Introduction
Dry eye disease (DED) is a chronic and progressive
ocular disorder that is frequently encountered in ophthalmic
practice (1,2). According to the definition and
classification proposed by the Dry Eye Workshop II, dry eye is a
multifactorial disease of the ocular surface characterized by loss
of homeostasis of the tear film and accompanied by ocular symptoms,
such as ocular surface disease index (3). Tear film instability, hyperosmolarity,
ocular surface inflammation and damage, and neurosensory
abnormalities serve major roles in the etiology of the condition
(4,5).
The pathology of DED is closely associated with inflammation in the
cornea and conjunctiva, which primarily involves T cells (5-8).
Current treatment for DED includes artificial tears,
immunomodulatory agents and corticosteroids (9-12).
Cyclosporine A (CsA) 0.05% ophthalmic emulsions is one of the
standard treatments for inflammatory DED; however, it does not
completely relieve inflammation on the ocular surface (13).
Thymosin β4 (Tβ4) is a 43-amino acid peptide that is
a major constituent protein found in platelets, macrophages and
polymorphonuclear cells (14,15). Tβ4 downregulates inflammatory
mediators by inhibiting activation of nuclear factor-κB (NF-κB)
(16). Additionally, Tβ4 regulates
pro-inflammatory signaling in microglia and controls inflammatory
processes in the brain (17). In
ophthalmology, application of topical Tβ4 significantly improves
clinical signs and symptoms in patients with DED (18,19).
Glycine-Tβ4 (Gly-Tβ4) is a small peptide which is a
single glycine terminal residue addition to Tβ4. Gly-Tβ4 promotes
corneal epithelial repair by increasing the migration of corneal
epithelial cells and reducing the production of inflammatory
cytokines in a rabbit model of ocular alkali burn (20).
Although topical Tβ4 was shown to improve tear film
parameters in clinical DED, there are no studies evaluating the
effect of Tβ4 on inflammatory molecules or cells in the ocular
surface of DED, to the best of our knowledge. In the present study,
the effects of topical 0.1% Gly-Tβ4 on inflammation, apoptosis and
conjunctival gobleT cell density, as well as tear film and ocular
surface parameters were determined, and the treatment efficacy of
Gly-Tβ4 was compared with 0.05% CsA in a mouse model of
experimental dry eye (EDE).
Materials and methods
Animal model of EDE
The research protocol used in the present study was
approved by the Chonnam National University School Research
Institutional Animal Care and Use Committee. All animals were
treated in accordance with ARVO Statement for the Use of Animals in
Ophthalmic and Vision Research (arvo.org/About/policies/statement-for-the-use-of-animals-in-ophthalmic-and-vision-research/).
Female C57BL/6 mice (n=35) aged 6-8 weeks were used in the
following experiments. EDE was induced by subcutaneous injection of
0.5 mg/0.2 ml scopolamine hydrobromide (Sigma Aldrich; Merck KGaA)
three times a day (9 a.m., 1:30 p.m. and 6 p.m.) with exposure to
an air draft and 30% ambient humidity, as previously described
(13,21). During these experiments, animal
behavior, and food and water intake were not restricted. Mice were
randomly divided into five groups according to the topical
treatment administered as follows: i) Untreated control mice that
were not exposed to desiccating stress or topical treatment (UT
group); ii) EDE mice that were exposed to desiccating stress but
were not administered eye drops (EDE group); iii) EDE mice treated
with balanced salt solution (BSS; obtained from Alcon) (BSS group);
iv) EDE mice treated with 0.05% CsA (Restasis®,
Allergan, Ltd.) (CsA group); and v) EDE mice treated with 0.1%
Gly-Tβ4. Gly-Tβ4 was prepared at 0.1% by Huons Co., Ltd. based on
the results of preliminary experiments (Fig. S1; Gly-Tβ4 group). A total of 2 µl eye
drops was applied topically to both eyes three times a day in BSS
and 0.1% Gly-Tβ4 groups, and twice a day in 0.05% CsA group until
they were euthanized. Clinical parameters, including tear volume,
tear film break-up time (TBUT) and corneal staining scores (CSS)
were measured 7 and 14 days after treatment. The clinical
measurements were taken 3 h after the last scopolamine injection
and application of eye drops. After measurement of the clinical
parameters, the mice were euthanized, and periodic acid-Schiff
(PAS) staining, TUNEL assay, multiplex immunobead assay, and flow
cytometry were performed as described below. Each group consisted
of six animals, and the experiments were performed on three
independent sets of mice.
Evaluation of tear film and ocular
surface parameters
Tear volume was measured using phenol
red-impregnated cotton threads (Zone-Quick; Oasis Medical, Inc.) 3
h after the last scopolamine injection, as previously described
(22,23). The thread was placed on the lower
conjunctival fornix at approximately one-third of the lower eyelid
distance from the lateral canthus for 20 seconds. The length of the
wet red thread was measured in millimeters under a photomicroscope
(magnification, x1; SMZ 1500; Nikon Corporation). A standard curve
was plotted to convert distance into volume.
A total of 1 µl 1% sodium fluorescein was instilled
into the inferior conjunctival sac using a micropipette. After
three blinks, TBUT was recorded in sec using slit lamp
biomicroscopy (magnification, x16; BQ-900; Haag-Streit) under
cobalt blue light. After a total of 90 sec, punctate staining of
the corneal surface was evaluated by a researcher who was blinded
to the therapeutic conditions. Each cornea was divided into four
quadrants, which were scored individually. CSS was calculated using
a 4-point scale, based on a previous study (24): 0, absent; 1, slightly punctate
staining <30 spots; 2, punctate staining >30 spots, but not
diffuse; 3, severe diffuse staining but no positive plaque; and 4,
positive fluorescein plaque. The four scores were added to generate
a final grade; the maximum possible score was 16 points.
Histology
The eye and the adnexa were surgically excised,
fixed in 4% paraformaldehyde overnight at 4˚C, dehydrated in a
gradient concentration of ethanol (70-100%), and embedded in
paraffin. Serial sections were cut from the lateral and medial
borders of each paraffin block (6 µm thick slices) and stained with
PAS reagent (cat. no. 395B-1 KT; Sigma-Aldrich Corporation) for 15
min at room temperature. Sections obtained from four animals in
each group were examined and imaged with a light microscope
(magnification, x10; Olympus Corporation) equipped with a digital
camera. GobleT cell density in the superior and inferior
conjunctiva was measured in three sections from each eye using
Image-Pro version 10.0.5; Medial Cybernetics, Inc.) and was
expressed as the number of goble T cells per 100 µm.
TUNEL staining
A TUNEL assay was used to detect the 3' hydroxyl
ends of fragmented DNA, an early event in the apoptotic cascade,
and used to identify apoptotic cells. The eye and the adnexa were
surgically excised, fixed in 4% paraformaldehyde overnight at 4˚C
and embedded in paraffin. Staining was performed using a
DeadEnd™ Fluorometric TUNEL system (Promega
Corporation), according to the manufacturer's protocol. Stained
tissues were mounted on slides, the nuclei were visualized with
DAPI present in the ProLong Gold Antifade Mounting Medium
(Invitrogen; Thermo Fisher Scientific, Inc.) and the tissues were
observed using a Leica TCS SP5 AOBS laser scanning confocal
microscope (Leica Microsystems, GmbH) under a Leica x63 (N.A. 1.4)
oil objective. Cell images were obtained separately with the
following fluorescence excitation and emission settings: Excitation
at 405 and 488 nm and emission between 424-472 and 502-550 nm for
TUNEL assay and DAPI, respectively. TUNEL positive cells and
nuclear staining with DAPI in the cornea were viewed under a
fluorescent microscope (magnification, x20).
Multiplex immunobead assay
A multiplex immunobead assay (Luminex 200; Luminex
Corporation) was used to measure the concentrations of interleukin
(IL)-1β, IL-6, tumor necrosis factor (TNF)-α and interferon (IFN)-γ
(all from Milliplex®, EMD Millipore; cat. no.
MCYTOMAG-70K) in the conjunctiva, as previously described (21). The tissues were collected and pooled
in TissueLyser lysis buffer (Qiagen, Inc.) containing protease
inhibitors for 30 min. The cell extracts were centrifuged at 14,000
x g for 15 min at 4˚C, and the supernatants were stored at -70˚C
until use. After centrifugation, each sample (10 µg/25 µl/well) was
added to a 96-well plate and incubated overnight at 4˚C in the
dark, with 25 µl 1x beads coupled to mouse
cytokine/chemokine-specific antibodies. Serial dilutions of each
cytokine/chemokine were also added to wells in the same plate, to
generate a standard curve. The following day, the beads were washed
and mixed with 25 µl 1x biotinylated secondary cytokine/chemokine
antibody mixture for 1 h at room temperature, followed by washing
and subsequent incubation with 25 µl streptavidin-phycoerythrin for
30 min at room temperature (both steps performed in the dark).
After a final wash, the wells were resuspended with 100 µl assay
buffer. The reactions were detected after addition of
streptavidin-phycoerythrin using an analysis system (xPONENT;
Luminex Corporation). Concentrations of the cytokines in the
tissues were calculated from standard curves of known
concentrations of recombinant mouse cytokines.
Flow cytometry
Flow cytometry was performed to measure the
proportion of CD4+/CCR5+ T cells from
conjunctiva using a previously described method (25). Tissues from each group were harvested,
dipped in PBS, teased apart with scissors, and shaken at 37˚C for
60 min in the presence of 0.5 mg/ml collagenase type D (Roche
Applied Science). After incubation, the tissues were homogenized by
grinding with a syringe plunger and passed through a cell strainer
with a pore size of 100 µm. Cells were centrifuged at 450 x g at
4˚C for 7 min and re-suspended in PBS with 1% BSA. The samples were
incubated with monoclonal antibodies. For detecting
CD4+/CCR5+ double-stained cells, the samples
were incubated with fluorescein-conjugated anti-mouse CD4 antibody
(0.5 mg/ml; cat. no. 553651; BD Biosciences) and
phycoerythrin-conjugated anti-mouse CCR5 antibody (0.5 mg/ml; cat.
no. 559923; BD Biosciences), or isotype control antibody (cat. nos.
553929 and 559841; BD Biosciences) at 4˚C for 30 min. After 30 min
of incubation, the cells were washed in PBS. The cells were then
centrifuged three times in 1 ml PBS and resuspended. The number of
CD4+/CCR5+ T cells were counted using a
FACSCalibur flow cytometer (BD Biosciences) with CellQuest software
(version 5.2.1; BD Biosciences).
Statistical analysis
SPSS version 18.0; SPSS, Inc.) was used for all
statistical analyses. Results are presented as the mean ± standard
deviation. Statistical differences in tear volume, TBUT and CSS
among the groups were determined using a one-way ANOVA with a
post-hoc Tukey's test. A Kruskal-Wallis test followed by a Dunn's
multiple comparisons post-hoc test was used to compare the cytokine
levels, flow cytometry, goblet cell density and apoptotic cell
density between the groups. P<0.05 was considered to indicate a
statistically significant difference.
Results
Clinical parameters in the ocular
surface
Mean tear volumes were 0.036±0.004 µl in the UT
group, 0.014±0.013 µl in the EDE group, 0.015±0.004 µl in the BSS
group, 0.020±0.001 µl in the CsA group and 0.021±0.003 µl in the
Gly-Tβ4 group after 7 days. The mean tear volume after 14 days was
0.035±0.003 µl (UT group), 0.013±0.003 µl (EDE group), 0.015±0.003
µl (BSS group), 0.023±0.003 µl (CsA group) and 0.022±0.003 µl
(Gly-Tβ4 group), respectively. The Gly-Tβ4 and CsA groups showed a
significant improvement in the tear volume compared with the EDE
and BSS groups after 7 and 14 days (EDE vs. Gly-Tβ4 and CsA,
P<0.05; BSS vs. Gly-Tβ4 and CsA, P<0.05; Fig. 1A).
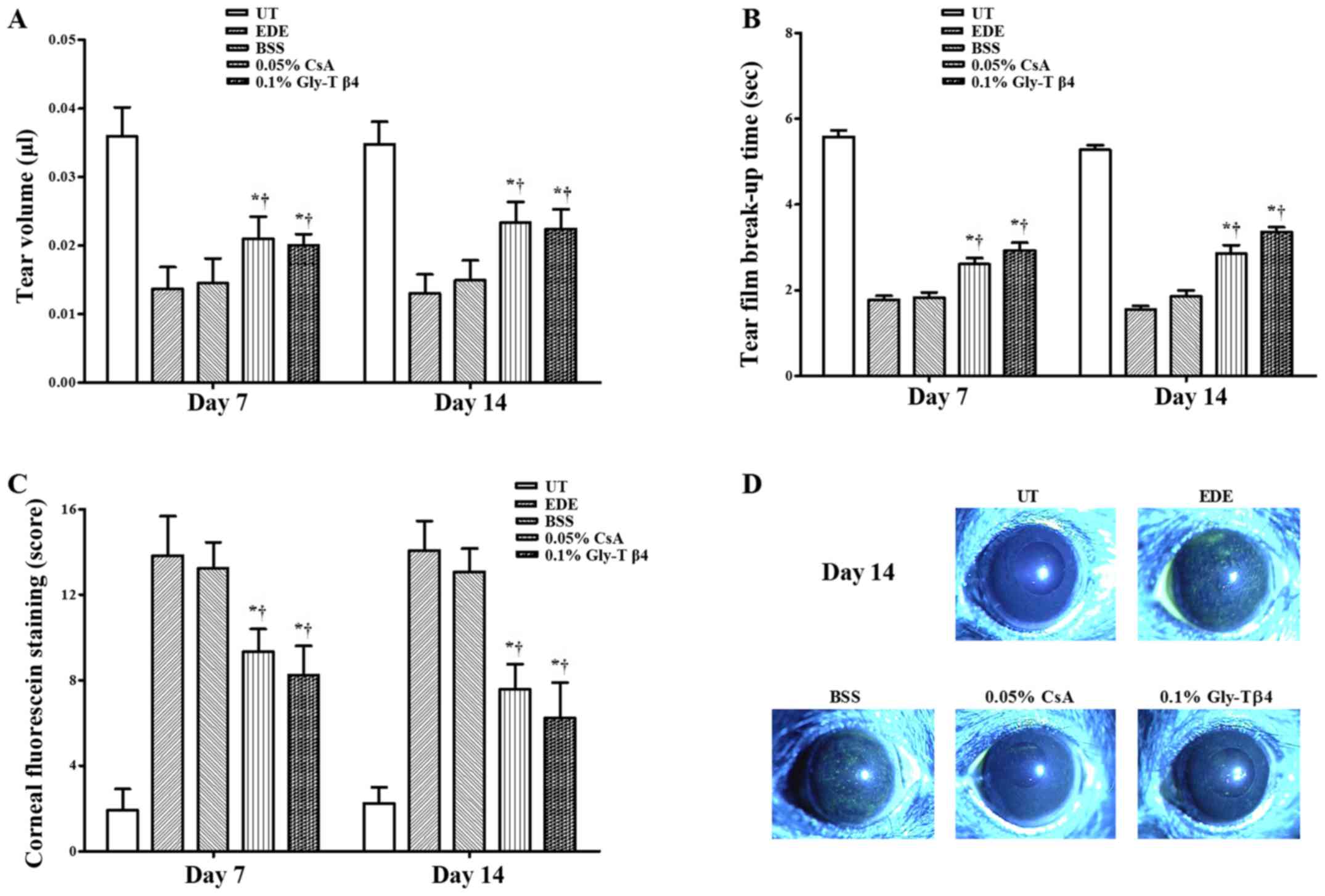 | Figure 1Tear film and ocular surface
parameters. (A) Mean tear volumes, (B) tear film break-up time, (C)
corneal staining scores and (D) representative images of corneal
staining in the UT, EDE, BSS, 0.05% CsA and 0.1% Gly-Tβ4 groups.
The Gly-Tβ4 and CsA groups showed an improvement in all clinical
parameters compared with the EDE and PSS groups. There were no
significant differences between the two treatment groups after 7
and 14 days. *P<0.05 vs. EDE group;
†P<0.05 vs. BSS group. UT, untreated control; EDE,
experimental dry eye; BSS, balanced salt solution; CsA,
Cyclosporine A; Gly-Tβ4, glycine-thymosin β4. |
Mean TBUTs were 5.58±0.52 sec in the UT group,
1.79±0.34 sec in the EDE group, 1.83±0.41 sec in the BSS group,
2.57±0.52 sec in the CsA group and 2.91±0.66 sec in the Gly-Tβ4
group after 7 days. Mean TBUTs after 14 days were 5.28±0.39 sec (UT
group), 1.56±0.24 sec (EDE group), 1.85±0.51 sec (BSS group),
2.86±0.66 sec (CsA group) and 3.36±0.39 sec (Gly-Tβ4 group). The
Gly-Tβ4 and CsA groups showed a significantly higher TBUT compared
with the EDE and BSS groups after 7 and 14 days (EDE vs. Gly-Tβ4
and CsA, P<0.05; BSS vs. Gly-Tβ4 and CsA, P<0.05; Fig. 1B).
A total of 7 days after induction, the mean CSS was
1.92±1.00 in the UT group, 13.83±1.85 in the EDE group, 13.25±1.22
in the BSS group, 9.33±1.07 in the CsA group and 8.25±1.35 in the
Gly-Tβ4 group. The mean CSS after 14 days was 2.25±0.75 (UT group),
14.08±1.38 (EDE group), 13.08±1.08 (BSS group), 7.58±1.16 (CsA
group) and 6.25±0.65 (Gly-Tβ4 group). The Gly-Tβ4- and CsA-treated
groups showed a significant improvement in the CSS after 7 and 14
days (EDE vs. Gly-Tβ4 and CsA, P<0.05; BSS vs. Gly-Tβ4 and CsA,
P<0.05; Fig. 1C).
There were no significant differences in all the
clinical parameters measured between the two treatment groups
(Gly-Tβ4 vs. CsA, all P>0.05).
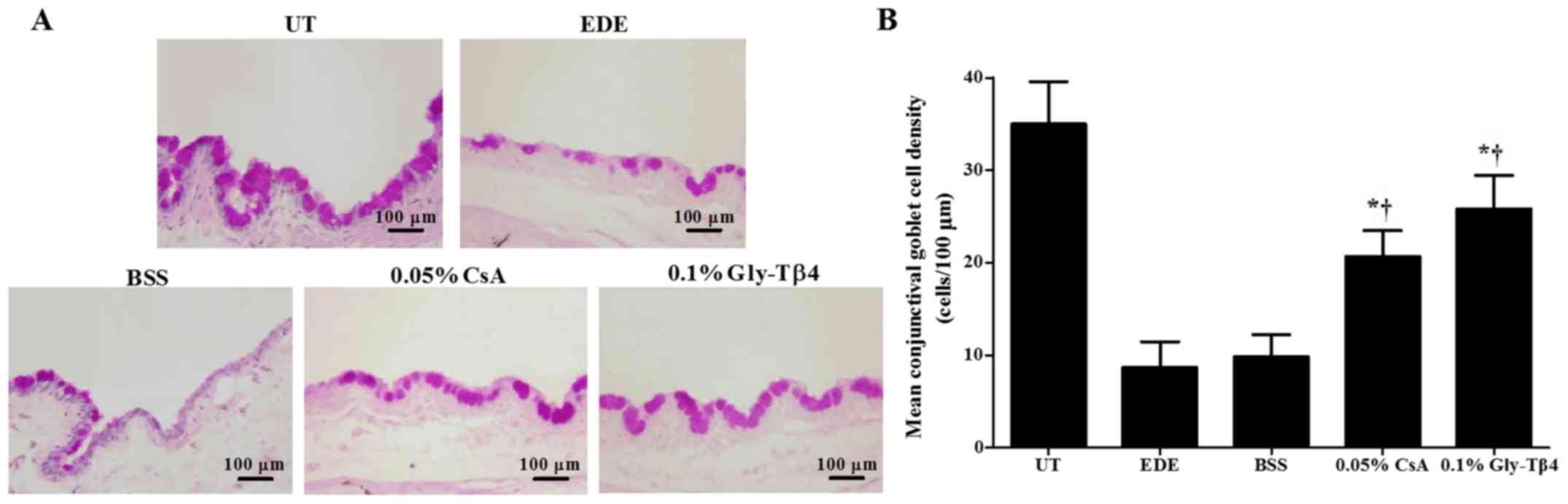
Conjunctival goblet cell density
Mean goblet cell densities were 35.00±4.56 cells/100
µm in the UT group, 8.67±2.80 cells/100 µm in the EDE group,
9.83±2.40 cells/100 µm in the BSS group, 20.67±2.80 cells/100 µm in
the CsA group and 25.83±3.60 cells/100 µm in the Gly-Tβ4 group.
Mice in the two treatment groups exhibited significantly higher
conjunctival gobleT cell densities compared with the EDE and BSS
groups (EDE vs. Gly-Tβ4 and CsA, P<0.05; BSS vs. Gly-Tβ4 and
CsA, P<0.05; Fig. 2). There was no
significant difference in goblet cell density between the Gly-Tβ4
and CsA treatment groups.
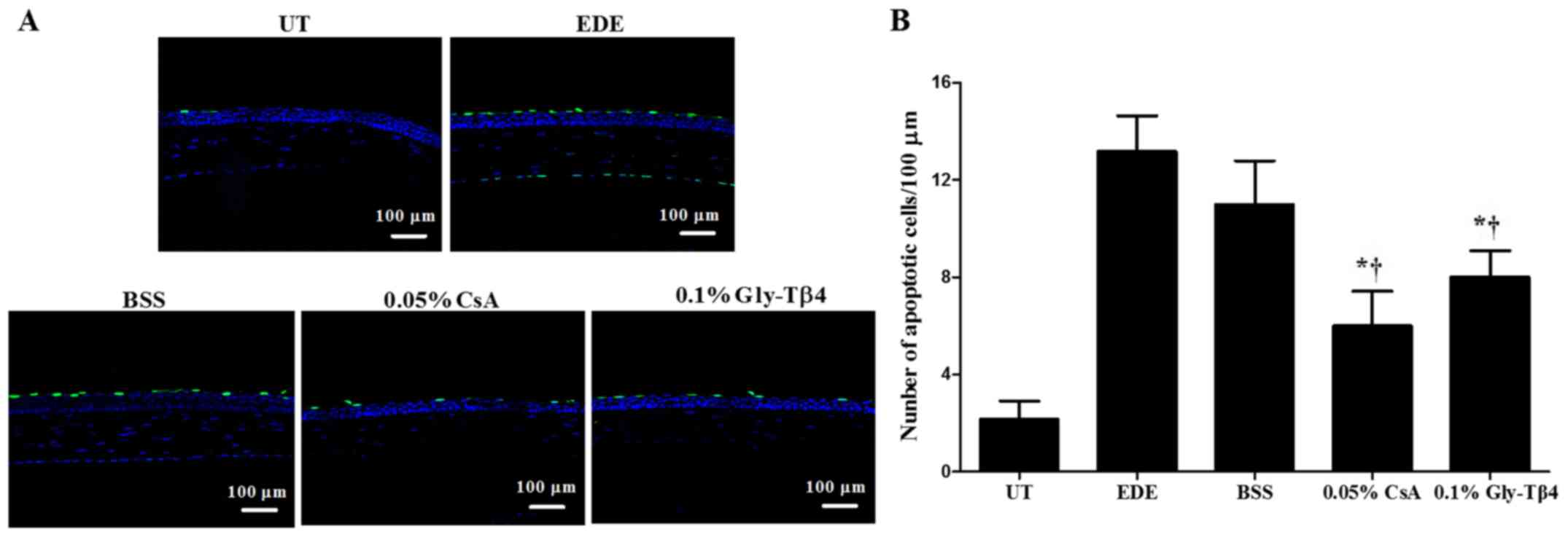
TUNEL staining
Mean apoptotic cell counts in the corneal epithelium
were 2.17±0.75 cells/100 µm in the UT group, 13.17±1.47 cells/100
µm in the EDE group, 11.00±1.79 cells/100 µm in the BSS group,
6.00±1.41 cells/100 µm in the CsA group and 8.00±1.10 cells/100 µm
in the Gly-Tβ4 group. Representative magnified images of the
corneal sections stained with TUNEL are presented in Fig. 3. There was a significant decrease in
the number of apoptotic cells observed in the Gly-Tβ4 and CsA
groups when compared with both the EDE and BSS groups (EDE vs.
Gly-Tβ4 and CsA, P<0.05; BSS vs. Gly-Tβ4 and CsA, P<0.05),
but there was no significant difference between the Gly-Tβ4 and CsA
treatment groups.
Inflammatory cytokine levels in
conjunctival tissues
There was a significant decrease in the levels of
IL-1β, IL-6, TNF-α and IFN-γ observed in the conjunctiva of mice in
the Gly-Tβ4 and CsA treatment groups when compared with the EDE and
BSS groups (EDE vs. Gly-Tβ4 and CsA, P<0.05; BSS vs. Gly-Tβ4 and
CsA, P<0.05). There was no significant difference observed
between the two treatment groups (Fig.
4).
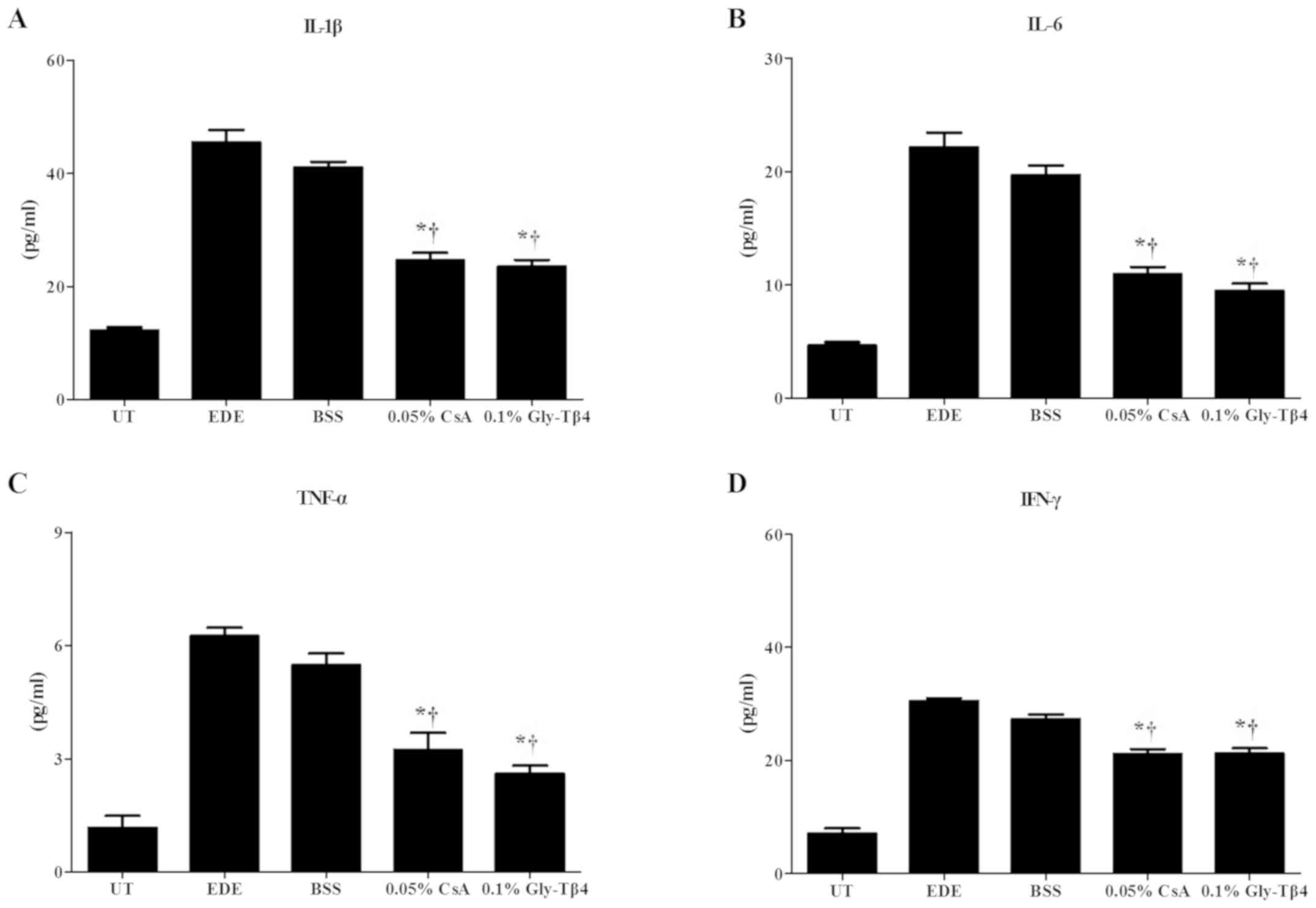 | Figure 4Inflammatory molecular levels in the
conjunctiva. Levels of (A) IL-1β, (B) IL-6, (C) TNF-α and (D) IFN-γ
in the UT, EDE, BSS, 0.05% CsA and 0.1% Gly-Tβ4 groups after 14
days. Significantly decreased levels of IL-1β, IL-6, TNF-α, and
IFN-γ were observed in the Gly-Tβ4- and CsA-treated groups when
compared with the EDE and BSS groups. There were no significant
differences found between the two treatment groups.
*P<0.05 vs. EDE group; †P<0.05 vs. BSS
group. Il, interleukin; TNF, tumor necrosis factor; IFN,
interferon; UT, untreated control; EDE, experimental dry eye; BSS,
balanced salt solution; CsA, Cyclosporine A; Gly-Tβ4,
glycine-thymosin β4. |
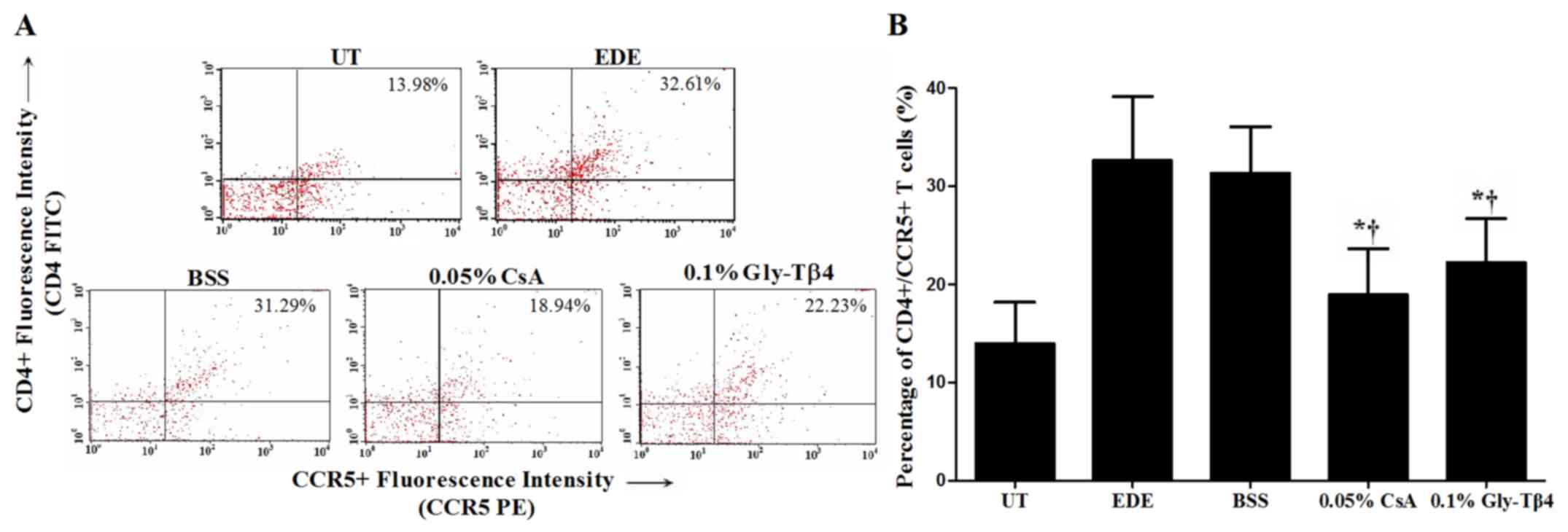
Flow cytometry analysis
The percentage of CD4+/CCR5+ T cells in the
conjunctiva was 13.98±4.18% in the UT group, 32.61±6.54% in the EDE
group, 31.29±4.77% in the BSS group, 18.94±4.77% in the CsA group
and 22.23±4.47% in the Gly-Tβ4 group. The Gly-Tβ4- and CsA-treated
groups both showed a significantly lower percentage of
CD4+/CCR5+ T cells in the conjunctiva
compared with the EDE and BSS groups (EDE vs. Gly-Tβ4 and CsA,
P<0.05; BSS vs. Gly-Tβ4 and CsA, P<0.05), with no differences
between the two treatment groups (Fig.
5).
Discussion
DED is a chronic and progressive ocular surface
disorder in which ocular surface inflammation and damage serve an
important role in the pathogenesis of the disease. In addition,
severe DED may result in epithelial defects of the ocular surface,
corneal ulcers and serious loss of vision, which result in
discomfort to patients, and reduce their quality of life. Various
treatments have proven to be useful for the treatment of DED, and
topical CsA has become one of the standard treatments for
inflammatory DED (1,13). However, no single agent or combination
of agents successfully results in the resolution of DED and ocular
surface healing.
Tβ4 is a natural peptide found in high
concentrations in the majority of tissues and cells, such as blood
platelets, macrophages and other lymphoid tissues (14). Tβ4 effectively downregulates the
levels of inflammatory mediators by inhibiting NF-κB activity and
promoting cell migration (16).
Gly-Tβ4 is a small 44-amino acid peptide with a single glycine
terminal residue added to Tβ4 that can bind to G-actin, and
consequently affects cell migration (14,15).
Topical application of Gly-Tβ4 results in reduced release of
inflammatory molecules and improved corneal epithelial recovery in
an animal model of alkali burn injury (20).
Previous studies have reported that Tβ4 regulated
the inflammatory process by reducing the production of inflammatory
cytokines and chemokines in acute myocardial infarction, alcoholic
liver disease and neurodegenerative diseases (17,26-28).
In ophthalmology, several studies have reported that topical Tβ4
application improved clinical dry eye parameters, including tear
volume, TBUT and corneal staining scores in human and experimental
DED (18,19). However, the effects of topical Tβ4 on
inflammatory or apoptotic changes in DED have not been
investigated.
Ocular surface inflammation, which is characterized
by increased expression of inflammatory cytokines and T cells,
serves a critical role in the pathogenesis of DED. It has
demonstrated that increased levels of inflammatory molecules and
increased number of Th1 cells on the ocular surface may
specifically induce the expression of chemokine receptors, such as
CCR5 and CXCR3 (4,29,30). In
the present study, the effects of topical 0.1% Gly-Tβ4 on
inflammatory cytokines, Th1 cells, apoptotic cells and conjunctival
gobleT cells, as well as on tear film and ocular surface parameters
were investigated using a mouse model of EDE. Regarding Th1 cells,
the percentage of CD4+/CCR5+ cells in the
conjunctiva was measured using flow cytometry, similar to
previously reported studies (31,32). The
anti-inflammatory and anti-apoptotic characteristics of Gly-Tβ4
were clearly shown in our results. Topical instillation of 0.1%
Gly-Tβ4 significantly decreased the levels of inflammatory
cytokines (IL-1β, IL-6, TNF-α and IFN-γ) and the percentage of Th1
cells in the conjunctiva, to a similar degree as 0.05% CsA. In
addition, a decrease in the number of TUNEL positive cells in the
cornea and increased conjunctival gobleT cell density were observed
with treatment of 0.1% Gly-Tβ4 equivalent to that observed with
0.05% CsA.
Anti-inflammatory medicines for the treatment of
DED, such as steroids and CsA, primarily focus on the improvement
of ocular surface inflammation and tear secretion (33,34).
However, ocular surface injury is a risk factor for severe DED that
intensifies the ocular surface inflammatory response, and results
in corneal ulcers and a serious impairment to vision (35). Tβ4 has been shown to improve cellular
epithelium repair by enhancing the expression of laminin-5,
promoting cell migration, and consequently downregulating
inflammatory responses (36).
Additionally, Gly-Tβ4 eye drops have been shown to inhibit corneal
neovascularization and improve epithelial wound healing in a rabbit
model of alkali burn (20). In the
present study, topical 0.1% Gly-Tβ4 treatment resulted in a
significant reversal of corneal epithelial damage, which was
indicated by the reduction in CSS. Thus, it is hypothesized that
the protective effects of topical Gly-Tβ4 on corneal epithelial
healing may be more effective in improving tear film parameters and
ocular surface damage, including tear volume, TBUT and CSS.
Based on the results of the present and previous
studies, topical 0.1% Gly-Tβ4 therapy significantly improves tear
film parameters, ocular surface damage and corneal epithelial
apoptosis in DED, by reducing ocular surface inflammation and
promoting corneal epithelial repair and shows a similar therapeutic
efficacy as 0.05% CsA emulsions. Therefore, Gly-Tβ4 may be used as
a supplementary agent for effective treatment of DED, particularly
for patients with ocular surface defects.
Supplementary Material
Figure S1. Preliminary experiments.
(A) Mean tear volumes, (B) corneal staining scores and (C)
percentage of CD4+/CCR5+ T cells in the conjunctiva measured by
flow cytometry in the EDE, BSS, 0.001% Gly-Tβ4, 0.01% Gly-Tβ4 and
0.1% Gly-Tβ4 groups. *P<0.05 vs. EDE group; †P<0.05 vs. BSS
group. EDE, experimental dry eye; BSS, balanced salt solution;
Gly-Tβ4, glycine-thymosin β4.
Acknowledgements
Not applicable.
Funding
This study was supported by Huons Co., Ltd., the
Basic Research Program through the National Research Foundation of
Korea and funded by the Ministry of Science, ICT & Future
Planning (grant no. 2017R1A2B4003367), and the Chonnam National
University Hospital Biomedical Research Institute (grant nos.
CRI18093-1 and BCRI 19038).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
KCY designed the experiment and revised the
manuscript. RJ, YL, LL, JHC and DHK performed the experiments. RJ,
YL, HJY, CDY and HSS analyzed and interpreted the data. RJ, YL,
DHK, CDY and HSS drafted the manuscript. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
The research protocol used in the present study was
approved by the Chonnam National University Medical School Research
Institutional Animal Care and Use Committee. Maintenance of animals
and all in vivo experiments were performed in accordance
with the Association for Research in Vision and Ophthalmology
statement for the Use of Animals in Ophthalmic and Vision
Research.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
The epidemiology of dry eye disease:
Report of the epidemiology subcommittee of the international dry
eye workshop (2007). Ocul Surf 5: 93-107, 2007.
|
|
2
|
Farrand KF, Fridman M, Stillman IÖ and
Stillman IO: Prevalence of diagnosed dry eye disease in the united
states among adults aged 18 years and older. Am J Ophthalmol.
182:90–98. 2017.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Wolffsohn JS, Arita R, Chalmers R,
Djalilian A, Dogru M, Dumbleton K, Gupta PK, Karpecki P, Lazreg S,
Pult H, et al: TFOS DEWS II diagnostic methodology report. Ocul
Surf. 15:539–574. 2017.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Yoon KC, De Paiva CS, Qi H, Chen Z, Farley
WJ, Li DQ and Pflugfelder SC: Expression of Th-1 chemokines and
chemokine receptors on the ocular surface of C57BL/6 mice: Effects
of desiccating stress. Invest Ophthalmol Vis Sci. 48:2561–2569.
2007.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Craig JP, Nichols KK, Akpek EK, Caffery B,
Dua HS, Joo CK, Liu Z, Nelson JD, Nichols JJ, Tsubota K and
Stapleton F: TFOS DEWS II definition and classification report.
Ocul Surf. 15:276–283. 2017.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Stern ME and Pflugfelder SC: Inflammation
in dry eye. Ocul Surf. 2:124–130. 2004.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Pflugfelder SC, De Paiva CS, Li DQ and
Stem ME: Epithelial-immune cell interaction in dry eye. Cornea.
27:S9–S11. 2008.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Bucolo C, Musumeci N, Musumeci S and Drago
F: Acidic mammalian chitinase and the eye: Implications for ocular
inflammatory diseases. Front Pharmacol. 2(43)2011.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Bucolo C, Fidilio A, Fresta CG, Lazzara F,
Platania CBM, Cantarella G, Di Benedetto G, Burgaletto C,
Bernardini R, Pizza C, et al: Ocular pharmacological profle of
hydrocortisone in dry eye disease. Front Pharmacol.
10(1240)2019.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Li Y, Cui L, Lee HS, Kang YS, Choi W and
Yoon KC: Comparison of 0.3% hypotonic and isotonic sodium
hyaluronate eye drops in the treatment of experimental dry eye.
Curr Eye Res. 42:1108–1114. 2017.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Yang JM, Choi W, Kim N and Yoon KC:
Comparison of topical cyclosporine and diquafosol treatment in dry
eye. Optom Vis Sci. 92:e296–e302. 2015.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Kim HS, Kim TI, Kim JH, Yoon KC, Hyon JY,
Shin KU and Choi CY: Evaluation of clinical efficacy and safety of
a novel cyclosporine A nanoemulsion in the treatment of dry eye
syndrome. J Ocul Phamacol Ther. 33:530–538. 2017.PubMed/NCBI View Article : Google Scholar
|
|
13
|
De Oliveira and Wilson SE: Practical
guidance for the use of cyclosporine ophthalmicsolutions in the
management of dry eye disease. Clin Ophthalmol. 13:1115–1122.
2019.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Crockford D, Turjman N, Allan C and Angel
J: Thymosin beta4: Structure, function, and biological properties
supporting current and future clinical applications. Ann NY Acad
Sci. 1194:179–189. 2010.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Huff T, Müller CS, Otto AM, Netzker R and
Hannappel E: beta-Thymosins, small acidic peptides with multiple
functions. Int J Biochem Cell Biol. 33:205–220. 2001.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Sosne G, Qiu P, Christopherson PL and
Wheater MK: Thymosin beta 4 suppression of corneal NFkappaB: A
potential anti-inflammatory pathway. Exp Eye Res. 84:663–669.
2007.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Pardon MC: Anti-inflammatory potential of
thymosin β4 in the central nervous system: Implications for
progressive neurodegenerative diseases. Expert Opin Biol Ther. 18
(Suppl 1):S165–S169. 2018.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Sosne G and Ousler GW: Thymosin beta 4
ophthalmic solution for dry eye: A randomized, placebo-controlled,
phase II clinical trial conducted using the controlled adverse
environment (CAE™) model. Clin Ophthalmol. 9:877–884.
2015.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Sosne G, Dunn SP and Kim C: Thymosin β4
significantly improves signs and symptoms of severe dry eye in a
phase 2 randomized trial. Cornea. 34:491–496. 2015.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Zhang W, Nie L, Du L, Chen W, Wu Z and Jin
Y: Topical treatment of corneal alkali burns with Gly-thymosin
β4 solutions and in situ hydrogels via inhibiting
corneal neovascularization and improving corneal epidermal recovery
in experimental rabbits. Burns. 43:1742–1747. 2017.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Yoon KC, De Paiva CS, Qi H, Chen Z, Farley
WJ, Li DQ, Sterm ME and Pflugfelder SC: Desiccating environmental
stress exacerbates autoimmune lacrimal keratoconjunctivitis in
non-obese diabetic mice. J Autoimmun. 30:212–221. 2008.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Yoon KC, Ahn KY, Choi W, Li Z, Choi JS,
Lee SH and Park SH: Tear production and ocular surface changes in
experimental dry eye after elimination of desiccating stress.
Invest Ophthalmol Vis Sci. 52:7267–7273. 2011.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Villareal AL, Farley W and Pflugfelder SC:
Effect of topical ophthalmic epinastine and olopatadine on tear
volume in mice. Eye Contact Lens. 32:272–276. 2006.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Pauly A, Brignole-Baudouin F, Labbė A,
Liang H, Warnet JM and Baudouin C: New tools for the evaluation of
toxic ocular surface changes in the rat. Invest Ophthalmol Vis Sci.
48:5473–5483. 2007.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Yoon KC, Park CS, You IC, Choi HJ, Lee KH,
Im SK, Park HY and Pflugfelder SC: Expression of CXCL9, -10, -11,
and CXCR3 in the tear film and ocular surface of patients with dry
eye syndrome. Invest Ophthalmol Vis Sci. 51:643–650.
2010.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Shah R, Reyes-Gordillo K, Cheng Y,
Varatharajalu R, lbrahin J and Lakshman MR: Thymosin β4 prevents
oxidative stress, inflammation, and fibrosis in ethanol- and
LPS-induced liver injury in mice. Oxid Med Cell Longev.
2018(9630175)2018.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Cacasin MA: Therapeutic potential of
thymosin-beta4 and its derivative
N-acetyl-seryl-aspartyl-lysyl-proline (Ac-SDKP) in cardiac healing
after infarction. Am J Cardiovasc Drugs. 6:305–311. 2006.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Goldstein AL, Hannappel E, Sosne G and
Kleinman HK: Thymosin β4: A multi-functional regenerative peptide.
Basic properties and clinical applications. Expert Opin Biol Ther.
12:37–51. 2012.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Gulati A, Sacchetti M, Bonini S and Dana
R: Chemokine receptor CCR5 expression in conjunctival epithelium of
patients with dry eye syndrome. Arch Ophthalmol. 124:710–716.
2006.PubMed/NCBI View Article : Google Scholar
|
|
30
|
EI Annan J, Chauhan SK, Ecoiffier T, Zhang
Q, Saban DR and Dana R: Characterization of effector T cells in dry
eye disease. Invest Ophthalmol Vis Sci. 50:3802–3807.
2009.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Li Y, Jin R, Li L, Hsu HH, You IC, Yoon HJ
and Yoon KC: Therapeutic effect of topical adiponectin-derived
short peptides compared with globular adiponectin in experimental
dry eye and alkali burn. J Ocul Phamacol Ther. 36:88–96.
2020.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Choi W, Li Z, Oh HJ, Im SK, Lee SH, Park
SH, You IC and Yoon KC: Expression of CCR5 and its ligands CCL3,
-4, and -5 in the tear film and ocular surface of patients with dry
eye disease. Curr Eye Res. 37:12–17. 2012.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Boboridis KG and Konstas AGP: Evaluating
the novel application of cyclosporine 0.1% in ocular surface
disease. Expert Opin Pharmacother. 19:1027–1039. 2018.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Yin J, Kheirkhah A, Dohiman T, Saboo U and
Dana R: Reduced efficacy of low-dose topical steroids in dry eye
disease associated with graft-versus-host disease. Am J Ophthalmol.
190:17–23. 2018.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Pflugfelder SC: Integrating restasis into
the management of dry eye. Int Ophthlamol Clin. 46:101–103.
2006.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Sosne G, Xu L, Prach L, Mrock LK, Kleinman
HK, Letterio JJ, Hazlett LD and Kurpakus-Wheater M: Thymosin beta 4
stimulates laminin-5 production independent of TGF-beta. Exp Cel
Res. 293:175–183. 2004.PubMed/NCBI View Article : Google Scholar
|