Introduction
Helicobacter pylori (H. pylori) is
present in the stomach of ~50% of the population worldwide
(1) and is considered to be the
primary cause of chronic gastritis, gastric cancer and peptic
ulcers (2). Around 89% of non-cardia
gastric cancer cases, representing 78% of all gastric cancer case,
are now estimated to be attributable to chronic H. pylori
infection (3). Gastric cancer is
among the five most common types of malignant tumors, and has the
second highest cause of cancer-associated death worldwide (4). However, only 1-3% of the individuals
with an H. pylori infection develop gastric cancer (5), as this pathology is dependent on the
virulence of the bacteria, the environment and genetic factors of
the host (6,7). For instance, common variable
immunodeficiency (CVID) syndrome is associated with a 45-fold
increase in the risk of gastric cancer and a 30-fold increase in
the risk of gastric lymphoma (8).
Although the etiology of CVID is not completely understood, in
adults it is associated with deletion of a gene which encodes the
inducible T-cell co-stimulator ICOS (9), which is expressed by T-cells when
activated by their antigen. The only known ligand of ICOS (ICOS-L)
is expressed constitutively by B lymphocytes (10). The interaction between ICOS:ICOS-L
serves an important role in mediating the cooperation between T and
B cells, as well as promoting the terminal differentiation of B
cells to plasma B cells. ICOS activation induces the secretion of
IL-4, IL-5, IL-6, IL-10, IL-21, tumor necrosis factor-a and
interferon gamma (IFN-γ). In doing so, ICOS co-induces the
secretion of interleukins and activates the function of Th1, Th2
and Th17 cells (11,12). Patients with deletion of ICOS have a
reduced number of naïve B cells and memory cells, and low levels of
serum antibodies, but do not exhibit a change in antibody isotype
(9).
Another molecule which modulates the immune response
is programmed death-ligand 1 (PD-L1; also known as B7 homolog 1).
Encoded by the CD274 gene, PD-L1 activates a membrane receptor of
programmed death 1 (PD-1). The PD-L1:PD-1 axis maintains the
balance between tolerance and autoimmunity. A deficiency or excess
in the function of PD-1 can result in various diseases, for example
arthritis and lupus (13). One
mechanism of regulating the expression of PD-L1, is binding of
STAT3 to its promoter (14). Research
has shown that in patients with tumors, upregulation of
hypoxia-inducible factor 1α is associated with elevated levels of
PD-L1(15). PD-L1 functions primarily
in a microenvironment enriched with lactate (16).
In relation to the virulence of H. pylori,
the most studied molecule is the cytotoxin associated gene (CagA),
which is translocated by the type IV secretion system of H.
pylori into gastric cells, generating intracellular signals
that facilitate malignancy (17).
Individuals have an increased risk of developing gastric cancer if
they express cagA+ instead of cagA-, and the strains of H.
pylori that carry CagA are associated with an increased risk of
developing chronic gastritis or peptic ulcers, as demonstrated in a
meta-analysis (18). CagA is
considered the primary virulence factor of H. pylori, and
results in the downregulation of the inflammatory modulator ICOS-L,
the attenuation of which occurs through the P70-S6 kinase signaling
pathway in gastric epithelial cells (19).
Vacuolating cytotoxin A is another virulence
mechanism of H. pylori correlated with gastric cancer
(20,21). The DNA sequence analysis of the vacA
gene shows a mosaic structure comprising allelic variations with
different biological activity, resulting in the s1 and m1 regions
as the two regions most frequently associated with peptic ulcers
and an increased risk of gastric cancer (22). One of the mechanisms attributed to
VacA is its interference with IL-2 production and IL-2 receptor
(IL-2R) expression, which in turn reduces the proliferation of T
lymphocytes (23). In mice, purified
VacA results in the loss of gastric epithelial cells in vivo
and in vitro by increasing apoptosis, which is initiated
through the release of mitochondrial cytochrome C and the
activation of Caspase 3(24).
A meta-analysis recently suggested that the presence
of the vacA-s1 and vacA-m1 genotype results in a
greater risk of gastric cancer and account for a 33.4% and
2.08-fold increase, respectively, with an age-standardized rate
(ASR) of 11-19 cases per 100,000 individual. A 40.2 and 66.6%
increase, respectively, of gastric cancer was reported for a group
with an ASR <10 per 100,000. Therefore, the vacA-m1
genotype of H. pylori appears to be more potent than
vacA-s1 for inducing gastric cancer (25).
In the present study, the differences in the mRNA
expression levels of cytokines, master transcription factors
associated with the T-cell profile, and the co-immunomodulatory
molecules PD-L1 and ICOS-L in two groups of patients infected with
H. pylori, those with and without gastric cancer were
examined. Additionally, the correlation between each group, the
polymorphisms of vacA and the presence of cagA was
assessed. The aim of the present study was to determine effect the
co-modulating molecules, ICOS-L and PD-L1, conferred on the immune
response of patients infected with H. pylori with or without
gastric cancer.
Materials and methods
Patients
The present study was performed in the National
Medical Center (Centro Médico Nacional 20 de November) of the
ISSSTE Medical Service (a health plan for government workers)
between June 2016 and August 2017. A total of 1,462 endoscopies
were performed during this period on adults (>18 years of age),
and 46 patients exhibited signs of a gastric pathology. These
patients were referred to our group. The patients were given a
detailed description of the study and all patients provided written
informed consent, in accordance with the Helsinki Declaration and
the Ethics Committee of the National Medical Center (Centro Médico
Nacional 20 de November) of the ISSSTE Medical Service. The
protocol used in the present study was approved by the Bioethics
Committee of the Autonomous University of Aguascalientes (approval
no. CIB-UAA-26).
The patients were divided into two groups; those
with cancer (n=18), males (n=10, 55.56%), female (n=8, 44.44%),
mean age 64.72±2.54 years (age range 48-82), and those without
cancer (n=28) males (n=16, 21.42%), female (n=22, 78.58%), age mean
55.68±2.59 years (age range 33-77). Of the patients without gastric
cancer, a duodenal ulcer was present in 10 patients, whereas the
other 18 patients exhibited various other types of gastritis
(epidemiological variables shown in Table
I).
 | Table IClinicopathological characteristics
of the patients recruited in the present study. |
Table I
Clinicopathological characteristics
of the patients recruited in the present study.
| Clinicopathological
characteristics | With cancer,
n=18 | Without cancer,
n=28 | P-value |
|---|
| Age, mean ±
standard deviation (age range) | 64.72±2.547
(48-82) | 55.68±2.594
(33-77) | 0.0227a |
| Males, n (%) | 10 (55.56) | 6 (21.42) | 0.0172a |
| Female, n (%) | 8 (44.44) | 22 (78.58) | 0.0271a |
| Smoker, n (%) | 8 (44.45) | 9 (32.14) | 0.4102 |
| Atrophy, n (%) | 5 (27.78) | 6 (21.42) | 0.6314 |
| Non-Atrophy, n
(%) | 13 (72.22) | 22 (78.58) | 0.6314 |
| Type 2 diabetes, n
(%) | 3 (16.66) | 7(25) | 0.7172 |
| High blood
pressure, n (%) | 3 (16.66) | 10 (35.71) | 0.1968 |
| Duodenal ulcer, n
(%) | 2 (11.11) | 12 (42.85) | 0.0465a |
Extraction of gastric biopsies
The endoscopy procedure was handled by an
experienced endoscopist on a Jaw Radial Endoscopy apparatus (160.24
cm in length with a 6 mm opening; Boston Scientific) and a 590 EG
ZW (with a working channel 2.8 mm in diameter) (Fujinon; FUJIFILM,
Inc.) with an EPX 4400 processor. By using the conventional
brightfield endoscopy procedure, the mucus was carefully observed
to detect visible alterations indicative of a gastropathy (for
examples gastritis, gastric ulcers or duodenal ulcers) in patients
with symptoms suggestive of these disorders. In individuals
suspected of having gastric cancer, the neoplasm was examined to
determine its characteristics and location. Gastric biopsies were
obtained from the pyloric antrum and were placed in small vials
containing TRIzol (Invitrogen; Thermo Fisher Scientific, Inc.) and
ethanol for extraction of RNA and DNA, respectively. The samples
were stored at -40˚C until required. Additional biopsies of the
pyloric antrum were extracted and placed in 4% formaldehyde for
histopathological confirmation using the Sydney protocol (26). In the event of a gastric neoplasm, a
biopsy of the lesion was taken to perform histopathological
analysis using hematoxylin and eosin staining (27).
DNA purification from gastric
tissue
DNA was extracted using phenol-chloroform (28). Gastric tissue was homogenized with 500
µl lysis buffer (2 mM Tris-HCl, 10% SDS, 1 mM EDTA and 1.5 µg/µl
proteinase K) and the sample was incubated at 65˚C for 24 h.
Subsequently, 500 µl phenol:isoamyl alcohol solution (24:1) was
added and the mixture was centrifuged at 15,800 x g for 15 min at
25˚C. The aqueous phase was separated, and a second extraction was
performed using the supernatant with 500 µl chloroform:isoamyl
alcohol solution (24:1). The sample was centrifuged at 15,800 x g
for 15 min at 25˚C and the aqueous phase was collected. After
precipitating the DNA present in the aqueous phase with 500 µl
isopropanol and centrifuging at 15,800 x g for 5 min at 25˚C, the
pellet was washed with 70% ethanol at -20˚C and resuspended in
nuclease-free water free of chelating agents. DNA was quantified by
spectrophotometry at 260 nm and its integrity was evaluated by
electrophoresis on a 1.25% agarose gel.
Sequence typing of vacA and
determination of cagA positivity
The genotypes of H. pylori with the
presence/absence of cagA and distinct polymorphic regions
for vacA (s1s2 or m1m2) were analyzed by
endpoint PCR from DNA samples extracted from the gastric tissue.
PCR was performed using a Platinum® PCR SuperMix
(Invitrogen; Thermo Fisher Scientific, Inc.) on a Techne 3 Prime
Base/02 thermocycler with the following thermocycling conditions:
Denaturation at 95˚C for 1 min, annealing at 58˚C for 30 sec and
extension at 72˚C for 30 sec for 40 cycles. The sequences the
primers used are presented in Table
II (29-32).
 | Table IIPrimer sequences used to identify
Helicobacter pylori positivity based on the presence of
cagA and for analysis of the vacA polymorphisms. |
Table II
Primer sequences used to identify
Helicobacter pylori positivity based on the presence of
cagA and for analysis of the vacA polymorphisms.
| Author, year | Gene | Forward | Reverse | Product length | (Refs.) |
|---|
| Abdi et al,
2016 | cag2-4 | GGAACCCTAGTC
GGTAATG |
ATCTTTGAGCTTGTCTATCG | 450/558 | (25) |
| Sipponen et
al, 2011 | cagA1 | TGGCAGTGGGTTAG
TCATAGCAG | AGGACTCTTGCAGGCGTT
GGTG | 481 | (26) |
| | cagA2 | ATAATGCTAAATTA
GACAACTTGAGCGA |
TTAGAATAATCAACAAACAT CACGCCAT | 298 | |
| Luna, 1968 | G-Hp1/2 | AAGCTTTTAGG
GGTGTTAGGGGTTT |
AAGCTTACTTTCTAACACTA AACGC | 294 | (27) |
| | G-H3/4 | CTTTCTTCTC
AAGCGGTTGTC |
CAAGCCATCGCCGGTTTTAGC | 252 | |
| Sambrook and Green,
2012 | s1 vac1 | GAAATACAAC
AAACACACCGC |
GGCTTGTTTGAGCCCCCAG | 201 | (28) |
| | s2 vac1 | GAAATACAAC
AAACACACCGC |
GGCTTGTTTGAGCCCCCAG | 228 | |
| | m1 Vac3 | GGTCAAAAT
GCGGTCATGG |
CATCAGTATTTCGCACCACA | 388 | |
| | m2 Vac4 | CCAGGAAAC
ATTGCCGGCAAA |
CATAACTAGCGCCTTGCAC | 346 | |
RNA extraction and reverse
transcription (RT)
Total RNA was extracted using the organic protocol
with TRIzol (33). To eliminate
genomic DNA contamination, digestion of DNA was performed using 2 U
DNase I (DNase amplification grade) (Invitrogen; Thermo Fisher
Scientific, Inc.) per µg RNA. The sample was incubated at room
temperature for 15 min. Subsequently, the enzyme was inactivated
with 1 µl EDTA (25 mM) and 10 min of heating at 65˚C. Total RNA was
quantified by spectrophotometry at 260 nm and the integrity of the
RNA was assessed using electrophoresis on a 1.2% agarose gel.
RT of total RNA was performed using a Superscript
VILO kit (Invitrogen; Thermo Fisher Scientific, Inc.), according to
the manufacturer's protocol. The resulting cDNA was quantified by
spectrophotometry at 260 nm and the samples were diluted to a
concentration of 400 ng/µl. The samples were stored at -20˚C until
further use.
Analysis of RNA expression in gastric
biopsies
The RNA obtained from gastric tissue was evaluated
by RT-quantitative (q)PCR using Express SYBR qPCR SuperMix
Universal (Invitrogen; Thermo Fisher Scientific, Inc.). The
expression levels of cytokines (IL-4, IL-10, IL-12, IL-17, IFN-γ
and TGF-β), the co-modulating molecules of the immune system (PD-L1
and ICOS-L), and the master transcription factors, including
forkhead box P3 (Foxp3), GATA3, and the retinoid orphan receptor γt
(RORγt). Analysis was performed on a StepOne™ Real-Time PCR system
(Applied Biosystems; Thermo Fisher Scientific, Inc.). The
thermocycling conditions were: Initial denaturation, 50˚C for 2 min
and 95˚C for 10 min; followed by 50 cycles of denaturation at 95˚C
for 15 sec, and annealing and an extension at 60˚C for 60 sec. All
primers were acquired from T4 Oligo. The sequences of the primers
used are presented in Table III
(34-38).
GAPDH was used as the normalization control. The differential
expression of genes was calculated using the 2-∆∆Cq
method (39).
 | Table IIIPrimer sequences used to analyze the
expression of the genes encoding transcription factors, cytokines
and immunomodulatory molecules. |
Table III
Primer sequences used to analyze the
expression of the genes encoding transcription factors, cytokines
and immunomodulatory molecules.
| Author, year | Gene | Forward | Reverse | Product length | (Refs.) |
|---|
| Wagih et al,
2015 | GATA3 |
GGAGGTGGATGTGCTTTTTAACA |
ACCTGGCTCCCGTGGTG | 116 | (30) |
| | RORγt |
AGGAAGTGACTGGCTACCAGAGG |
GAACTCCACCACGTACTGAATG | 94 | |
| | IL10 |
TTCCCTGTGAAAACAAGAGC |
TCACTCATGGCTTTGTAGATGC | 90 | |
| | IL17A |
CTCATTGGTGTCACTGCTACTG |
CCTGGATTTCGTGGGATTGTG | 77 | |
| Osman et al,
2015 | IFN-γ |
GTGTGGAGACCATCAAGGAAGACA |
TTGGACATTCAAGTCAGTTACC | 112 | (31) |
| | TGF-β1 |
GGTGGAAACCCACAACGAAAT |
TCTCGGAGCTCTGATGTGTTGA | 86 | |
| | GAPDH |
TGGTCTCCTCTGACTTCAACA |
AGCCAAATTCGTTGTCATACC | 116 | |
| | Foxp3 |
GAGAAGGGCAGGGCACAAT |
GTGGGCCTGCATGGCAC | 101 | |
| Rhead et al,
2007 | IL-4 |
CACAGGCACAAGCAGCTGAT |
CCTTCACAGGACAGGAATTCAAG | 87 | (32) |
| | IL-12p40 |
CATCTCTTGGTTTTCCCTGGTT |
CATAAACATCTTTCTTCAGTTCCCATAT | 73 | |
| Chomczynski and
Sacchi, 1987 | PD-L1 |
ACAGAGGGCCCGGCTGTTGA |
AGCGGTACACCCCTGCATCCT | 94 | (33) |
| Yilmaz et
al, 2015 | ICOS-L |
GCAAACCAGTGAGTCGAAAACC |
GGTGACATCAGGGCTCGGT | 101 | (34) |
Statistical analysis
The results from qPCR were examined on DataAssist™
Software version 3.01 (Applied Biosystems; Thermo Fisher
Scientific, Inc.) using the Cq values for relative expression. The
relative expression of mRNA was compared between patients with and
without cancer to determine the significance of differences using a
Mann-Whitney U test and SPSS version 20 (IBM Corp.) and multiple
comparisons were evaluated using a Kruskal-Wallis test with a
Dunn's post-hoc. This test is suitable for comparison of two
unpaired groups of continuous variables that are not normally
distributed. P<0.05 was considered to indicate a statistically
significant difference. To analyze if there was a relationship
between the mRNA expression levels of the various genes within each
group, a Spearman's Rho (ρ) value was calculated (40). The correlation was considered either
positive/negative and either high/medium/low (high, ρ value of
0.7-0.9; medium, ρ value of 0.5-0.7; low ρ value <0.5) according
to the scale described by Mukaka (41).
Results
Prevalence of the cagA and vacA
genotype in gastric biopsies
All 46 biopsies were positive for ureC (a
marker for H. pylori) and vacA. Only 18 biopsies were
positive for cagA (39.1%). Regarding vacA, the
frequency of polymorphisms in the subregions s1 and
m1 in the group of patients with gastric cancer was higher
compared with the rest of the subregions (Table IV). It has been reported that
polymorphisms in these subregions increase the risk of gastric
cancer (25). The possible
association between these polymorphisms with the expression of the
mRNA of cytokines, nuclear transcription factors and co-modulating
molecules related to the gastric pathology caused by H.
pylori was further analyzed.
 | Table IVFrequency of two vacA
polymorphisms and of positivity for cagA and ureC in
patients with and without gastric cancer. |
Table IV
Frequency of two vacA
polymorphisms and of positivity for cagA and ureC in
patients with and without gastric cancer.
| | | | s regions
of vacA | m regions of
vacA |
|---|
| Groups | ureC | cagA | s1 | s2 | m1 | m2 |
|---|
| Cancer | 18 | 10 | 14 | 1 | 9 | 4 |
| Without Cancer | 28 | 8 | 16 | 2 | 14 | 12 |
| Total | 46 | 18 | 30 | 3 | 23 | 16 |
Analysis of mRNA expression in gastric
biopsies
The expression of cytokines, transcription factors
and co-modulating molecules of the immune system was determined
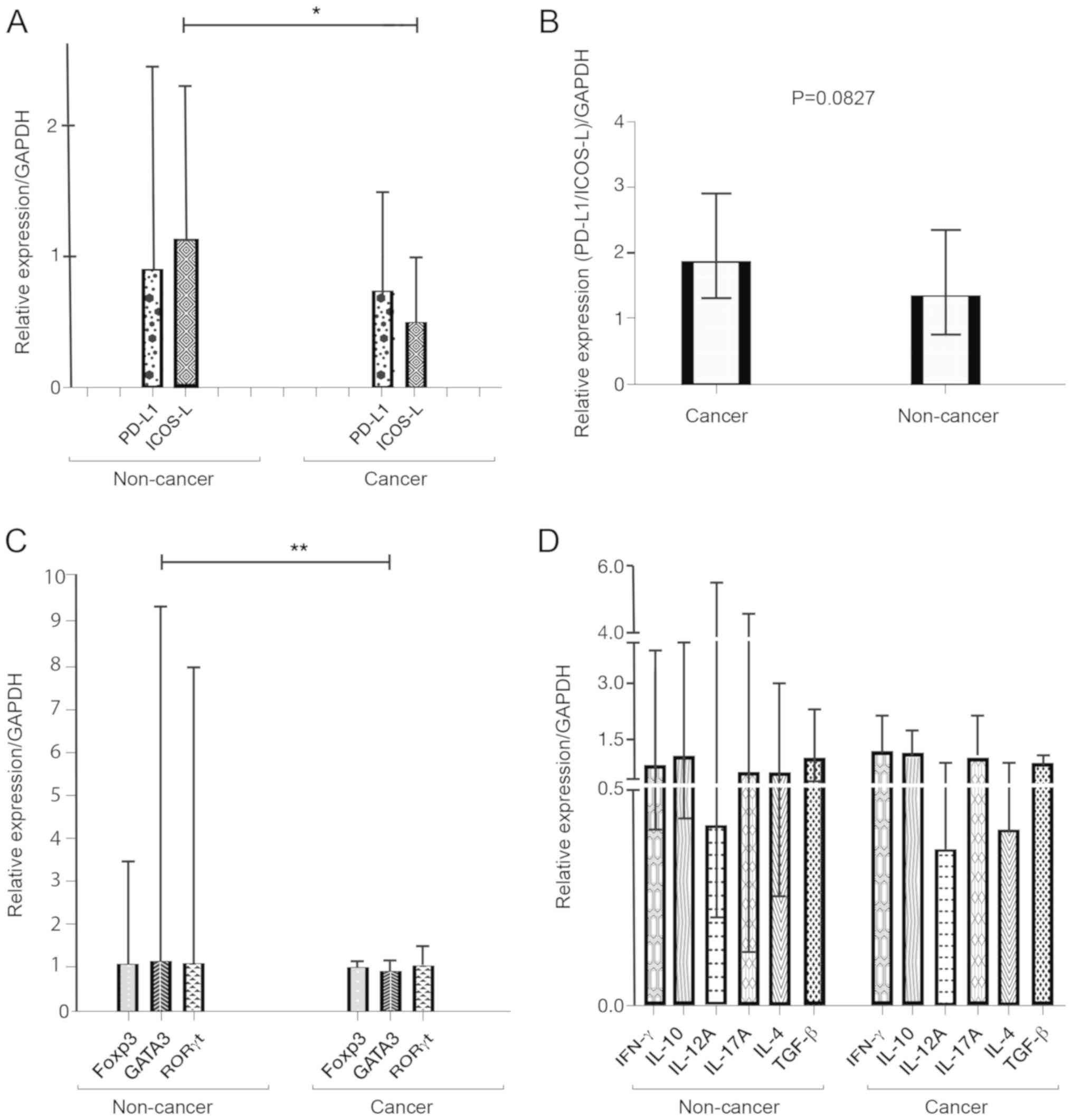
using RT-qPCR. The expression levels of ICOS-L were significantly
reduced in patients with cancer (Fig.
1A). Additionally, there was a lower level of ICOS-L relative
to PD-L1, represented by the ratio PD-L1/ICOS-L, although this
decrease was not significant (Fig.
1B).
The relative expression of the master transcription
factors was higher with the phenotype of regulatory T cell (Treg),
Th17 and GATA3 in the patients without cancer. The difference in
the expression of Foxp3 (P=0.063) and RORγt (P=0.060) between
patients with and without gastric cancer was not significant;
however, the difference in the expression of GATA3 was significant
between the two groups. (P<0.01; Fig.
1C).
The expression levels of IL-4, IL-10, IL-12 and
IL-17, IFN-γ and TGF-β were similar between the two groups. The
expression levels of IL-4 in the Th2 profile were decreased in
patients with gastric cancer, but the difference was not
significant (P=0.07; Fig. 1D).
Spearman's rank correlation
coefficient
Spearman's bivariate correlation analysis of immune-
and virulence-related variables in patients with and without cancer
are presented in Table V. Patients
without cancer showed a high positive correlation between ICOS-L
and PD-L1 (ρ=0.707), IL-4 (ρ=0.719) and IL-12A (ρ=0.712); a
moderate positive correlation with RORγt (ρ=0.687), GATA-3
(ρ=0.561) and Foxp3 (ρ=0.530); and low positive correlation with
TGF-β (ρ=0.481) and IL-17 (ρ=0.411). Patients with cancer exhibited
a high positive correlation between ICOS-L and IL-12 (ρ=0.772),
RORγt (ρ=0.760) and IL-10 (ρ=0.708); a moderate positive
correlation with GATA3 (ρ=0.689), IFN-γ (ρ=0.637), PD-L1 (ρ=0.610),
vacA-m2 (ρ=0.567) and IL-4 (ρ=0.566); and a low positive
correlation factor with Foxp3 (ρ=0.490).
 | Table Vρ values from correlation analysis
between immune- and virulence-related variables in patients with
and without gastric cancer. |
Table V
ρ values from correlation analysis
between immune- and virulence-related variables in patients with
and without gastric cancer.
| A, Without
cancer |
|---|
| Factor | IL-4 | IL-10 | IL-12 | IL-17 | IFN-γ | TGF-β | ICOS-L | PD-L1 | RORγt | GATA3 | FoxP3 | vacA-m1 | vacA-m2 | CagA |
|---|
| ICOS-L | 0.719 | <0.4 | 0.712 | 0.411 | <0.4 | 0.481 | - | 0.707 | 0.687 | 0.561 | 0.53 | - | <0.4 | - |
| PD-L1 | 0.668 | 0.428 | 0.675 | 0.570 | 0.454 | 0.525 | 0.707 | - | - | <0.4 | 0.551 | - | | - |
| IL-12 | 0.785 | 0.509 | - | 0.530 | 0.437 | - | 0.712 | 0.675 | 0.772 | 0.509 | 0.784 | - | <0.4 | - |
| IL-17 | - | 0.690 | 0.530 | - | - | - | 0.411 | 0.570 | 0.603 | - | - | - | - | <0.4 |
| RORγt | 0.737 | - | 0.772 | - | - | - | 0.687 | - | - | - | 0.708 | <0.4 | <0.4 | - |
| vacA-m2 | <0.4 | <0.4 | <0.4 | - | - | - | <0.4 | - | <0.4 | - | - | - | - | - |
| B, Cancer |
| Factor | IL-4 | IL-10 | IL-12 | IL-17 | IFN-γ | TGF-β | ICOS-L | PD-L1 | RORγt | GATA3 | FoxP3 | vacA-m1 | vacA-m2 | CagA |
| ICOS-L | 0.566 | 0.708 | 0.772 | <0.4 | 0.637 | <0.4 | - | 0.610 | 0.760 | 0.689 | 0.490 | - | 0.567 | - |
| PD-L1 | <0.4 | 0.789 | 0.789 | <0.4 | 0.485 | <0.4 | 0.610 | - | - | 0.757 | 0.483 | - | - | - |
| IL-12 | 0.593 | 0.696 | - | <0.4 | 0.547 | - | - | 0.789 | 0.623 | 0.762 | 0.659 | - | 0.567 | - |
| IL-17 | - | 0.493 | - | - | - | - | - | - | <0.4 | - | - | - | - | -0.537 |
| RORγt | 0.426 | - | 0.623 | <0.4 | - | - | 0.760 | - | - | - | <0.4 | -0.756 | - | - |
| vacA-m2 | 0.567 | 0.630 | 0.567 | - | - | - | 0.567 | - | 0.598 | - | - | - | - | - |
Patients without cancer exhibited a high positive
correlation between PD-L1 and ICOS-L (ρ=0.707); a moderate positive
correlation with IL-4 (ρ=0.668), IL-17A (ρ=0.570), Foxp3 (ρ=0.551)
and TGF-β (ρ=0.525); and a low positive correlation with IFN-γ
(ρ=0.454) and IL-10 (ρ=0.428). For the patients with cancer, there
was a high positive correlation between PD-L1 and IL-10 (ρ=0.789),
IL-12A (ρ=0.789) and GATA3 (ρ=0.757); and a low positive
correlation with IFN-γ (ρ=0.485) and Foxp3 (ρ=0.483).
Patients without cancer demonstrated a high positive
correlation between RORγt and IL-12A (ρ=0.772), IL-4 (ρ=0.737) and
Foxp3 (ρ=0.708); a moderate positive correlation with IL-17A
(ρ=0.603) and PD-L1 (ρ=0.549); and a low positive correlation with
TGF-β (ρ=0.408). Patients with cancer exhibited a high positive
relation of RORγt; a moderate positive correlation with IL-12A
(ρ=0.623), vacA-m2 (ρ=0.598) and GATA3 (ρ=0.522); and a low
positive correlation with IL-10 (ρ=0.488). A high negative
correlation was observed between RORγt and vacA-m1
(ρ=-0.756).
In the patients without cancer, there was a high
positive correlation between Foxp3 and IL-12A (ρ=0.784) and IL-4
(ρ=0.715); a moderate positive correlation with IL-17A (ρ=609),
IFN-γ (ρ=0.585) and IL-10 (ρ=0.579); and a low positive correlation
with TGF-β (ρ=0.457). In patients with cancer, there was a moderate
correlation between Foxp3 and IL-12 (ρ=0.659) and IL-10 (ρ=0.569);
and a low positive correlation with GATA3 (ρ=0.493).
Patients without cancer had a moderate positive
correlation between IL-17A and IL-10 (ρ=0.690), TGF-β (ρ=0.637),
IFN-γ (ρ=0.572), IL-4 (ρ=0.565) and IL-12A (ρ=0.530). In patients
with cancer, there was only a low positive correlation between
IL-17A and IL-10 (ρ=0.493) and a moderate negative correlation with
CagA (ρ=-0.537).
Patients without cancer exhibited a moderate
positive correlation between TGF-β with IFN-γ (ρ=0.676) and IL-10
(ρ=0.621); and a low positive correlation with IL-4 (ρ=0.413).
Patients with cancer only showed a moderate positive correlation
between TGF-β and IFN-γ (ρ=0.581).
Patients without cancer exhibited a high positive
correlation between IL-10 with IFN-γ (ρ=0.775); a moderate positive
correlation with TGF-β (ρ=0.621), Foxp3 (ρ=0.579). Patients with
cancer demonstrated a high positive correlation between IL-10 a
moderate positive correlation with IL-12A (ρ=0.696), GATA3
(ρ=0.672) and vacA-m2 (ρ=0.630).
Discussion
In the present study, the presence of H.
pylori in gastric biopsies from patients with and without
gastric cancer was examined, as well as the correlation between the
development of this disorder and the presence of virulence factors,
CagA and VacA (42), including the
polymorphisms of vacA (s1, s2, m1 and
m2) (22). The s1m1
polymorphism of vacA was found to be the most frequently
observed polymorphism in the participants, consistent with a
previous study on an adult population in Mexico (43).
The host factors which may affect the elimination or
proliferation of H. pylori (44) were also analyzed in the present study.
Gastric biopsies were obtained to determine the mRNA expression
levels of interleukins, master transcription factors and
co-modulators of the immune response. RT-qPCR analysis showed
significantly lower relative mRNA expression levels of the
co-activating molecule ICOS-L in patients with cancer compared with
patients without cancer. ICOS-L is the only known ligand for ICOS,
a receptor which activates various inflammatory pathways implicated
in the elimination of H. pylori (11).
In the patients with cancer, the mRNA expression
levels of the master transcription factor GATA3 was significantly
reduced. GATA3 promotes the differentiation of T-cells into Th2
cells (45). The reported negative
association between allergies and H. pylori infection can be
explained, at least in part, by hygiene theory, which is based on
the fact that microbial infections protect against allergic
processes by suppressing T-helper 2 immune responses (46). The inflammation caused by H.
pylori can induce an imbalance in T helper cells between the
Th1- and Th2-types in the gastric mucosa (47). When stimulated ex vivo with
H. pylori, dendritic cells of peripheral blood (derived from
mononuclear cells) exhibit increased production of IL-12(48).
The differentiation to the Th1 phenotype was
determined based on the expression of IL-12A and IFN-γ which
constitute the most important cytokines produced by Th1 cells and
underlie the suppression of GATA-3 in T-cells (49); a result observed in the present study
as well in the patients with cancer. The suppression of GATA-3 may
be explained by the fact that the Helicobacter pylori
neutrophil-activating protein HP-NAP is an antagonist of TLR2 and
stimulates neutrophils and monocytes to produce inflammatory
cytokines associated with Th1 lymphocytes (50). Bagheri et al (51-53)
showed that in the evolution of gastric pathologies caused by H.
pylori infection, there is a dynamic change in the phenotypes
of inflammatory cells of the innate and adaptive immune system, as
well as in the underlying mechanisms of regulation and damage
repair, performed by cells of the immune system and their
mediators, with significant differences in the levels of expression
of inflammatory and anti-inflammatory cytokines between healthy
patients (no H. pylori infection) compared with patients
infected with H. pylori, and at different stages of gastric
infection.
To improve our understanding of the microenvironment
in the gastric tissues, Spearman's rank correlation coefficient
analysis was performed between various factors of the immune
response: Co-modulating molecules, cytokines, transcription factors
and variants of the virulent factors of H. pylori. In the
participants without cancer, there was a positive correlation
between ICOS-L and IL-17A. The latter cytokine represents the Th17
group, which is associated with the elimination of H. pylori
(54). This correlation did not exist
in the patients with cancer, perhaps due to the phenomenon
described by Downs (55); the
microenvironment of the tumor has a mechanism for evading the
immune response through the transdifferentiation of the lymphocytes
from a Th17+/Foxp3- phenotype to a
Th17+/Foxp3+ phenotype, resulting in an
increase in the population of Treg cells as well as
anti-inflammatory cytokines.
Unlike the patients without cancer, those with
cancer showed a positive correlation between ICOS-L and IL-10
(Treg) and IFN-γ (Th1). It has been reported that signaling through
ICOS-L can have a direct effect on dendritic cells via
phosphorylation of p38-MAPK and decrease the expression of
IL-10/FoxP3 in cell lines (56). In
the results of the present study, the high correlation between
IL-10 and ICOS-L observed in patients with cancer suggests a
possible alteration of the p38-MAPK pathway.
The expression of the transcription factor GATA3 was
significantly different between the two groups. The most notable
positive correlation of GATA 3 was with IL-4 (the primary cytokine
of the Th2 inflammatory phenotype) in the patients without cancer.
For the other transcription factors analyzed by RT-qPCR (RORγt and
Foxp3), the relative expression levels of their mRNA was lower in
the patients with cancer, but the difference was not significant. A
key correlation, reported for the first time in the present study,
to our knowledge, was the high negative correlation between the ROR
transcription factor and the m1 vacA polymorphism, additionally a
negative correlation between IL-17A and CagA was identified.
Establishing that both proteins interfere with the Th17
inflammatory profile activation axis. The previous result indicates
a possible synergy of both proteins to interfere with the axis of
activation of the Th17 inflammatory profile. The synergy between
CagA-VacA has only been documented to generate damage to gastric
cells and facilitate iron acquisition (57).
Additionally, there was a moderate negative
correlation between IL-17A and the oncoprotein CagA in the patients
with (but not without) cancer. The same inverse association has
been explained as a reduction in ICOS-L expression in gastric
epithelial cells through CagA-induced activation of the mTOR kinase
p70 S6 signaling pathway, associated with a reduction in Th17 cells
(19). The mechanism of evasion used
by H. pylori may be coordinated by the polymorphism of the
middle region of vacA (m1) and the presence of the
CagA protein to decrease the Th17 phenotype, and thus allow the
bacteria to survive and proliferate in the host. The genotypes of
H. pylori with these characteristics thus constitute an
increased risk of gastric cancer. A meta-analysis revealed that
m1 of vacA is the greatest risk factor for gastric
cancer (25).
In the participants without cancer, IL-17A exhibited
a high correlation with IL-10, TGF-β, Foxp3 and RORγt. Downs-Canner
et al (55) showed that Th17
lymphocytes are dependent on TGF-β for their differentiation.
Furthermore, plasticity has been described for the
transdifferentiation of Th17 cells to
Th17-IL-17neg-Foxp3+ cells, which is induced by the
microenvironment of tumoral tissue (55). The significant correlation shown
between IL-17A and Foxp3 in healthy tissue establishes a balance in
the inflammatory/anti-inflammatory response, whereas in patients
with cancer, the expression of Foxp3 with anti-inflammatory
function, predominates in the tumor microenvironment.
Similarly, in the patients without (but not with)
cancer, there a high positive correlation between RORγt and Foxp3.
There is an antagonism between tumor tissue and Foxp3 T-cells,
which has been reported for RORγt in mice (58). Recently it was documented that in the
microenvironment of tumor tissue, a reduced number of
Foxp3+ T-cells resulted in a change in the phenotype of
Foxp3+ T-cells by the gene of the master transcription
factor RORγt (55).
There was a very high correlation between TGF-β and
IFN-γ and a high correlation between TGF-β and IL-17A in the
patients without stomach cancer. The co-cultivation of
CD4+ T-cells and macrophages was previously demonstrated
to increase the secretion of IFN-γ and IL-17A. When infected with
H. pylori, these cells exhibited upregulated expression of
RORγt and an increase in the number of Th17 cells (59). The correlation between TGF-β and
RORγt, IL-17A, Foxp3 and IL-10 was only observed in the patients
without cancer. Additionally in the patients without cancer, a
correlation was also observed between TGF-β and the two
co-modulating molecules of the inflammatory response (ICOS-L and
PD-L1).
RORγt is the master transcription factor for the
differentiation of T-cells to Th17. Similarly, IL-6 and TGF-β
concomitantly serve an important roles in initiating
differentiation (58). It is thus
hypothesized that if a chronic infection results in the
transformation of tumor cells, the correlation between TGF-β and
cytokines, including transcription factors, may be lost. In the
patients with cancer in the present study, TGF-β was only
correlated with cytokines, and moderately with IFN-γ.
Of the polymorphisms of vacA, only the
m2 polymorphism showed a high positive correlation with
RORγt, IL-12A, IL-4 and the co-stimulatory molecule ICOS-L, in
patients with cancer. Notably, a meta-analysis showed an inverse
correlation between the vacA-m2 polymorphism and the
risk of gastric cancer (25). In the
present study, cytokines and transcription factors were positively
correlated with the vacA-m2 polymorphism, suggesting that
the cytokines were associated with elimination of H. pylori
and protection against gastric cancer.
In patients with gastric cancer, IL-10 was the
anti-inflammatory cytokine which exhibited the highest correlation
with the co-inhibitory molecule PD-L1 and with IL-12A; whereas in
the patients without cancer, IFN-γ and IL-17A exhibited the highest
correlations with IL-10.
According to a previous study, H. pylori
infection deregulates the expression of PD-L1, and the primary
cytokine secreted during infection (principally by dendritic cells)
is IL-10. The levels of IL-12A and IFN-γ are increased only if the
dendritic cells are treated with CD40L (60). H. pylori is capable of
stimulating IL-23 secretion, which belongs to the family of IL-12A,
and stimulates the production of IL-17 from TH-17 cells. However,
following prolonged stimulation, the capacity of dendritic cells to
produce IL-12A is reduced (61).
The results of the present study suggest that the
evasion of the immune system by H. pylori occurs
predominantly through the downregulation of the expression of
ICOS-L in individuals with gastric cancer. These results suggest a
coordination of the virulence factors cagA and vacA
of H. pylori (particularly the m1 polymorphism of
vacA) to inhibit the differentiation of cells to the
inflammatory Th17 phenotype.
In conclusion, the relative mRNA expression levels
of the co-activating molecules ICOS-L and GATA3 (the master
transcription factor for Th2 phenotype) were significantly lower in
patients with cancer compared with patients without cancer. To the
best of our knowledge, the present study is the first to show a
high inverse correlation between the m1 polymorphism of
vacA and the master transcription factor RORγt.
Additionally, a previously described negative correlation between
cagA and IL-17A was confirmed (55). Based on these results, it is
hypothesized that vacA-m1 and cagA coordinate the
inhibition of the inflammatory response of Th17 cells. There was no
correlation between TGF-β and other cytokines or transcription
factors in the patients with cancer. TGF-β and IL-6 are essential
for the differentiation of the Th17 phenotype. The present study
focused on correlations between the factors involved in the immune
system, H. pylori and gastric cancer, showing novel
associations of bacterial virulence factors and possible points of
modulation of the inflammatory response. These results may
highlight potential avenues for the design, diagnosis and
therapeutics in oncological pathologies
Acknowledgements
We would like to thank Dr Ricardo Leopoldo Guido
Bayardo of the Centro Médico Nacional 20 de Noviembre ISSSTE
(Ciudad de México) for providing the facilities to collect clinical
samples.
Funding
This study was funded by the Universidad Autónoma de
Aguascalientes (Institutional registration no. PIBB17-3) in
collaboration with the Centro Médico Nacional 20 de Noviembre
ISSSTE.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
The present study was performed by RGS and JGEC.
Patient management was performed by EVMC, JTL, MITR, LAWG and RGJJ.
Samples analysis was performed by MECV and JVJ. PCR analysis was
performed by JGEC, MHMO and MEVC. Statistics analysis was performed
by JELR and JGEC. The literature review, manuscript and references
was performed by JGEC and RGS. All authors have read and approved
the final version of the manuscript.
Ethics approval and consent to
participate
All patients provided written informed consent, in
accordance with the Helsinki Declaration and the Ethics Committee
of the National Medical Center (Centro Médico Nacional 20 de
November) of the ISSSTE Medical Service. The protocol used in the
present study was approved by the Bioethics Committee of the
Autonomous University of Aguascalientes (approval no.
CIB-UAA-26)
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Hooi JKY, Lai WY, Ng WK, Suen MMY,
Underwood FE, Tanyingoh D, Malfertheiner P, Graham DY, Wong VWS, Wu
JCY, et al: Global prevalence of helicobacter pylori
infection: Systematic review and meta-analysis. Gastroenterology.
153:420–429. 2017.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Wang C, Yuan Y and Hunt RH: The
association between Helicobacter pylori infection and early
gastric cancer: A meta-analysis. Am J Gastroenterol. 102:1789–1798.
2007.PubMed/NCBI View Article : Google Scholar
|
|
3
|
IARC Helicobacter pylori Working
Group: Helicobacter pylori eradication as a strategy for
preventing gastric cancer. IARC Working Group Reports, No. 8. IARC,
Lyon, 2014.
|
|
4
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Yakirevich E and Resnick MB: Pathology of
gastric cancer and its precursor lesions. Gastroenterol Clin North
Am. 42:261–284. 2013.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Salama NR, Hartung ML and Muller A: Life
in the human stomach: Persistence strategies of the bacterial
pathogen Helicobacter pylori. Nat Rev Microbiol. 11:385–399.
2013.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Cover TL and Blaser MJ: Helicobacter
pylori in health and disease. Gastroenterology. 136:1863–1873.
2009.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Kinlen LJ, Webster AD, Bird AG, Haile R,
Peto J, Soothill JF and Thompson RA: Prospective study of cancer in
patients with hypogammaglobulinaemia. Lancet. 1:263–266.
1985.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Salzer U, Maul-Pavicic A,
Cunningham-Rundles C, Urschel S, Belohradsky BH, Litzman J, Holm A,
Franco JL, Plebani A, Hammarstrom L, et al: ICOS deficiency in
patients with common variable immunodeficiency. Clin Immunol.
113:234–240. 2004.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Hutloff A, Dittrich AM, Beier KC,
Eljaschewitsch B, Kraft R, Anagnostopoulos I and Kroczek RA: ICOS
is an inducible T-cell co-stimulator structurally and functionally
related to CD28. Nature. 397:263–266. 1999.PubMed/NCBI View
Article : Google Scholar
|
|
11
|
Burugu S, Dancsok AR and Nielsen TO:
Emerging targets in cancer immunotherapy. Semin Cancer Biol.
52:39–52. 2018.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Wikenheiser DJ and Stumhofer JS: ICOS
Co-stimulation: Friend or foe? Front Immunol. 7(304)2016.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Wang Y, Wang H, Yao H, Li C, Fang JY and
Xu J: Regulation of PD-L1: Emerging routes for targeting tumor
immune evasion. Front Pharmacol. 9(536)2018.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Marzec M, Zhang Q, Goradia A, Raghunath
PN, Liu X, Paessler M, Wang HY, Wysocka M, Cheng M, Ruggeri BA and
Wasik MA: Oncogenic kinase NPM/ALK induces through STAT3 expression
of immunosuppressive protein CD274 (PD-L1, B7-H1). Proc Natl Acad
Sci USA. 105:20852–20857. 2008.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Noman MZ, Desantis G, Janji B, Hasmim M,
Karray S, Dessen P, Bronte V and Chouaib S: PD-L1 is a novel direct
target of HIF-1α, and its blockade under hypoxia enhanced
MDSC-mediated T cell activation. J Exp Med. 211:781–790.
2014.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Feng J, Yang H, Zhang Y, Wei H, Zhu Z, Zhu
B, Yang M, Cao W, Wang L and Wu Z: Tumor cell-derived lactate
induces TAZ-dependent upregulation of PD-L1 through GPR81 in human
lung cancer cells. Oncogene. 36:5829–5839. 2017.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Chang WL, Yeh YC and Sheu BS: The impacts
of H. pylori virulence factors on the development of
gastroduodenal diseases. J Biomed Sci. 25(68)2018.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Matos JI, de Sousa HA, Marcos-Pinto R and
Dinis-Ribeiro M: Helicobacter pylori CagA and VacA genotypes
and gastric phenotype: A meta-analysis. Eur J Gastroenterol
Hepatol. 25:1431–1441. 2013.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Lina TT, Pinchuk IV, House J, Yamaoka Y,
Graham DY, Beswick EJ and Reyes VE: CagA-dependent downregulation
of B7-H2 expression on gastric mucosa and inhibition of Th17
responses during Helicobacter pylori infection. J Immunol.
191:3838–3846. 2013.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Vacanti NM, Cheng H, Hill PS, Guerreiro
JD, Dang TT, Ma M, Watson S, Hwang NS, Langer R and Anderson DG:
Localized delivery of dexamethasone from electrospum fibers reduces
the foreign body response. Biomacromolecules. 13:3031–3038.
2012.PubMed/NCBI View Article : Google Scholar
|
|
21
|
de Figueiredo Soares T, de Magalhães
Queiroz DM, Mendes EN, Rocha GA, Rocha Oliveira AM, Alvares Cabral
MM and de Oliveira CA: The interrelationship between
helicobacter pylori vacuolating cytotoxin and gastric
carcinoma. Am J Gastroenterol. 93:1841–1847. 1998.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Sugimoto M, Zali MR and Yamaoka Y: The
association of vacA genotypes and Helicobacter
pylori-related gastroduodenal diseases in the middle east. Eur
J Clin Microbiol Infect Dis. 28:1227–1236. 2009.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Cover TL and Blanke SR: Helicobacter
pylori VacA, a paradigm for toxin multifunctionality. Nat Rev
Microbiol. 3:320–332. 2005.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Galmiche A, Rassow J, Doye A, Cagnol S,
Chambard JC, Contamin S, de Thillot V, Just I, Ricci V, Solcia E,
et al: The N-terminal 34 kDa fragment of Helicobacter pylori
vacuolating cytotoxin targets mitochondria and induces cytochrome c
release. EMBO J. 19:6361–6370. 2000.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Abdi E, Latifi-Navid S, Latifi-Navid H and
Safarnejad B: Helicobacter pylori vacuolating cytotoxin
genotypes and preneoplastic lesions or gastric cancer risk: A
meta-analysis. J Gastroenterol Hepatol. 31:734–744. 2016.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Sipponen P and Price AB: The sydney system
for classification of gastritis 20 years ago. J Gastroenterol
Hepatol. 1 (Suppl 26):31–34. 2011.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Luna LG: Manual of histologic staining
methods of the Armed Forces Institute of Pathology. Blakiston
Division, McGraw-Hill, New York, NY, 1968.
|
|
28
|
Sambrook J and Green MR: Molecular
cloning: A laboratory manuals. Cold Spring Harbor Laboratory Press,
Cold Spring Harbor, NY, 2012.
|
|
29
|
Rudi J, Kolb C, Maiwald M, Kuck D, Sieg A,
Galle PR and Stremmel W: Diversity of Helicobacter pylori
vacA and cagA genes and relationship to VacA and CagA protein
expression, cytotoxin production, and associated diseases. J Clin
Microbiol. 36:944–948. 1998.PubMed/NCBI
|
|
30
|
Wagih HM, El-Ageery SM and Alghaithy AA: A
study of RUNX3, E-cadherin and β-catenin in CagA-positive
Helicobacter pylori associated chronic gastritis in Saudi
patients. Eur Rev Med Pharmacol Sci. 19:1416–1429. 2015.PubMed/NCBI
|
|
31
|
Osman EY, El-Eragi AM, Musa AM, El-Magboul
SB, A/Rahman MB and Abdo AE: Detection of Helicobacter
pylori glmM gene in bovine milk using Nested polymerase chain
reaction. Vet World. 8:913–917. 2015.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Rhead JL, Letley DP, Mohammadi M, Hussein
N, Mohagheghi MA, Eshagh Hosseini M and Atherton JC: A new
Helicobacter pylori vacuolating cytotoxin determinant, the
intermediate region, is associated with gastric cancer.
Gastroenterology. 133:926–936. 2007.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Chomczynski P and Sacchi N: Single-step
method of RNA isolation by acid guanidinium
thiocyanate-phenol-chloroform extraction. Anal Biochem.
162:156–159. 1987.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Yilmaz V, Oflazer P, Aysal F, Durmus H,
Poulas K, Yentur SP, Gulsen-Parman Y, Tzartos S, Marx A, Tuzun E,
et al: Differential Cytokine Changes in Patients with Myasthenia
Gravis with Antibodies against AChR and MuSK. PLoS One.
10(e0123546)2015.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Moraes-Vieira PM, Takenaka MC, Silva HM,
Monteiro SM, Agena F, Lemos F, Saitovitch D, Kalil J and Coelho V:
GATA3 and a dominant regulatory gene expression profile
discriminate operational tolerance in human transplantation. Clin
Immunol. 142:117–126. 2012.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Schnopp C, Rad R, Weidinger A, Weidinger
S, Ring J, Eberlein B, Ollert M and Mempel M: Fox-P3-positive
regulatory T cells are present in the skin of generalized atopic
eczema patients and are not particularly affected by medium-dose
UVA1 therapy. Photodermatol Photoimmunol Photomed. 23:81–85.
2007.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Wang L, Qian J, Lu Y, Li H, Bao H, He D,
Liu Z, Zheng Y, He J, Li Y, et al: Immune evasion of mantle cell
lymphoma: Expression of B7-H1 leads to inhibited T-cell response to
and killing of tumor cells. Haematologica. 98:1458–1466.
2013.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Bao H, Lu P, Li Y, Wang L, Li H, He D,
Yang Y, Zhao Y, Yang L, Wang M, et al: Triggering of toll-like
receptor-4 in human multiple myeloma cells promotes proliferation
and alters cell responses to immune and chemotherapy drug attack.
Cancer Biol Ther. 11:58–67. 2011.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Scheller HV, Jensen JK, Sørensen SO and
Harholt J: Biosynthesis of pectin. Physiologia Plantarum.
129:283–295. 2006.
|
|
41
|
Mukaka MM: Statistics Corner: A guide to
appropriate use of Correlation coefficient in medical research.
Malawi Med J. 24:69–71. 2012.PubMed/NCBI
|
|
42
|
Amieva M and Peek RM Jr: Pathobiology of
Helicobacter pylori-induced gastric cancer.
Gastroenterology. 150:64–78. 2016.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Gonzalez-Valencia G, Atherton JC, Munoz O,
Dehesa M, la Garza AM and Torres J: Helicobacter pylori vacA
and cagA genotypes in mexican adults and childre. J Infect Dis.
182:1450–1454. 2000.PubMed/NCBI View
Article : Google Scholar
|
|
44
|
Liu C, Zhang Z and Zhu M: Immune responses
mediated by Th17 cells in Helicobacter pylori infection.
Integrative Med Int. 3:57–63. 2016.
|
|
45
|
Yu S, Kim HY, Chang YJ, DeKruyff RH and
Umetsu DT: Innate lymphoid cells and asthma. J Allergy Clin
Immunol. 133:943–950. 2014.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Brooks C, Pearce N and Douwes J: The
hygiene hypothesis in allergy and asthma: An update. Curr Opin
Allergy Clin Immunol. 13:70–77. 2013.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Kayhan B, Arasli M, Eren H, Aydemir S,
Kayhan B, Aktas E and Tekin I: Analysis of peripheral blood
lymphocyte phenotypes and Th1/Th2 cytokines profile in the systemic
immune responses of Helicobacter pylori infected
individuals. Microbiol Immunol. 52:531–538. 2008.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Guiney DG, Hasegawa P and Cole SP:
Helicobacter pylori preferentially induces interleukin 12
(IL-12) rather than IL-6 or IL-10 in human dendritic cells. Infect
Immun. 71:4163–4166. 2003.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Hafsi N, Voland P, Schwendy S, Rad R,
Reindl W, Gerhard M and Prinz C: Human dendritic cells respond to
Helicobacter pylori, promoting NK cell and Th1-effector
responses in vitro. J Immunol. 173:1249–1257. 2004.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Amedei A, Cappon A, Codolo G, Cabrelle A,
Polenghi A, Benagiano M, Tasca E, Azzurri A, D'Elios MM, Del Prete
G and de Bernard M: The neutrophil-activating protein of
Helicobacter pylori promotes Th1 immune responses. J Clin
Invest. 116:1092–1101. 2006.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Bagheri N, Razavi A, Pourgheysari B,
Azadegan-Dehkordi F, Rahimian G, Pirayesh A, Shafigh M,
Rafieian-Kopaei M, Fereidani R, Tahmasbi K and Shirzad H:
Up-regulated Th17 cell function is associated with increased peptic
ulcer disease in Helicobacter pylori-infection. Infect Genet
Evol. 60:117–125. 2018.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Bagheri N, Shirzad H, Elahi S,
Azadegan-Dehkordi F, Rahimian G, Shafigh M, Rashidii R, Sarafnejad
A, Rafieian-Kopaei M, Faridani R, et al: Downregulated regulatory T
cell function is associated with increased peptic ulcer in
Helicobacter pylori-infection. Microb Pathog. 110:165–175.
2017.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Bagheri N, Shirzad H, Mirzaei Y,
Nahid-Samiei M, Sanaei M, Rahimian G, Shafigh M, Zandi F, Tahmasbi
K and Razavi A: T-bet cells polarization in patients infected with
Helicobacter pylori increase the risk of peptic ulcer
development. Arch Med Res. 50:113–121. 2019.PubMed/NCBI View Article : Google Scholar
|
|
54
|
D'Elios MM and Czinn SJ: Immunity,
inflammation, and vaccines for Helicobacter pylori.
Helicobacter. 19 (Suppl 1):19–26. 2014.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Downs-Canner S, Berkey S, Delgoffe GM,
Edwards RP, Curiel T, Odunsi K, Bartlett DL and Obermajer N:
Suppressive IL-17A(+)Foxp3(+) and ex-Th17 IL-17A(neg)Foxp3(+) Treg
cells are a source of tumour-associated Treg cells. Nat Commun.
8(14649)2017.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Dianzani C, Minelli R, Gigliotti CL,
Occhipinti S, Giovarelli M, Conti L, Boggio E, Shivakumar Y,
Baldanzi G, Malacarne V, et al: B7h triggering inhibits the
migration of tumor cell lines. J Immunol. 192:4921–4931.
2014.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Tan S, Noto JM, Romero-Gallo J, Peek RM Jr
and Amieva MR: Helicobacter pylori perturbs iron trafficking
in the epithelium to grow on the cell surface. PLoS Pathog.
7(e1002050)2011.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Yang XO, Nurieva R, Martinez GJ, Kang HS,
Chung Y, Pappu BP, Shah B, Chang SH, Schluns KS, Watowich SS, et
al: Molecular antagonism and plasticity of regulatory and
inflammatory T cell programs. Immunity. 29:44–56. 2008.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Zhuang Y, Shi Y, Liu XF, Zhang JY, Liu T,
Fan X, Luo J, Wu C, Yu S, Chen L, et al: Helicobacter
pylori-infected macrophages induce Th17 cell differentiation.
Immunobiology. 216:200–207. 2011.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Das S, Suarez G, Beswick EJ, Sierra JC,
Graham DY and Reyes VE: Expression of B7-H1 on gastric epithelial
cells: Its potential role in regulating T cells during
Helicobacter pylori infection. J Immunol. 176:3000–3009.
2006.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Mitchell P, Germain C, Fiori PL, Khamri W,
Foster GR, Ghosh S, Lechler RI, Bamford KB and Lombardi G: Chronic
exposure to Helicobacter pylori impairs dendritic cell
function and inhibits Th1 development. Infect Immun. 75:810–819.
2007.PubMed/NCBI View Article : Google Scholar
|