepatol. 8:457–459.
2014.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Shah SC, Colombel JF, Sands BE and Narula
N: Mucosal healing is associated with improved long-term outcomes
of patients with ulcerative colitis: A systematic review and
meta-analysis. Clin Gastroenterol Hepatol. 14:1245–1255.e8.
2016.PubMed/NCBI View Article : Google Scholar
|
|
26
|
D'Haens G, Sandborn WJ, Feagan BG, Geboes
K, Hanauer SB, Irvine EJ, Lémann M, Marteau P, Rutgeerts P,
Schölmerich J and Sutherland LR: A review of activity indices and
efficacy end points for clinical trials of medical therapy in
adults with ulcerative colitis. Gastroenterology. 132:763–786.
2007.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Rosenberg L, Nanda KS, Zenlea T, Gifford
A, Lawlor GO, Falchuk KR, Wolf JL, Cheifetz AS, Goldsmith JD and
Moss AC: Histologic markers of inflammation in patients with
ulcerative colitis in clinical remission. Clin Gastroenterol
Hepatol. 11:991–996. 2013.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Mazzuoli S, Guglielmi FW, Antonelli E,
Salemme M, Bassotti G and Villanacci V: Definition and evaluation
of mucosal healing in clinical practice. Dig Liver Dis. 45:969–977.
2013.PubMed/NCBI View Article : Google Scholar
|