Introduction
Ulcerative colitis (UC) and Crohn's disease (CD),
collectively known as inflammatory bowel disease (IBD), are
chronic, relapsing, immune-mediated disorders (1). CD is characterized by patchy
granulomatous inflammation that may affect any part of the
gastrointestinal tract, from the mouth to the anus (2). UC is characterized by a continuous
pattern of inflammation that is restricted to the colon (3). The prevalence of IBD has rapidly
increased in Europe and North America in the second half of the
twentieth century and is becoming more common in the rest of the
world, as different countries adopt a Western based diet and
lifestyle (4).
The pathogenesis underlying IBD is complex and
results from the interaction of environmental factors, genetic
variations and intestinal microbiota with the innate and adaptive
immune responses (5). Altered immune
responses are considered the cornerstone of the pathogenesis
underlying IBD (5). For example, in
both forms of IBD, the numbers of macrophages and dendritic cells
in the lamina propria increase and attain an activated phenotype
(5). Furthermore, the production of
pro-inflammatory cytokines and chemokines is also enhanced
(5). The analysis of the inflamed
mucosa from patients with IBD shows an increase in the expression
of several cytokines, such as interleukin (IL)-1, IL-6, IL-8 and
tumor necrosis factor (TNF)-α (5).
These cytokines are hypothesized to subsequently direct the
development of an adaptive immune response which is primarily
mediated by T and B lymphocytes (6).
The cumulative effect of the above processes eventually leads to
IBD.
The production of cytokines serves a central role in
the pathogenesis of IBD. Another hallmark of IBD is the dysmotility
of the muscular layers of the bowel (7). The specific mechanism underlying the
IBD-mediated changes in contractility are currently unknown but may
be directly or indirectly associated with the increased production
of cytokines. The neurotrophic factor, brain derived neurotrophic
factor (BDNF), has been shown to be secreted by smooth muscle cells
of the rat colon in a dextran sodium sulphate induced colitis model
(8), which enhances the cholinergic
contraction of the smooth muscle cells of the colon (9). Taken together, it is hypothesized that
cytokines produced from the inflammation of the bowel observed in
IBD, may directly stimulate the expression of BDNF in the smooth
muscle cells of the colon. Secreted BDNF acts in an autocrine
manner and affects the contractility of the smooth muscle cells
themselves. These observations demonstrate a tentative link between
the increased production of inflammatory cytokines in bowel tissues
and the ensuing changes in contractility. To support this
hypothesis, the aim of the present study was to test the hypothesis
that direct treatment of colon smooth muscle cells with
inflammatory cytokines increased the synthesis and secretion of
BDNF.
Materials and methods
Animal experiments
All experiments were performed in accordance with
the Institutional Animal Care and Use Committee at Jordan
University of Science and Technology (approval no. 2019/0023). Male
adult Sprague-Dawley rats, weighing 150-200 g, were maintained at
the University animal house under with a 12-h light/dark cycle, in
polyethylene cages at -22˚C and 50% humidity.
A total of 20 rats were euthanized using 100%
CO2. The colons were dissected, emptied of their
contents and placed in cold smooth muscle buffer (120 mM NaCl, 4 mM
KCl, 2.6 mM KH2PO4, 2.0 mM CaCl2,
0.6 mM MgCl2, 25 mM HEPES, 14 mM glucose and 2.1%
essential amino mixture; pH 7.4). Sections (2-3 cm) of the colon
were removed and mounted onto a glass rod. The fat and mesenteric
regions were removed, and the longitudinal muscle was separated
from the circular layer by radial abrasion with a Kim wipe. The
muscle layers were released from the mucosal/submucosal layers
using micro dissection and cut into small sections using surgical
scissors. Equal amounts (1.5 g/well) were placed in 6-well plates
containing DMEM with penicillin (200 U/ml), streptomycin (200
µg/ml), gentamycin (100 µg/ml1) and amphotericin B (2.5
µg/ml), and placed in a 37˚C incubator.
TNF-α and IL-1β exposure
Longitudinal smooth muscle tissues from rat colons
were exposed for 24 h to either medium alone (control, n=5) or
medium supplemented with 10 ng/ml recombinant human TNF-α (n=3;
R&D Systems, Inc.; cat. no. 210-TA-005) or 10 ng/ml IL-1β (n=3;
Sigma-Aldrich; Merck KGaA; cat. no. 11457756001) for 24 h. The
roles of Ca2+ and PKA were tested by pre-treating smooth
muscle tissues with 1 µM BABTA-AM (n=3; R&D Systems, Inc.) or 1
µM PKA inhibitor 6-22 (n=3; EMD Millipore).
Protein extraction
Smooth muscle tissues were homogenized with
solubilization buffer [50 mM Tris-HCL, 150 mM NaCL, 1 mM EDTA, 1%
Triton X-100, 100 mM NaF and protease/phosphatase inhibitor
cocktail (100 µg ml-1 PMSF, 10 µg ml-1
aprotinin, 10 µg ml-1 leupeptin, 30 mM sodium fluoride
and 3 mM sodium vanadate)]. After sonication for 15 sec, and
centrifugation at 2,000 x g for 10 min at 4˚C, protein
concentrations in the supernatant were determined using a DC
protein assay kit according to the manufacturer's protocol (Bio-Rad
Laboratories, Inc).
ELISA
Secreted BDNF and BDNF protein levels in smooth
muscle tissues were measured using sandwich ELISA (Promega BDNF
Emax immunoassay; Promega Corporation; cat. no. G7611) according to
the manufacturer's protocol. The samples were acidified to a
pH<3.0 with 1 M HCI for 15 min and then neutralized to pH 7.6
prior to use for ELISA. The antibody used was specific for BDNF
with <3% cross reactivity with nerve growth factor, NT-3 and
NT-4, with no cross reactivity with PACAP, SP, VIP, secretin and
somatostatin according to manufacturer's protocol. The limit for
detection with ELISA is 4 ng/ml and the range is 4-500 ng/ml.
Briefly, ELISA plates were coated with anti-BDNF mAb (1:1,000) and
incubated overnight at 4˚C. The following day, the plate was washed
and blocked with blocking buffer (Promega Corporation). A total of
100 µl BDNF standard or sample was added to each well and incubated
for 2 h at room temperature. The plate was washed, and 100 µl
anti-BDNF pAb (1:500) was added to each well and incubated at room
temperature for 2 h. After washing, 100 µl of diluted
anti-immunoglobulin Y-horseradish peroxidase conjugate (1:200) was
added to each well and developed with TMB solution and 1 M HCI. The
absorbance was measured at 450 nm using an ELISA microplate reader
(elx-800; BioTek Instruments, Winooski, VT, USA), and the
concentration of BDNF in the samples was calculated using a
standard curve. Data are expressed relative to the total protein
concentration and compared with the control, TNF-α and IL-1β
treated groups.
Statistical analysis
Data are presented as the mean ± the standard error
of the mean. Each experiment used at least three animals and was
repeated three times. GraphPad PRISM version 8 (GraphPad Software,
Inc.) was used for statistical analysis. ANOVA with a post hoc
Tukey's test was used for multiple comparisons. P<0.05 was
considered to indicate a statistically significant difference.
Results
Effect of TNF-α and IL-1β on BDNF
expression in the longitudinal muscles of the rat colon
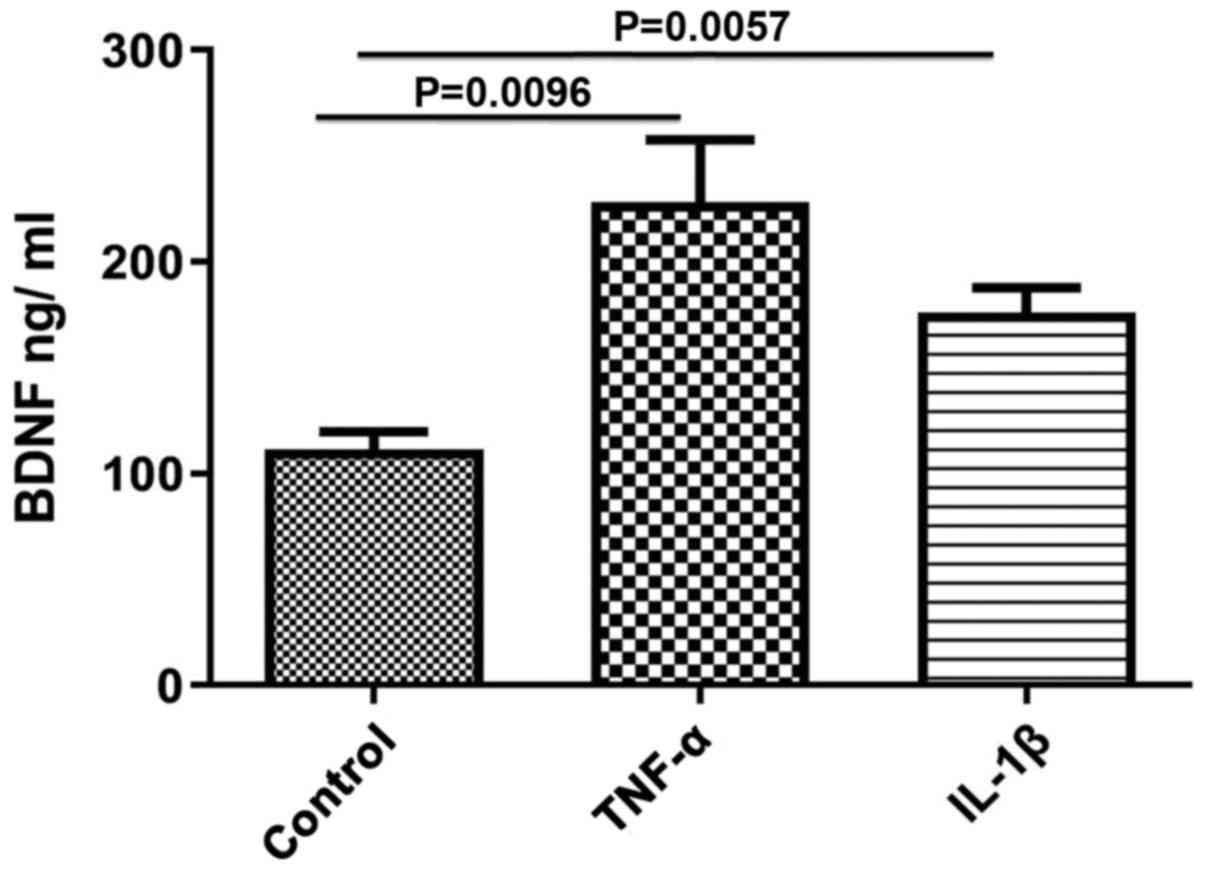
The effect of TNF-α and IL-1β on BDNF protein
content in smooth muscle tissues was evaluated. Both TNF-α and
IL-1β significantly upregulated BDNF protein expression levels
compared with the control (control, 111.7±8.11; TNF-α, 228±29.51,
P=0.0096; IL-1β, 175.8±12.03, P=0.0057; Fig. 1). The effect of TNF-α was greater than
that of IL-1β, but was not statistically significant.
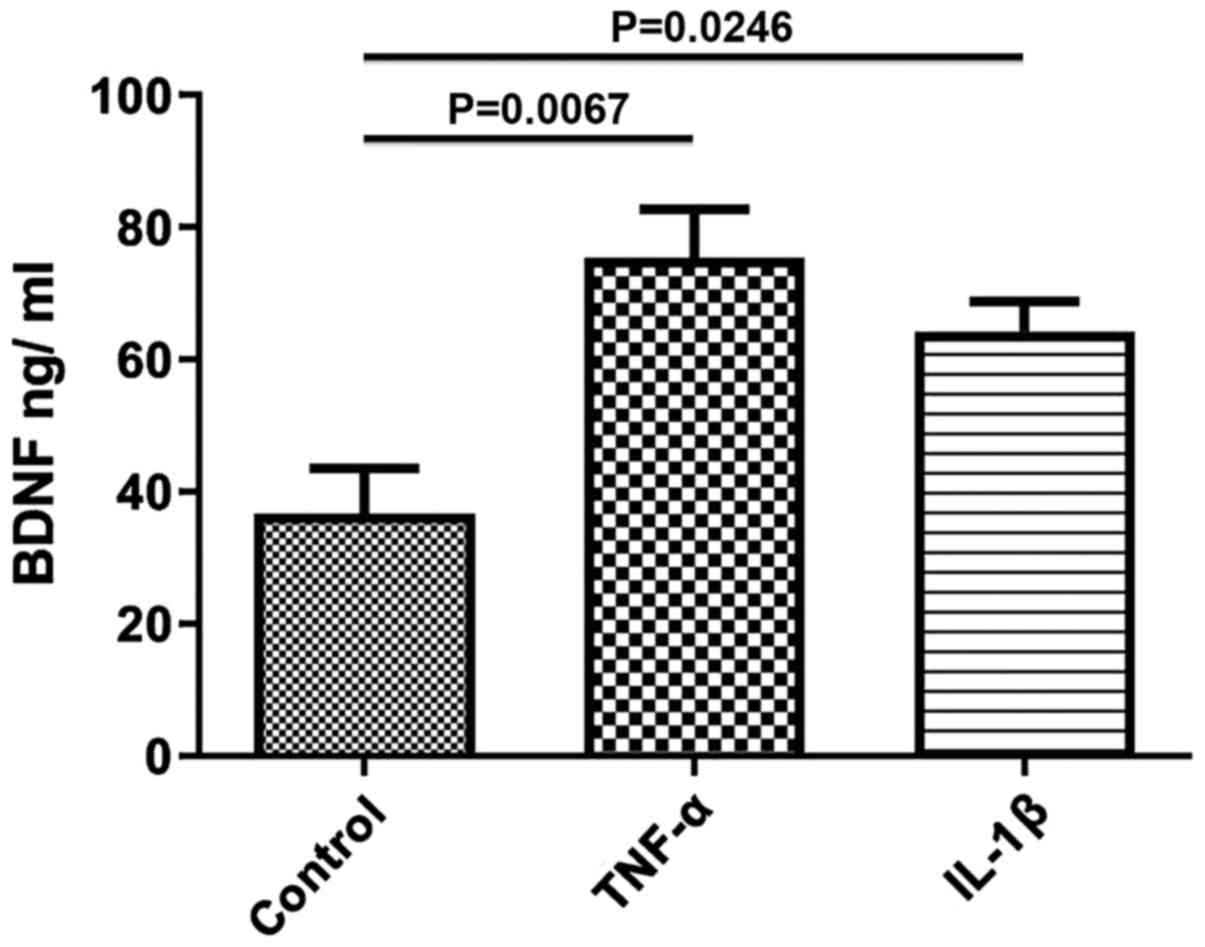
Effect of TNF-α and IL-1β on BDNF
secretion in the longitudinal smooth muscle of rat colons
To study the effects of TNF-α and IL-1β on the
secretion of BDNF, longitudinal smooth muscle tissues were treated
with TNF-α and IL-1β for 24 h and the quantity of BDNF release into
the media was determined using ELISA. After 24 h of incubation with
either of the cytokines, secretion of BDNF was significantly
increased compared with the control (control, 36.67±6.888; TNF-α,
75.33±7.42, P=0.0067; IL-1β, 64.25±4.54, P=0.0246; Fig. 2). The increase in BDNF secretion
induced by TNF-α was greater than that of IL-1β, but was not
statistically significant.
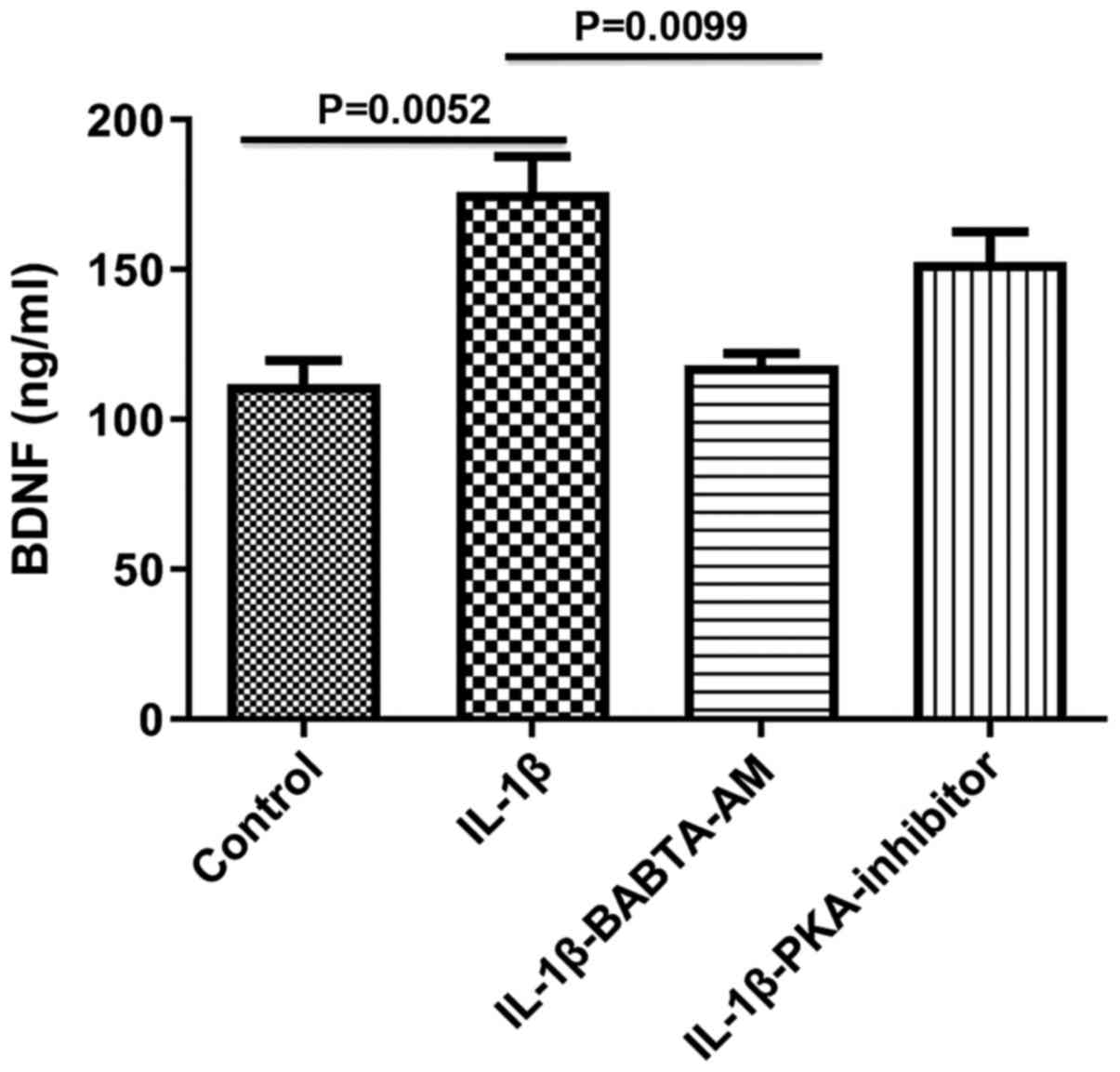
Role of Ca2+ and PKA in
mediating the effects of IL-1β on BDNF expression in longitudinal
muscle tissues
To determine the mechanism of action underlying the
IL-1β-mediated increase in BDNF expression, the roles of
Ca2+ and PKA were both investigated, as both are
involved in the responses evoked by IL-1β, and are known activators
of BDNF gene transcription (10,11).
Incubation of longitudinal muscle tissues with IL-1β for 24 h in
the presence of 1 µM BAPTA-AM to chelate Ca2+, abrogated
the ability of IL-1β to increase BDNF expression (control,
111.7±8.11; IL-1β, 175.8±12.03, P=0.0052 vs. control;
IL-1β-BABTA-AM, 118.0±4.041, P=0.913 vs. control; P=0.0099, IL-1β
vs. IL-1β-BABTA-AM; Fig. 3). However,
the effect of 1 µM of PKA inhibitor 6-22 Amide did not
significantly alter the effect of IL-1β on BDNF expression
(control, 111.7±8.11; IL-1β, 175.8±12.03; IL-1β-PKA inhibitor,
152.3±10.48; P=0.3720, IL-1β vs. IL-1β-PKA).
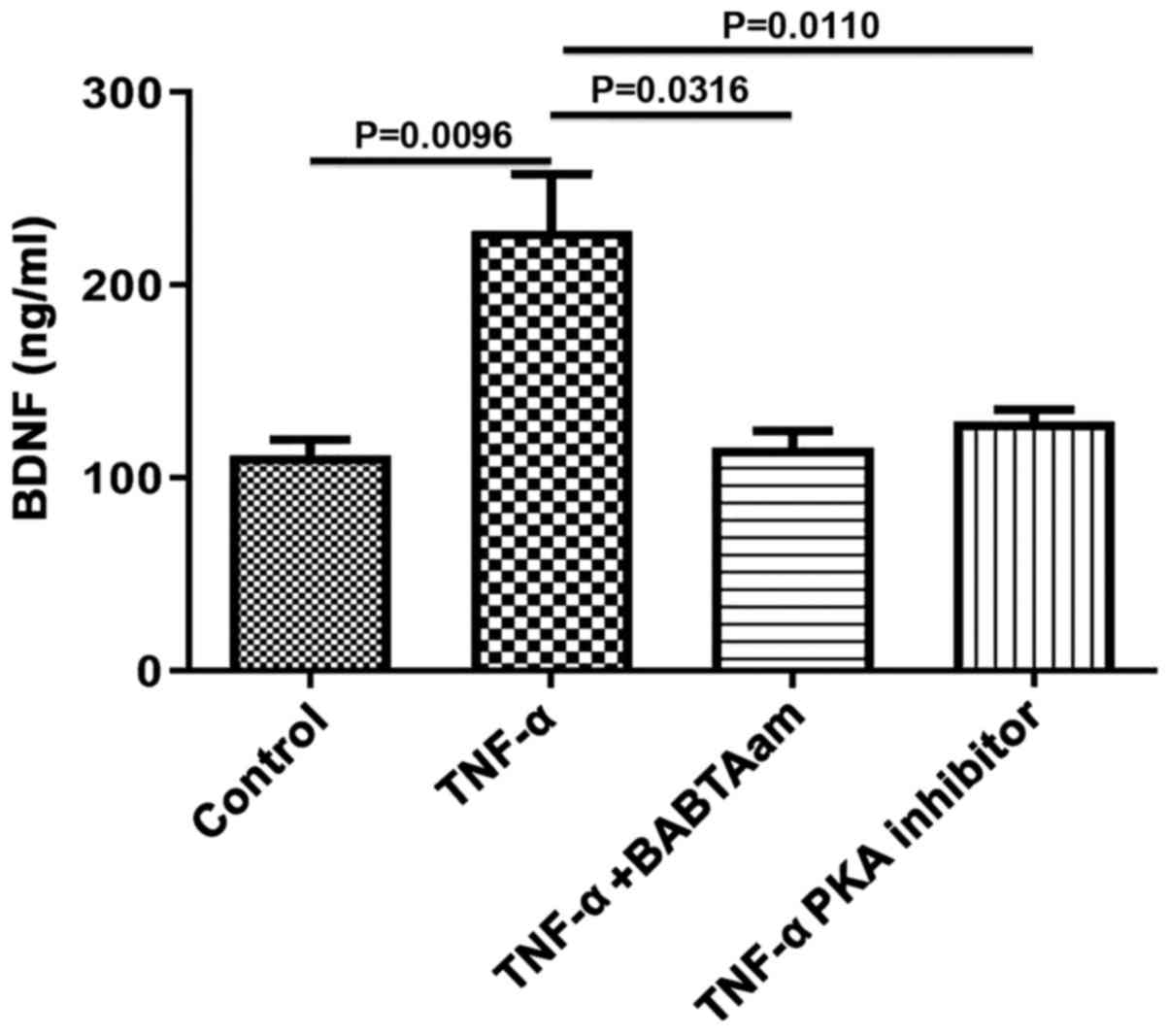
Role of Ca2+ and PKA in
mediating the effect of TNF-α on BDNF expression in longitudinal
muscle tissues
To determine the mechanism of action underlying
TNF-α on BDNF expression, the role of Ca2+ and PKA was
investigated, as both are associated with TNF-α-mediated effects.
Incubation of longitudinal muscle tissues with 10 ng/ml TNF-α for
24 h in the presence of 1 µM BAPTA-AM to chelate Ca2+
abrogated the ability of TNF-α to increase BDNF expression
(control, 111.7±8.11; TNF-α, 228±29.51, P=0.0096 vs. control;
TNF-α-BAPTA-AM, 115.7±8.686, P=0.6934 vs. control; P=0.0316, TNF-α
vs. TNF-α-BAPTA-AM; Fig. 4).
Furthermore, PKA inhibition with 1 µM of 6-22 Amide abolished the
effects of TNF-α on BDNF expression (control, 111.7±8.11; TNF-α,
23±29.51, P=0.0096 vs. control; TNF-α-PKA inhibitor, 129±6.35,
P=0.9834 vs. control; P=0.0110, TNF-α vs. TNF-α-PKA).
Role of Ca2+ and PKA in
mediating the effect of IL-1β on BDNF secretion from longitudinal
muscle tissues
As BDNF is secreted in an activity dependent manner
in response to elevated intracellular Ca2+, the role of
general Ca2+ on BDNF secretion in response to IL-1β was
assessed. Incubation of rat smooth muscle tissue for 24 h with
IL-1β in the presence of 1 µm of BAPTA-AM resulted in complete
inhibition of IL-1β on BDNF secretion (control, 36.67±6.89; IL-1β,
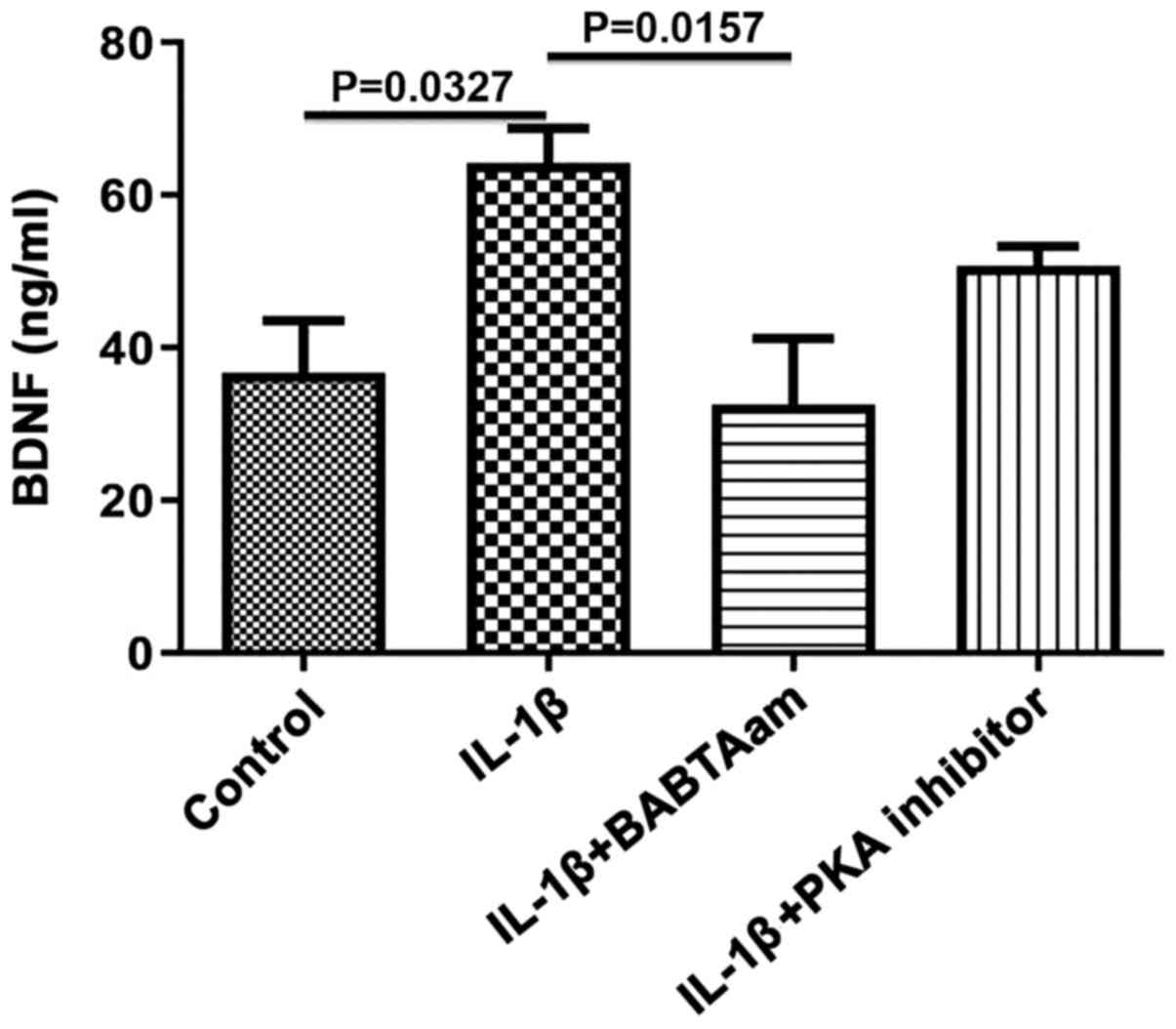
64.25±4.54, P=0.0327 vs. control; IL-1β-BAPTA-AM, 32.67±8.57,
P=0.9656 vs. control; P=0.0157, IL-1β vs. IL-1β-BAPTA-AM; Fig. 5). Treatment with PKA did not
significantly decrease BDNF secretion (control, 36.67±6.888; IL-1
β, 64.25±4.54; IL-1β-PKA inhibitor, 50.67±2.60; P=0.3882, IL-1β vs.
IL-1β-PKA).
Role of Ca2+ and PKA in
mediating the effect of TNF-α on BDNF secretion from longitudinal
muscle tissues
Incubation of rat smooth muscle tissues for 24 h
with TNF-α in the presence of 1 µm of BAPTA-AM abolished the
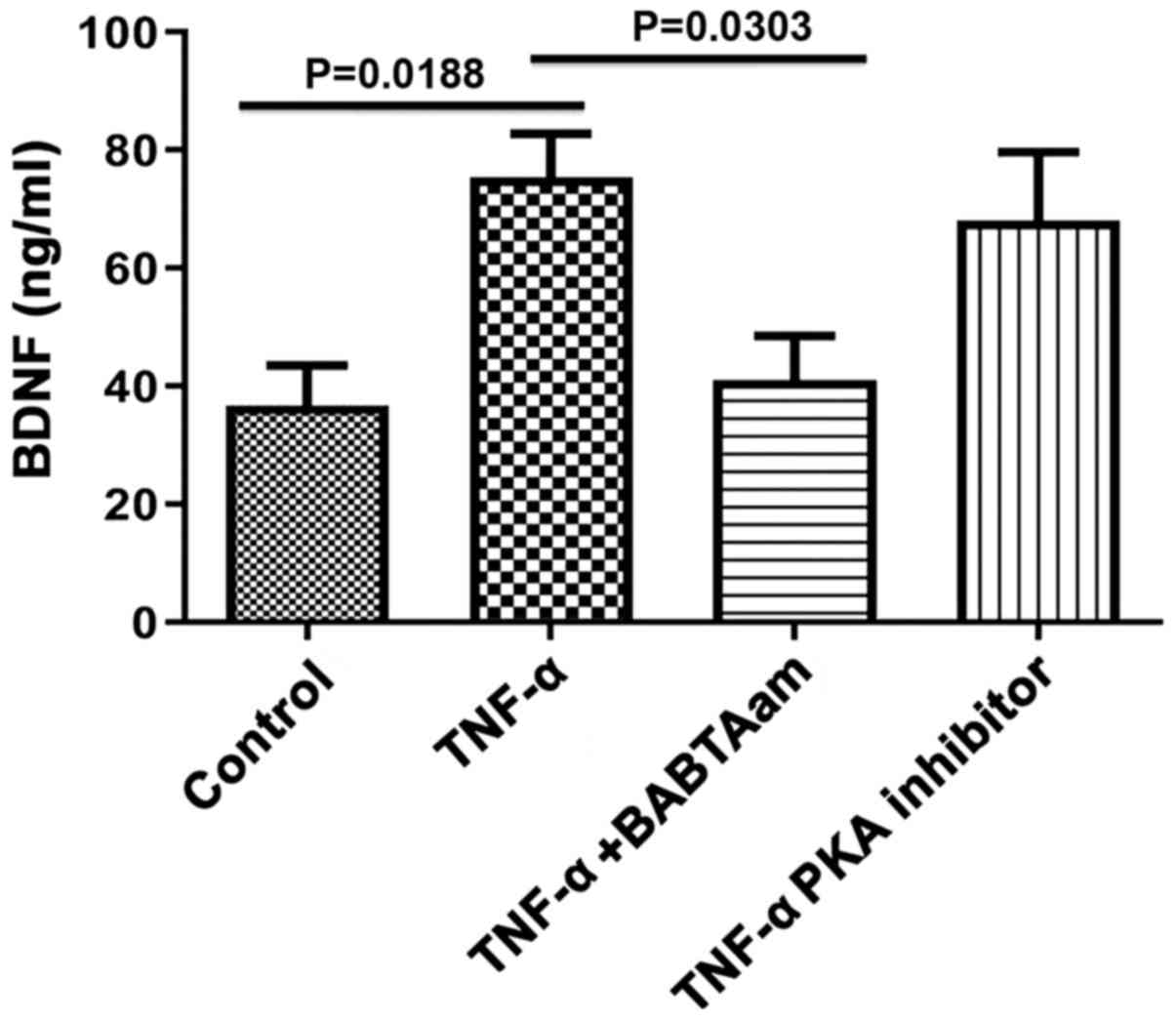
ability of TNF-α to increase BDNF secretion (control, 36.67±6.89;
TNF-α, 75.33±7.42, P=0.0188 vs. control; TNF-α-BAPTA-AM, 41±7.55,
P=0.0.6934 vs. control; P=0.0303, TNF-α vs. TNF-α-BAPTA-AM;
Fig. 6). However, 1 µM of 6-22 Amide
did not significantly inhibit BDNF secretion induced by TNF-α
(control, 36.67±6.89; TNF-α, 75.33±7.42, P=0.0188 vs. control;
TNF-α-PKA inhibitor, 68.00±11.68, P=0.0785 vs. control; P=0.9283,
TNF-α vs. TNF-α-PKA).
Discussion
In the present study, it was demonstrated that the
exogenous proinflammatory cytokines, TNF-α and IL-1β, upregulated
BDNF protein expression and secretion in rat colon smooth muscle
tissues. Furthermore, the expression and secretion of BDNF by TNF-α
and IL-1β were likely Ca2+-dependent. Finally the effect
of TNF-α on the expression and secretion of BDNF was regulated by
the cAMP/PKA pathway as well.
Previously, it has been shown that BDNF and its
receptors are present in adult rat colon smooth muscle, and its
expression is increased in inflammatory diseases such as UC
(8,12). Furthermore, exogenous BDNF enhances
cholinergic contraction of the smooth muscle cells associated with
IBD (9). In the present study, it was
demonstrated that TNF-α and IL-1β significantly upregulated BDNF
levels in rat colon smooth muscle tissues. In agreement with the
results of the present study, inflammatory cytokines enhanced BDNF
expression and secretion in airway smooth muscle cells (13). The effect of these cytokines may
underlie functional and structural changes which take place during
bowel inflammation. For example, the reported changes in gut
motility during colitis and in response to inflammatory cytokines
(14) may be due to the increase in
expression/secretion of BDNF which was induced by TNF-α and IL-1β,
as demonstrated in the present study. The effect of BDNF on gut
motility is well established. BDNF enhances stool frequency in
patients treated with a higher dose of BDNF, enhances
gastrointestinal and colonic transit in human subjects, accelerates
myoelectric activity in the gastrointestinal tract and enhances
peristalsis in the rat colon (15-18).
Furthermore, BDNF may account for the abdominal pain and visceral
hypersensitivity experienced by patients with gut inflammation
(19). Intraperitoneal BDNF
injections results in increased pain sensation in response to colon
distension in healthy rats, and administration of BDNF antibodies
inhibited visceral hypersensitivity in experimental colitis
(19), and BDNF heterogeneous
knockout animals experienced notably less pain compared with wild
type mice in a model of colitis (20). Furthermore, BDNF increases the
expression of calcitonin gene-related peptide during colitis, which
is considered a pain mediator (21).
The results of the present study suggest that TNF-α and IL-1β may
underlie the increase in BDNF expression.
An additional aim of the present study was to
delineate the mechanisms underlying the increase in BDNF expression
and secretion mediated by proinflammatory cytokines. BDNF
expression by both TNF-α and IL-1β was Ca2+ and PKA
dependent. BDNF gene expression is under the control of
intracellular Ca2+ and PKA levels (22). Stimuli that result in an increase in
the intracellular Ca2+ levels activate BDNF
transcription and synthesis by activating
Ca2+/calmodulin, which phosphorylates
calmodulin-dependent protein kinase I (CaMKII). CaMKII, in turn,
phosphorylates cAMP response element-binding protein (CREB) which
results in BDNF transcription and translation (23). Although in the present study,
Ca2+ levels were not measured in response to TNF-α and
IL-1β, it has previously been shown that these cytokines may
increase intracellular Ca2+ levels in various types of
cells, including smooth muscle cells (24). In agreement with the results of the
present study, TNF-α upregulates BDNF protein in primary astrocytes
(25), monocytes (26), neurons (27) and smooth muscle cells (13). In airway smooth muscle cells,
elevation of intracellular Ca2+ and PKA are essential
for TNF-α induced BDNF synthesis (13).
BDNF is secreted in an activity dependent manner
that is regulated by intracellular Ca2+ levels (28). In the present study, it was shown that
the TNF-α and IL-1β induced BDNF release was inhibited by the
chelation of intracellular and extracellular Ca2+ using
BAPTA-AM. As the source of Ca2+ was not investigated,
future studies should use different pharmacological inhibitors of
intracellular and extracellular Ca2+ routes, to
elucidate the pathways involved in cytokine induced BDNF
release.
BDNF may act in a positive feedback loop to induce
its synthesis and release (28). BDNF
activates three primary signaling pathways; ERK, PI3K and
phospholipase C, resulting in increases in intracellular
Ca2+ levels (29). Thus in
future studies the effects of these cytokines in the presence and
absence of tropomyosin-related kinase B should be investigated to
assess this possibility. In airway smooth muscle cells,
cytokine-mediated BDNF release was Ca2+ dependent and
involved a positive feedback mechanism mediated by BDNF, acting in
an autocrine and paracrine manner (13), which may also apply to colon smooth
muscle cells.
The reported role of cAMP/PKA in cytokine-induced
BDNF synthesis and release is supported by several previous
studies. It was previously shown that substance P (SP) and
pituitary adenylate cyclase activating peptide (PACAP) induced BDNF
synthesis in intestinal smooth muscle cells in a cAMP/PKA dependent
manner (30). PKA inhibition resulted
in reduced BDNF synthesis in response to SP or PACAP treatment
(30). In neurons from rat
hippocampi, BDNF expression was under the control of PKA activation
and the subsequent phosphorylation of CREB (31-33)
these data demonstrate that cytokines stimulation of BDNF synthesis
in colon smooth muscle cells is PKA dependent as well.
In conclusion, TNF-α and IL-1β upregulated BDNF
synthesis and release in rat colon smooth muscle cells. Both
cytokines depend on Ca2+ for their action on BDNF
synthesis and release; however, PKA may have been more involved in
BDNF synthesis rather than release. These data may highlight novel
potential avenues for treatment of IBD, by targeting BDNF.
Acknowledgements
Not applicable.
Funding
The present study was supported by a grant from the
Jordan University of Science and Technology (grant no.
2019/0102).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
MAQ conceived the study and wrote the manuscript. MA
designed the study and collected the data. OAS generated the
figures and supervised the study. RS performed the statistical
analysis. AAD designed the experiments and revised the manuscript.
All authors have read and approved the final manuscript.
Ethics approval and consent to
participate
All experiments were performed in accordance with
the Institutional Animal Care and Use Committee at Jordan
University of Science and Technology (approval no. 2019/0023).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Hanauer SB: Inflammatory bowel disease:
Epidemiology, pathogenesis, and therapeutic opportunities. Inflamm
Bowel Dis. 12 (Suppl 1):S3–S9. 2006.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Loftus EV, Schoenfeld P and Sandborn WJ:
The epidemiology and natural history of Crohn's disease in
population-based patient cohorts from North America: A systematic
review. Aliment Pharmacol Ther. 16:51–60. 2002.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Sloan WP Jr, Bargen FA and Gage RP: Life
histories of patients with chronic ulcerative colitis: A review of
2,000 cases. Gastroenterology. 16:25–38. 1950.PubMed/NCBI
|
|
4
|
Loftus EV Jr: Clinical epidemiology of
inflammatory bowel disease: Incidence, prevalence, and
environmental influences. Gastroenterology. 126:1504–1517.
2004.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Sobolewska-Włodarczyk A and Włodarczyk M:
Pathogenesis of IBD. In: Introduction to Gastrointestinal Diseases
Vol. 1. Springer, pp 83-93, 2017.
|
|
6
|
Műzes G, Molnár B, Tulassay Z and Sipos F:
Changes of the cytokine profile in inflammatory bowel diseases.
World J Gastroenterol. 18:5848–5861. 2012.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Vermillion DL, Huizinga JD, Riddell RH and
Collins SM: Altered small intestinal smooth muscle function in
Crohn's disease. Gastroenterology. 104:1692–1699. 1993.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Al-Qudah M, Shammala DA, Al-Dwairi A,
Al-Shboul O and Mustafa AG: Dextran sodium sulphate (DSS)-induced
colitis alters the expression of neurotrophins in smooth muscle
cells of rat colon. Physiol Res. 66:1009–1020. 2017.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Al-Qudah M, Anderson CD, Mahavadi S,
Bradley ZL, Akbarali HI, Murthy KS and Grider JR: Brain-derived
neurotrophic factor enhances cholinergic contraction of
longitudinal muscle of rabbit intestine via activation of
phospholipase C. Am J Physiol Liver Physiol. 306:G328–G337.
2013.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Zheng F, Zhou X, Luo Y, Xiao H, Wayman G
and Wang H: Regulation of brain-derived neurotrophic factor exon IV
transcription through calcium responsive elements in cortical
neurons. PLoS One. 6(e28441)2011.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Xue W, Wang W, Gong T, Zhang H, Tao W, Xue
L, Sun Y, Wang F and Chen G: PKA-CREB-BDNF signaling regulated long
lasting antidepressant activities of Yueju but not ketamine. Sci
Rep. 6(26331)2016.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Al-Qudah M, Shammala DA, Al-Dwairi A and
Al-Shboul O: Differential expression of neurotrophins in
(DSS)-induced colitis in smooth muscle of rat colon. J Teknol 78,
2016.
|
|
13
|
Aravamudan B, Thompson MA, Pabelick CM and
Prakash YS: Mechanisms of BDNF regulation in asthmatic airway
smooth muscle. Am J Physiol Lung Cell Mol Physiol. 311:L270–L279.
2016.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Nalli AD, Kumar DP, Mahavadi S, Al-Shboul
O, Alkahtani R, Kuemmerle JF, Grider JR and Murthy KS:
Hypercontractility of intestinal longitudinal smooth muscle induced
by cytokines is mediated by the nuclear factor-kappaB/AMP-activated
kinase/myosin light chain kinase pathway. J Pharmacol Exp Ther.
350:89–98. 2014.PubMed/NCBI View Article : Google Scholar
|
|
15
|
A controlled trial of recombinant
methionyl human BDNF in ALS: The BDNF Study Group (Phase III).
Neurology 52: 1427-1433, 1999.
|
|
16
|
Coulie B, Szarka LA, Camilleri M, Burton
DD, McKinzie S, Stambler N and Cedarbaum JM: Recombinant human
neurotrophic factors accelerate colonic transit and relieve
constipation in humans. Gastroenterology. 119:41–50.
2000.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Chai NL, Dong L, Li ZF, Du KX, Wang JH,
Yan LK and Dong XL: Effects of neurotrophins on gastrointestinal
myoelectric activities of rats. World J Gastroenterol. 9:1874–1877.
2003.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Grider JR, Piland BE, Gulick MA and Qiao
LY: Brain-derived neurotrophic factor augments peristalsis by
augmenting 5-HT and calcitonin gene-related peptide release.
Gastroenterology. 130:771–780. 2006.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Delafoy L, Gelot A, Ardid D, Eschalier A,
Bertrand C, Doherty AM and Diop L: Interactive involvement of brain
derived neurotrophic factor, nerve growth factor, and calcitonin
gene related peptide in colonic hypersensitivity in the rat. Gut.
55:940–945. 2006.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Xia CM, Gulick MA, Yu SJ, Grider JR,
Murthy KS, Kuemmerle JF, Akbarali HI and Qiao LY: Up-regulation of
brain-derived neurotrophic factor in primary afferent pathway
regulates colon-to-bladder cross-sensitization in rat. J
Neuroinflammation. 9(30)2012.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Hashmi F, Liu M, Shen S and Qiao LY:
Phospholipase C gamma mediates endogenous brain-derived
neurotrophic factor-regulated calcitonin gene-related peptide
expression in colitis-induced visceral pain. Mol Pain.
12(1744806916657088)2016.PubMed/NCBI View Article : Google Scholar
|
|
22
|
West AE, Chen WG, Dalva MB, Dolmetsch RE,
Kornhauser JM, Shaywitz AJ, Takasu MA, Tao X and Greenberg ME:
Calcium regulation of neuronal gene expression. Proc Natl Acad Sci
USA. 98:11024–11031. 2001.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Yan X, Liu J, Ye Z, Huang J, He F, Xiao W,
Hu X and Luo Z: CaMKII-mediated CREB phosphorylation is involved in
Ca2+-induced BDNF mRNA transcription and neurite outgrowth promoted
by electrical stimulation. PLoS One. 11(e0162784)2016.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Prakash YS, Thompson MA and Pabelick CM:
Brain-derived neurotrophic factor in TNF-α modulation of Ca2+ in
human airway smooth muscle. Am J Respir Cell Mol Biol. 41:603–611.
2009.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Saha RN, Liu X and Pahan K: Up-regulation
of BDNF in astrocytes by TNF-α: A case for the neuroprotective role
of cytokine. J Neuroimmune Pharmacol. 1:212–222. 2006.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Schulte-Herbrüggen O, Nassenstein C,
Lommatzsch M, Quarcoo D, Renz H and Braun A: Tumor necrosis
factor-alpha and interleukin-6 regulate secretion of brain-derived
neurotrophic factor in human monocytes. J Neuroimmunol.
160:204–209. 2005.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Murphy PG, Borthwick LA, Altares M,
Gauldie J, Kaplan D and Richardson PM: Reciprocal actions of
interleukin-6 and brain-derived neurotrophic factor on rat and
mouse primary sensory neurons. Eur J Neurosci. 12:1891–1899.
2000.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Al-Qudah MA and Al-Dwairi A: Mechanisms
and regulation of neurotrophin synthesis and secretion.
Neurosciences. 21(306)2016.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Aravamudan B, Thompson MA, Pabelick C and
Prakash YS: Secretion of brain derived neurotrophic factor is
regulated by inflammation-induced signals in asthmatic airway
smooth muscle cells. In: A109. REMODELING AND THE MATRIX. American
Thoracic Society, pp. A2839, 2016.
|
|
30
|
Al-Qudah M, Alkahtani R, Akbarali HI,
Murthy KS and Grider JR: Stimulation of synthesis and release of
brain-derived neurotropic factor from intestinal smooth muscle
cells by substance P and pituitary adenylate cyclase-activating
peptide. Neurogastroenterol Motil. 27:1162–1174. 2015.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Zhong Y, Chen J, Li L, Qin Y, Wei Y, Pan
S, Jiang Y, Chen J and Xie Y: PKA-CREB-BDNF signaling pathway
mediates propofol-induced long-term learning and memory impairment
in hippocampus of rats. Brain Res. 1691:64–74. 2018.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Zhen JL, Chang YN, Qu ZZ, Fu T, Liu JQ and
Wang WP: Luteolin rescues pentylenetetrazole-induced cognitive
impairment in epileptic rats by reducing oxidative stress and
activating PKA/CREB/BDNF signaling. Epilepsy Behav. 57:177–184.
2016.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Park H and Kaang BK: Balanced actions of
protein synthesis and degradation in memory formation. Learn Mem.
26:299–306. 2019.PubMed/NCBI View Article : Google Scholar
|