Introduction
The immunostimulatory effects of herbal medicines,
such as Kampo in Japan and Chinese herbal medicines, have been
extensively reported. Herbal medicines are prescribed for infection
and tumor, and one of their properties is strong immunostimulatory
effects, including increased phagocytosis and antibody production
(1,2).
However, limited information is currently available regarding the
immunostimulant components of these herbal medicines. In our
previous, it was demonstrated using electron microscopy that cell
wall-based nanoparticles were universally present in boiled water
herbal extracts and these nanoparticles were isolated using
ultracentrifugation (3). In the
present study, the immune effects of cell wall-based nanoparticles
isolated from boiled Glycyrrhizae radix, the root and stolon
of Glycyrrhiza uralensis Fischer (4) water extracts were primarily
investigated, as this is a commonly used herb in traditional herbal
medicines (5). The isolated
nanoparticles are taken up by mouse macrophages (RAW-blue cells)
via phagocytosis, triggering immunostimulatory effects and inducing
the expression of the inflammatory cytokines, interleukin-6 (IL-6)
and tumor necrosis factor-α (TNF-α) in RAW-blue cells (3). However, the molecular mechanisms
underlying these immunostimulatory effects have not yet been
elucidated as these nanoparticles have only recently been
discovered. Macrophages are a type of phagocytotic immune cell
which primarily exert immunostimulatory effects, such as
inflammatory responses (6). The
inflammatory response mediated by macrophages are induced by
pattern recognition receptors, such as Toll-like receptors (TLRs)
(7,8)
and Dectin-1 (9,10). RAW-blue cells are a macrophage cell
line stably transfected with secreted embryonic alkaline
phosphatase (SEAP) reporter gene construct which is inducible by
NF-κB (11). Therefore, RAW-blue
cells were used for monitoring the activation of NF-κB, which is
part of the downstream signaling pathway of TLRs and Dectin-1
(7,8).
The aim of the present study was to determine the signaling pathway
underlying the immunostimulatory effects of nanoparticles from
boiled herbal water extracts of Glycyrrhizae radix and to
identify the receptors involved.
Materials and methods
Antibodies and reagents
A phospho-specific antibody against NF-κB p65
(Ser-536; cat. no. 93H1) was purchased from Cell Signaling
Technology, Inc. Antibodies against β-actin (C-11; cat. no.
sc-1615) and NF-κB p65 (C-20; cat. no. sc-372) were obtained from
Santa Cruz Biotechnology, Inc. Secondary antibodies used were
horseradish peroxidase-conjugated anti-rabbit (cat. no. P0448) or
anti-mouse (cat. no. P0260) immunoglobulin G (Dako; Agilent
Technologies, Inc.). Negative control small interfering (si)RNA and
TLR4-siRNA were purchased from Santa Cruz Biotechnology. Inc.
Dectin-1 siRNA was purchased from Thermo Fisher Scientific, Inc.
Lipofectamine® RNAiMAX transfection reagent was obtained
from Thermo Fisher Scientific, Inc. Normocin, Zeocin and
Quanti-Blue were purchased from InvivoGen. Dexamethasone (Dex), an
NF-κB inhibitor, was purchased from Wako Pure Chemical Industries,
Ltd. Block Ace for western blotting was obtained from DS Pharma
Medical Glycyrrhizae radix was obtained from Tochimoto
Tenkaido, Co., Ltd.
Cell line and culture
RAW-blue cells were cultured in DMEM, High Glucose
with L-glutamine, phenol red and sodium pyruvate (Wako Pure
Chemical Industries, Ltd.) supplemented with 10% heat-inactivated
FBS, 50 U/ml penicillin, 50 µg/ml streptomycin and 100 µg/ml
normocin at 37˚C in a humidified incubator with 5%
CO2.
Isolation of nanoparticles from boiled
Glycyrrhizae radix water extracts
Boiled herbal water extracts were prepared by gently
boiling 100 g Glycyrrhizae radix in 500 ml water for 50 min
at 95˚C and then filtering the decoction. The decoction was
centrifuged at 3,000 x g for 5 min at 4˚C (Kubota 6800; Kubota
Corporation) and the supernatant was collected and then centrifuged
at 20,000 x g for 20 min at 4˚C. The supernatant (60 ml) was
collected again and ultra-centrifuged at 140,000 x g for 50 min
twice at 4˚C, according to a previously described method for
preparation of exosomes (8). After
removal of the supernatant, the transparent pellet was dispersed in
distilled water (40 ml) and freeze-dried.
Scanning electron microscopy
(SEM)
Negative staining was performed as follows:
Collodion mesh (Nisshin EM Co., Ltd.) was placed on a 20 µl droplet
of nanoparticle solution (100 µg/ml) for 30 sec and the solution
was absorbed with filter paper. Subsequently, the mesh was placed
on a droplet of 2% uranyl acetate for 5 sec, which was absorbed
with filter paper, and dried. Nanoparticles which were negatively
stained were observed using a JSM-6700F SEM (JEOL, Ltd.) operated
using PCSEM software version 1 (JEOL, Ltd.) at a calibrated
magnification of x50,000.
NF-kB-SEAP reporter assay
A total of 0, 1, 1.5, 5 and 10 µg/ml nanoparticles
were added to the cell culture medium of RAW-blue cells and
incubated for 20 h, where 0 mg/ml was used as the control. The cell
culture supernatant was collected and transferred to QUANTI-Blue
medium (InvivoGen) and SEAP expression was then measured after 1 h
or 90 min at 620 nm using a spectrophotometer.
Western blotting
Whole cell lysates were extracted using lysis buffer
(20 mM HEPES-NaOH, 0.3 M NaCl, 1.5 mM MgCl2, 0.2 mM
EDTA, 0.001% Triton X-100, 1 mM DTT, 1 mM sodium orthovanadate, 20
mM β-glycerophosphate disodium salt hydrate, 10 µg/ml aprotinin, 10
µg/ml leupeptin and 1 mM PMSF). Protein concentrations in lysates
were quantified using a Bradford assay, (Bio-Rad Laboratories,
Inc.). The lysates were mixed with an equivalent volume of SDS
sample buffer (100 mM Tris-HCl, pH 6.8; 2.0% SDS; 70 mM DTT; 10%
glycerol; and 0.10% bromophenol blue) and heated at 95˚C for 5 min.
Samples were loaded onto a 9% gel, resolved using SDS-PAGE and
subsequently transferred onto an Immobilon-P nylon membrane (EMD
Millipore). The membrane was blocked using 4% Block Ace overnight
at 4˚C, and probed with primary antibodies (NF-κB p65, rabbit
anti-phospho NF-κB p65 and β-actin; all at 1:1,000), for 90 min at
room temperature. Primary antibodies were detected using the
horseradish peroxidase-conjugated anti-rabbit antibody (1:2,000)
and visualized using an ECL system (GE Healthcare). The density of
the blots was quantified using ImageJ version 1.8.0_172 (National
Institutes of Health). Experiments were repeated at least three
times and representative results are shown.
RNA interference
Mouse TLR4-siRNA (cat. no. sc-40261), mouse
Dectin-1-siRNA (cat. no. sc-63277) and negative control (cat. no.
sc-37007) were purchased from Santa Cruz Biotechnology, Inc.
RAW-blue cells were transfected with siRNAs at a final
concentration of 100 nM using Lipofectamine® RNAiMAX
transfection reagent (Thermo Fisher Scientific, Inc.) according to
the manufacturer's protocol. After 12 h, the medium was replaced
with fresh medium and the cells were cultured in the presence of
nanoparticles for a further 24 h. TLR4 siRNA (cat. no. sc-40261) is
a pool of 3 different siRNA duplexes. sc-40261A sense,
CUAGCCUUCUUCAAUCUUAtt and antisense, UAAGAUUGAAGAAGGCUAGtt;
sc-40261B sense, CCGUUGGUGUAUCUUUGAAtt and antisense,
UUCAAAGAUACACCAACGGtt; and sc-40261C sense, GAAGGCCCAUAUUUGACUAtt
and antisense, UAGUCAAAUAUGGGCCUUCtt. Dectin-1 siRNA sequences were
as follows: Dectin-1 sense, GACAACUUCCUAUCAAGAAtt and antisense,
UUCUUGAUAGGAAGUUGUCtt. The sequences of the negative control siRNAs
used were not disclosed by the manufacturer.
Reverse transcription-quantitative
(RT-q)PCR
Total RNA from the RAW 264.7 cells was extracted
using RNeasy Mini kit (Qiagen, Inc.) according to the
manufacturer's protocol. First-strand cDNA synthesis was performed
using the RNA as the template (2 µg) using oligo(dT)18 primer and
SuperScript III reverse transcriptase (Invitrogen; Thermo Fisher
Scientific Inc.). Reverse transcription was performed at 42˚C for
50 min and then at 70˚C for 15 min. qPCR amplification was
performed using a FastStart Essential DNA Green Master mix (Roche
Diagnostics). The thermocycling conditions were: Denaturation at
94˚C for 5 sec, annealing at 60˚C for 5 sec and extension at 72˚C
for 10 sec for 28 cycles qPCR was performed using a Lightcycler
nano system (Roche Diagnostics) according to the manufacturer's
protocol. β-actin was used as the internal control. The relative
quantification of mRNA expression was calculated as a ratio of the
target gene to β-actin (12). Primer
sequences were as follows: TLR4 forward, 5'-GGACTCTGATCATGGCACTG-3'
and reverse, 5'-CTGATCCATGCATTGGTAGGT-3'; Dectin-1 forward,
5'-TTGTGTCGCCAAAATGCTAGG-3' and reverse,
5'-CTGATCCATGCATTGGTAGGT-3'; IL-6 forward,
5'-GCTACCAAACTGGATATATAATCAGGA-3' and reverse
5'-GGTCTGGGCCATAGAACTGA-3'; TNF-α forward
5'-TCTTCTCATTCCTGCTTGTTG-3' and reverse,
5'-GGTCTGGGCCATAGAACTGA-3'; and β-actin forward,
5'-CTAAGGCCAACCGTGAAAAG-3' and reverse,
5'-ACCAGAGGCATACAGGGACA-3'.
Statistical analysis
Data are presented as the mean ± standard deviation
of at least 3 independent experiments. Differences between groups
were compared using an ANOVA with a post-hoc Tukey-Kramer test.
Statistical analyses were performed using JMP Pro software version
13 (SAS Institute). P<0.05 was considered to indicate a
statistically significance difference.
Results
SEM of nanoparticles
Freeze-dried nanoparticles from boiled
Glycyrrhizae radix water extracts were visualized using SEM.
The diameter of the nanoparticles were 80-100 nm (Fig. 1).
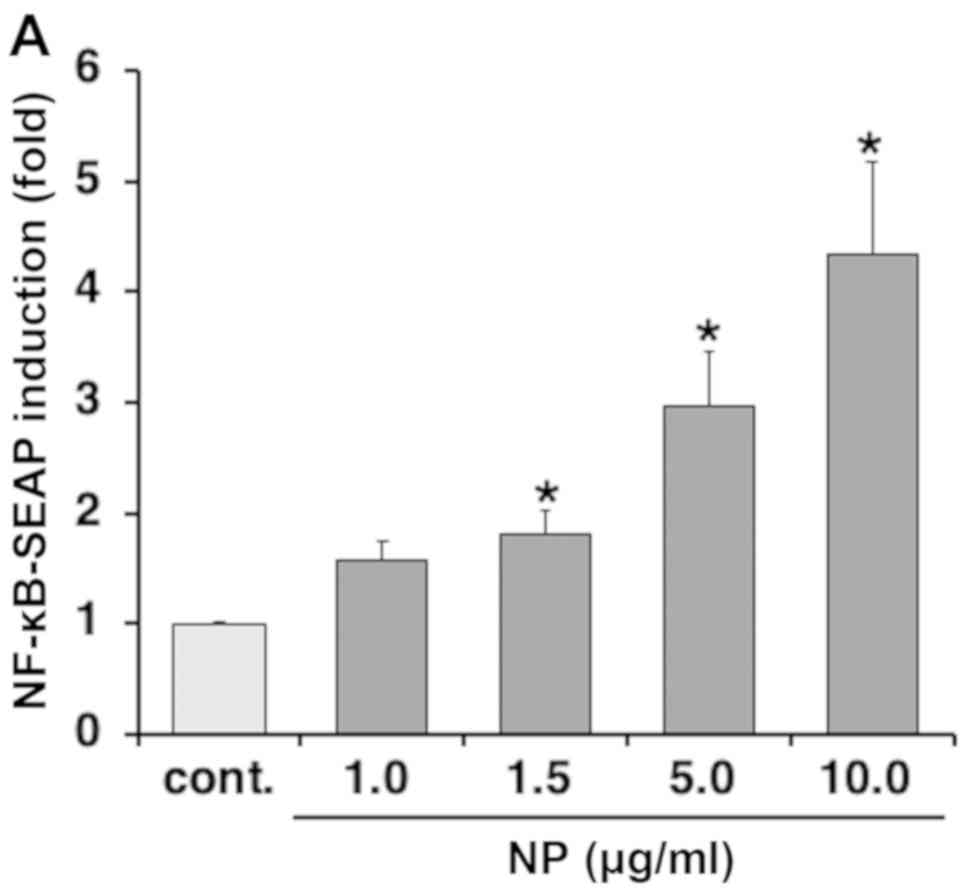
NF-κB activation by nanoparticles
The effects of the nanoparticles on RAW-blue cells
were investigated using a reporter gene assay with NF-κB. NF-κB
activation in RAW-blue cells was significantly higher when treated
with nanoparticles compared with the control, and NF-κB activation
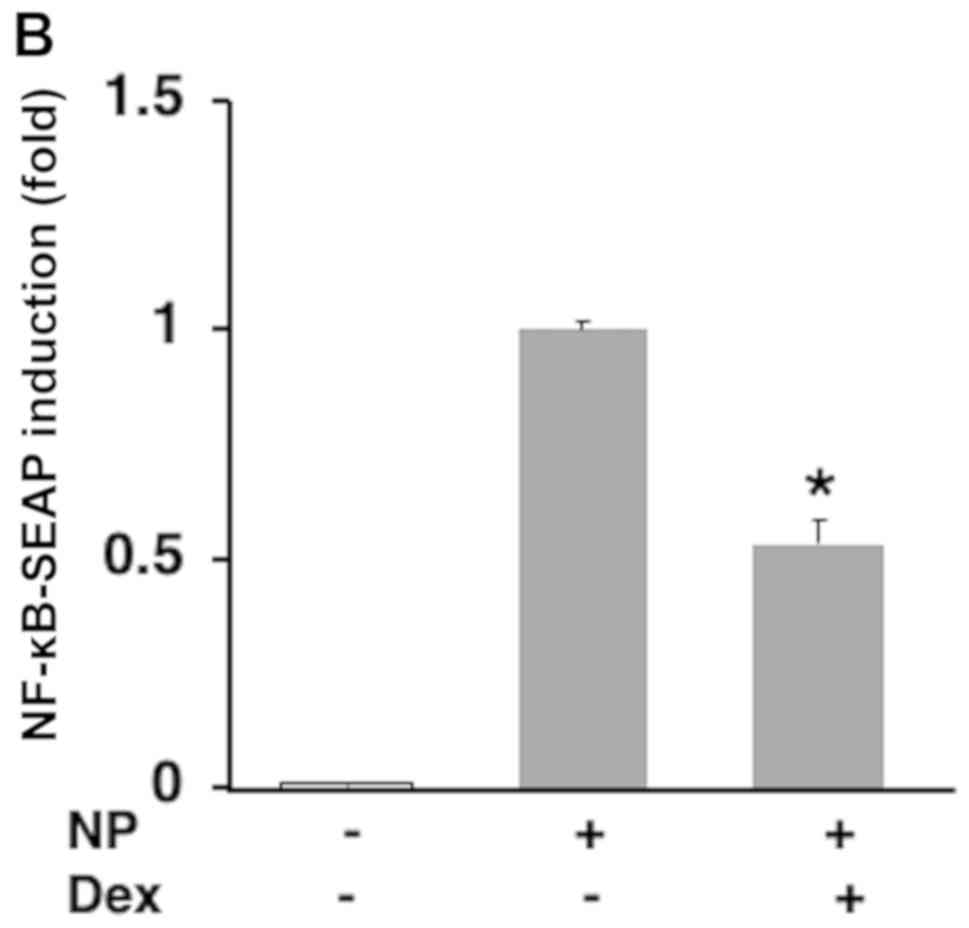
was increased in a dose-dependent manner (Fig. 2A). This activation was suppressed by
the NF-κB inhibitor Dex (Fig. 2B).
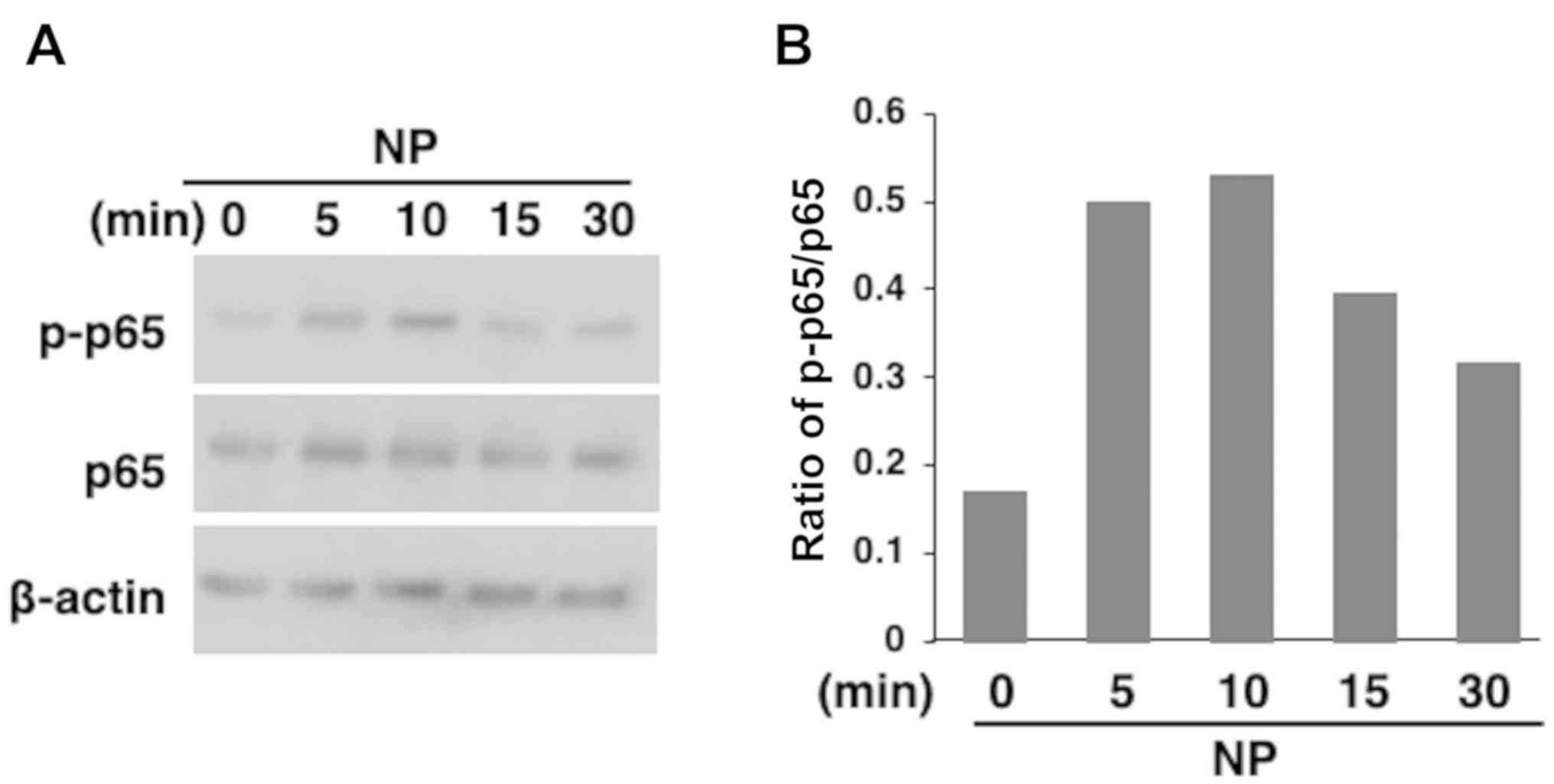
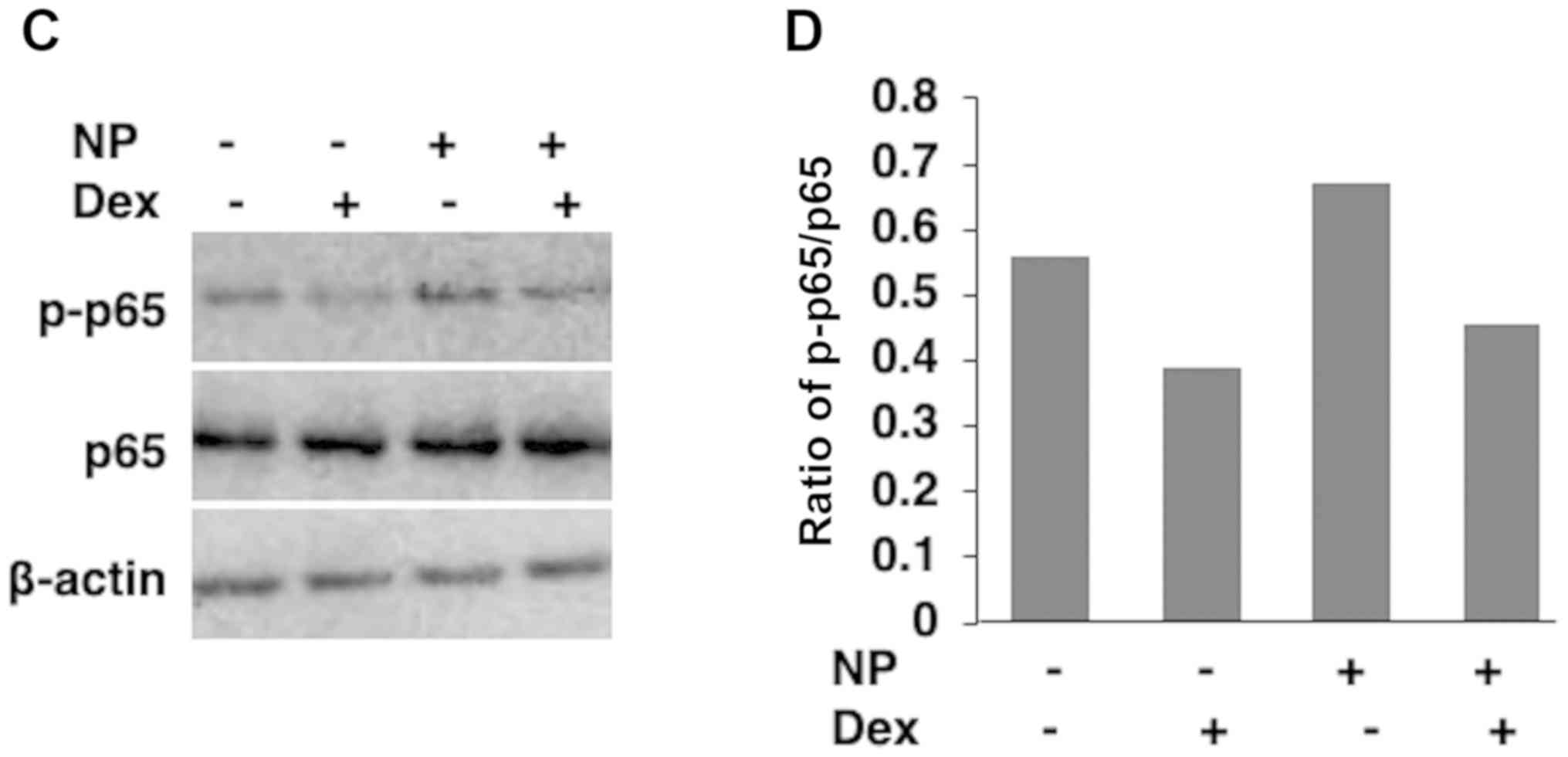
Furthermore, nanoparticles induced the phosphorylation of the NF-κB
subunit p65 (Fig. 3A and B) and this phosphorylation was suppressed by
Dex in the western blotting analysis (Fig. 3C and D).
Expression levels of inflammatory
cytokines are increased by nanoparticles
Based on the activation of NF-κB, cytokine
production induced by nanoparticles in RAW-blue cells was examined.
The mRNA expression levels of the inflammatory cytokines IL-6 and
TNF-α, which are regulated by NF-κB, were increased in RAW-blue
cells exposed to nanoparticles compared with the control cells
(Fig. 4).
Identification of receptors of
nanoparticles
Based on the activation of NF-κB by nanoparticles,
the receptors the nanoparticles bound to, to exert their effect in
RAW-blue cells, were determined (Fig.
5A). Activation of NF-κB by nanoparticles was significantly
suppressed when cells were transfected with TLR4 siRNA, but not by
Dectin-1 siRNA. The reduction in SEAP activity incells transfected
with si-TLR4 was ~50% (Fig. 5B).
Together, these results suggest that the signaling
pathway by which nanoparticles obtained from boiled
Glycyrrhizae radix water extracts at least partially
involved TLR4, and signal transmission induced the expression of
the inflammatory cytokines IL-6 and TNF-α in RAW-blue cells.
Discussion
The immunostimulatory effects of herbal medicines
are widely known (1,2). In our previous study, it was
demonstrated that juzentaihoto, a Japanese herbal medicine,
increased and prolonged antibody production following an influenza
vaccination in a human clinical experiment (13). However, the immunostimulatory
components of herbal medicines and the underlying molecular
mechanisms remain unclear. On the other hand, studies investigating
the sugar chains in herbal medicines have previously been performed
(14,15). In future studies, similarities between
these reported sugar chains and these nanoparticles should be
examined.
TLRs and dectin-1 are pattern-recognition receptors,
which exogenous ligands [pathogen-associated molecular patterns
(PAMPs)] and endogenous ligands (damage-associated molecular
patterns) and subsequently induce an immune response (16).
Dectin-1 is a β-glucan receptor and β-glucans are
polysaccharide triplet chains composed of D-glucose units via β-1,3
glycosidic bonds (17). In the
present study, the nanoparticles were not recognized by Dectin-1,
even though the primary constituent of these nanoparticles is
glucose (3). A possible explanation
for this may be that the polymerized glucose constituents of the
nanoparticles may not be recognized as a polysaccharide triplet
chain equipped with β-1,3 glycosidic bonds by Dectin-1.
Nanoparticles obtained from boiled
Glycyrrhizae radix water extracts induced NF-κB activation
via the TLR4 signaling pathway. These results suggest that the
activation of NF-κB in RAW-blue cells by nanoparticles is partially
mediated by TLR4. TLR4, a TLR, and its ligand axis has been
reported to activate several transcription factors, such as NF-κB,
in order to induce immune responses (18).
In our previous study, it was demonstrated that
nanoparticles obtained from boiled Glycyrrhizae radix water
extracts where composed of the cell wall components arabinogalactan
and cellulose, suggesting that they are a semi-artificial assembled
form of plant cell wall degradants (3). The typical TLR4 ligands of PAMPs are
cell wall components of bacteria (lipopolysaccharide) and fungi
(mannan), which may provide insight into the immunological function
of nanoparticles as TLR4 ligands (18,19). In
the present study, nanoparticles from boiled Glycyrrhizae
radix water extracts induced the phosphorylation of the NF-κB
subunit p65. It is hypothesized that the nanoparticles function as
TLR4 ligands, initiating signal transduction and increasing the
expression of the inflammatory cytokines TNF-α and IL-6 via
NF-κB.
TNF-α is an immunostimulatory cytokine that prevents
infection by bacteria and viruses and eliminates tumor cells
(20). IL-6 is an immunostimulatory
cytokine that serves an essential role in acquired immunity through
its regulation of antibody production (21,22). As
previously discussed, limited information is currently available
concerning the immunostimulant components of herbal medicines. The
present study suggests that nanoparticles from boiled
Glycyrrhizae radix water extracts are novel immunostimulant
components via TLR4.
The TLR4-mediated signaling pathway is regarded as
an attractive pharmaceutical target, particularly for cancer
therapy (23). TLR4 ligands have
advanced through pre-clinical and clinical stages and two agents,
Bacillus Calmette-Guérin (BCG) and monophosphoryl lipid A, have
been approved for immunotherapy of in situ bladder carcinoma
by the Food and Drug Administration (24).
In the present study, in vivo experiments
were not performed. Therefore, in future studies, the anti-cancer
effects of nanoparticles from boiled Glycyrrhizae radix
water extracts will need to be investigated using animal models to
confirm their efficacy.
In conclusion, nanoparticles obtained from boiled
Glycyrrhizae radix water extracts were demonstrated to
activate the NF-κB signaling pathway and increase the expression of
inflammatory cytokines via TLR4.
BCG, which is a TLR4 agonist and a classical
immunostimulants prepared from Mycobacterium tuberculosis, is an
efficient type of immunotherapy used to treat patients with bladder
carcinoma for >40 years (25).
Given that nanoparticles obtained from boiled Glycyrrhizae
radix water extracts are a TLR4 agonist prepared from a plant, the
results of the present study may aid in the development of novel
anti-cancer drugs such as BCG.
Acknowledgements
We would like to thank the Daicel Corporation
(Osaka, Japan) and ROHTO Pharmaceutical Co., Ltd. (Osaka, Japan)
for their technical support.
Funding
The present study was supported by a Grant-in-Aid
for the Cooperative Research Project from the Institute of Natural
Medicine, University of Toyama, Toyama, Japan (grant nos. 2014Y and
2015Y).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
HI, KK and NS designed the research. HI, KK, MS, YO
and MJ performed the experiments and prepared all the figures. HI,
KK and NS wrote the paper. All authors discussed and agreed on the
results, read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Liu H, Wang J, Sekiyama A and Tabira T:
Juzen-taiho-to, an herbal medicine, activates and enhances
phagocytosis in microglia/macrophages. Tohoku J Exp Med. 215:43–54.
2008.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Munakata K, Takashima K, Nishiyama M,
Asano N, Mase A, Hioki K, Ohnishi Y, Yamamoto M and Watanabe K:
Microarray analysis on germfree mice elucidates the primary target
of a traditional Japanese medicine juzentaihoto: Acceleration of
IFN-α response via affecting the ISGF3-IRF7 signaling cascade. BMC
Genomics. 13(30)2012.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Iitsuka H, Koizumi K, Inujima A, Suzaki M,
Mizuno Y, Takeshita Y, Eto T, Otsuka Y, Shimada R, Liu M, et al:
Discovery of a sugar-based nanoparticle universally existing in
boiling herbal water extracts and their immunostimulant effect.
Biochem Biophys Rep. 16:62–68. 2018.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Guo ZZ, Wu YL, Wang RF, Wang WQ, Liu Y,
Zhang XQ, Gao SR, Zhang Y and Wei SL: Distribution patterns of the
contents of five active components in taproot and stolon of
Glycyrrhiza uralensis. Biol Pharm Bull. 37:1253–1258.
2014.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Nose M, Tada M, Kojima R, Nagata K, Hisaka
S, Masada S, Homma M and Hakamatsuka T: Comparison of glycyrrhizin
content in 25 major kinds of Kampo extracts containing
Glycyrrhizae Radix used clinically in Japan. J Nat Prod.
71:711–722. 2017.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Doster RS, Rogers LM, Gaddy JA and Aronoff
DM: Macrophage extracellular traps: A scoping review. J Innate
Immun. 10:3–13. 2018.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Ozinsky A, Underhill DM, Fontenot JD,
Hajjar AM, Smith KD, Wilson CB, Schroeder L and Aderem A: The
repertoire for pattern recognition of pathogens by the innate
immune system is defined by cooperation between toll-like
receptors. Proc Natl Acad Sci USA. 97:13766–13771. 2000.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Takeda K, Kaisho T and Akira S: Toll-like
receptors. Annu Rev Immunol. 21:335–376. 2003.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Borriello F, Zanoni I and Granucci F:
Cellular and molecular mechanisms of antifungal innate immunity at
epithelial barriers: The role of C-type lectin receptors. Eur J
Immunol. 50:317–325. 2020.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Fesel PH and Zuccaro A: β-glucan: Crucial
component of the fungal cell wall and elusive MAMP in plants.
Fungal Genet Biol. 90:53–60. 2016.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Hansen FC, Kalle-Brune M, van der Plas MJ,
Strömdahl AC, Malmsten M, Mörgelin M and Schmidtchen A: The
thrombin-derived host defense peptide GKY25 inhibits
endotoxin-induced responses through interactions with
lipopolysaccharide and macrophages/monocytes. J Immunol.
194:5397–5406. 2015.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Saiki I, Koizumi K, Goto H, Inujima A,
Namiki T, Raimura M, Kogure T, Tatsumi T, Inoue H, Sakai S, et al:
The long-term effects of a kampo medicine, juzentaihoto, on
maintenance of antibody titer in elderly people after influenza
vaccination. Evid Based Complement Alternat Med.
2013(568074)2013.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Kiyohara H, Uchida T, Takakiwa M,
Matsuzaki T, Hada N, Takeda T, Shibata T and Yamada H: Different
contributions of side-chains in beta-D-(1->3,6)-galactans on
intestinal Peyer's patch-immunomodulation by polysaccharides from
Astragalus mongholics Bunge. Phytochemistry. 71:280–293.
2010.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Kiyohara H, Matsuzaki T and Yamada H:
Intestinal Peyer's patch-immunomodulating glucomannans from
rhizomes of Anemarrhena asphodeloides Bunge. Phytochemistry.
96:337–346. 2013.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Martínez A, Bono C, Megías J, Yáñez A,
Gozalbo D and Gil ML: PRR signaling during in vitro macrophage
differentiation from progenitors modulates their subsequent
response to inflammatory stimuli. Eur Cytokine Netw. 28:102–110.
2017.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Nakashima A, Yamada K, Iwata O, Sugimoto
R, Atsuji K, Ogawa T, Ishibashi-Ohgo N and Suzuki K: β-Glucan in
foods and its physiological functions. J Nutr Sci Vitaminol
(Tokyo). 64:8–17. 2018.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Takeda K, Kaisho T and Akira S: Toll-like
receptors. Annu Rev Immunol. 21:335–376. 2003.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Akira S, Uematsu S and Takeuchi O:
Pathogen recognition and innate immunity. Cell. 124:783–801.
2006.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Casale TB and Carolan EJ: Cytokine-induced
sequential migration of neutrophils through endothelium and
epithelium. Inflamm Res. 48:22–27. 1999.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Dienz O and Rincon M: The effects of IL-6
on CD4 T cell responses. Clin Immunol. 130:27–33. 2009.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Hunter CA and Jones SA: IL-6 as a keystone
cytokine in health and disease. Nat Immunol. 16:448–457.
2015.PubMed/NCBI View
Article : Google Scholar
|
|
23
|
Roy A, Srivastava M, Saqib U, Liu D,
Faisal SM, Sugathan S, Bishnoi S and Baig MS: Potential therapeutic
targets for inflammation in toll-like receptor 4 (TLR4)-mediated
signaling pathways. Int Immunopharmacol. 40:79–89. 2016.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Vacchelli E, Eggermont A, Sautès-Fridman
C, Galon J, Zitvogel L, Kroemer G and Galluzzi L: Trial Watch:
Toll-like receptor agonists for cancer therapy. Oncoimmunology.
2(e25238)2013.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Camargo JA, Passos GR, Ferrari KL, Billis
A, Saad MJA and Reis LO: Intravesical immunomodulatory imiquimod
enhances bacillus Calmette-Guérin downregulation of
nonmuscle-invasive bladder cancer. Clin Genitourin Cancer.
16:e587–e593. 2018.PubMed/NCBI View Article : Google Scholar
|