Introduction
Carbon tetrachloride (CCl4) is an organic solvent
extensively used in cleaning reagents. It has been used in animal
models to investigate synthetic poison-induced inner organ damage.
When this lethal compound is introduced in to the body by
ingestion, inward breath or skin assimilation, it is disseminated
throughout the body, often accumulating in the liver, cerebrum,
kidney, muscle, fat and blood (1).
There are several studies demonstrating CCl4-induced kidney
(2), liver and testicular damage
(3), as well as blood disorders
(4). CCl4 intoxication in animals is
used experimentally to induce oxidative stress under various
physiological conditions (5).
Prolonged exposure to CCl4 induces histopathological features such
as inflammatory leukocyte infiltration, necrosis, fibrosis,
cirrhosis, and may also result in the development of cancer
(6).
Nephrotoxicity is a widely recognized health issue
and is often the result of exposure to medication or toxins
(7). Renal failure results in a
severe decline in the excretory capacity of the kidney, resulting
in buildup of nitrogenous waste in the blood (8). Renal failure is a common
pathophysiological disturbance caused by CCl4 that leads to death,
and is categorized into acute and chronic renal failure (9). One of the principal causes of acute
kidney injury is oxidative stress that subsequently results in the
formation of reactive oxygen species (ROS). Direct nephrotoxic
effects of toxins such as CCl4, including phospholipid damage,
mitochondrial dysfunction, increased intracellular calcium
concentration and lysosomal hydrolase inhibition result in the
formation of ROS and thus increases oxidative stress, causing
proximal tubular toxicity (10). ROS
contributes to the progression of fibrosis either directly or
indirectly by promoting inflammation. Fibrosis and inflammation
together may further augment ROS formation or stimulate the
production of cytokines and growth factors (11). Reactive oxygen metabolites are
postulated to underlie the pathogenesis of CCl4 nephrotoxicity
(12). Thus, the buildup of free
radicals in cells can induce lipid peroxidation and the oxidative
breakdown of membrane polyunsaturated fats results in readjustments
to cell membrane permeability and viscosity (13). In vivo and in vitro
reports have shown that CCl4 increases lipid peroxidation, reduces
oxidized glutathione levels in the kidney cortex and causes a
reduction in the activity of enzymes which would result in
decreased lipid peroxidation (14).
CCl4 can sub-lethally induce proximal tubular damage in the kidney
and cause changes to the granular pneumocytes (15).
Several medicinal plants are known for their
remedial properties when used to treat renal disorders, due to the
presence of various multifaceted therapeutic chemical compounds
(16). When medicinal plants with
nephroprotective properties are administered alongside various
nephrotoxic agents, they may attenuate toxicity (8). Punicagranatum L.
(Punicaceae), commonly known as pomegranate, is often used
in folk lore medicine for the management of various diseases
(17). Pomegranate peel extract
(PPE) exhibits marked antioxidant properties (18). It has been shown to reduce oxidative
stress mediators indicating its antioxidant capacity, which is
attributed to the presence of diverse phenolic compounds, such as
gallic acids, ellagic acids, ellagitannins, catechins,
gallotannins, anthocyanins, quercetins and ferulic acids (19,20).
These polyphenols possess antioxidant properties in scavenging free
radicals and inhibiting lipid oxidation (21). Furthermore, studies on animals have
shown PPE does not exhibit any toxic effects (22). Additionally, the anti-inflammatory
(23) as well as the anticancer
properties of PPE have also been established (24), and PPE may be a potent
nephroprotective agent (25,26).
The aim of the present study was to evaluate the
protective properties of pomegranate peel aqueous extract against
CCl4 induced kidney damage in mice. Three major endogenous
antioxidant enzymes, superoxide dismutase (SOD), catalase (CAT) and
glutathione peroxidase (GPx) expression levels were assessed,
alongside biochemical, histopathological and immunohistochemical
changes, to evaluate the antioxidant capacity of the PPE.
Materials and methods
Preparation of the plant extract
The dried peel of pomegranates was obtained from
local markets. To prepare a water extract of the pomegranate peel,
the peels were cleaned with distilled water, desiccated and crushed
to a fine powder. A total of 1 kg peel powder was added to L
boiling water, after which the mixture was kept in a bolted vessel
for cooling. The solvent was filtered and concentrated in a water
bath until the extract was reduced to a volume of 100 ml. The
herb/extract ratio was 10/1. PPE was finally diluted to 10% as
described previously (27).
Gas chromatography-mass spectrometry
(GC-MS) analysis
GC-MS analysis of the aqueous peel extract of P.
granatum was performed using a GC-MS examination system (Trace
GC Ultra and ISQ Single Quadruple MS; Thermo Fisher Scientific,
Inc.) at a flow rate of 1.5 ml/min. as described previously
(28). The database of the National
Institute Standard and Technology (NIST, chemdata.nist.gov/) was consulted for the
identification of mass spectrum GC-MS.
Experimental animals
A total of 40 adult male CD1 albino mice weighing
20-30 g were acquired from the Animal House of VACSERA, Co. The
mice were maintained under normal environmental conditions of
temperature and humidity and were given adequate food and water.
The mice were allowed to acclimatize for 1 week prior to beginning
the experiments. The present study was performed in accordance with
published guidelines (29) and
approved by the Internal Research Regulation and the Animal Ethics
Committee of the Department of Zoology, Faculty of Science, Helwan
University (Helwan, Egypt).
Experimental design
To study the effects of PPE on CCl4 mediated
nephrotoxicity, CCl4 was mixed with olive oil as a vehicle in a 1:1
proportion. The adult male mice were divided into four groups of 10
mice each. The first group was the control group. The second group
was treated with a daily oral dose of PPE (400 mg/kg) for two
weeks. Group three was injected with 1 ml/kg CCl4 dissolved in
olive oil twice a week for two weeks. The fourth group was injected
intraperitoneally (IP) with CCl4 and treated with PPE, both as
above. The dose of CCl4 and treatment period were based on previous
studies (30-32).
An equal quantity of olive oil was given IP to the control group. A
blank fifth group, not administered olive oil, did not exhibit any
differences compared with control group, and therefore the data are
not presented.
Biochemical analysis
Animals were anesthetized with inhalant isoflurane
(3%) and blood samples were collected and stored in vacuum tubes
with clot activator. These samples were centrifuged at 3,000 x g
for 10 min at room temperature to separate the serum, and the serum
was stored at -20˚C. The quantity of serum urea and creatinine was
assessed using commercial kits from Reflotron; Liquicolor analysis
according to the manufacturer's protocol. Serum urea (33) and creatinine concentrations (34) were measured as described
previously.
Histological examination
Mice were euthanized using isoflurane (6%) to avoid
stressing the mice (35). The 2 mm
thick mouse kidney tissues were fixed in 10% formalin for 48 h at
room temperature, and tissues were processed for microscopic
examination. The sections were dyed with Harris's hematoxylin and
eosin (36) and Mallory's trichrome
stain for collagen fibers as described previously (37). The kidney sections of the control and
experimental groups were observed using light microscope, and
images were captured for analysis.
Immunohistochemistry analysis
Immunohistochemical detection of Caspase-3 was
performed using an anti-Caspase3 primary antibody (Labvision;
Thermo Fisher Scientific, Inc.) as described previously (38), using a streptavidin-biotin system.
Positive reactions for Caspase 3 were observed as brown coloration
of the cytoplasm in treated cells. The mean optical pixel density
of the kidney tissue was analyzed by using Image Pro Plus version
6.0 (Media Cybernetics, Inc.) and is expressed as the mean optical
density (MOD). For each sample, 10 random fields of view were
averaged to determine the mean. MOD values were only determined for
Caspase-3 staining and not for the collagen staining.
Antioxidant enzyme expression
Quantitative analysis of endogenous antioxidant
enzymes, such as SOD, CAT and GPx was performed. A PureLink FFPE
RNA Isolation kit, (Thermo Fisher Scientific, Inc.) was used for
RNA isolation. A total of 6 slices of 10 µm thick kidney tissue
sections were fixed and preserved in formalin, embedded in
paraffin, placed into a sterile microcentrifuge tube and RNA was
extracted according to the manufacturer's protocol. Briefly, the
tissue was separated from the melted paraffin by centrifugation at
3,000 x g for 5 min at 4˚C and digested with Proteinase K. The
tissue lysate was then processed by selective binding of RNA to a
silica-based membrane in the Spin Cartridge. Wash Buffer was used
to remove impurities by thorough washing. The final product was
eluted as total RNA in RNase-Free water. Quantity and purity of RNA
were measured using a NanoDrop ND-1000 spectrophotometer (Nanodrop
Technologies; Thermo Fisher Scientific, Inc.). Eluted RNAs with an
absorbance ratio OD 260/280 >2.0 were used for further analysis.
RNA quality was determined using agarose gel electrophoresis on
1.5% gel, and the RNA was frozen at -20˚C for further use.
The primers were designed using the nucleotide
sequence alignment NCBI BLAST tool (blast.ncbi.nlm.nih.gov/Blast.cgi). The sequences of
the primers were: CAT forward, 5'-TCCGGGATCTTTTTAACGCCATTG-3' and
reverse, 5'-TCGAGCACGGTAGGGACAGTTCAC-3'; SOD forward,
5'-AGCTGCACCACAGCAAGCAC-3' and reverse 5'-TCCACCACCCTTAGGGCTGA-3';
GPx forward 5'-GGCAAGGTACTACTTATCGAG-3' and reverse,
5'-GTTCACCTCGCACTTCTCGAAG-3'; and GAPDH forward
5'-GGATTTGGTCGTATTGGG-3' and reverse 5'-CGACATACTCAGCACCGG-3'.
GAPDH was used as the house keeping gene. Primers were purchased
from Macrogen, Inc.
Reverse transcription-quantitative
(RT-q)PCR
First-strand cDNA was synthesized from 2 µg total
RNA with primers for each gene. Briefly, 20 µl reverse
transcription reaction mix was prepared using M-MLV Reverse
Transcriptase system (Thermo Fisher Scientific, Inc.). cDNA was
synthesized by incubating the reaction mix at 42˚C for 1 h and
stored at -20˚C or used immediately. qPCR was performed using a
7500 Applied Biosystems RT-PCR system (Applied Biosystems; Thermo
Fisher Scientific, Inc. For each sample, a 20 µl reaction mixture
consisting of 1 µl diluted cDNA (1:20), 5 pmol each of the forward
and reverse primers, and 10 µl 2x SYBR Premix Ex Taq II (Takara
Bio, Inc.). Expression in each sample was assessed in triplicate.
Relative expression was calculated using the 2-ΔΔCq
method (39).
Statistical analysis
Data are presented as the mean ± standard deviation
and were analyzed using GraphPad Prism version 7 (GraphPad
Software, Inc.). A two-way ANOVA was used for multiple comparisons
with a post-hoc Bonferroni correction. A Student's t-test was used
to compare each treated sample with the control. P<0.05 was
considered to indicate a statistically significant difference.
Results
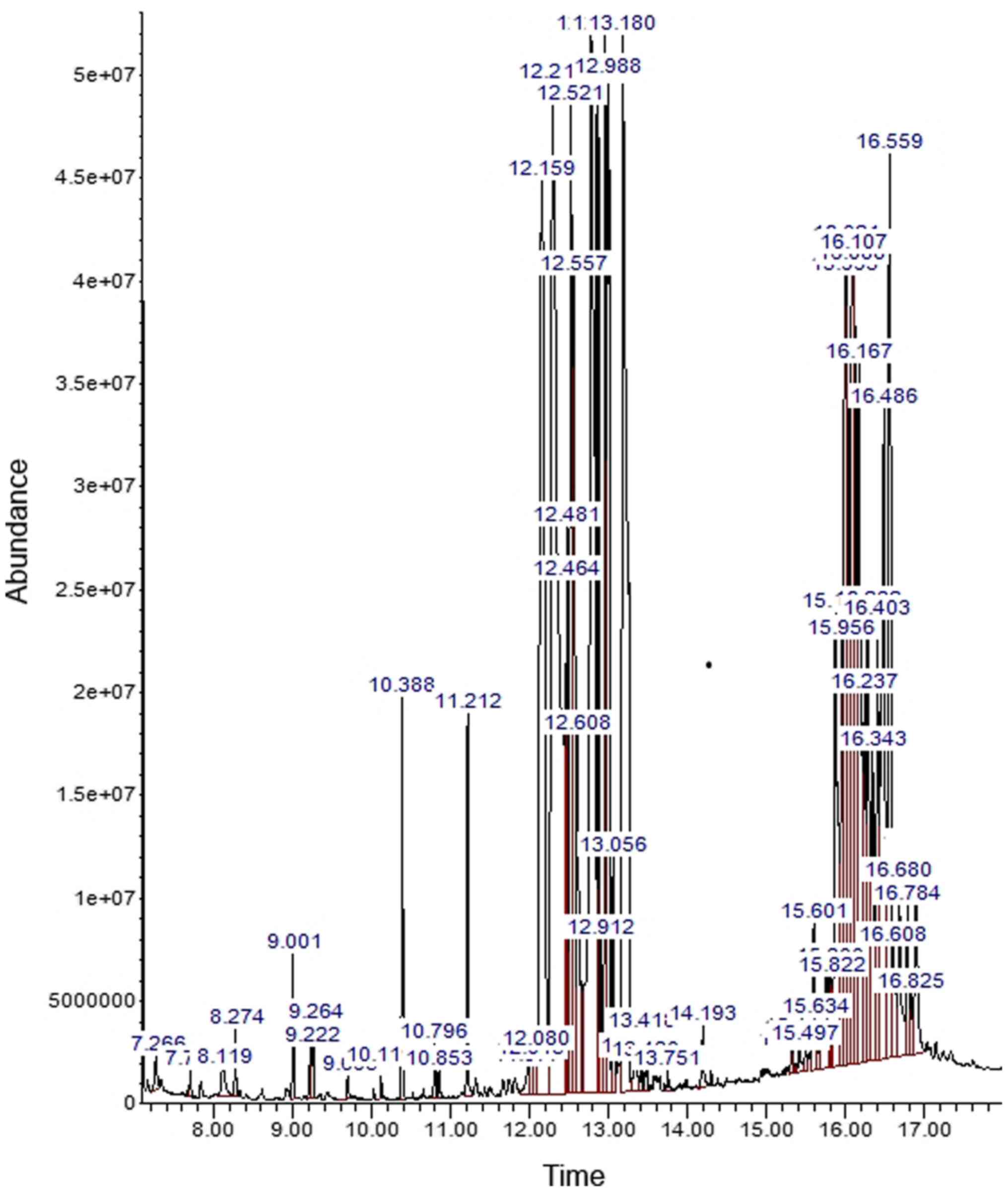
GC-MS analysis
Analysis of aqueous extract of pomegranate peel
using GC-MS showed the presence of a variety of phenolic compounds,
heterocyclic compounds, organic acids, fatty acids and sugars
(Fig. 1). The peaks in the
chromatogram were assimilated and equated using the NIST Mass
Spectral Library database of known compounds stored in the GC-MS
library. The phytoconstituents were identified and are presented in
Table I. The extract contained a
total of 23 peaks characteristic of phenolic components which are
carbohydrates; gallotannins and gallotannin derivatives
(1,2,3-Tri-O-galloyl-glucopyranose); phenolic acids (predominantly
gallic acid); and organic acids such as citric acid and malic acid.
In addition, fatty acids (octadecatrienoic acid and palmitic acid)
were present in small concentrations.
 | Table IPhytochemicals identified in the
aqueous extract peel of P. granatum by gas
chromatography-mass spectrometry. |
Table I
Phytochemicals identified in the
aqueous extract peel of P. granatum by gas
chromatography-mass spectrometry.
| Number of
peaks | Retention time | Area
percentage | Compound name |
|---|
| | | | Trisiloxane,
octamethyl- |
| | | | Pyrazol-5(4H)-one,
3-(4-methoxyphenyl)- |
| | | | 3-Methoxyphenol,
TMS derivative |
| | | |
4-Pentamethyldisilyloxyhexadecane |
| | | | Silanol,
trimethyl-, phosphate (3:1) |
| | | | Butanedioic acid,
bis(trimethylsilyl) ester |
| | | | 2-Propenoic acid,
2-[(trimethylsilyl)oxy]-, trimethylsilyl ester |
| 1 | 10.391 | 0.7193 | Malic acid |
| | | |
Isoindole-1,3(1H,3H)-dione,
2-[2-(4-methylphenylhydrazono)propyl]- |
| 2 | 11.2118 | 0.8076 | D-(-)-Ribofuranose,
tetrakis(trimethylsilyl) ether (isomer 1)a |
| | | | Arabinonic acid,
2,3,5-tris-O-(trimethylsilyl)-, .γ.-lactone, d- |
| | | | L-(-)-Arabitol,
5TMS derivative |
| 3 | 12.1597 | 7.1061 |
D-(-)-Tagatofuranose,
pentakis(trimethylsilyl) ether (isomer 2) |
| 4 | 12.2921 | 12.0624 | D-Psicofuranose,
pentakis(trimethylsilyl) ether (isomer 2) |
| 5 | 12.4615 | 1.1313 | Citric acid, 4TMS
derivative |
| 6 | 12.5198 | 3.9131 | D-Allofuranose,
pentakis(trimethylsilyl) ether |
| 7 | 12.5568 | 2.5652 |
.β.-D-Galactofuranose,
1,2,3,5,6-pentakis-O-(trimethylsilyl)- |
| 8 | 12.6098 | 1.2826 | Erythritol, 4TMS
derivative |
| 9 | 12.7792 | 9.0392 |
1,2,3-Tri-O-galloyl- -glucopyranose |
| 10 | 12.8692 | 1.9619 | D-(+)-Xylose, 4TMS
derivative |
| | | |
2-Hydroxybenzimidazole, 2TBDMS
derivative |
| 11 | 12.9593 | 2.7779 | 2(1H)Naphthalenone,
3,5,6,7,8,8a-hexahydro-4,8a-dimethyl-6-(1-methylethenyl)- |
| 12 | 12.9857 | 3.6912 | 2-.α.-Mannobiose,
octakis(trimethylsilyl) ether, methyloxime (isomer 1) |
| 13 | 13.0546 | 5.8949 | Gallic acid, 4TMS
derivative (6.4499) |
| 14 | 13.1817 | 8.3094 | Mannopyranoside,
trimethylsilyl 2,3,4,6-tetrakis-O-(trimethylsilyl)- |
| | | | 1,5-Anhydrohexitol,
4TMS derivative |
| | | | Palmitic Acid, TMS
derivative |
| | | | 2,4-Hexadienedioic
acid, (E, E)-, 2TMS derivative |
| | | | 11-Octadecenoic
acid, (E)-, TMS derivative |
| | | | Arabinonic acid,
2,3,5-tris-O-(trimethylsilyl)-, .γ.-lactone, d- |
| | | | Sucrose, 8TMS
derivative |
| | | | Lactulose,
octakis(trimethylsilyl) ether (isomer 2) |
| 15 | 15.8717 | 2.5257 |
[4-Bromo-2-(hydrazono-phenyl-methyl)-phenyl]-carbamic
acid, ethyl ester |
| 16 | 15.9564 | 1.4316 | Ethyl
2-[4-chlorophenyl]-7,8-benzocinchoninate |
| 17 | 15.9935 | 3.1568 | D-Fructose, 5TMS
derivative |
| 18 | 16.0888 | 3.3861 | D-Psicofuranose,
pentakis(trimethylsilyl) ether (isomer 2) |
| 19 | 16.1047 | 3.2436 | Sucrose, 8TMS
derivative |
| 20 | 16.1682 | 4.3471 | D-(-)-Ribofuranose,
tetrakis(trimethylsilyl) ether (isomer 2) |
| 21 | 16.2371 | 1.2581 | D-Fructose, 5TMS
derivative |
| 22 | 16.4859 | 4.0291 | Acrylonitrile,
2-chloro-3,3-bis-(4-nitrophenoxy)- |
| 23 | 16.5601 | 3.7202 | Molybdenum,
tricarbonyl[(1,2,3,4,5,6-.eta.)-1,4-dimethylbenzene]- |
| | | |
2-(2-Bromo-4-methylphenoxy)-N'-([1-(4-nitrophenyl)-2-pyrrolidinyl]
methylene)acethydrazide |
| | | | D-Psicopyranose,
5TMS derivative (isomer 2) |
Biochemical analysis
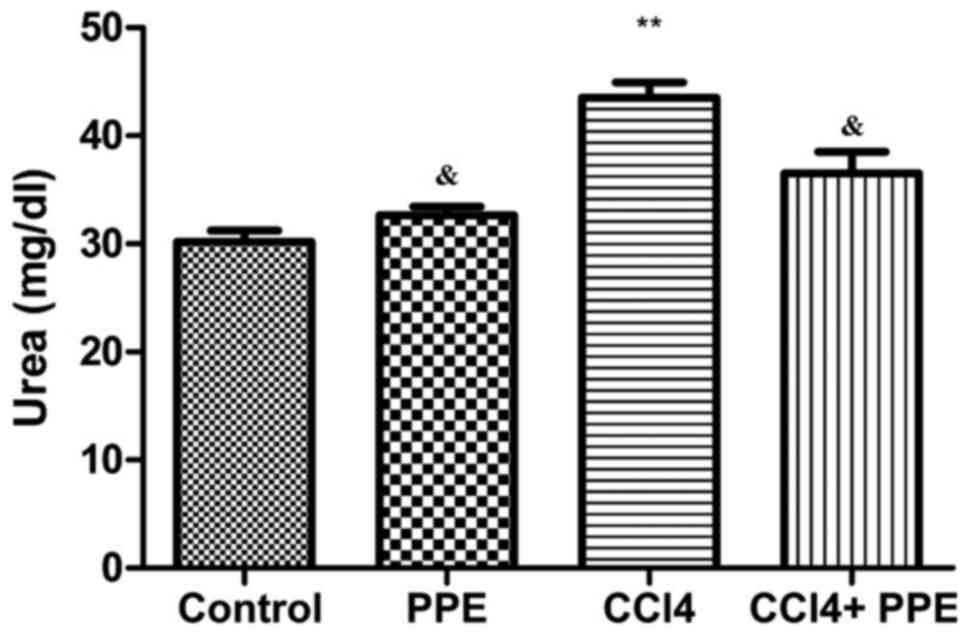
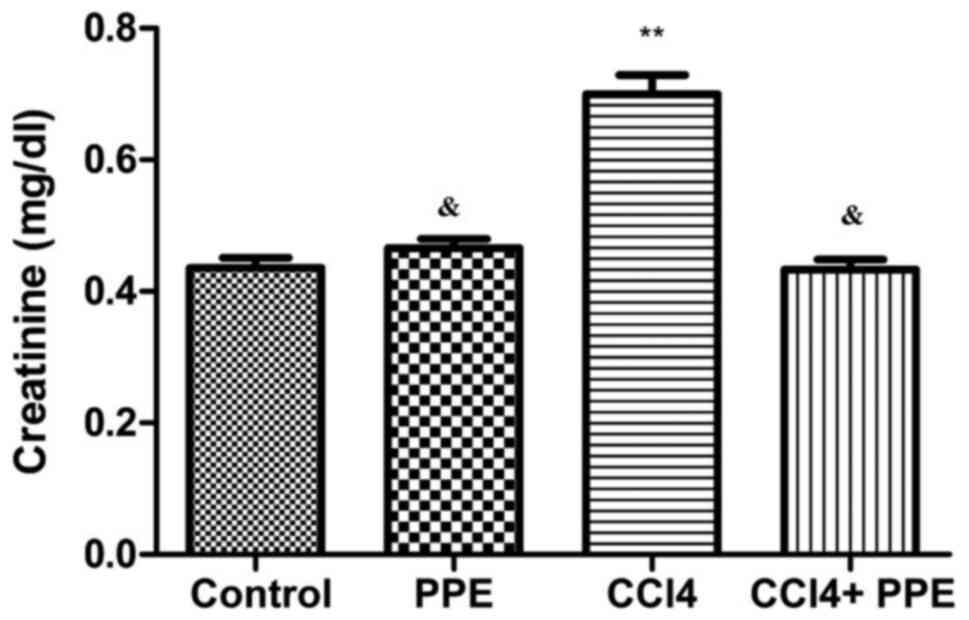
Changes in the urea and creatinine concentrations in
serum of adult male mice in the control and experimental groups are
presented in Figs. 2 and 3. The data show that administration of CCl4
for 15 days induced a significant increase in serum urea and
creatinine levels compared with the control group (P<0.001).
Treatment of the CCl4-treated group with PPE resulted in a
significant reduction in the level of serum urea and creatinine
concentrations compared with the CCl4 treated group (P<0.05),
and did not differ significantly compared with the mice in the
control group (P>0.05; Figs. 3
and 4). In addition, there were no
notable psychological differences observed in any of the mice. Food
and water consumption were regularly monitored and did not deviate
noticeably throughout the duration of the experiments (data not
shown).
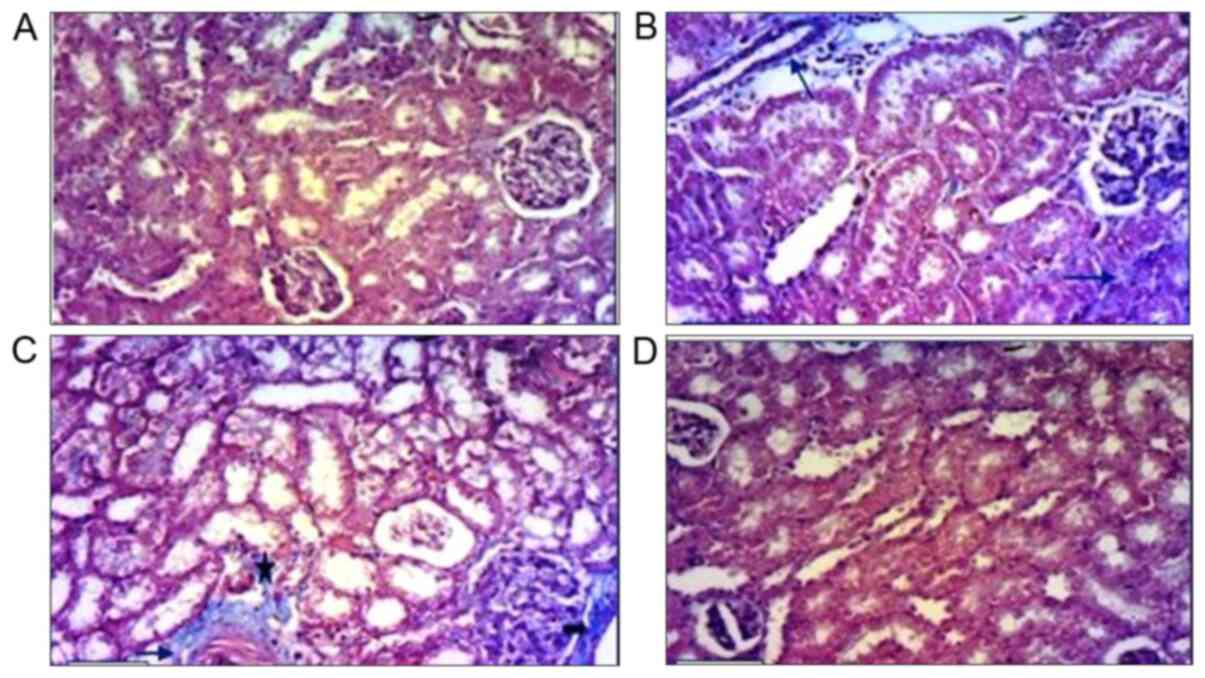
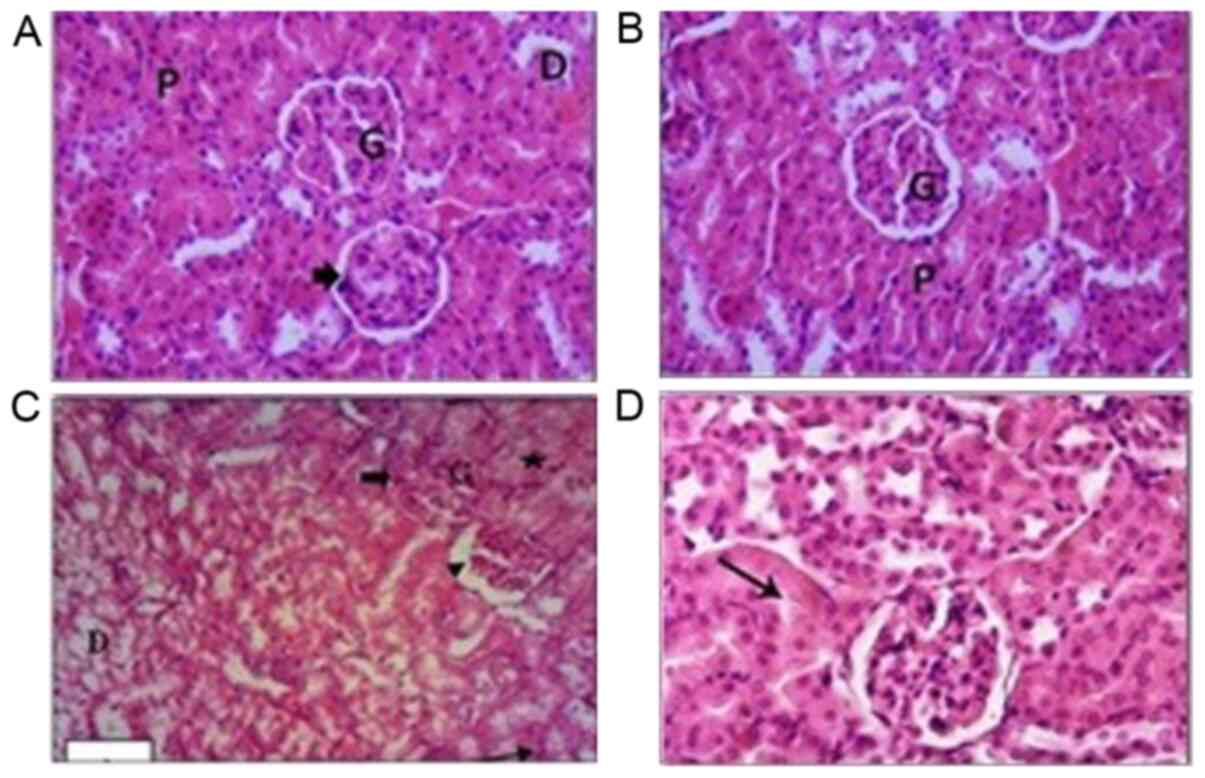 | Figure 4Images of kidney tissues stained with
hematoxylin and eosin. (A) Kidney tissues in the control group
showed normal histological architecture of the mouse kidney cortex,
normal glomeruli, normal Bowman's space (arrowhead), and normal
distal and proximal tubules. (B) Kidney tissues of the PPE-treated
group showed normal glomeruli, renal tubules and normal proximal
tubules. (C) Kidney tissue of the CCl4-treated group showed
hypercellularity of glomeruli (thick arrow) and widening of the
Bowman's space (arrowhead). Some tubules exhibited swollen cells in
the epithelial lining that narrowed the lumen (star). Some renal
tubules exhibited extensive vacuolar degeneration and their lumens
were filled with cellular debris (arrow). (D) Kidney tissues in the
CCl4 + PPE treated group. Glomeruli exhibited slight congestion and
some of the cells of the renal tubules appeared slightly swollen
(arrow). Magnification, x100. G, glomeruli; D, distal tubules; P,
proximal tubules; PPE, pomegranate peel extract; CCl4, carbon
tetrachloride. |
Histological analysis
Kidney sections from control mice stained using
hematoxylin and eosin showed normal renal cortex architecture. The
proximal convoluted tubules and the distal convoluted tubules
appeared normal (Fig. 4A). The renal
tissues of PPE treated mice showed several normal glomeruli and
renal tubules, and low levels of inflammatory cells in the
intertubular spaces (Fig. 4B).
The renal architecture in CCl4 treated mice was
notably affected. The majority of the glomeruli exhibited
hypercellularity severe glomerular congestion. Bowman's spaces in
the glomeruli were narrowed, whereas in other glomeruli, there was
an increase in the Bowman's spaces. The epithelial cells which
constituted the lining of tubules were severely swollen with a
decreased lumen volume. Some of the renal tubules showed extensive
vacuolar degeneration and their lumens were filled with cellular
debris. Cellular infiltration in the renal tissues was visible and
notably increased (Fig. 4C).
Administration of PPE to CCl4 treated mice resulted
in marked improvements, and reduced the renal damage caused by
CCl4. The kidney tissues of the CCl4 + PPE group retained intact
histological architectures with reduced damaged areas compared with
the CCl4 group; however some glomeruli appeared to be slightly
congested, and a few tubules showed slightly swollen epithelial
cells but with normal nuclei (Fig.
4D).
Alteration in collagen fibers
There were notable histopathological changes
observed in the collagen fiber content in renal tissues between the
control and treated sections. Mallory's triple stain of the renal
tissues in the control sections showed that there was less
interstitial connective tissue, and where present, it was primarily
concentrated around the blood vessels (Fig. 5). The collagen content surrounding
the glomeruli was scant (Fig. 5A).
The collagen fiber content in renal tissues of the PPE group was
similar to that of the control group; a moderate increase in the
connective tissue was observed in both glomerular and peritubular
areas (Fig. 5B). The renal tissues
of CCl4 treated mice exhibited an extensive increase in the
collagen fiber content primarily in the intra-glomerulus, around
the renal corpuscles, in the intertubular spaces and around the
blood vessels (Fig. 5C). The renal
tissue of the CCl4 and PPE treated mice showed a moderate decrease
in the collagen fiber content when compared with the CCl4 group
(Fig. 5D).
Immunohistochemical observations
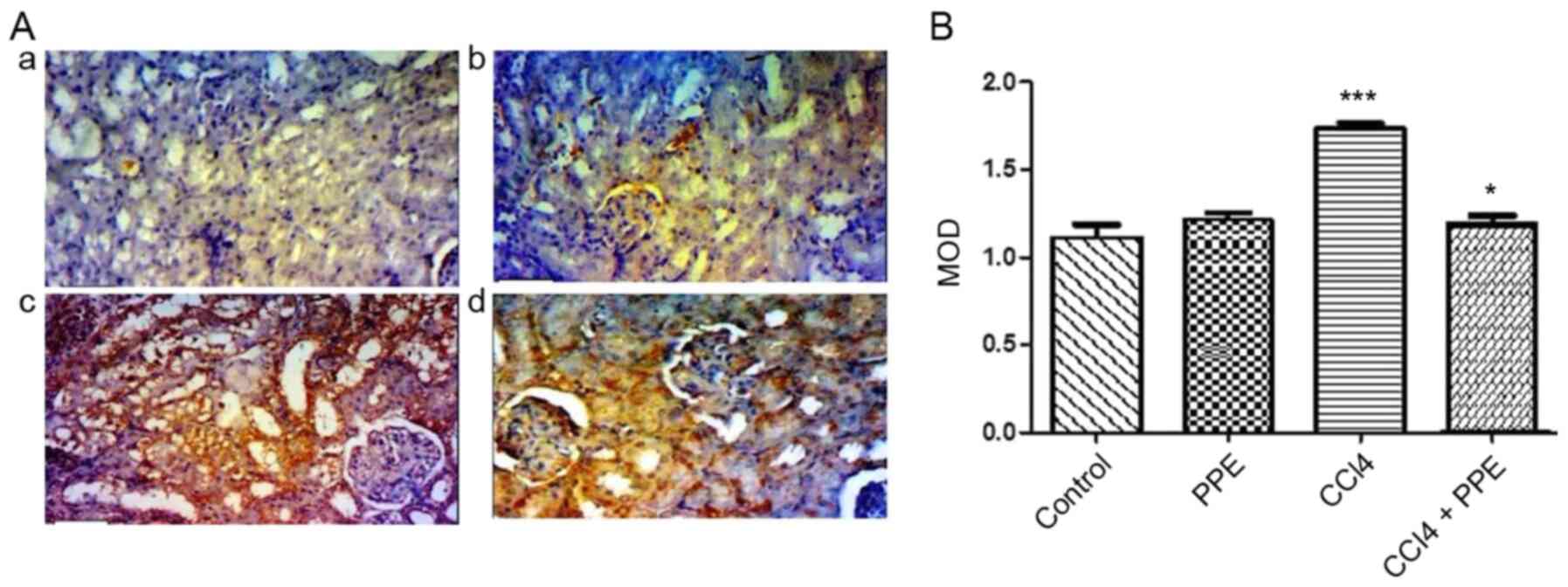
The results obtained by image analysis for Caspase-3
expression showed that the CCl4-treated group exhibited strong
cytoplasmic immunoreactivity, indicated by the intense brown color
in renal tissues of mice and the MOD values were significantly
increased compared with the control group (P<0.001; Fig. 6Aa, Ac and B). The PPE treated mice
exhibited relatively normal levels of Caspase-3 immunoreactivity
(Fig. 6Ab). There was a significant
decrease in the intensity of Caspase-3 expression in the renal
tissue, when treated with PPE combined with CCl4 compared with the
CCl4 group (P<0.05; Fig.
6Ad).
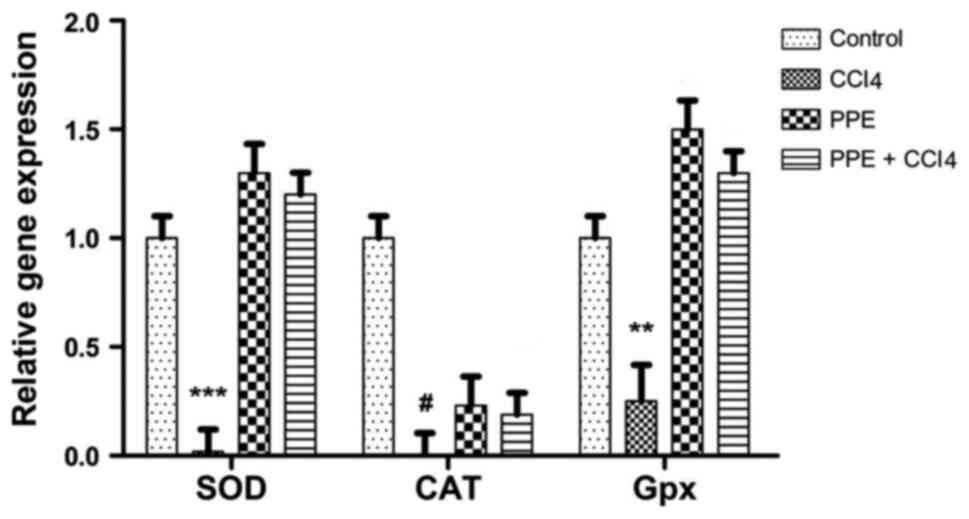
RT-qPCR
RT-qPCR analysis of SOD, CAT and GPx mRNA expression
levels are shown in Fig. 7. The
results showed a significant increase in the gene expression levels
of these genes in the PPE and PPE + CCl4 treated groups compared
with the control group. A significant increase in gene expression
levels were observed in all three genes compared with the CCl4
treated group. The CAT gene expression levels were increased both
in PPE and PPE + CCl4 group compared with the CCl4 group. However,
the expression of CAT in the CCl4, PPE and PPE + CCl4 groups were
notably lower than the control, unlike the expression of SOD and
GPx. The difference between the expression levels of all the genes
in the CCl4 and PPE + CCl4 groups was significant when compared
with the control (P<0.001).
Discussion
Kidneys are responsible for the removal of various
chemicals and toxins from the blood stream, and thus are likely to
be subjected to consequent damage. Functional impairment of the
kidneys is a significant public health problem (8). The present study demonstrated the
ameliorative effects of PPE on CCl4 induced renal toxicity in mice.
The results show that treatment with CCl4 resulted in
nephrotoxicity, as indicated by a rise in serum levels of urea and
creatinine along with detectable histopathological changes in the
kidney tissue which manifested as morphological changes to the
glomerulus, renal tubules and vacuolization of the cells. The
results of the present study are similar to previous studies
examining the histopathological alterations in the kidney tissue
induced by administration of CCl4, and the increase changes in the
serum urea and creatinine levels (40-42).
These changes are caused by the formation of free radicals, which
results in lipid peroxidation and breakdown of the membrane
structure, and thus subsequently in the damage of nephron
structural integrity (40,43). Additionally, the oxidation of lipids,
proteins, carbohydrates, DNA and other biological molecules by
noxious ROS can result in DNA mutations, which contribute to
impairment of the target cell's function and often results in cell
senescence and death (42,44). The capacity of tubular absorption is
altered and the nephrons are overloaded, resulting in subsequent
renal dysfunction (45,46).
The animals treated with PPE alone for 15 days did
not show any toxicity in the histopathological evaluations nor any
significant changes in serum urea and creatinine levels when
compared with the control. These results are in agreement with a
previous study (47), showing that
pomegranates exhibit potent antioxidative effects without any
noticeable toxic effects. Furthermore, the present study suggested
that PPE reduced the CCl4-induced effects on renal physiology. The
reduction in tissue damage was attributed to the high antioxidant
and nephroprotective effects of PPE, possibly due to the high
levels of phenolic compounds (26,48-50).
Administration of the PPE is reported to attenuate the toxicity and
oxidative stress induced by chlorpyrifos-ethyl treatment in animals
(51). The presence of kidney
fibrosis is generally considered as endpoint organ failure
proceeding to loss of function (49). In the present study, in the
CCl4-treated group, there was a substantial increase in collagen
content in the intra-glomerular and the intertubular spaces as well
as around the renal corpuscles and the blood vessels. CCl4-induced
nephrotoxicity is suggested to impair renal function, increase
inflammation and fibrosis, with notably increased collagen
deposition as a downstream outcome of increased free radical levels
(52). However, the treatment of
mice with CCl4 + PPE resulted in a notable decrease in collagen
fiber content; this protective effect may be attributed to the
antioxidant and antifibrotic properties of PPE (48,49,53). The
histopathological changes described in the present study are
limited in their value, due to an unavailability of the
high-resolution electron microscopic images. Caspase-3 is the key
protein involved in programmed cell death (54) In the present study,
immunohistochemical results of kidney tissue from CCl4-administered
mice showed a substantial increase in Caspase-3 production,
suggesting an increase in apoptosis compared with the control
group. CCl4 has been shown to induce acute nephrotoxicity with
apoptotic renal damage by increasing the renal levels of active
Caspases-3. Furthermore, CCl4 is capable of increasing oxidative
stress and apoptosis in other organs such as the liver (55). Co-administration of PPE with CCl4
resulted in a decrease in Caspase-3 levels, further highlighting
the protective effects of PPE against oxidative stress-induced
apoptosis, as a result of diethylnitrosamine and phenobarbital
mediated apoptosis in the kidney (29). These results suggest that PPE
protects against CCl4-induced renal tissue damage by enhancing the
anti-apoptotic activity as well as by reducing the expression of
apoptotic proteins. As only total Caspase-3 expression levels were
analyzed, it is not possible to concretely say whether apoptosis
was increased without assessing cleaved Caspase 3 levels. However,
the increase in Caspase 3 levels do suggest a potential increase in
apoptosis, and thus, the antiapoptotic effects of PPE.
In the CCl4 treated mice, the expression levels of
SOD, CAT and GPx were significantly decreased, whereas an increase
in expression of SOD and GPx was observed in the PPE treated mice
group compared with the control group, suggesting an overall
improvement in the antioxidative state, even in the absence of
injury. In the CCl4 + PPE treated mice compared with the
CCl4-treated group, there was a significant 1.3 fold increase in
SOD and GPx expression. In addition, there was a slight increase in
CAT expression. These results highlight the protective effects of
PPE against oxidative stress. A previous study showed that phenolic
compounds from PPE showed strong antioxidant properties as gauged
by the Gallic acid monohydrate, 2,2-diphenyl-1-picrylhydrazyl
scavenging activity and ferric reduction tests (56). The results of the present study are
in agreement with the previous study, suggesting the protective
effects of pomegranate against stress-induced tissue damage. A
significant protective effect was observed when
Streptozotocin-nicotinamide induced diabetic rats were subjected to
21 days of oral gavage with pomegranate seed-juice (57). Treatment with pomegranate seed-juice
significantly increased the activity of SOD and CAT. The qPCR
results performed in the present study further support the
biochemical and histopathological data, showing a reduction in
tissue injury of the Bowman's capsule and glomerular cells.
In conclusion, the results of the present study
demonstrate the nephroprotective role of the aqueous PPE against
CCl4-induced nephrotoxicity at the biochemical, histopathological
and molecular level. The histopathological improvement may be
attributed to the antioxidant properties of PPE. The present study
further validates the folk based use of aqueous PPE for the
treatment of toxicity-related renal injuries. Pomegranate
peel-based tea may protect against diabetes, or against kidney
damage in patients who regularly use nephrotoxic medicines.
Pomegranate fruit peels are a waste material of the food industry,
and the present study highlights its potential clinical value.
Acknowledgements
We would like to thank Dr Shazina Kanwal (Guangzhou
Institutes of Biomedicine and Health, China) for reviewing our
manuscript and for the valuable suggestions, and Miss Angel Weaver
for assistance with revising the language of the manuscript.
Funding
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
MARI and HAO performed the in vivo
experiments. HAO conducted the biochemical analysis and the plant
extract analysis. NME and MARI performed the histopathology and
immunohistochemical analysis. SA and TA performed the molecular
experiments. All authors contributed equally in experimental design
and to writing the manuscript. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Internal
Research Regulation and the Animal Ethics Committee of the
Department of Zoology, Faculty of Science, Helwan University
(Helwan, Egypt).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Agency for Toxic Substances and Disease
Registry: Toxicological profile for arsenic. PB/2000/108021. US
Department of Health and Human Services, Atlanta, 2000.
|
|
2
|
Balahoroğlu R, Dülger H, Özbek H, Bayram İ
and Şekeroğlu MR: Protective effects of antioxidants on the
experimental liver and kidney toxicity in mice. Eur J Gen Med.
5:157–164. 2008.
|
|
3
|
Manjrekar AP, Jisha V, Bag PP, Adhikary B,
Pai MM, Hegde A and Nandini M: Effect of phyllanthus niruri Linn.
treatment on liver, kidney and testes in CCl4 induced hepatotoxic
rats. Indian J Exp Biol. 46:514–520. 2008.PubMed/NCBI
|
|
4
|
Soliman AM and Fahmy SR: Protective and
curative effects of the 15 KD isolated protein from the peganum
harmala L: Seeds against carbon tetrachloride induced oxidative
stress in brain, tests and erythrocytes of rats. Eur Rev Med
Pharmacol Sci. 15:888–899. 2011.PubMed/NCBI
|
|
5
|
Benjamin IJ and Schneider MD: Learning
from failure: Congestive heart failure in the postgenomic age. J
Clin Invest. 115:495–499. 2005.PubMed/NCBI View
Article : Google Scholar
|
|
6
|
Qiu DK, Hua J, Li JQ and Li EL: CD14
expression on Kupffer cells during the course of carbon
tetrachloride-mediated liver injury. Chin J Dig Dis. 6:137–141.
2005.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Porter GA and Bennett WM: Nephrotoxic
acute renal failure due to common drugs. Am J Physiol. 241:F1–F8.
1981.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Lakshmi SM, Reddy U and Rani S: A review
on medicinal plants for nephroprotective activity. Asian J Pharm
Clin Res. 5:8–14. 2012.
|
|
9
|
Shirwaikar A, Issac D and Malini S: Effect
of Aerva lanata on cisplatin and gentamicin models of acute renal
failure. J Ethnopharmacol. 90:81–86. 2004.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Hosohata K: Role of oxidative stress in
drug-induced kidney injury. Int J Mol Sci. 17(E1826)2016.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Siddik ZH: Cisplatin: Mode of cytotoxic
action and molecular basis of resistance. Oncogene. 22:7265–7279.
2003.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Rahmat AA, Dar FA and Choudhary IM:
Protection of CCl4-induced liver and kidney damage by phenolic
compounds in leaf extracts of cnestis ferruginea (de Candolle).
Pharmacognosy Res. 6:19–28. 2014.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Baud L and Ardaillou R: Reactive oxygen
species: Production and role in the kidney. Am J Physiol.
251:F765–F776. 1986.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Khan MR and Siddique F: Antioxidant
effects of citharexylum spinosum in CCl4 induced nephrotoxicity in
rat. Exp Toxicol Pathol. 64:349–355. 2012.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Rajesh MG and Latha MS: Preliminary
evaluation of the antihepatotoxic activity of kamilari, a
polyherbal formulation. J Ethnopharmacol. 91:99–104.
2004.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Priyadarsini G, Kumar A, Anbu J, Anjana A
and Ayyasamy S: Nephroprotective activity of decoction of
indigofera tinctoria (avuri kudineer) against cisplatin-induced
nephropathy in rats. Int J Life Sci Pharma Res. 2:56–62. 2012.
|
|
17
|
Moneim AEA: Antioxidant activities of
Punica granatum (pomegranate) peel extract on brain of rats.
J Med Plants Res. 6:195–199. 2012.
|
|
18
|
Negi P, Jayaprakasha G and Jena B:
Antioxidant and antimutagenic activities of pomegranate peel
extracts. Food Chemistry. 80:393–397. 2003.
|
|
19
|
Seeram NP, Adams LS, Henning SM, Niu Y,
Zhang Y, Nair MG and Heber D: In vitro antiproliferative, apoptotic
and antioxidant activities of punicalagin, ellagic acid and a total
pomegranate tannin extract are enhanced in combination with other
polyphenols as found in pomegranate juice. J Nutr Biochem.
16:360–367. 2005.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Wu S and Tian L: Diverse phytochemicals
and bioactivities in the ancient fruit and modern functional food
pomegranate (Punica granatum). Molecules.
22(E1606)2017.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Salama SM, AlRashdi AS, Abdulla MA,
Hassandarvish P and Bilgen M: Protective activity of panduratin a
against thioacetamide-induced oxidative damage: Demonstration with
in vitro experiments using WRL-68 liver cell line. BMC Complement
Alternative Med. 13(1)2013.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Mansouri E, Basgen J and Saremy S: The
effects of pomegranate extract on normal adult rat kidney: A
stereological study. Vet Res Forum. 7:1–6. 2016.PubMed/NCBI
|
|
23
|
Adams LS, Seeram NP, Aggarwal BB, Takada
Y, Sand D and Heber D: Pomegranate juice, total pomegranate
ellagitannins, and punicalagin suppress inflammatory cell signaling
in colon cancer cells. J Agric Food Chem. 54:980–985.
2006.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Lansky EP and Newman RA: Punica
granatum (pomegranate) and its potential for prevention and
treatment of inflammation and cancer. J Ethnopharmacol.
109:177–206. 2007.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Hamouda AF and Shaban NZ: Short and long
term effects of pomegranate (Punica granatum) extracts on
apoptosis in rat kidney induced by diethylnitrosamine and
phenobarbital. J Pharm Pharmacol. 4:52–63. 2016.
|
|
26
|
Ahmed MM and Ali SE: Protective effect of
pomegranate peel ethanol extract against ferric nitrilotriacetate
induced renal oxidative damage in rats. J Cell Mol Biol. 7:35–43.
2010.
|
|
27
|
Kamali M, Tavakoli H, Khodadoost M,
Daghaghzadeh H, Kamalinejad M, Gachkar L, Mansourian M and Adibi P:
Efficacy of the Punica granatum peels aqueous extract for
symptom management in ulcerative colitis patients. A randomized,
placebo-controlled, clinical trial. Complement Ther Clin Pract.
21:141–146. 2015.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Abdel AM: The neuroprotective effects of
purslane (Portulaca oleracea) on rotenone-induced biochemical
changes and apoptosis in brain of rat. CNS Neurol Disord Drug
Targets. 12:830–841. 2013.PubMed/NCBI View Article : Google Scholar
|
|
29
|
National Research Council (US) Institute
for Laboratory Animal Research: Guide for the Care and Use of
Laboratory Animals. National Academies Press (US), Washington, DC,
1996.
|
|
30
|
Fahmy MA, Diab KA, Abdel-Samie NS, Omara
EA and Hassan ZM: Carbon tetrachloride induced hepato/renal
toxicity in experimental mice: Antioxidant potential of egyptian
salvia officinalis L essential oil. Environ Sci Pollut Res Int.
25:27858–27876. 2018.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Safhi MM: Nephroprotective effect of
zingerone against CCl4-induced renal toxicity in Swiss albino mice:
Molecular mechanism. Oxid Med Cell Longev.
2018(2474831)2018.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Ghasemi M, Azarnia M, Jamali M,
Mirabolghasemi G, Nazarian S, Naghizadeh MM, Rajabi M and Tahamtani
Y: Protective effects of ephedra pachyclada extract on mouse models
of carbon tetrachloride-induced chronic and acute liver failure.
Tissue Cell. 46:78–85. 2014.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Price CP and Koller PU: A multicentre
study of the new reflotron system for the measurement of urea,
glucose, triacylglycerols, cholesterol, gamma-glutamyltransferase
and haemoglobin. J Clin Chem Clin Biochem. 26(4):233–250.
1988.PubMed/NCBI
|
|
34
|
Koller PU: The
reflotron®-system-principles and practical experiences.
Upsala Journal of Medical Sciences. 91(2):135–138. 1986.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Marquardt N, Feja M, Hünigen H, Plendl J,
Menken L, Fink H and Bert B: Euthanasia of laboratory mice: Are
isoflurane and sevoflurane real alternatives to carbon dioxide?
PLoS One. 13(e0203793)2018.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Bancroft J and Gamble M: Theory and
practicle of histological techniques. 6th edition. Churkhil
Livinstone, London, pp433-469, 2008.
|
|
37
|
Avwioro G: Histochemical uses of
haematoxylin-a review. JPCS. 1:24–34. 2011.
|
|
38
|
Abdel-Wahab BA and Metwally ME:
Clozapine-induced cardiotoxicity: Role of oxidative stress, tumour
necrosis factor alpha and NF-κβ. Cardiovasc Toxicol. 15:355–365.
2015.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Sakr SA and Lamfon HA: Protective effect
of rosemary (Rosmarinus officinalis) leaves extract on carbon
tetrachloride-induced nephrotoxicity in albino rats. Life Sci J.
9:779–785. 2012.
|
|
41
|
Olagunju J, Adeneye A, Fagbohunka B,
Bisuga N, Ketiku A, Benebo A, Olufowobic O, Adeoyec A, Alimic M and
Adelekec A: Nephroprotective activities of the aqueous seed extract
of carica papaya linn. in carbon tetrachloride induced renal
injured wistar rats: A dose-and time-dependent study. Biol Med.
1:11–19. 2009.
|
|
42
|
Venkatanarayana G, Sudhakara G, Sivajyothi
P and Indira P: Protective effects of curcumin and vitamin E on
carbon tetrachloride-induced nephrotoxicity in rats. EXCLI J.
11:641–650. 2012.PubMed/NCBI
|
|
43
|
Ozturk F, Ucar M, Ozturk IC, Vardi N and
Batcioglu K: Carbon tetrachloride-induced nephrotoxicity and
protective effect of betaine in sprague-dawley rats. Urology.
62:353–356. 2003.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Shyur LF, Tsung JH, Chen JH, Chiu CY and
Lo CP: Antioxidant properties of extracts from medicinal plants
popularly used in Taiwan. Int J Appl Sci Eng. 3:195–202. 2005.
|
|
45
|
Khan MR, Rizvi W, Khan GN, Khan RA and
Shaheen S: Carbon tetrachloride-induced nephrotoxicity in rats:
Protective role of digera muricata. J Ethnopharmacol. 122:91–99.
2009.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Khan RA, Khan MR and Sahreen S: Evaluation
of launaea procumbens use in renal disorders: A rat model. J
Ethnopharmacol. 128:452–461. 2010.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Moneim AEA, Dkhil MA and Al-Quraishy S:
Studies on the effect of pomegranate (Punica granatum) juice
and peel on liver and kidney in adult male rats. J Med Plants Res.
5:5083–5088. 2011.
|
|
48
|
Cekmen M, Otunctemur A, Ozbek E, Cakir SS,
Dursun M, Polat EC, Somay A and Ozbay N: Pomegranate extract
attenuates gentamicin-induced nephrotoxicity in rats by reducing
oxidative stress. Ren Fail. 35:268–274. 2013.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Abdel Moneim AE and El-Khadragy MF: The
potential effects of pomegranate (Punica granatum) juice on
carbon tetrachloride-induced nephrotoxicity in rats. J Physiol
Biochem. 69:359–370. 2013.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Atawodi S, Adekunle O and Bala I:
Antioxidant, organ protective and ameliorative properties of
methanol extract of anogeissus leiocarpus stem bark against carbon
tetrachloride-induced liver injury. Int J Pharmaceutical Sci Res.
2(1443)2011.
|
|
51
|
Ahmed MM and Zaki NI: Assessment the
ameliorative effect of pomegranate and rutin on
chlorpyrifos-ethyl-induced oxidative stress in rats. Nat Sci.
7:49–61. 2009.
|
|
52
|
Hamed MA, Ali SA and El-Rigal NS:
Therapeutic potential of ginger against renal injury induced by
carbon tetrachloride in rats. ScientificWorldJournal.
2012(840421)2012.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Wei XL, Fang RT, Yang YH, Bi XY, Ren GX,
Luo AL, Zhao M and Zang WJ: Protective effects of extracts from
Pomegranate peels and seeds on liver fibrosis induced by carbon
tetrachloride in rats. BMC Complement Altern Med.
15(389)2015.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Lossi L, Castagna C and Merighi A:
Caspase-3 mediated cell death in the normal development of the
mammalian cerebellum. Int J Mol Sci. 19(E3999)2018.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Hassan MH, Bahashawan SA, Abdelghany TM,
Abd-Allah GM and Ghobara MM: Crocin abrogates carbon
tetrachloride-induced renal toxicity in rats via modulation of
metabolizing enzymes and diminution of oxidative stress, apoptosis,
and inflammatory cytokines. J Biochem Mol Toxicol. 29:330–339.
2015.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Shiban MS, Al-Otaibi MM and Al-Zoreky NS:
Antioxidant activity of pomegranate (Punica granatum L.)
fruit peels. Food Nutr Sci. 3:991–996. 2012.
|
|
57
|
Aboonabi A, Rahmat A and Othman F:
Antioxidant effect of pomegranate against
streptozotocin-nicotinamide generated oxidative stress induced
diabetic rats. Toxicol Rep. 1:915–922. 2014.PubMed/NCBI View Article : Google Scholar
|