Introduction
Immunotherapy has been established as the fourth
mode of cancer treatment with the advent of immune checkpoint
inhibitors, which have become new therapeutic targets for various
tumors such as malignant melanoma (1), non-small cell lung cancer (2,3), gastric
cancer (4,5), malignant mesothelioma (6) and Hodgkin's lymphoma (7). Recent reports have suggested the
clinical benefits of immune checkpoint inhibitors in combination
with chemotherapy (8,9). Therapeutic cancer vaccine immunotherapy
is a therapy that induces cellular immune responses against the
target molecule to elicit clinical anti-tumor effects (10). Although cancer vaccine immunotherapy
has not been established as a monotherapy, cancer vaccines may be
efficiently combined with other modalities, including immune
checkpoint inhibitors (10,11). It is essential to monitor cellular
immune responses against the target molecule to evaluate the
induction and maintenance of antigen-specific cellular immune
responses during the vaccination period; analysis of
antigen-specific cellular immune responses includes in vivo
testing and DTH skin reaction test as well as ex vivo tests
such as flow cytometric multimer, proliferation and enzyme-linked
immunospot (ELISPOT) assays (12).
The ELISPOT assay detects cytokine-producing cells in the
antigen-stimulation conditions. Therefore, it is possible to
analyze not only the frequency of antigen-specific immune cells,
but also the effector functions of immune cells as determined by
cytokine production/secretion (12).
In addition, the ELISPOT assay is adaptable to human leukocyte
antigen (HLA) class II binding helper T lymphocyte epitopes, for
which qualified multimers for flow cytometric assay are not
currently available to the best of our knowledge. This assay is
also capable of multi-sample measurement since the procedures are
simple and the assay is commonly performed in 96-well plates.
Therefore, the ELISPOT assay is widely used as a monitoring tool
for cellular immune response in clinical trials for infectious
diseases (13,14) and cancer immunotherapy (15-17).
The Wilms' tumor 1 (WT1) gene was originally
isolated as a gene responsible for pediatric kidney neoplasm and
had been regarded as a tumor suppressor gene (18). However, a number of researchers
consider the WT1 gene to serve an oncogenic role in leukemia
(19-22)
and a wide variety of solid tumors (23-25)
based on the results reported by our group and other groups
(26-28).
The WT1 protein is highly immunogenic (29). Immunotherapies targeting WT1 have been
developed in a number of countries as novel, promising therapeutic
strategies for various types of cancer such as leukemia,
glioblastoma and pancreatic cancer (30-39).
The aim of the present study was to test a simple
Reader-free ELISPOT assay method for reproducibility and apply it
to the analysis of cytokine production/secretion of PBMCs in
healthy volunteers and patients with cancer, including those who
were treated with WT1 peptide-based vaccine immunotherapy.
Materials and methods
Peripheral blood mononuclear cells
(PBMCs)
PBMCs were obtained with written informed consent
from 17 patients with cancer (12 male and 5 female; median age, 45
years; age range, 21-72 years) and six healthy individuals (2 male
and 4 female; median age, 24 years; age range, 23-52 years). The
types of cancer included seven cases of glioblastoma, seven cases
of anaplastic glioma, one case of lung cancer, one case of salivary
gland cancer and one case of rhabdomyosarcoma. Of the 17 patients,
one patient with lung cancer and two patients with salivary gland
cancer and glioblastoma were enrolled in clinical trials of WT1
peptide vaccine cancer immunotherapy registered as UMIN#000002001
and UMIN#000023579, respectively. In the clinical trials, WT1
peptide vaccine was administered weekly (40) or biweekly for three months. Peripheral
blood was collected before and one, two, and three months after the
initiation of the treatment. PBMCs were isolated from heparinized
whole blood using the Ficoll-Paque method (GE Healthcare) according
to the manufacturer's instructions and cryopreserved in liquid
nitrogen until use. The present study was performed under the
approval of the Ethical Review Board of the Faculty of Medicine,
Osaka University (Suita, Japan).
Peptide synthesis
Peptides for the ELISPOT assay were synthesized by
PH Japan. The amino acid sequences were as follows: WT1-235
peptide, CMTWNQMNL; WT1-126 peptide, RMFPNAPYL.
ELISPOT assay
Following hydrophilization treatment with 35%
ethanol for 1 min and three washes with PBS, a membrane of each
well in a 96-well filtration plate (Merck KGaA) was incubated with
capture antibodies, anti-human interferon-γ (IFN-γ) monoclonal
antibody (cat. no. 3420-3-250; Mabtech AB; final concentration, 15
µg/ml in PBS) and anti-human tumor necrosis factor-α (TNF-α)
monoclonal antibody (cat. no. 3510-3-250; Mabtech AB; final
concentration, 7.5 µg/ml in PBS) at 4˚C overnight. Following four
washes with PBS, the membrane was incubated with 200 µl 1X Blocking
one (cat. no. 03953-95; Nacalai Tesque, Inc.) for 2 h and washed
three times with PBS. Thawed PBMCs were suspended in FBS-free
RPMI-1640 medium (Nacalai Tesque, Inc.) and 5x104 cells
per 100 µl were seeded in each well in triplicate and incubated
with 5% CO2 in a humidified atmosphere at 37˚C for 48 h.
To stimulate PBMCs, an antigen peptide was added to each well at a
final concentration of 10 µg/ml. Following removal of the cell
suspension, each membrane was washed with 200 µl PBS containing
0.05% Tween-20 for 10 min and treated with 100 µl ACCUMAX™
(Sigma-Aldrich; Merck KGaA) at room temperature for 15 min with
gentle agitation. After three washes with PBS containing 0.05%
Tween-20, each membrane was incubated at 4˚C overnight with the
corresponding detection antibodies in PBS containing 1% BSA and
0.05% Tween 20: Biotinylated anti-human IFN-γ monoclonal antibody
(cat. no. 3420-6-250; Mabtech AB; final concentration, 3 µg/ml) and
biotinylated-anti-human TNF-α monoclonal antibody (cat. no.
3510-6-250; Mabtech AB; final concentration, 1.5 µg/ml). Following
four washes with PBS, each membrane was incubated with alkaline
phosphatase-conjugated streptavidin (cat. no. 3310-8; Mabtech AB;
diluted 1:500 with 0.05% Tween-20 in phosphate buffered saline
without magnesium and calcium [PBS (-)]) at room temperature for 1
h. After washing both sides of the membranes with deionized water
for 3 min, the spots were stained with BCIP/NBT solution (Nacalai
Tesque, Inc.) for 3 min followed by washing with deionized water.
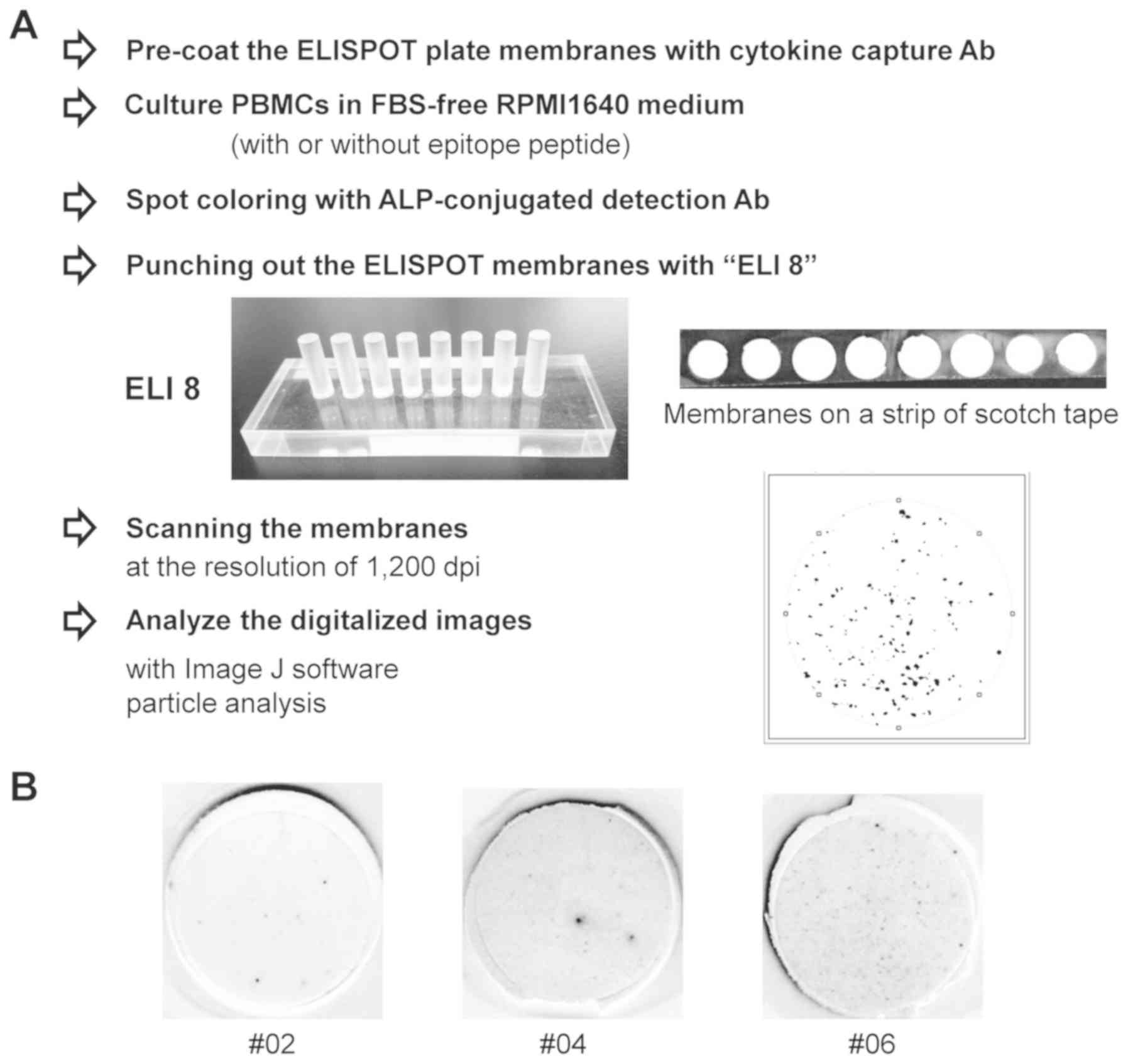
Following drying at 4˚C overnight, a strip of clear adhesive tape
was attached to the back of the membranes of 8 wells in a single
row. The membranes were punched out with an acrylic device ELI 8
(Create Ltd.). The membranes were subsequently sandwiched a second
strip of adhesive tape and scanned at the resolution of 1,200 dpi.
The generated digital images were analyzed by spot counting using
particle analysis by ImageJ 1.45 software (National Institutes of
Health) (Fig. 1).
In concordance analysis, two different examiners
with the experience of ELISPOT assay of >6 months performed spot
counting with the assistance of ImageJ software. Scanned images of
colored membranes from six wells of ELISPOT assay with variable
numbers of spots were used. Examiner-1 performed spot counting of
six images on two different days for analysis of inter-assay
concordance.
Statistical analysis
Difference in IFN-γ and TNF-α secretion by PBMCs
between patients with cancer and healthy individuals was analyzed
by Welch's t-test using Statcel 3 software (OMS Publisher).
Individual values are presented. P<0.05 was considered to
indicate a statistically significant difference.
Results
Inter-assay and inter-examiner
concordance of the ImageJ software-assisted spot counting step in
the ELISPOT assay
First, a reader-free ELIPSOT assay was developed
(Fig. 1). For the membrane
preparation step, the present study developed a handy acrylic
punching device ELI 8. With this device, the membranes of eight
wells were easily punched out at once in an array on a strip of
adhesive tape. Since the counting reproducibility of spot numbers
is an important factor in the ELISPOT assay, the inter-assay and
inter-examiner concordances in the spot-counting step of the
ELISPOT assay were examined using six sample membranes with a
variable number of spots. The number of spots detected on each
sample membrane was scored as follows: -, no spot; 1+, 1-9 spots;
2+, 10-29 spots; 3+, 30-89 spots; 4+, ≥90 spots. Concordance was
defined as follows: i) Scores for one sample judged by one examiner
on different days or by two or more examiners are identical; ii)
scores for one sample are different, but are in a range across the
border number between two score categories. For example, 5-15
(10±5) spots, 1+ and 2+; 20-40 (30±10) spots, 2+ and 3+; 75-105
(90±15) spots, 3+ and 4+.
First, inter-assay concordance was examined by
Examiner 1. As presented in Table I,
the scores judged on two different days were identical in all six
examined samples. Subsequently, inter-examiner concordance was
examined. The scores judged by Examiner 2 were also identical in
all six examined samples (Table I).
These results indicated that ImageJ software-assisted spot counting
was reproducible with good inter-assay and inter-examiner
concordance.
 | Table IInter-assay and inter-examiner
concordance of spot counting. |
Table I
Inter-assay and inter-examiner
concordance of spot counting.
| | Score (spot
count) |
|---|
| Sample no. | Examiner 1 Day
1 | Examiner 1 Day
8 | Examiner 2 Day
1 |
|---|
| 1 | 1+ (9) | 1+ (8) | 1+ (8) |
| 2 | 2+ (12) | 2+ (17) | 2+ (13) |
| 3 | 2+ (29) | 2+ (27) | 2+ (16) |
| 4 | 3+ (36) | 3+ (37) | 3+ (33) |
| 5 | 3+ (50) | 3+ (50) | 3+ (40) |
| 6 | 4+ (176) | 4+ (143) | 4+ (152) |
Increased spontaneous
production/secretion of Th1 type cytokines by PBMCs in patients
with cancer
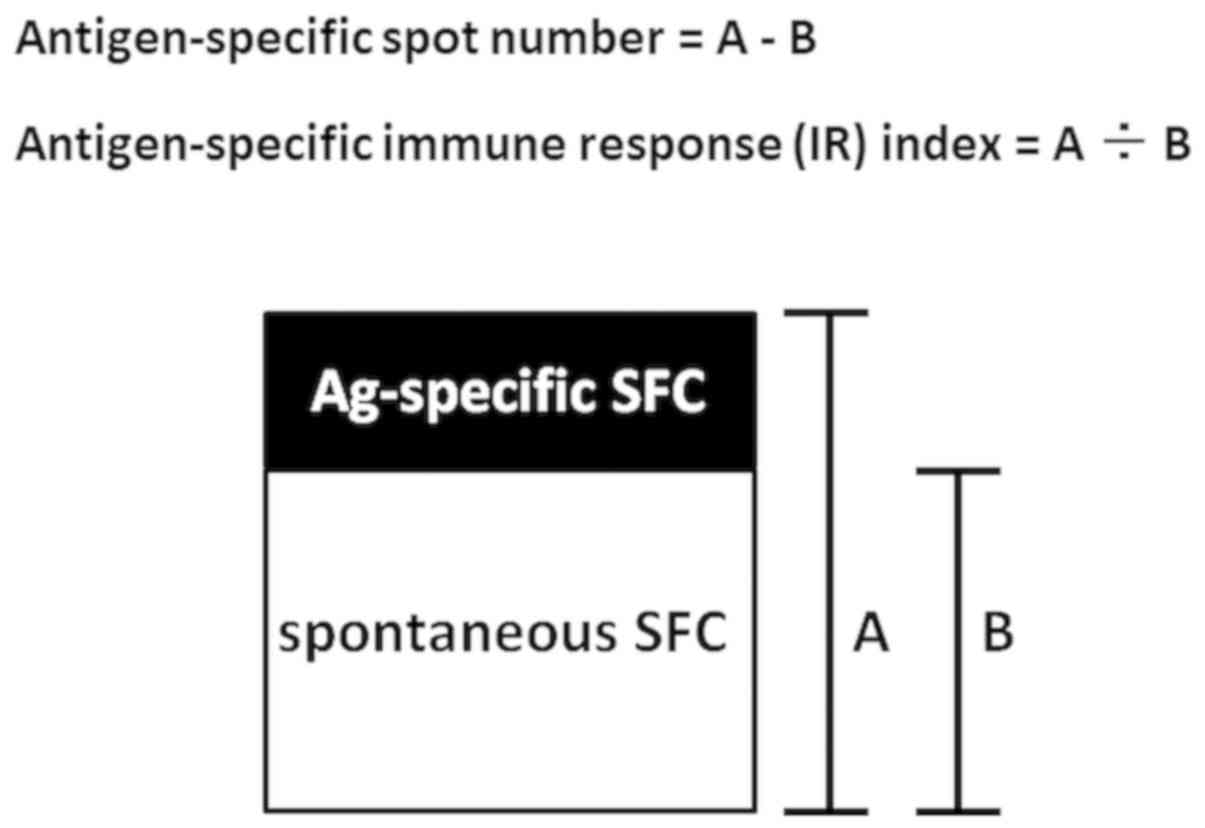
Counts of spot-forming cells (SFCs) are defined as
the number of SFCs in the respective antigen-stimulated test
conditions minus the number of SFCs in antigen-free control
conditions. Thus, spontaneous production and secretion of Th1 type
cytokines IFN-γ and TNF-α by PBMCs in the absence of antigen
peptides was analyzed by the ELISPOT assay in 17 patients with
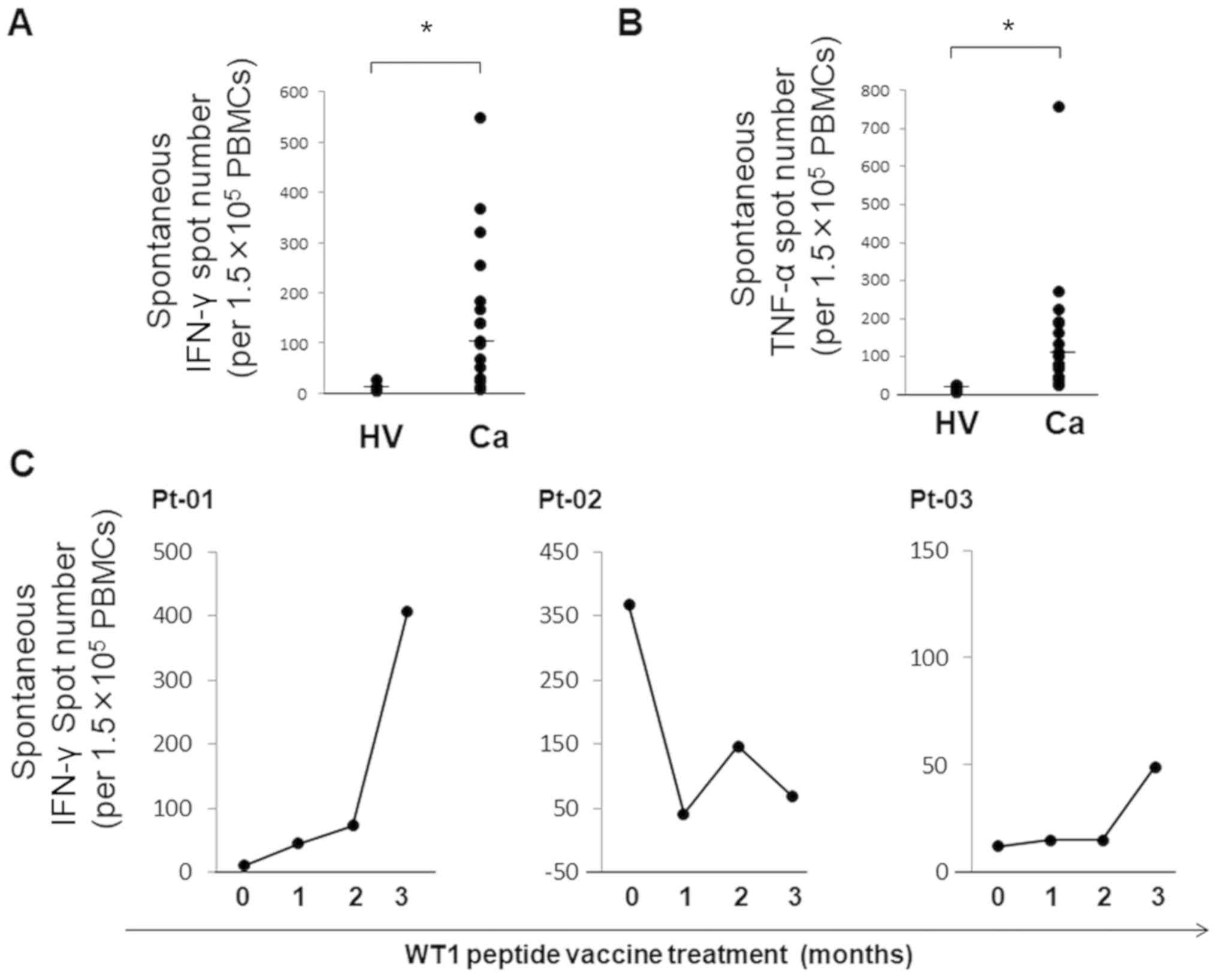
cancer and six healthy subjects. The numbers of cells that
spontaneously produced IFN-γ and TNF-α were between 8 and 548
(median, 103) and between 23 and 756 (median, 100), respectively,
per 1.5x105 PBMCs in 17 patients. By contrast, the
numbers of IFN-γ and TNF-α producing cells in healthy subjects were
between 5 and 28 (median, 7) and between 5 and 26 (median, 11.5),
respectively, per 1.5x105 PBMCs (Fig. 2A). To investigate the spontaneous
cytokine secretion by immune cells in cancer vaccine-treated
patients, spontaneous production of IFN-γ by PBMCs was analyzed in
patients with cancer treated with WT1 peptide vaccine cancer
immunotherapy at different time points during three months of
treatment. Pt-01 was a patient with lung cancer treated with the
WT1-235 peptide vaccine 12 times. Pt-02 and Pt-03 were patients
with salivary gland cancer and glioblastoma, respectively, who were
treated with WT1 Trio peptide vaccine composed of three WT1
peptides including WT1-126 and WT1-235 seven times. WT1-126 and
WT1-235 are HLA class I-binding CTL peptides specific for
HLA-A*02:01 and HLA-A* 24:02, respectively.
SFCs with spontaneous secretion of IFN-γ increased 40.7- and
4.1-fold in two patients, but decreased 0.2-fold in one patient
after three months of WT1 peptide vaccine cancer immunotherapy
(Fig. 2B).
These results indicated that the changes in the
numbers of spontaneous cytokine-producing immune cells should be
taken into consideration in the monitoring of antigen-specific
cellular immune responses.
Antigen-specific immune response (IR)
index as a marker for antigen-specific cellular immune
response
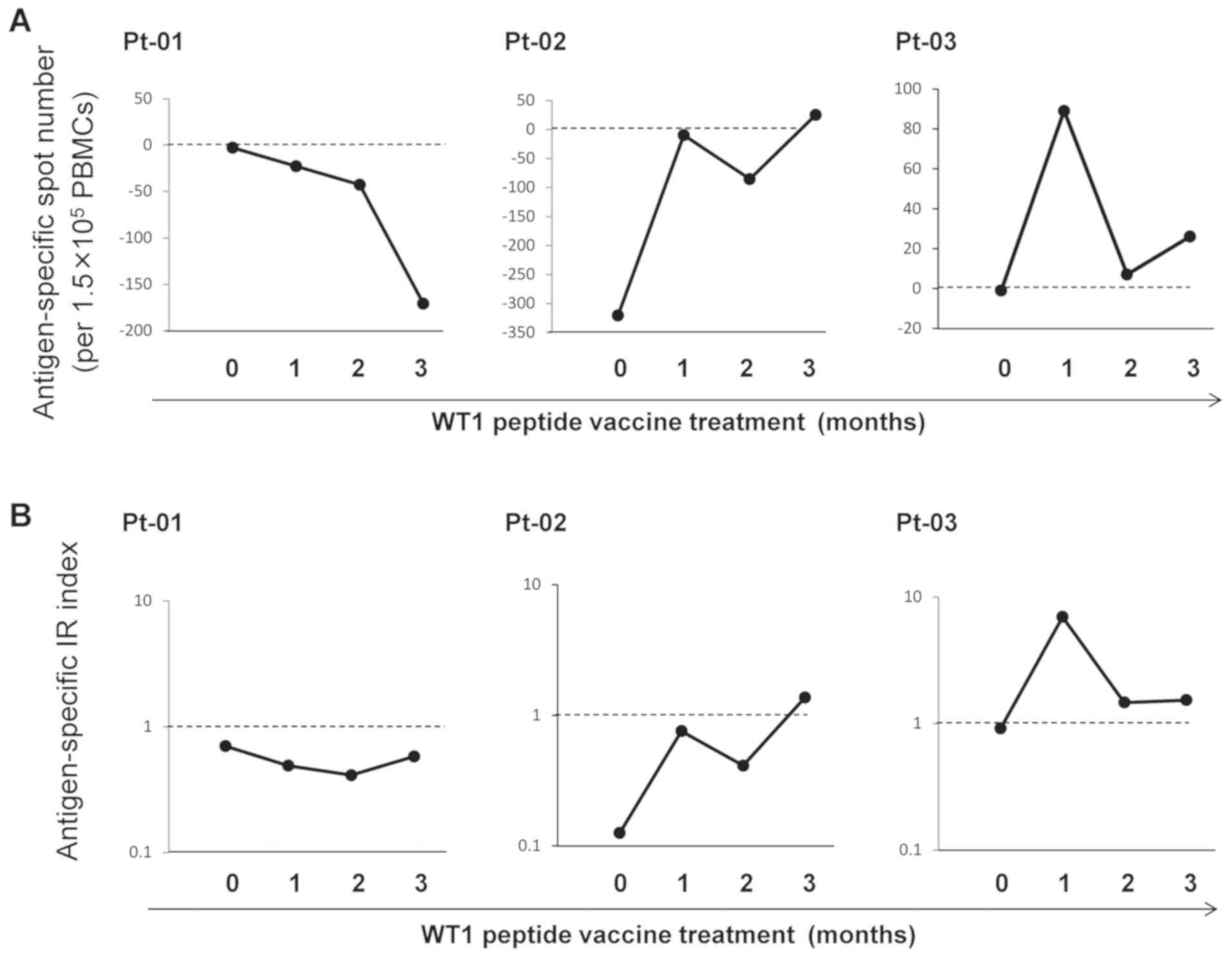
Secretion of IFN-γ by PBMCs was analyzed by ELISPOT
assay in three patients with cancer at the indicated time points
during three months of WT1 peptide vaccine cancer immunotherapy.
WT1-235 and WT1-126 peptides were used for antigen-stimulation of
HLA-A*24:02 patients (Pt-01 and Pt-03) and
HLA-A*02:01 patient (Pt-02), respectively. First, WT1
antigen-specific IFN-γ secretion by PBMCs was described as
antigen-specific spot number: (Number of SFCs in antigen-stimulated
test conditions)-(number of SFCs in antigen-free control
conditions) (Fig. 3). As presented in
Fig. 3A, masked by the number of
spontaneous cytokine-producing cells, antigen-specific
IFN-γ-secreting spot numbers became negative at multiple time
points. In addition, the number of SFCs provided no information
about the frequency of antigen-specific cytokine-secreting cells in
the total pool of cytokine-producing cells. Therefore, when the
number of antigen-specific SFCs increased after vaccine
immunotherapy, it remained unclear whether this increase was due to
antigen-non-specific effects or the induction of antigen-specific
cellular immune responses. Therefore, WT1 antigen-specific IFN-γ
secretion by PBMCs was also described as antigen-specific IR index:
(Number of SFCs in antigen-stimulated test conditions)/(number of
SFCs in antigen-free, control conditions). With this index, the
direction of the cellular immune responses to the targeted antigen
WT1 was successfully detected (Fig.
3B).
Discussion
In the present study, a reader-free ELISPOT assay
was developed using a membrane-punching device ELI 8. Using
particle analysis by ImageJ, the results of spot counting were
reproducible with good inter-assay and inter-examiner concordance.
ELISPOT analysis demonstrated that immune cells that produced and
secreted Th1 cytokines without antigen-peptide stimulation were
present in PBMCs, and that their frequencies in patients with
cancer were significantly higher compared with those in healthy
individuals. These frequencies varied between individuals or time
points during the course of cancer vaccine immunotherapy. Due to
the variability in spontaneous cytokine production/secretion by
PBMCs, the present study proposed an antigen-specific IR index
rather than the number of spot-forming cells as a marker for the
cellular immune responses in patients treated with cancer vaccine
immunotherapy. This index successfully detected the induction of
WT1-specific cellular immune responses in patients with cancer
treated with WT1 peptide vaccine immunotherapy.
The ELISPOT assay is performed for various
immuno-monitoring purposes including clinical trials for infectious
diseases (13,14) and cancer immunotherapy (15-17).
For reader-free ELISPOT assay, the preparation of ELISPOT membrane
for spot counting can be a time-consuming process. A single-well
punch kit, ELIPUNCH (EMD Millipore) is not currently available. To
the best of our knowledge, Eli.Punch (A.EL.VIS GmbH) is the only
available punching tool for the ELISPOT assay. In addition, since
Eli.Punch is a device specifically designed for 96-well punching,
it does not allow flexibility in well numbers. In the present
study, a handy acrylic punching device ELI 8 was developed. With
this device, membranes of eight wells may be easily punched out at
once in an array on a strip of adhesive tape, allowing increased
flexibility in the scale of the assay compared with commercially
available methods. In addition, punching with ELI 8 is economical
due to minimal requirements such as adhesive tape.
In the present study, digital images of scanned
membranes were converted to binary images and analyzed using free
ImageJ particle analysis software provided by the National
Institutes of Health. In addition to saving labor by
semi-automation, analysis using ImageJ demonstrated that spot
counting in the ELISPOT assay achieved high inter-assay and
inter-examiner concordance. In the spot counting process, the
setting of the threshold for determining a spot is a crucial step;
as an examiner typically sets the threshold value, recording this
value makes the analysis process traceable. Therefore, the
threshold setting data may be useful for education purposes to
match the criteria of threshold setting among multiple examiners,
including beginners.
In the ELISPOT assay, antigen-specific cytokine
secretion of immune cells is often reported as the number of SFCs
in the respective antigen-stimulated test conditions minus the
number of SFCs in antigen-free control conditions (41,42). The
results of the present clearly demonstrated that there is a
statistically significant difference between spontaneous cytokine
production/secretion in patients with cancer and healthy
individuals, and that spontaneous cytokine production/secretion
changed over time in the three patients treated with WT1 peptide
vaccine. Despite a small sample size, these results demonstrated
that spontaneous production/secretion of cytokines in immune cells
varied between individuals and over time during the course of
cancer vaccine immunotherapy. Since the number of antigen-specific
SFCs does not provide information about the frequency of
antigen-specific cytokine-secreting cells in the total pool of
cytokine-producing cells, changes in the number of spontaneous
cytokine-producing immune cells should be taken into consideration
in monitoring antigen-specific cellular immune responses. In
addition, detection of antigen-specific cytokine secretion by
immune cells in an ELISPOT assay may be better reported not only in
terms of antigen-specific SFC numbers, but additionally with regard
to a supplementary antigen-specific IR index (Fig. 4). One advantage of the
antigen-specific IR index is its robustness in measuring error as
an indicator of antigen-specific cytokine secretion. The number of
spontaneous IFN-γ-secreting PBMCs changes widely even within the
same patient. As demonstrated by the concordance analysis in the
present study, as the number of spots increases, it is expected
that the measurement error also increases. Assuming that the true
numbers of SFCs for antigen-stimulation and antigen-free control
conditions are 160 and 150, respectively, with a measurement error
of 10%, SFCs for the two conditions would be counted as 154-176 and
135-165, respectively. Thus, antigen-specific cytokine secretion
would be reported as between -11 and 41 with respect to
antigen-specific SFC number, which is a wide range of variation;
however, it would be reported as between 0.93 and 1.303 with
respect to antigen-specific IR index. This simulation indicates
that the antigen-specific IR index may be more resistant to
measurement error compared with the antigen-specific SFC number as
an indicator of antigen-specific cytokine secretion.
Acknowledgements
Not applicable.
Funding
This work was supported in part by Grants-in-Aid for
Scientific Research from the Ministry of Education, Culture,
Sports, Science and Technology, Japan (grant no.
KAKENHI#15K09476).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
YuO, SH, SM, FF and HS contributed to the study
conception and design. SH, RI, MA and SI performed the ELISPOT
analysis. YuO, JN, SN, AT, NH, HN, KH and YoO contributed to the
acquisition of the patient samples and conception of the study. YuO
and SH drafted the manuscript. All authors have read and approved
the final manuscript.
Ethics approval and consent to
participate
Blood samples were obtained with written informed
consent. The present study was approved by the Ethics Committee of
Osaka University Hospital (approval nos. 13110 and 11293).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Hamid O, Robert C, Daud A, Hodi FS, Hwu
WJ, Kefford R, Wolchok JD, Hersey P, Joseph RW, Weber JS, et al:
Safety and tumor responses with lambrolizumab (anti-PD-1) in
melanoma. N Engl J Med. 369:134–144. 2013.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Brahmer J, Reckamp KL, Baas P, Crinò L,
Eberhardt WE, Poddubskaya E, Antonia S, Pluzanski A, Vokes EE,
Holgado E, et al: Nivolumab versus docetaxel in advanced
squamous-cell non-small-cell lung cancer. N Engl J Med.
373:123–135. 2015.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Carbone DP, Reck M, Paz-Ares L, Creelan B,
Horn L, Steins M, Felip E, van den Heuvel MM, Ciuleanu TE, Badin F,
et al: First-line nivolumab in stage IV or recurrent non-small-cell
lung cancer. N Engl J Med. 376:2415–2426. 2017.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Togasaki K, Sukawa Y, Kanai T and Takaishi
H: Clinical efficacy of immune checkpoint inhibitors in the
treatment of unresectable advanced or recurrent gastric cancer: An
evidence-based review of therapies. Onco Targets Ther.
11:8239–8250. 2018.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Kelly RJ: Immunotherapy for esophageal and
gastric cancer. Am Soc Clin Oncol Educ Book. 37:292–300.
2017.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Forde PM, Scherpereel A and Tsao AS: Use
of immune checkpoint inhibitors in mesothelioma. Curr Treat Options
Oncol. 20(18)2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Matsuki E and Younes A: Checkpoint
inhibitors and other immune therapies for hodgkin and non-hodgkin
lymphoma. Curr Treat Options Oncol. 17(31)2016.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Addeo A, Banna GL, Metro G and Di Maio M:
Chemotherapy in combination with immune checkpoint inhibitors for
the first-line treatment of patients with advanced non-small cell
lung cancer: A systematic review and literature-based
meta-analysis. Front Oncol. 9(264)2019.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Gamerith G, Kocher F, Rudzki J and Pircher
A: ASCO. 2018 NSCLC highlights-combination therapy is key. Memo.
11:266–271. 2018.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Tran T, Blanc C, Granier C, Saldmann A,
Tanchot C and Tartour E: Therapeutic cancer vaccine: Building the
future from lessons of the past. Semin Immunopathol. 41:69–85.
2019.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Hu Z, Ott PA and Wu CJ: Towards
personalized, tumour-specific, therapeutic vaccines for cancer. Nat
Rev Immunol. 18:168–182. 2018.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Lamano JB, Ampie L, Choy W, Kesavabhotla
K, DiDomenico JD, Oyon DE, Parsa AT and Bloch O: Immunomonitoring
in glioma immunotherapy: Current status and future perspectives. J
Neurooncol. 127:1–13. 2016.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Ogunjimi B, Smits E, Hens N, Hens A,
Lenders K, Ieven M, Van Tendeloo V, Van Damme P and Beutels P:
Exploring the impact of exposure to primary varicella in children
on varicella-zoster virus immunity of parents. Viral Immunol.
24:151–157. 2011.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Blume J, Kostler J and Weissert R: Benefit
of ELISpot in early diagnosis of tuberculous meningoencephalitis:
Case report and literature review. eNeurologicalSci. 1:51–53.
2015.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Butterfield LH: The society for
immunotherapy of cancer biomarkers task force recommendations
review. Semin Cancer Biol. 52:12–15. 2018.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Smith SG, Harris SA, Satti I, Bryan D,
Walker KB, Dockrell HM, McShane H and Ho MM: Assay optimisation and
technology transfer for multi-site immuno-monitoring in vaccine
trials. PLoS One. 12(e0184391)2017.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Scheibenbogen C, Romero P, Rivoltini L,
Herr W, Schmittel A, Cerottini JC, Woelfel T, Eggermont AM and
Keilholz U: Quantitation of antigen-reactive T cells in peripheral
blood by IFNgamma-ELISPOT assay and chromium-release assay: A
four-centre comparative trial. J Immunol Methods. 244:81–89.
2000.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Call KM, Glaser T, Ito CY, Buckler AJ,
Pelletier J, Haber DA, Rose EA, Kral A, Yeger H and Lewis WH:
Isolation and characterization of a zinc finger polypeptide gene at
the human chromosome 11 Wilms' tumor locus. Cell. 60:509–520.
1990.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Inoue K, Sugiyama H, Ogawa H, Nakagawa M,
Yamagami T, Miwa H, Kita K, Hiraoka A, Masaoka T and Nasu K: WT1 as
a new prognostic factor and a new marker for the detection of
minimal residual disease in acute leukemia. Blood. 84:3071–3079.
1994.PubMed/NCBI
|
|
20
|
Inoue K, Tamaki H, Ogawa H, Oka Y, Soma T,
Tatekawa T, Oji Y, Tsuboi A, Kim EH, Kawakami M, et al: Wilms'
tumor gene (WT1) competes with differentiation-inducing signal in
hematopoietic progenitor cells. Blood. 91:2969–2976.
1998.PubMed/NCBI
|
|
21
|
Ito K, Oji Y, Tatsumi N, Shimizu S, Kanai
Y, Nakazawa T, Asada M, Jomgeow T, Aoyagi S, Nakano Y, et al:
Antiapoptotic function of 17AA(+)WT1 (Wilms' tumor gene) isoforms
on the intrinsic apoptosis pathway. Oncogene. 25:4217–4229.
2006.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Algar EM, Khromykh T, Smith SI, Blackburn
DM, Bryson GJ and Smith PJ: A WT1 antisense oligonucleotide
inhibits proliferation and induces apoptosis in myeloid leukaemia
cell lines. Oncogene. 12:1005–1014. 1996.PubMed/NCBI
|
|
23
|
Oji Y, Miyoshi S, Maeda H, Hayashi S,
Tamaki H, Nakatsuka S, Yao M, Takahashi E, Nakano Y, Hirabayashi H,
et al: Overexpression of the Wilms' tumor gene WT1 in de novo lung
cancers. Int J Cancer. 100:297–303. 2002.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Oji Y, Suzuki T, Nakano Y, Maruno M,
Nakatsuka S, Jomgeow T, Abeno S, Tatsumi N, Yokota A, Aoyagi S, et
al: Overexpression of the Wilms' tumor gene W T1 in primary
astrocytic tumors. Cancer Sci. 95:822–827. 2004.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Kanai T, Ito Z, Oji Y, Suka M, Nishida S,
Takakura K, Kajihara M, Saruta M, Fujioka S, Misawa T, et al:
Prognostic significance of Wilms' tumor 1 expression in patients
with pancreatic ductal adenocarcinoma. Oncol Lett. 16:2682–2692.
2018.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Jomgeow T, Oji Y, Tsuji N, Ikeda Y, Ito K,
Tsuda A, Nakazawa T, Tatsumi N, Sakaguchi N, Takashima S, et al:
Wilms' tumor gene WT1 17AA(-)/KTS(-) isoform induces morphological
changes and promotes cell migration and invasion in vitro. Cancer
Sci. 97:259–270. 2006.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Tatsumi N, Oji Y, Tsuji N, Tsuda A,
Higashio M, Aoyagi S, Fukuda I, Ito K, Nakamura J, Takashima S, et
al: Wilms' tumor gene WT1-shRNA as a potent apoptosis-inducing
agent for solid tumors. Int J Oncol. 32:701–711. 2008.PubMed/NCBI
|
|
28
|
Wagner KD, Wagner N, Wellmann S, Schley G,
Bondke A, Theres H and Scholz H: Oxygen-regulated expression of the
Wilms' tumor suppressor Wt1 involves hypoxia-inducible factor-1
(HIF-1). FASEB J. 17:1364–1366. 2003.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Oka Y, Udaka K, Tsuboi A, Elisseeva OA,
Ogawa H, Aozasa K, Kishimoto T and Sugiyama H: Cancer Immunotherapy
targeting Wilms' tumor gene WT1 product. J Immunol. 164:1873–1880.
2000.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Gao L, Bellantuono I, Elsässer A, Marley
SB, Gordon MY, Goldman JM and Stauss HJ: Selective elimination of
leukemic CD34(+) progenitor cells by cytotoxic T lymphocytes
specific for WT1. Blood. 95:2198–2203. 2000.PubMed/NCBI
|
|
31
|
Oka Y, Tsuboi A, Taguchi T, Osaki T, Kyo
T, Nakajima H, Elisseeva OA, Oji Y, Kawakami M, Ikegame K, et al:
Induction of WT1 (Wilms' tumor gene)-specific cytotoxic T
lymphocytes by WT1 peptide vaccine and the resultant cancer
regression. Proc Natl Acad Sci USA. 101:13885–13890.
2004.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Tawara I, Kageyama S, Miyahara Y, Fujiwara
H, Nishida T, Akatsuka Y, Ikeda H, Tanimoto K, Terakura S, Murata
M, et al: Safety and persistence of WT1-specific T-cell receptor
gene-transduced lymphocytes in patients with AML and MDS. Blood.
130:1985–1994. 2017.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Keilholz U, Letsch A, Busse A, Asemissen
AM, Bauer S, Blau IW, Hofmann WK, Uharek L, Thiel E and
Scheibenbogen C: A clinical and immunologic phase 2 trial of Wilms
tumor gene product 1 (WT1) peptide vaccination in patients with AML
and MDS. Blood. 113:6541–6548. 2009.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Anguille S, Van de Velde AL, Smits EL, Van
Tendeloo VF, Juliusson G, Cools N, Nijs G, Stein B, Lion E, Van
Driessche A, et al: Dendritic cell vaccination as postremission
treatment to prevent or delay relapse in acute myeloid leukemia.
Blood. 130:1713–1721. 2017.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Van Tendeloo VF, Van de Velde A, Van
Driessche A, Cools N, Anguille S, Ladell K, Gostick E, Vermeulen K,
Pieters K, Nijs G, et al: Induction of complete and molecular
remissions in acute myeloid leukemia by Wilms' tumor 1
antigen-targeted dendritic cell vaccination. Proc Natl Acad Sci
USA. 107:13824–13829. 2010.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Oji Y, Oka Y, Nishida S, Tsuboi A,
Kawakami M, Shirakata T, Takahashi K, Murao A, Nakajima H, Narita
M, et al: WT1 peptide vaccine induces reduction in minimal residual
disease in an imatinib-treated CML patient. Eur J Haematol.
85:358–360. 2010.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Nishida S, Ishikawa T, Egawa S, Koido S,
Yanagimoto H, Ishii J, Kanno Y, Kokura S, Yasuda H, Oba MS, et al:
Combination gemcitabine and WT1 peptide vaccination improves
progression-free survival in advanced pancreatic ductal
adenocarcinoma: A phase II randomized study. Cancer Immunol Res.
6:320–331. 2018.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Oji Y, Inoue M, Takeda Y, Hosen N,
Shintani Y, Kawakami M, Harada T, Murakami Y, Iwai M, Fukuda M, et
al: WT1 peptide-based immunotherapy for advanced thymic epithelial
malignancies. Int J Cancer. 142:2375–2382. 2018.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Zauderer MG, Tsao AS, Dao T, Panageas K,
Lai WV, Rimner A, Rusch VW, Adusumilli PS, Ginsberg MS, Gomez D, et
al: A randomized phase ii trial of adjuvant galinpepimut-S, WT-1
analogue peptide vaccine, after multimodality therapy for patients
with malignant pleural mesothelioma. Clin Cancer Res. 23:7483–7489.
2017.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Oji Y, Hashimoto N, Tsuboi A, Murakami Y,
Iwai M, Kagawa N, Chiba Y, Izumoto S, Elisseeva O, Ichinohasama R,
et al: Association of WT1 IgG antibody against WT1 peptide with
prolonged survival in glioblastoma multiforme patients vaccinated
with WT1 peptide. Int J Cancer. 139:1391–1401. 2016.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Cox MC, Castiello L, Mattei M, Santodonato
L, D'Agostino G, Muraro E, Martorelli D, Lapenta C, Di Napoli A, Di
Landro F, et al: Clinical and antitumor immune responses in
relapsed/refractory follicular lymphoma patients after intranodal
injections of IFNα-dendritic cells and rituximab: A phase I
clinical tril. Clin Cancer Res. 25:5231–5241. 2019.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Kalli KR, Block MS, Kasi PM, Erskine CL,
Hobday TJ, Dietz A, Padley D, Gustafson MP, Shreeder B,
Puglisi-Knutson D, et al: Folate receptor alpha peptide vaccine
generates immunity in breast and ovarian cancer patients. Clin
Cancer Res. 24:3014–3025. 2018.PubMed/NCBI View Article : Google Scholar
|