Introduction
Nerve injury forms the pathological basis of the
majority of neurological diseases, and is involved in spinal cord
injury, brain injury and neurodegenerative diseases. There are
several factors which result in nerve damage, among which oxidative
stress serves an important role (1).
Oxidative stress can result in the dysfunction of the mitochondria,
the endoplasmic reticulum, lipid peroxidation, protein oxidation
and subsequently lead to neuronal apoptosis or ferroptosis
(2-4).
Due to the ‘non-renewable’ nature of neurons, it is particularly
important to improve the anti-oxidative capacity of neurons during
potential crises. Apocynum venetum (sword-leaf dogbane) is a
traditional Chinese herb used to treat hypertension, nephritis and
neurasthenia, and has been shown to possess diuretic and sedative
effects (5). A. venetum has
become a popular herbal medicine in East Asia and North America
(6,7). Numerous additional pharmacological
properties of A. venetum leaf extract (AVLE) have been
discovered, several of which are closely associated with the
nervous system. In an in vitro model of ischemia-reperfusion
induced by oxygen and glucose deprivation, administration of AVLE
notably reduced apoptosis and morphological damage to neurons
(8). Although several studies have
shown that AVLE may protect neurons against injury and promote
recovery, the underlying mechanisms of these pharmacological
properties have not been shown, to the best of our knowledge.
Autophagy is an important mechanism of cell survival
in eukaryotic cells under stressful conditions (9), which consists of several steps, from
initiation of nucleation to formation of double membrane
autophagosomes and finally to autophagosome/lysosome fusion and
lysosomal enzyme-mediated degradation of the contents of the
autophagosome (10). Autophagy
involves degradation of long-lived proteins and damaged organelles,
such as mitochondria, the endoplasmic reticulum, peroxisomes, and
proteins damaged by oxidative stress, to prevent or slow down
initiation of apoptosis. Blocking autophagy allows toxic proteins
and damaged mitochondria to accumulate, which further aggravates
oxidative stress (11,12). The role of autophagy in the nervous
system has been widely investigated. Evidence from knockout mouse
has shown that autophagy exerts a protective effect against
neurodegeneration through clearance of intracytoplasmic
aggregate-prone proteins (13).
However, the effect of AVLE on autophagic activity has not been
studied. In the present study, whether AVLE exerted protective
effects on PC12 cells against H2O2-induced
oxidative stress was determined. Additionally, autophagic activity
was assessed to determine whether it was involved in the mechanism
underlying the protective effects of AVLE.
Materials and methods
Reagents and antibodies
AVLE was donated by the Department of Integrative
Medicine of Zhongshan Hospital, Shanghai, China.
H2O2 was purchased from Sigma-Aldrich; Merck
KGaA (cat. no. 323381). Cell Counting Kit-8 (CCK-8) was purchased
from Dojindo Molecular Technologies, Inc. (cat. no. CK04). A
live-dead detection kit was purchased from Thermo Fisher
Scientific, Inc. (cat. no. R37601). A DHE cell reactive oxygen
species (ROS) detection kit was purchased from Nanjing KeyGen
Biotech Co., Ltd. (cat. no. KGAF019). 3-Methyladenine (3-MA; cat.
no. S2767; Selleck Chemicals), was dissolved in double-distilled
water. Primary antibodies for Bax (cat. no. 14796), caspase-3 (cat.
no. 14220) and LC3-II (cat. no. 3868) were purchased from Cell
Signaling Technology, Inc. (all used at 1:1,000). The primary
antibody SQSTM1/p62 (cat. no. ab56416) was purchased from Abcam and
the GAPDH antibody was purchased from ProteinTech Group, Inc. (cat.
no. 60004-1-Ig; 1:5,000). The secondary antibodies peroxidase
AffiniPure goat anti-mouse IgG (cat. no. 33201ES60) and
peroxidase-conjugated goat anti-rabbit IgG (cat. no. 33101ES60)
were purchased from Shanghai Yeasen Biotechnology, Co., Ltd.
Cell culture and treatment
PC12 cells were purchased from The Cell Bank of Type
Culture Collection of the Chinese Academy of Sciences and were
cultured in high-glucose DMEM (Nanjing KeyGen Biotech Co., Ltd.)
supplemented with 10% FBS (cat. no. 16000-044; Gibco; Thermo Fisher
Scientific, Inc.) and 1% penicillin/streptomycin solution at 37˚C
and 5% CO2. The cells were digested with 0.25% trypsin
containing EDTA and passaged. When the cells reached 80% density,
following 10 h of serum starvation, different concentrations of
H2O2 or AVLE were added to the medium to
screen for the optimum concentration. To evaluate the effect of
AVLE on oxidative stress, the PC12 cells were pre-treated with AVLE
for 2 h before subsequent experimentation and continued with
treatment in the corresponding groups. To inhibit autophagy, cells
were treated with 3-methyladenine (3-MA, 5 mM) prior to AVLE
treatment.
Cell viability
A total of 1x103 PC12 cells/well were
added to a 96-well plate. The cells were treated with
H2O2 (0, 10, 20, 40, 80 or 160 µmol/l) for 2
h to determine a suitable concentration for induction of oxidative
stress. Subsequently, cells were treated with a series of
concentrations of AVLE (0, 1, 10, 100, 250, 500 or 1,000 µg/ml) for
24 or 48 h to determine the safe pharmacological range, as AVLE is
a herbal Chinese medicine with several complex constituents, thus
it may be challenging for cells to metabolise or clear the
compounds. The protective effects of AVLE against
H2O2 were also measured (0 µg/ml AVLE, 10
µg/ml AVLE, 100 µg/ml AVLE, 40 µM H2O2, 40 µM
H2O2 + 10 µg/ml AVLE and 40 µM
H2O2 + 100 µg/ml AVLE). Each group had six
repeat wells. After treatment, the cells were incubated with 10 µl
CCK-8 at 37˚C for 2 h to measure viability according to the
manufacturer's protocol. The absorbance values were determined at
460 nm using a spectrophotometer. Cell viability was calculated as
viability rate (%) = [optical density (OD) of the treatment
group-OD blank group)]/(OD control group-OD blank group) x100%.
Western blot analysis
Western blot analysis was performed as previously
described (14). The cells were
lysed with RIPA lysis buffer (cat. no. P0013B; Beyotime Institute
of Biotechnology, Inc.) containing a protease inhibitor cocktail. A
total of 30 µg protein was added to each lane of a 12.5% SDS gel,
resolved using SDS-PAGE (cat. no. PG113; Epizyme) and transferred
to a PVDF membrane (cat. no. ISEQ85R; EMD Millipore). The membrane
was blocked in non-fat milk and incubated with cleaved caspase-3
and Bax primary antibodies at 4˚C overnight. The membrane was
incubated with secondary antibodies (1:5,000) at room temperature
for 1 h. Enhanced chemiluminescence reagent was added for signal
detection (Tanon Science and Technology Co., Ltd.), and
densitometry analysis was performed using Quantity One software
version 4.6.2 (Bio-Rad Laboratories, Inc.). GAPDH was used as the
internal reference.
Immunofluorescence assay
The immunofluorescence assay was performed according
to the manufacturer's protocol. For live-dead detection, cells were
treated with H2O2 and AVLE (control, 10 µg/ml
AVLE, 100 µg/ml AVLE, 40 µM H2O2, 40 µM
H2O2 + 10 µg/ml AVLE, 40 µM
H2O2 + 100 µg/ml AVLE), immersed in live-dead
solution and incubated for 15 min at 20-25˚C, and then imaged using
a fluorescent microscope at x40 magnification (Nikon Corporation).
For ROS detection, cells were treated with different combinations
of H2O2, AVLE and 3-MA as follows: 0 µg/ml
AVLE, 10 µg/ml AVLE, 100 µg/ml AVLE, 40 uM
H2O2, H2O2 + 10 µg/ml
AVLE, H2O2 + 100 µg/ml AVLE and vehicle +
H2O2 + 100 µg/ml AVLE, 3-MA +
H2O2 + 100 µg/ml AVLE. Cell medium was
replaced with fresh medium containing 10 µM ROS detection solution,
the cells were incubated at 37˚C for 30 min and imaged using a
fluorescent microscope. The images were analysed using ImageJ
version 1.8.0 (National Institutes of Health) to compare the ROS
expression.
Statistical analysis
Data are presented as the mean ± standard deviation.
Analysis was performed using SPSS version 20.0 software (IBM,
Corp.) and GraphPad Prism version 5.0 (GraphPad Software, Inc.).
Comparisons between paired groups were determined using the
Student's t-test. A one-way ANOVA followed by a post hoc Tukey's
test was used for comparisons between multiple groups P<0.05 was
considered to indicate a statistically significant difference.
Results
AVLE improves cell viability in PC12
cells treated with H2O2
Oxidative stress was induced in PC12 cells using
different concentrations of H2O2 and a CCK-8
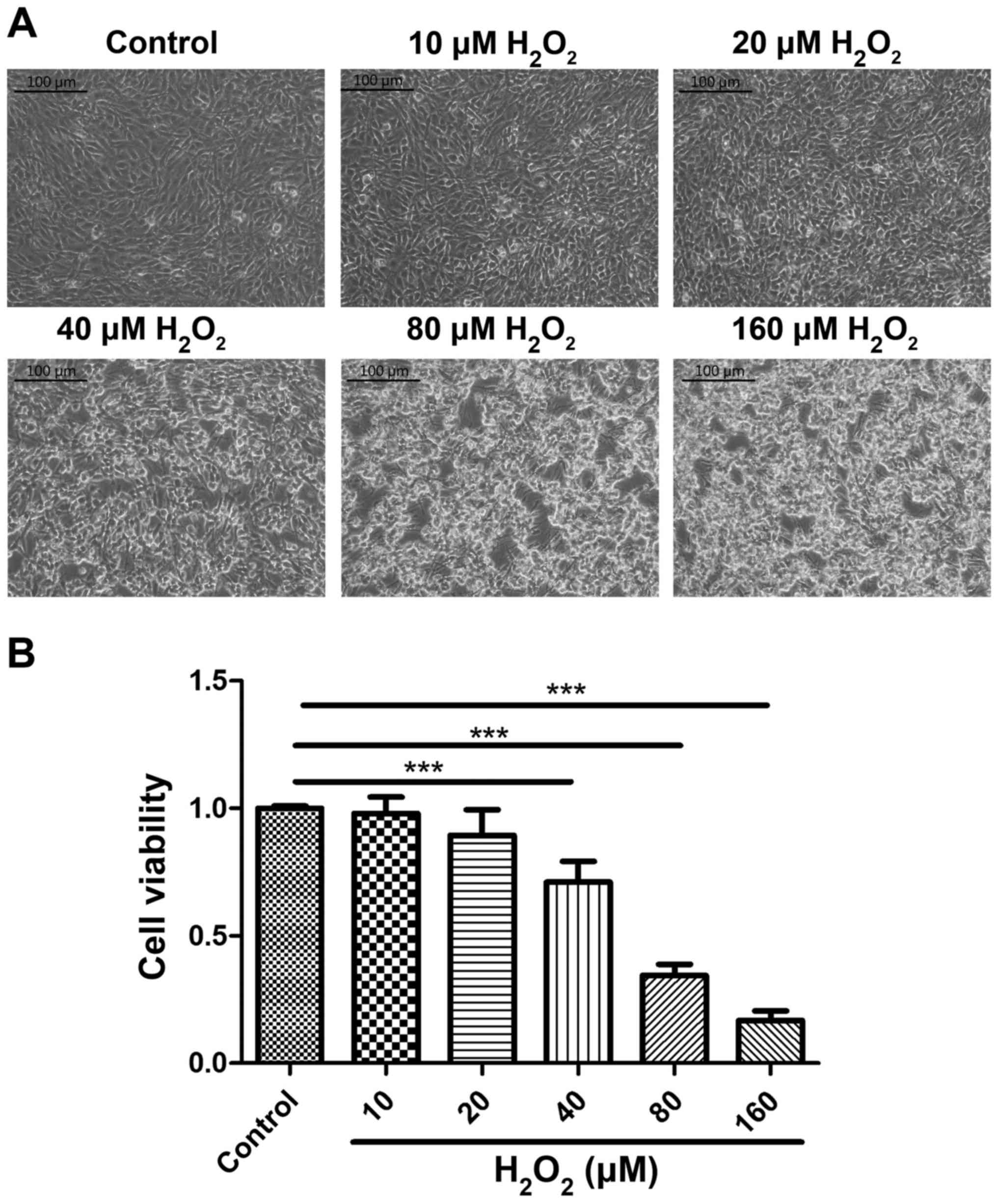
assay was used to detect cell viability. Cellular morphology was
altered by H2O2 treatment, and treatment with
40 µM of H2O2 for 2 h caused cells to lose
their adhesion and shrink (Fig. 1A).
The CCK-8 assay confirmed that the viability of cells treated with
H2O2 at concentrations >40 µM was
significantly lower compared with the control group (P<0.001;
Fig. 1B). Thus, the optimum
conditions for inducing oxidative stress were 40 µM
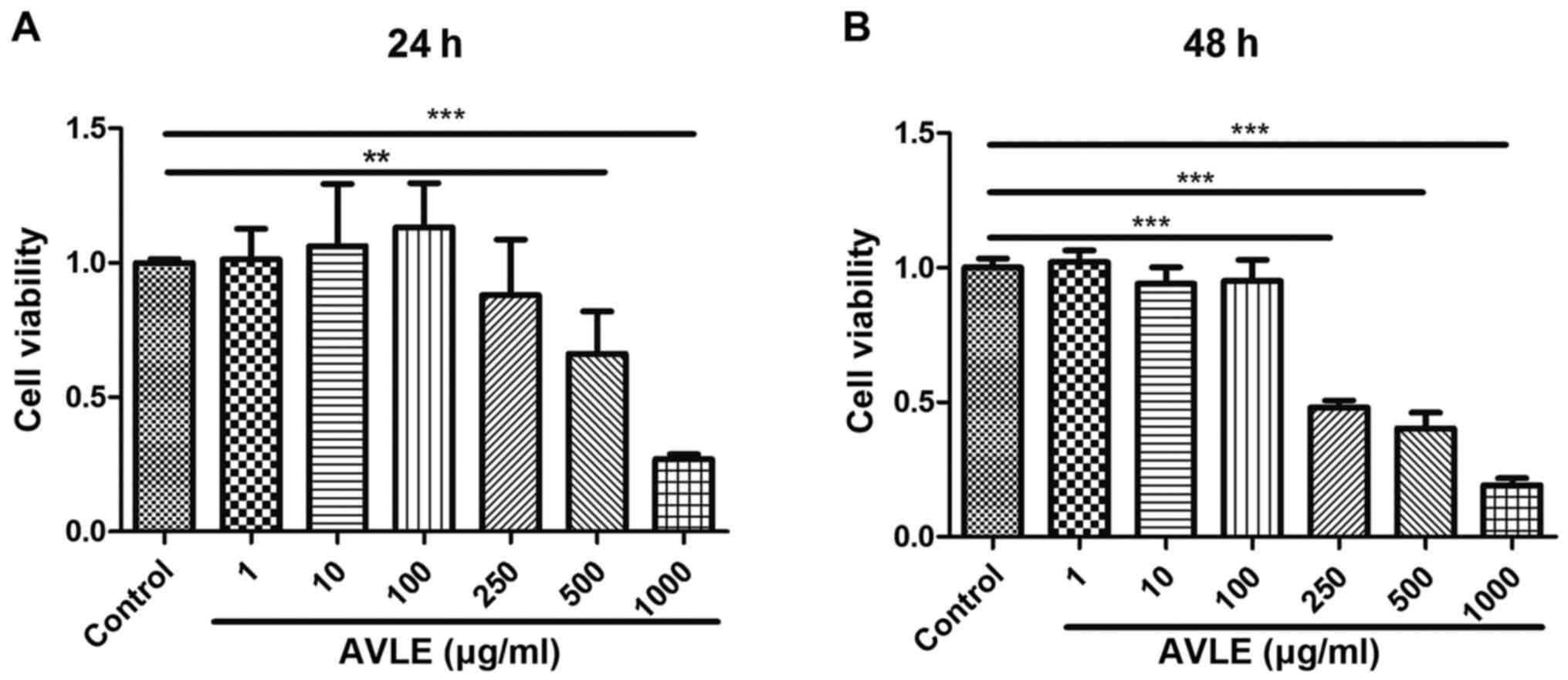
H2O2 treatment for 2 h. The optimum AVLE
concentration was also evaluated using a CCK-8 assay. Cell
viability decreased significantly following exposure to AVLE at
concentrations >500 µg/ml for 24 h (P<0.01), and cell
viability continued to decrease at AVLE concentrations >250
µg/ml for 48 h (P<0.001). Conversely, the cell viability was not
significantly affected by AVLE at concentrations <100 µg/ml
(P>0.05). Thus, the safe AVLE concentration range was determined
to be 1-100 µg/ml (Fig. 2). The
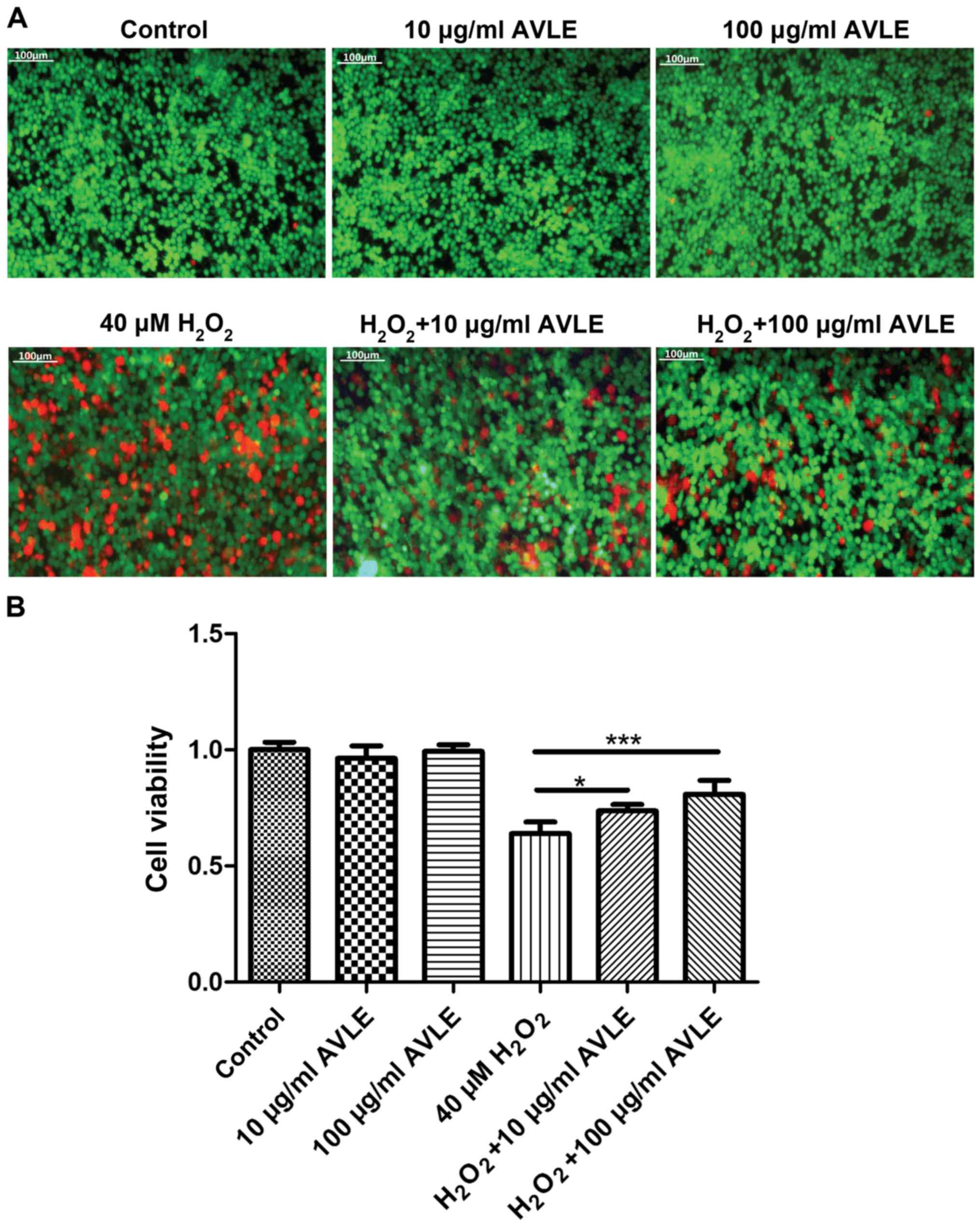
protective effects of AVLE against H2O2 were
measured. Red fluorescence (dead cells) decreased and green
fluorescence (live cells) increased significantly following AVLE
treatment of cells exposed to H2O2,
suggesting that AVLE reduced the number of dead cells, possibly
through reduction of oxidative stress (Fig. 3A). In addition, cell viability
increased significantly following AVLE treatment (0.64±0.05 for 40
µM H2O2 vs. 0.74±0.03 for 40 µM
H2O2 + 10 µg/ml AVLE; P<0.05; 0.64±0.05
for 40 µM H2O2 vs. 0.81±0.06 for 40 µM
H2O2 + 100 µg/ml AVLE; P<0.001; 0.35±0.04
for 80 µM H2O2 vs. 0.54±0.13 for 80 µM
H2O2 + 100 µg/ml AVLE; P<0.01; Figs. 3B and S1).
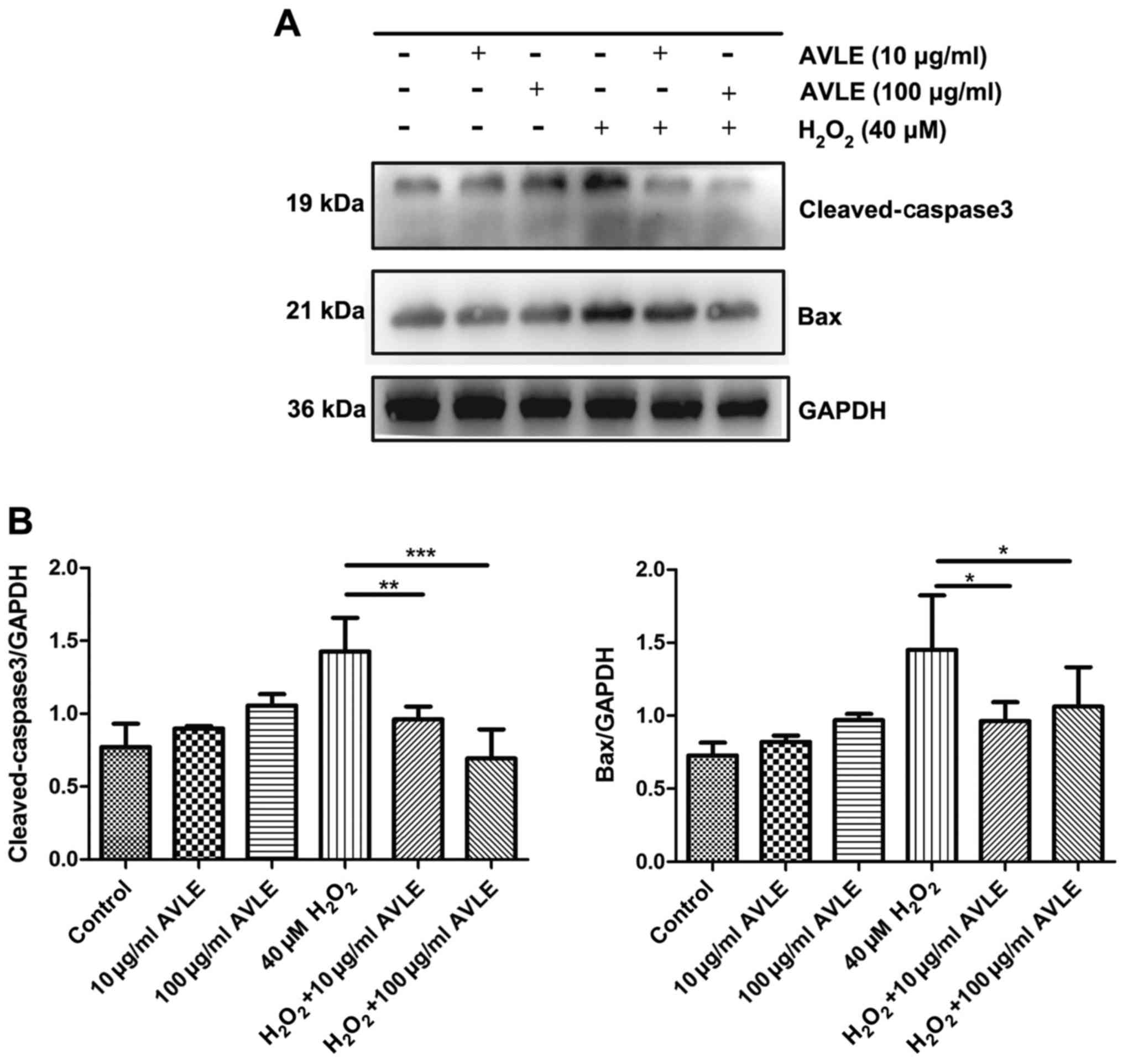
AVLE protects PC12 cells against
H2O2-induced apoptosis
Cells were treated with H2O2
and different concentrations of AVLE (0 µg/ml AVLE, 10 µg/ml AVLE,
100 µg/ml AVLE, 40 µM H2O2, 40 µM
H2O2 + 10 µg/ml AVLE, 40 µM
H2O2 + 100 µg/ml AVLE). Western blotting
showed that the expression of the apoptotic proteins Bax and
cleaved-caspase-3 increased following treatment with 40 µM
H2O2 (Fig. 4).
However, AVLE (10 or 100 µg/ml) significantly decreased apoptosis
by reducing the expression of Bax compared with 40 µM
H2O2 (both P<0.05) and reduced the
cleavage of the apoptosis-related protein caspase-3 (40 µM
H2O2 + 10 µg/ml AVLE vs. 40 µM
H2O2, P<0.01; 40 µM
H2O2 + 100 µg/ml AVLE vs. 40 µM
H2O2, P<0.001).
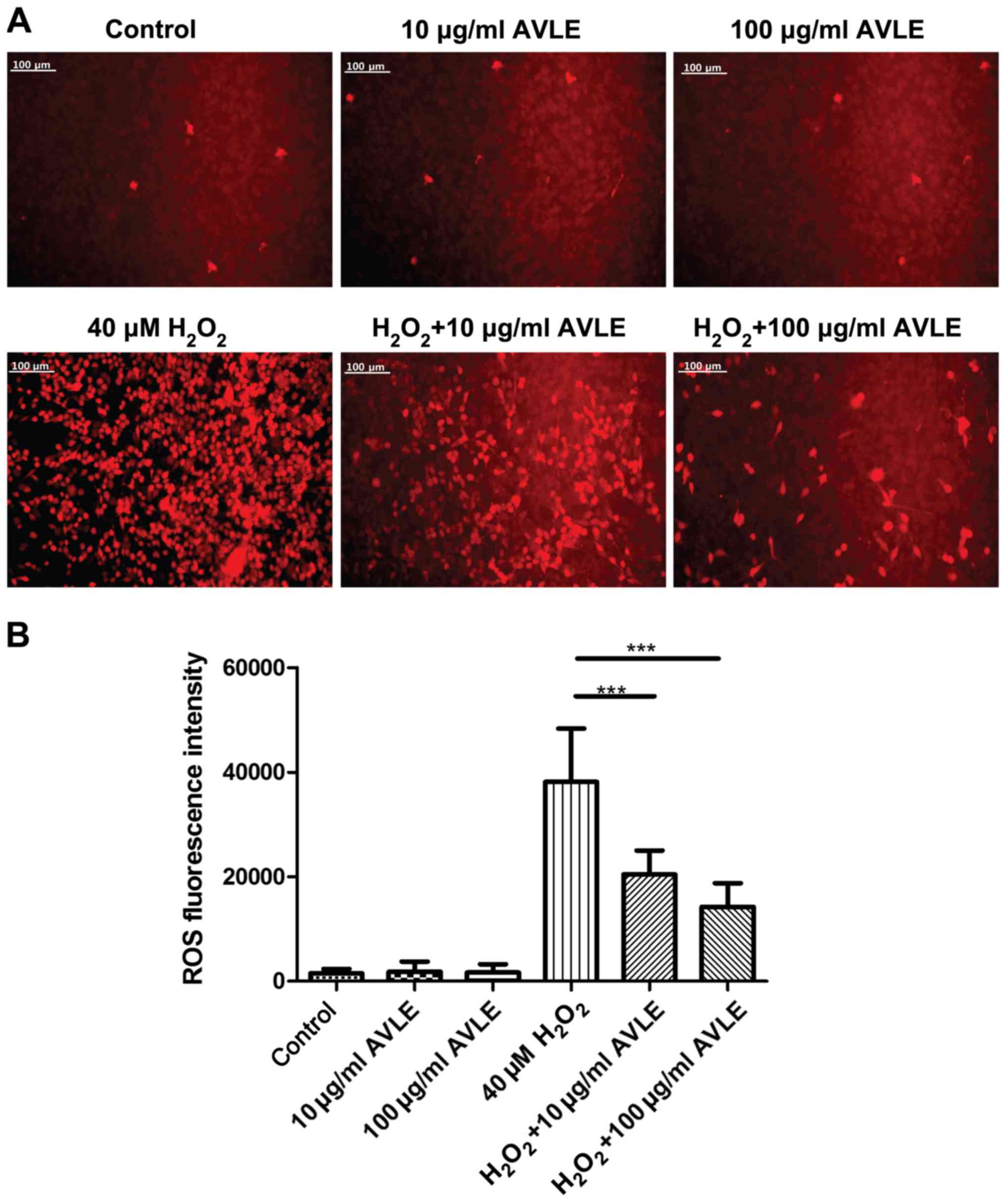
AVLE ameliorates ROS accumulation in
H2O2-treated PC12 cells
The production of ROS was determined following
H2O2 exposure and AVLE treatment (Fig. 5). Treatment with AVLE at 10 or 100
µg/ml alone did not increase ROS production, indicating that it was
safe for normal cells. ROS production was significantly increased
by 40 µM H2O2 compared with the control
cells, and treatment with 10 or 100 µg/ml AVLE significantly
reduced ROS production compared with 40 µM
H2O2 (P<0.001). The antagonistic effect of
AVLE on ROS increased as the concentration of AVLE used AVLE
increased.
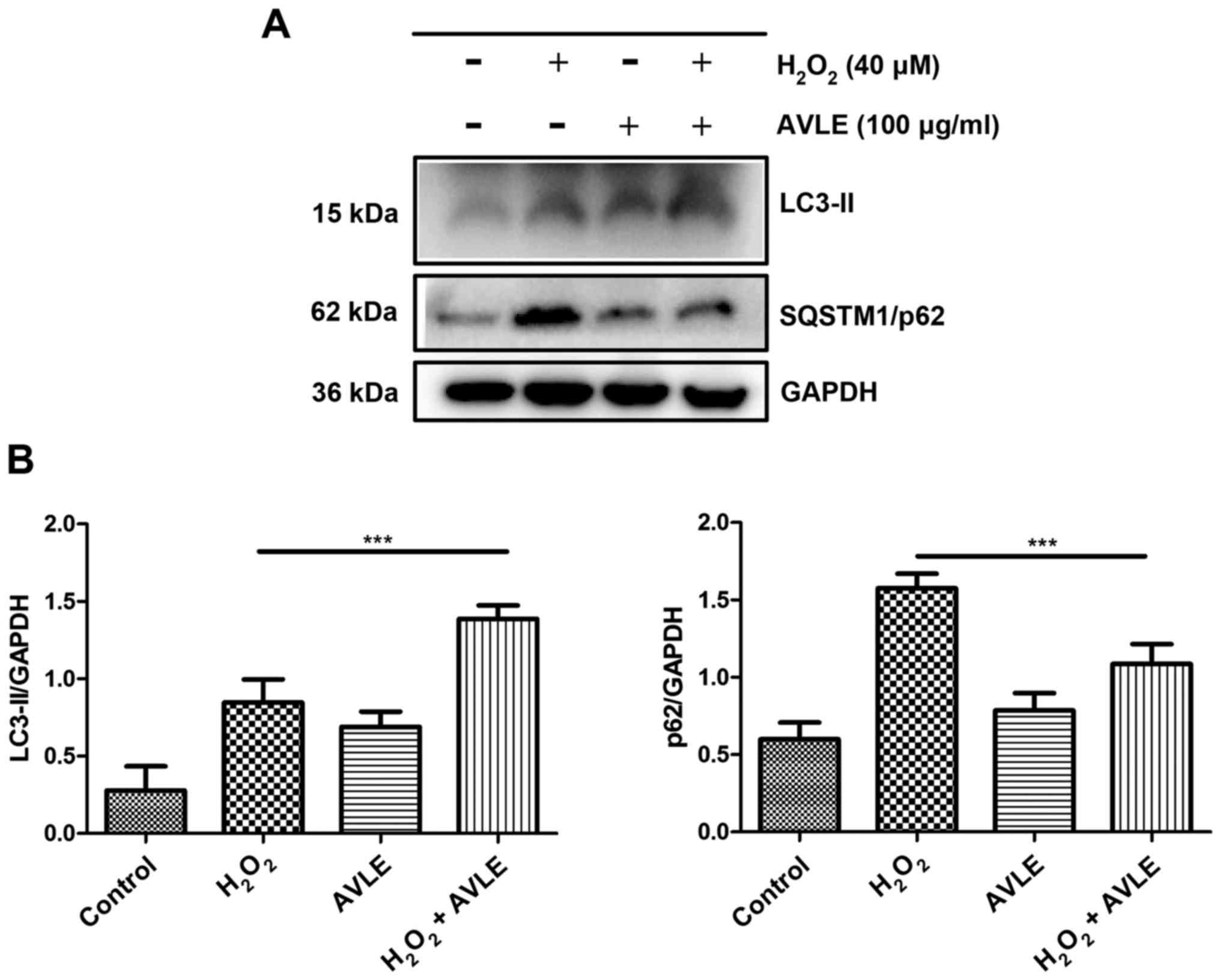
AVLE protects against
H2O2-induced oxidative stress by increasing
autophagy
To confirm the mechanism underlying the protective
effects of AVLE following H2O2 exposure, the
protein expression levels of LC3-II and SQSTM1/p62 were measured to
evaluate autophagic activity in the control, 40 µM
H2O2, 100 µg/ml AVLE and 100 µg/ml AVLE + 40
µM H2O2 groups. LC3-II protein expression was
significantly upregulated and SQSTM1/p62 expression was
downregulated in the cells treated with 100 µg/ml AVLE + 40 µM
H2O2 compared with those treated with 40 µM
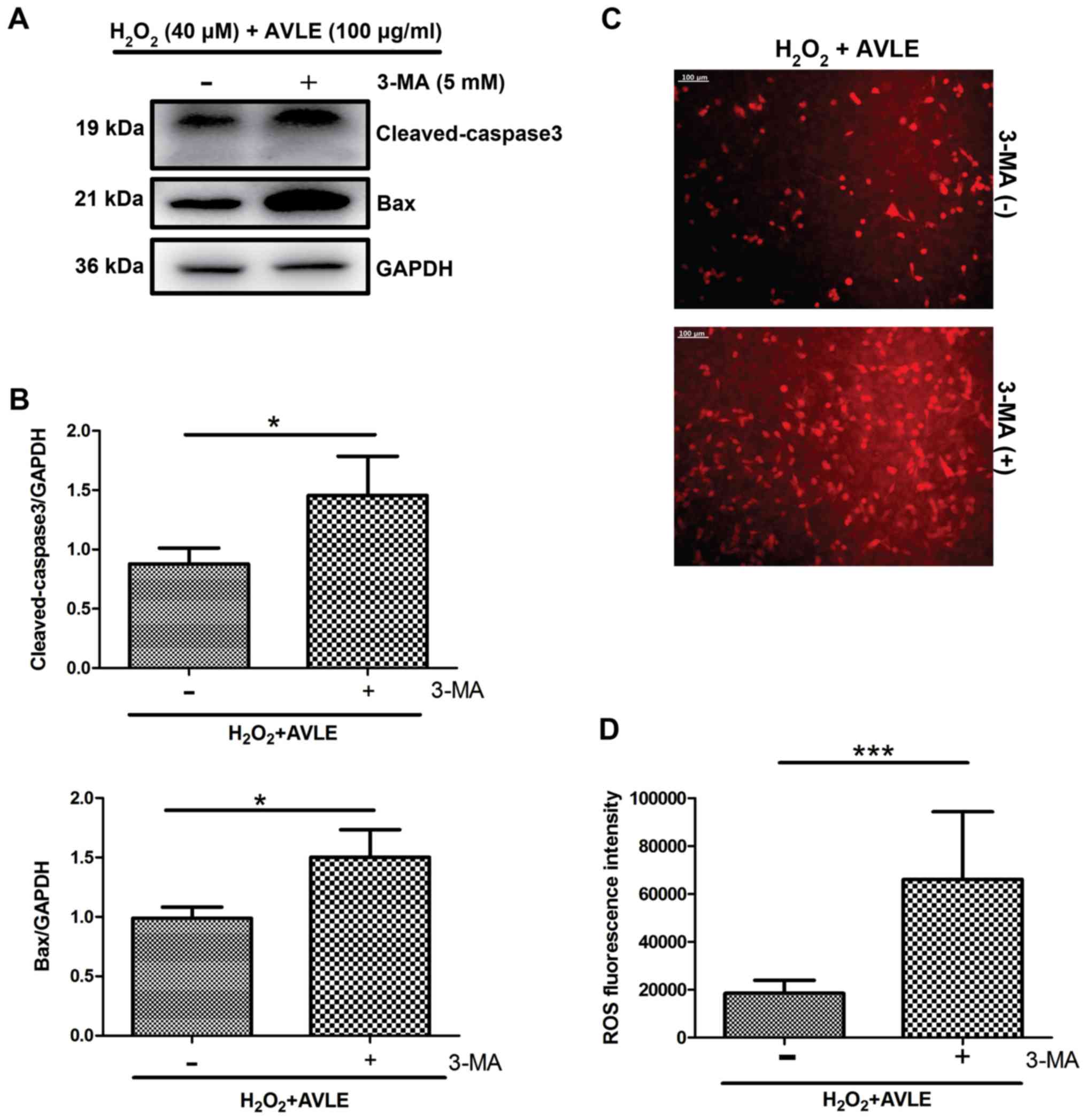
H2O2 (P<0.001; Fig. 6). Furthermore, administration of the
autophagic inhibitor 3-MA significantly increased the expression of
the apoptotic proteins cleaved caspase-3 and Bax in the 100 µg/ml
AVLE + 40 µM H2O2 group compared with the
control group (P<0.05; Fig. 7A
and B) and increased the ROS levels
compared with the control (P<0.001; Fig. 7C and D).
Discussion
AVLE has multiple pharmacological effects on the
nervous system, including antidepressant effects, anxiolytic
effects, and protective effects against stroke. Butterweck et
al (5) reported that male rats
treated with AVLE had significantly shorter immobility times in a
forced swimming test, suggesting possible antidepressant effects.
AVLE also showed antidepressant-like effects in a chronic
unpredictable mild stress model of depression in rats, and these
effects were possibly caused by the suppression of neuronal
apoptosis and increased expression of brain-derived neurotrophic
factor (15). The anxiolytic effects
of AVLE were measured in mice subjected to an elevated plus maze
test, and mice treated with AVLE entered and spent more time in the
open arms of the maze, whilst showing no other behavioural changes
or motor dysfunction (7). AVLE has
protective effects against neurological injuries such as stroke.
AVLE (500 mg/kg/day) significantly reduced the cerebral infarct
area by alleviating blood-brain barrier disruption in a rat model
of cerebral ischemia-reperfusion injury (16). In the present study, an oxidative
stress model was established by treating PC12 cells with 40 µM
H2O2 for 2 h. The safe pharmacological range
was established to be between 1-100 µg/ml. Since AVLE is a herbal
Chinese medicine consisting of several complex components, when the
drug concentration is too high, exceeding the metabolic or
clearance capacity of cells, it will exhibit toxic effects on the
cells, thus, 10 and 100 µg/ml AVLE were used as safe concentrations
in the present study. Cell viability detection and a live-dead
assay showed that AVLE reduced the number of injured PC12 cells.
Oxidative stress is involved in several pathological processes,
such as stroke, neurodegenerative disorders and spinal cord injury
(1,17). AVLE also mitigated myocardial
ischemia/reperfusion injury by inhibiting oxidative stress
(18). The findings of the present
study suggest that AVLE may exert similar effects on nervous system
diseases.
Oxidative stress induced by excessive accumulation
of ROS serves an important role in the development and progression
of nervous system diseases (3,19,20).
Excess intracellular ROS can initiate apoptotic pathways, and
following activation of the apoptotic pathway, the anti-apoptotic
protein Bcl2 is inhibited and the pro-apoptotic protein Bax
oligomerizes, which induces mitochondrial permeabilization and
release of cytochrome c. Subsequently, caspase-3 is activated,
leading to the fragmentation of PARP and ultimately apoptosis
(19,21). In the present study, AVLE
significantly inhibited H2O2-induced
apoptosis in PC12 cells, and western blotting showed that AVLE
reduced the expression of Bax and inhibited the cleavage of the
apoptosis-related protein, caspase-3. ROS expression in the
H2O2-treated cells was significantly reduced
by AVLE. These results suggest that AVLE may inhibit apoptosis of
neurons induced by oxidative damage, which may be achieved by
reducing the levels of ROS, and subsequently blocking the
activation of the pro-apoptotic proteins Bax and caspase-3.
ROS causes oxidative stress during apoptosis, and
~90% of ROS is produced by the mitochondrial membrane respiratory
chain (22). Excessive amounts of
ROS activates autophagy by inhibiting the PI3K-Akt-mTOR pathway,
and cells activate mitophagy to remove damaged mitochondria, and
thus maintain low ROS levels (23,24).
Natural antioxidants, including resveratrol, curcumin and apigenin,
reduce oxidative stress by increasing autophagic activity (24-28).
In the present study, in H2O2-treated cells,
AVLE upregulated LC3-II expression and downregulated p62
expression. LC3-II may be attached to the membrane of an
autophagosome, and is a labelled protein of the autophagosome,
whereas p62 can recruit ubiquitinated proteins for autophagic
degradation, and the aggregation of p62 often reflects disruption
of autophagic flux (29,30). The expression of LC3-II and p62
indicated that autophagic activity was activated, whereas
inhibition of autophagy with 3-MA increased the levels of ROS and
apoptotic proteins. Autophagy is also maintained at low levels in
normal cells and serves an important role in the physiological
functioning of cells. Under stressful conditions, such as oxidative
stress and inflammation, autophagy is readily activated, but
activation is kept limited. certain autophagy activators, such as
rapamycin and metformin, are often used to reduce stress in tissues
(31,32). The results of the present study
suggested that, under oxidative stress, AVLE increased autophagic
activity, similar to the effects of rapamycin or metformin, and
thus reduced intracellular ROS aggregation and cell apoptosis.
These effects may be the result of AVLE itself being rich in
antioxidants. Apocynum venetum contains an abundance of
flavonoids, including hyperoside, quercetin, kaempferol and rutin
(33,34), and hyperoside and quercetin have
protective effects against oxidative damage on injured cells
(35-38).
The present study provides further insights into the
effects of AVLE on neurons, and the mechanism underlying the
protective effects of AVLE against oxidative stress-induced
apoptosis. The results of the present study may facilitate the
development of novel treatments for nervous system diseases and
injuries. However, there are some limitations. First, PC12 cells
were used in the present study which are a highly differentiated
cell line. PC12 cells are used as model of neurons, and thus the
behaviour of PC12 cells may partially differ from that real neurons
in vivo. It will be of value to repeat these experiments
using primary cells and using in vivo animal models to
further confirm the effects of AVLE. In addition, the environment
of the cells in vitro differs from that of neurons in
vivo, necessitating the need for in vivo animal
experiments. Although the AMPK/mTOR pathway may participate in the
protective effects of AVLE (39),
the mechanism involved in the effects of AVLE in the nervous system
still require further investigation. Additionally, the composition
of AVLE is complex, thus it is difficult to distinguish or identify
which components served the major roles in protecting PC12 cells
against oxidative stress. Extraction of the functional components
and identification of the effects of the individual components on
the various signalling pathways is required to fully elucidate the
effects of AVLE, and to provide more clinically relevant
information. In future studies, the active ingredients will be
further clarified with the aim of reducing the side effects of
treatment with AVLE.
Supplementary Material
Figure S1. Effects of AVLE on higher
concentrations of H2O2 (80 μM). Cell Counting
Kit‑8 assay showed that cell viability improved with increasing
AVLE concentrations following H2O2 exposure.
The difference in viability between 80 μM
H2O2 treated cells and
H2O2 + 100 μg/ml AVLE treated cells was
significantly different (0.35±0.04 for 80 μM
H2O2 vs. 0.54±0.13 for 80 μM
H2O2 + 100 μg/ml AVLE; P<0.01). n=6. ns,
no significance; **P<0.01; Ctrl, control; AVLE, Apocynum
venetum leaf extract.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant no. 81301047).
Availability of data and materials
The data sets used and/or analyzed during the
current study are available from the corresponding author on
reasonable request.
Authors' contributions
ZL and ZC designed the study and drafted the
manuscript. FY conducted the experiments and analyzed the data. YF
and CJ contributed to the search of literature, analysis of data,
and performed experiments. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Salim S: Oxidative stress and the central
nervous system. J Pharmacol Exp Ther. 360:201–205. 2017.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Ratan RR: The chemical biology of
ferroptosis in the central nervous system. Cell Chem Biol: May 24,
2020 (Epub ahead of print). doi:
10.1016/j.chembiol.2020.03.007.
|
|
3
|
Islam MT: Oxidative stress and
mitochondrial dysfunction-linked neurodegenerative disorders.
Neurol Res. 39:73–82. 2017.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Höhn A, Tramutola A and Cascella R:
Proteostasis failure in neurodegenerative diseases: Focus on
oxidative stress. Oxid Med Cell Longev.
2020(5497046)2020.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Butterweck V, Nishibe S, Sasaki T and
Uchida M: Antidepressant effects of Apocynum venetum leaves
in a forced swimming test. Biol Pharm Bull. 24:848–851.
2001.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Kobayashi M, Saitoh H, Seo S, Butterweck V
and Nishibe S: Apocynum venetum extract does not induce
CYP3A and P-glycoprotein in rats. Biol Pharm Bull. 27:1649–1652.
2004.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Xie W, Zhang X, Wang T and Hu J: Botany,
traditional uses, phytochemistry and pharmacology of Apocynum
venetum L. (Luobuma): A review. J Ethnopharmacol. 141:1–8.
2012.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Xiang J, Tang YP, Zhou ZY, Wu P, Wang Z,
Mori M and Cai DF: Apocynum venetum leaf extract protects
rat cortical neurons from injury induced by oxygen and glucose
deprivation in vitro. Can J Physiol Pharmacol. 88:907–917.
2010.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Codogno P and Meijer AJ: Autophagy and
signaling: Their role in cell survival and cell death. Cell Death
Differ. 12 (Suppl 2):1509–1518. 2005.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Radad K, Moldzio R, Al-Shraim M, Kranner
B, Krewenka C and Rausch WD: Recent advances in autophagy-based
neuroprotection. Expert Rev Neurother. 15:195–205. 2015.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Wu D and Cederbaum AI: Inhibition of
autophagy promotes CYP2E1-dependent toxicity in HepG2 cells via
elevated oxidative stress, mitochondria dysfunction and activation
of p38 and JNK MAPK. Redox Biol. 1:552–565. 2013.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Wang T, Wang Q, Song R, Zhang Y, Zhang K,
Yuan Y, Bian J, Liu X, Gu J and Liu Z: Autophagy plays a
cytoprotective role during cadmium-induced oxidative damage in
primary neuronal cultures. Biol Trace Elem Res. 168:481–489.
2015.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Menzies FM, Fleming A, Caricasole A, Bento
CF, Andrews SP, Ashkenazi A, Füllgrabe J, Jackson A, Jimenez
Sanchez M, Karabiyik C, et al: Autophagy and neurodegeneration:
Pathogenic mechanisms and therapeutic opportunities. Neuron.
93:1015–1034. 2017.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Chen Z, Li Z, Jiang C, Jiang X and Zhang
J: MiR-92b-3p promotes neurite growth and functional recovery via
the PTEN/AKT pathway in acute spinal cord injury. J Cell Physiol.
234:23043–23052. 2019.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Li X, Wu T, Yu Z, Li T, Zhang J, Zhang Z,
Cai M, Zhang W, Xiang J and Cai D: Apocynum venetum leaf
extract reverses depressive-like behaviors in chronically stressed
rats by inhibiting oxidative stress and apoptosis. Biomed
Pharmacother. 100:394–406. 2018.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Xiang J, Lan R, Tang YP, Chen YP and Cai
DF: Apocynum venetum leaf extract attenuates disruption of
the blood-brain barrier and upregulation of matrix
metalloproteinase-9/-2 in a rat model of cerebral
ischemia-reperfusion injury. Neurochem Res. 37:1820–1828.
2012.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Scholpa NE and Schnellmann RG:
Mitochondrial-based therapeutics for the treatment of spinal cord
injury: Mitochondrial biogenesis as a potential pharmacological
target. J Pharmacol Exp Ther. 363:303–313. 2017.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Wang W, Liang X, Fu D, Tie R, Xing W, Ji
L, Liu F, Zhang H and Li R: Apocynum venetum leaf attenuates
myocardial ischemia/reperfusion injury by inhibiting oxidative
stress. Am J Chin Med. 43:71–85. 2015.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Jabbour E, Ottmann OG, Deininger M and
Hochhaus A: Targeting the phosphoinositide 3-kinase pathway in
hematologic malignancies. Haematologica. 99:7–18. 2014.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Slimen IB, Najar T, Ghram A, Dabbebi H,
Ben Mrad M and Abdrabbah M: Reactive oxygen species, heat stress
and oxidative-induced mitochondrial damage. A review. Int J
Hyperthermia. 30:513–523. 2014.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Sinha K, Das J, Pal PB and Sil PC:
Oxidative stress: The mitochondria-dependent and
mitochondria-independent pathways of apoptosis. Arch Toxicol.
87:1157–1180. 2013.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Mi Y, Xiao C, Du Q, Wu W, Qi G and Liu X:
Momordin Ic couples apoptosis with autophagy in human
hepatoblastoma cancer cells by reactive oxygen species
(ROS)-mediated PI3K/Akt and MAPK signaling pathways. Free Radic
Biol Med. 90:230–242. 2016.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Portal-Nunez S, Esbrit P, Alcaraz MJ and
Largo R: Oxidative stress, autophagy, epigenetic changes and
regulation by miRNAs as potential therapeutic targets in
osteoarthritis. Biochem Pharmacol. 108:1–10. 2016.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Yuan H, Perry CN, Huang C, Iwai-Kanai E,
Carreira RS, Glembotski CC and Gottlieb RA: LPS-induced autophagy
is mediated by oxidative signaling in cardiomyocytes and is
associated with cytoprotection. Am J Physiol Heart Circ Physiol.
296:H470–H479. 2009.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Cho B, Kim T, Huh YJ, Lee J and Lee YI:
Amelioration of mitochondrial quality control and proteostasis by
natural compounds in Parkinson's disease models. Int J Mol Sci.
20(E4208)2019.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Sebori R, Kuno A, Hosoda R, Hayashi T and
Horio Y: Resveratrol decreases oxidative stress by restoring
mitophagy and improves the pathophysiology of dystrophin-deficient
mdx mice. Oxid Med Cell Longev. 2018(9179270)2018.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Guo S, Long M, Li X, Zhu S, Zhang M and
Yang Z: Curcumin activates autophagy and attenuates oxidative
damage in EA.hy926 cells via the Akt/mTOR pathway. Mol Med Rep.
13:2187–2193. 2016.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Sun H, Wang Z and Yakisich JS: Natural
products targeting autophagy via the PI3K/Akt/mTOR pathway as
anticancer agents. Anticancer Agents Med Chem. 13:1048–1056.
2013.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Ichimura Y, Kirisako T, Takao T, Satomi Y,
Shimonishi Y, Ishihara N, Mizushima N, Tanida I, Kominami E, Ohsumi
M, et al: A ubiquitin-like system mediates protein lipidation.
Nature. 408:488–492. 2000.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Kabeya Y, Mizushima N, Ueno T, Yamamoto A,
Kirisako T, Noda T, Kominami E, Ohsumi Y and Yoshimori T: LC3, a
mammalian homologue of yeast Apg8p, is localized in autophagosome
membranes after processing. EMBO J. 19:5720–5728. 2000.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Sekiguchi A, Kanno H, Ozawa H, Yamaya S
and Itoi E: Rapamycin promotes autophagy and reduces neural tissue
damage and locomotor impairment after spinal cord injury in mice. J
Neurotrauma. 29:946–956. 2012.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Zhang D, Xuan J, Zheng BB, Zhou YL, Lin Y,
Wu YS, Zhou YF, Huang YX, Wang Q, Shen LY, et al: Metformin
improves functional recovery after spinal cord injury via autophagy
flux stimulation. Mol Neurobiol. 54:3327–3341. 2017.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Kamata K, Seo S and Nakajima J:
Constituents from leaves of Apocynum venetum L. J Nat Med.
62:160–163. 2008.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Song RJ and Zhou J: Microemulsion liquid
chromatographic method for simultaneous separation and
determination of six flavonoids of Apocynum venetum leaf
extract. J Chromatogr B Analyt Technol Biomed Life Sci.
995-996:8–14. 2015.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Hao XL, Kang Y, Li JK, Li QS, Liu EL and
Liu XX: Protective effects of hyperoside against H2O2-induced
apoptosis in human umbilical vein endothelial cells. Mol Med Rep.
14:399–405. 2016.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Liu Z, Tao X, Zhang C, Lu Y and Wei D:
Protective effects of hyperoside (quercetin-3-o-galactoside) to
PC12 cells against cytotoxicity induced by hydrogen peroxide and
tert-butyl hydroperoxide. Biomed Pharmacother. 59:481–490.
2005.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Rashidi Z, Aleyasin A, Eslami M, Nekoonam
S, Zendedel A, Bahramrezaie M and Amidi F: Quercetin protects human
granulosa cells against oxidative stress via thioredoxin system.
Reprod Biol. 19:245–254. 2019.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Xiao HB, Lu XY, Liu ZK and Luo ZF:
Kaempferol inhibits the production of ROS to modulate OPN-αvβ3
integrin pathway in HUVECs. J Physiol Biochem. 72:303–313.
2016.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Lu L, Zhang D, Sun B, Hu Y, Yan M, Liu K,
Li X and Ren L: Apocynum leaf extract inhibits the progress
of atherosclerosis in rats via the AMPK/mTOR pathway. Pharmazie.
72:41–48. 2017.PubMed/NCBI View Article : Google Scholar
|