Introduction
Rheumatoid arthritis (RA) is a common form of
arthritis which primarily affects multiple joints, but can also
cause damage to other organs, known as extra-articular
manifestations (1). The symptoms
associated with such conditions include pain, swollen joints,
stiffness with synovitis, and progressive cartilage and bone
erosion. RA can lead to serious functional disability if it is not
properly managed (2).
Although the etiology of RA remains incompletely
understood, previous studies have suggested that the activation of
T cells potentiates a subsequent activation of other immune cells
such as B cells, fibroblasts and macrophages resulting in a complex
network of continuously secreted pro-inflammatory cytokines
(3-5).
The overproduction of pro-inflammatory cytokines,
particularly tumor necrosis factor (TNF-α) and interleukin-1β
(IL-1β) are crucially involved in the pathogenesis and progression
of RA (6,7).
Nuclear factor-κB (NF-κB) activation has also been
shown to be associated with the pathogenesis of RA, resulting in
cartilage and bone destruction (8).
The NF-κB pathway was demonstrated to result in upregulation of
pro-inflammatory cyclooxygenase-2 (COX-2) levels and inducible
nitric oxide synthase (iNOS), leading to the subsequent production
of pro-inflammatory prostaglandins (PGs) and nitric oxide, which
lead to further articular damage and induced hyperalgesia (9-11).
The use of alternative natural products for
treatment of RA is gaining increasing interest. Bee venom (BV),
obtained from Apis mellifera, contains different peptides,
including melittin, apamin, adolapin and mast cell degranulating
peptide (12). BV is widely used in
traditional Chinese medicine for the treatment of inflammatory
diseases, such as RA and to alleviate the associated pain (13,14).
Additionally, BV has been reported to exert
promising anti-inflammatory and immunomodulatory properties
(15-17).
Thus, the aim of the present study was to evaluate
the anti-arthritic activity of BV, and the possible underlying
immunomodulatory mechanisms of systemic BV in RA using an in
vivo experimental model.
Materials and methods
Chemicals and solvents
Methotrexate was purchased from Mylan N.V. BV was
purchased from Apis Injeel® (Heel, GMBH). Complete
Freund's adjuvant (CFA) was purchased from Sigma-Aldrich; Merck
KGaA. TNF-α (cat. no. CSB-E11987r) and IL-1β (cat. no. CSB-E08055r)
ELISA kits were purchased from CUSABIO TECHNOLOGY LLC. COX
inhibitor screening assay kit (cat. no. 560131) was purchased from
Cayman Chemical Company. Other reagents used were purchased from
Sigma-Aldrich; Merck KGaA.
Animals
Studies were performed on adult male Wistar rats
weighing (150-200 g), and were obtained from the animal house of
VACSERA Co. Animals were maintained in a controlled environment at
the ambient temperature, with ad libitum access to food and
water. Animals were allowed to acclimatize for 7 days prior to
initiation of experiments. The study time plan was designed for 21
days during which the animals were housed in cages of suitable
sizes (maximum of 3 animals per cage) to ensure animal comfort.
Sodium pentobarbital was used to anesthetize the
animals before the induction of arthritis and at the end of the
study to euthanize the animals via intraperitoneal (i.p.) injection
(800 mg/kg) (18). Ethical approval
was obtained for all the procedures from the Ethics Committee of
the Faculty of Pharmacy, Damanhour University, (Damanhour, Egypt)
(approval no. 717PO5).
Induction of arthritis
Animals were anesthetized using sodium pentobarbital
(50 mg/kg, i.p.) (19). Arthritis
was induced by intra-articular injection of 0.3 ml CFA (1 mg/ml) to
the right knee joint, as described previously (20). As a control, 0.3 ml saline was
injected into the left knee joint. The point of injection was
marked to ensure consistency in the knee circumference follow up
readings. Rats were considered arthritic if redness and joint
swelling was observed in at least joint. The signs were assessed
according to a scoring system as described below.
Treatment protocol and experimental
design
A total of 20 rats were randomly assigned to one of
four groups (n=5 per group) as follows: i) Normal healthy rats; ii)
arthritic rats that were treated with saline as a negative control;
iii) arthritic rats treated with methotrexate (2 mg/kg/week, i.p.)
as a standard drug (21); and iv)
arthritic rats treated with BV (60 mg/kg/day, i.p.). All the
treatments with methotrexate or BV started one day after the
induction procedure was performed, and was performed consistently
for 21 days. BV dose was selected through screening of various
doses of BV (5, 10, 15, 30, 45 and 60 mg/kg) against the stable
standard methotrexate, and 60 mg/kg BV was found to the most
efficacious dose (Table S1).
Evaluation of knee joint edema
The circumferences of the knee joint were measured
at the previously marked points using flexible tape prior to and
following the induction of arthritis. Subsequently, the
circumference was measured periodically every week for 21 days
(22). On day 21; the rats were
euthanized. Blood and serum samples were collected for further
biochemical analysis. Knee joints were harvested and preserved in
buffered formalin-saline (10%) at room temperature for 48 h for
follow up histopathological examination.
Scoring index of arthritic
manifestations
Rats were evaluated every 6 days from day 1 for
symptoms of RA and other signs of inflammation. The severity of the
symptoms in each rat was evaluated by grading the four knee joints
on a scale of 0-3 according to the variations in erythema, edema,
presence of nodules and the involvement of other non-injected
joints with a total score of 12 per rat. The scores were defined as
follows: 0, erythema, no swelling with no nodules; 1, erythema,
mild swelling with no nodules. 2, erythema, moderate swelling with
or without nodules; and 3, erythema with severe swelling limiting
the overall movement and presence of nodules or lesions, as
described previously (23,24).
Erythrocyte sedimentation rate
(ESR)
ESR is a non-specific test that indirectly measures
the presence of inflammation in a whole blood sample. ESR was
evaluated using the Westergren method (25). Blood was drawn from each rat and was
placed into disposable vacuum Westergren ESR tubes containing
sodium citrate from Wei Hai Kangzhou Biotechnology Co. Ltd. The
tubes were allowed to stand vertically for 1 and 2 h. The drop in
erythrocyte level was measured and was considered to represent the
ESR value (26).
Measurement of the serum levels of
TNF-α and IL-1β using ELISA
TNF-α and IL-1β levels were assessed using ELISA. An
antibody specific for TNF-α or IL-1β had been pre-coated separately
onto microplates, then both the standard and the serum samples were
placed into wells to form an immobilized antibody. Subsequent
addition of biotin-conjugated antibody then avidin conjugated
horseradish peroxidase, followed by a substrate solution was added
to the wells resulting in the development of a color if the tested
antibody was present in the serum. The intensity of the color was
measured and was relative to the quantity of TNF-α or IL-1β bound
antibody (27).
Histopathological examination of the
knee joints in adjuvant-induced arthritic rats
The preserved knee joints were decalcified in EDTA
for 4 weeks, then embedded in paraffin blocks. The blocks were
sliced into sections 4 µm thick, and the joint sections were
stained with Mayer hematoxylin solution for 8 min and eosin Y
solution for 1 min, both at room temperature.
H&E stained sections were scored for changes in
cell infiltration, synovitis, synovial proliferation and cartilage
or bone erosion. The sections were assessed on a scale of 0-3, and
classified as follows: 0, No cell infiltration, no synovitis,
intact synovial lining and no damage to the cartilage or the bone;
1, small count of cell infiltration, mild synovitis, limited pannus
formation and no apparent damage to the cartilage or the bones; 2,
moderate density of infiltrating cells, moderate synovitis,
moderate pannus formation and moderate lesions in the cartilage or
the bone; and 3, large quantities of infiltrating cells, severe
synovitis, severe pannus formation and extensive damage to the
cartilage or the bones (28).
Immunostaining for NF-κB (p65)
expression in adjuvant-induced arthritic rats
CFA induced arthritic knee joints were subjected to
an NF-κB (p65) immunostaining kit obtained from Thermo Fisher
Scientific, Inc. (cat. no. RB-1638-R7), and performed according to
the manufacturer's protocol. Staining was assessed using a light
microscope at a magnification of x400. Images were analysed using
ImageJ; the percentage of area stained and the intensity of the
NF-κB (p65) staining were assessed (29).
Determination of in-vitro COX
activity
COX activity was evaluated using a COX (ovine)
Inhibitor Screening assay kit that included both ovine COX-1 and
human recombinant COX-2 enzymes. The assay was used to screen
isozyme-specific inhibitors by direct measurement of PGF2α which is
produced by the reduction of COX-derived PGH2 by SnCl2. Finally,
the yield was evaluated using enzyme immunoassay for
quantification, as described previously (30,31).
Carrageenan paw edema
Acute anti-inflammatory activity was evaluated using
a carrageenan-induced rat paw edema test. A total of 15 male Wistar
rats were divided into 3 groups. After injecting BV (60 mg/kg,
i.p.), rats were treated with diclofenac sodium as a standard drug
(5 mg/kg, i.p.) (32). The rats were
challenged by subcutaneous injection of 0.1 ml 1% carrageenan
solution into the plantar side of the right hind paw. The paw
volume was measured using a micrometer caliper, before, and 1, 3
and 4 h after the injection of the carrageenan solution (33,34).
Acetic acid writhing test
To measure the analgesic activity, 20 male albino
mice (weighting 20-25 g) were divided into 4 groups (n=5 per
group). One group contained healthy rats. Another group served as a
control that only received saline as a treatment. The other two
groups were treated with wither diclofenac (5 mg/kg, i.p.)
(32) or BV (60 mg/kg, i.p.), prior
to administration of 0.1 ml/10 g 1% acetic acid (i.p.). The number
of writhes or abdominal stretches were counted for 20 min in the
mice (35,36).
Acute toxicity study in mice
Acute toxicity in mice was assessed as described
previously (37). A total of 20 male
albino mice were sorted into 4 groups (n=5). The mice were housed 5
days prior to the start of the study, and the animals were
maintained as described above. BV was administrated as a single
i.p. dose (60, 600 or 1,200 mg/kg), respectively, to 3 of the
groups, and the remaining group served as the control. Following BV
treatment, the mice were observed continuously every 2 h for 6 h,
then daily for 3 days, for any changes in the general behavior and
any signs of toxic manifestations, such as tremors, convulsions,
loss of right reflex, muscle spasm, decreased motor activity,
sedation, writhing, respiration and mortalities. Blood samples were
collected after 3 days for further assessment of liver function
using an alanine aminotransferase (ALT) test using a
spectrophotometric assay with diagnostic kits purchased from
Sigma-Aldrich; Merck KGaA as described previously (38). In addition, kidney function tests
were guided by the determination of serum creatinine levels as
described previously (39).
Statistical analysis of the data
Results are expressed as the mean ± the standard
error of the mean. Analysis was performed using SPSS version 15.0
(SPSS, Inc.). One-way ANOVA followed by a Bonferroni post hoc test
was used to compare the differences between the groups. P<0.05
was considered to indicate a statistically significant
difference.
Analysis of immunostaining was analysed using ImageJ
version 1.45f 112_1.8.0 (National Institutes of Health). The
percentage of stained area was compared between groups using a
one-way ANOVA followed by a Bonferroni post hoc test. P<0.05 was
considered to indicate a statistically significant difference.
Results
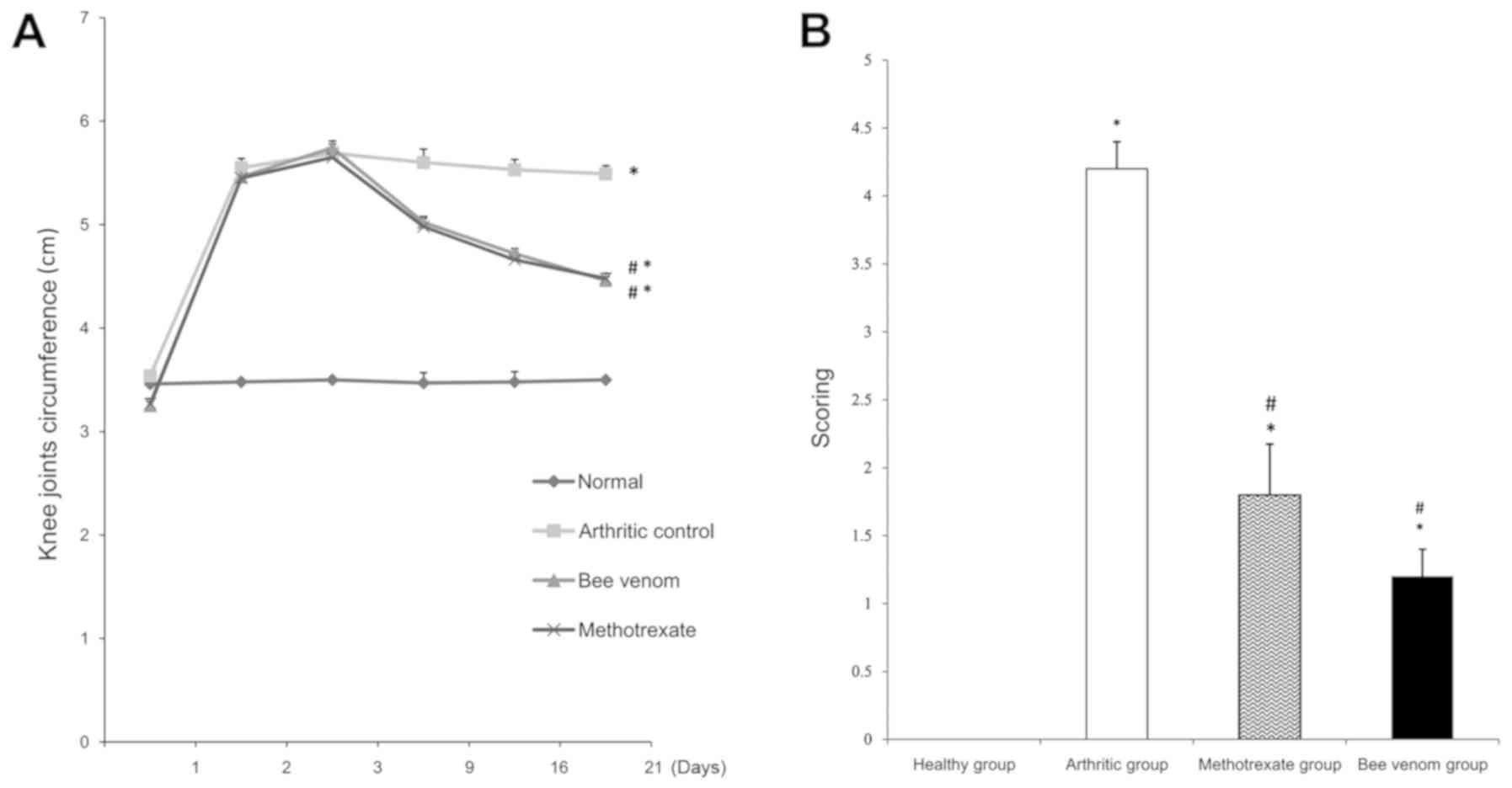
Knee joint circumference
Immunization of the rats with CFA resulted in the
induction of prominent arthritis, as shown by the significant
increase in knee joint swelling and an increase in the knee joint
circumferences compared with the healthy controls (P<0.05).
Individual treatments with methotrexate and BV significantly
reduced the knee joint swelling circumferences compared with the
control arthritic group (Fig. 1A;
both P<0.05).
Arthritic index
Rats injected with CFA showed a significant increase
in the arthritis index compared with the healthy rats (P<0.05).
Rats treated with methotrexate or BV showed a significant reduction
in the arthritis index at the end of the study (day 21) compared
with the arthritic control group (Fig.
1B; both P<0.05).
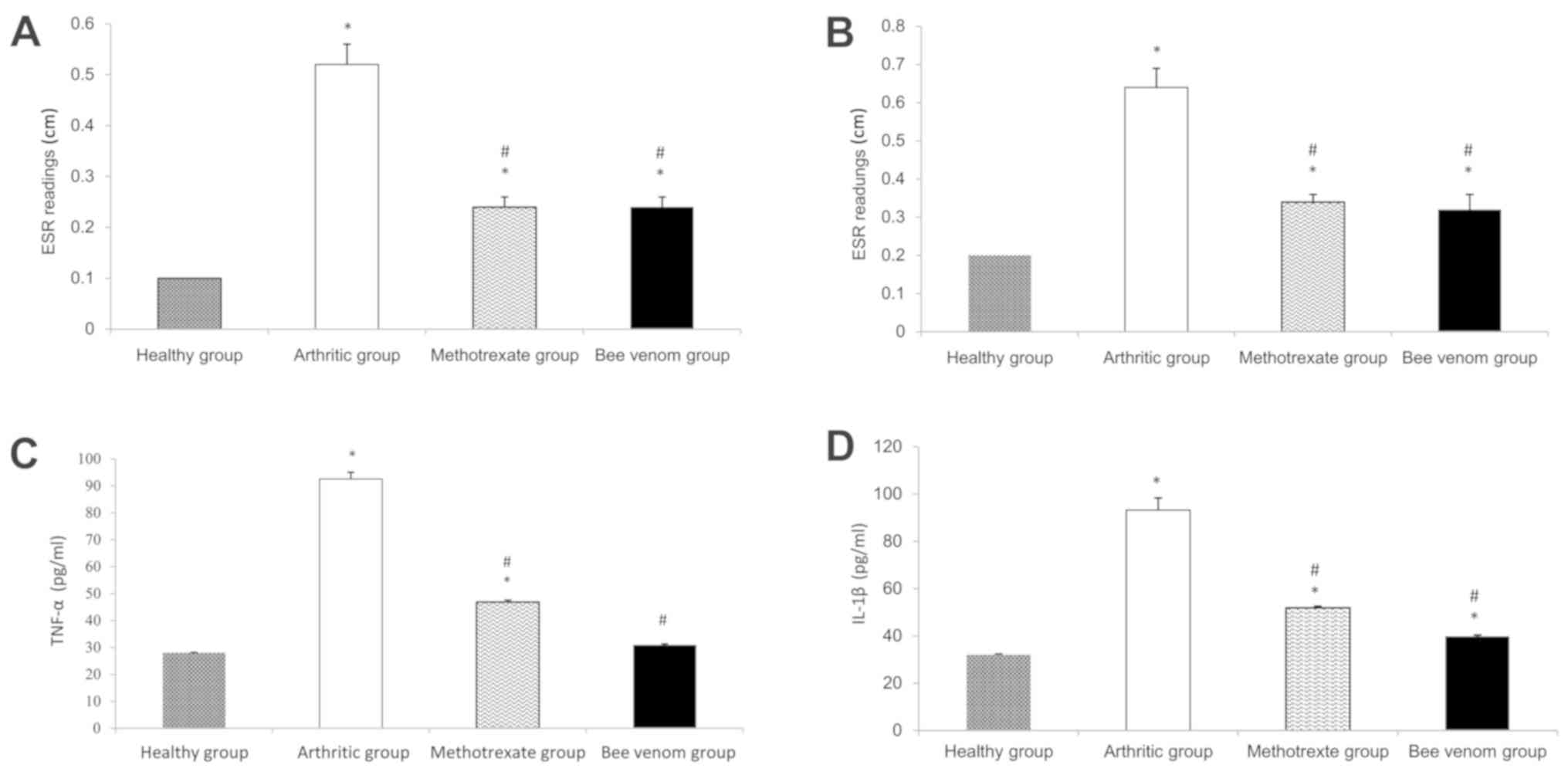
ESR
The arthritic group showed a significant increase in
ESR values compared with the normal control (P<0.05).
Methotrexate and BV significantly reduced the increase in ESR
levels (Fig. 2A and B; both P<0.05).
Serum TNF-α and IL-1β levels
The arthritic group showed a noticeable increase
(~3-fold) in both the levels of TNF-α and IL-1β, and this increase
was consistent throughout the entire duration of the study compared
with the normal control group. Treatment with methotrexate
significantly reduced TNF-α and IL-1β levels compared with the
arthritic control group. Similarly, treatment with BV resulted in a
reduction in serum TNF-α and IL-1β levels compared with the
methotrexate group, reaching the levels of the normal control rats
Fig. 2C and D.
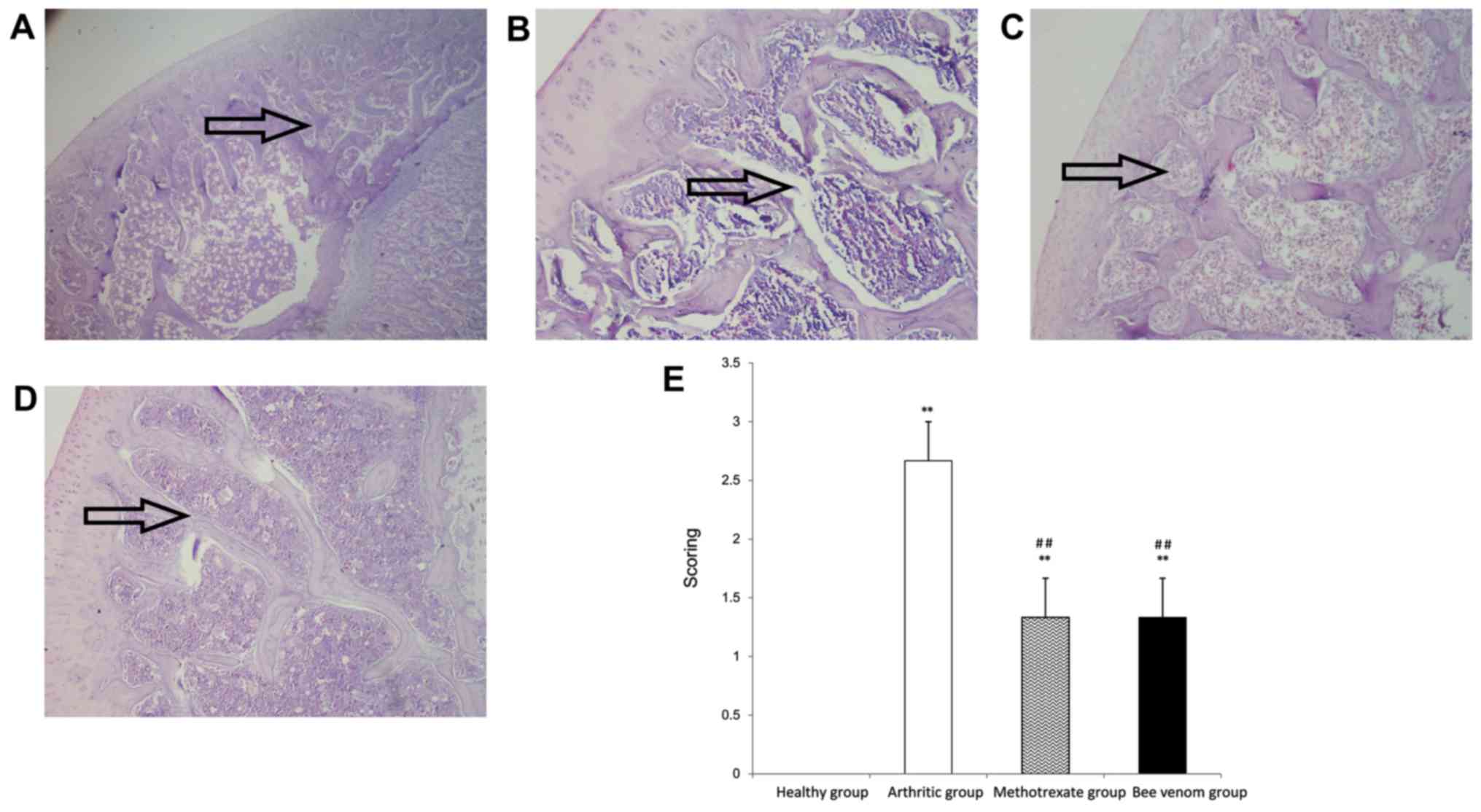
Histopathological examination
Histopathological examination of sections of the
knee joint showed apparent infiltration of mononuclear inflammatory
cells, and cell debris with a high incidence of cartilage
destruction and pannus development in the arthritic group.
Treatment with methotrexate or BV significantly reduced the
histopathological score (P<0.01), and the sections showed
notably reduced inflammatory cell infiltration and pannus formation
in the synovium and surrounding tissues, and the sections
maintained most of the normal joint and tissue construction and
integrity when compared with the arthritic control group (Fig. 3).
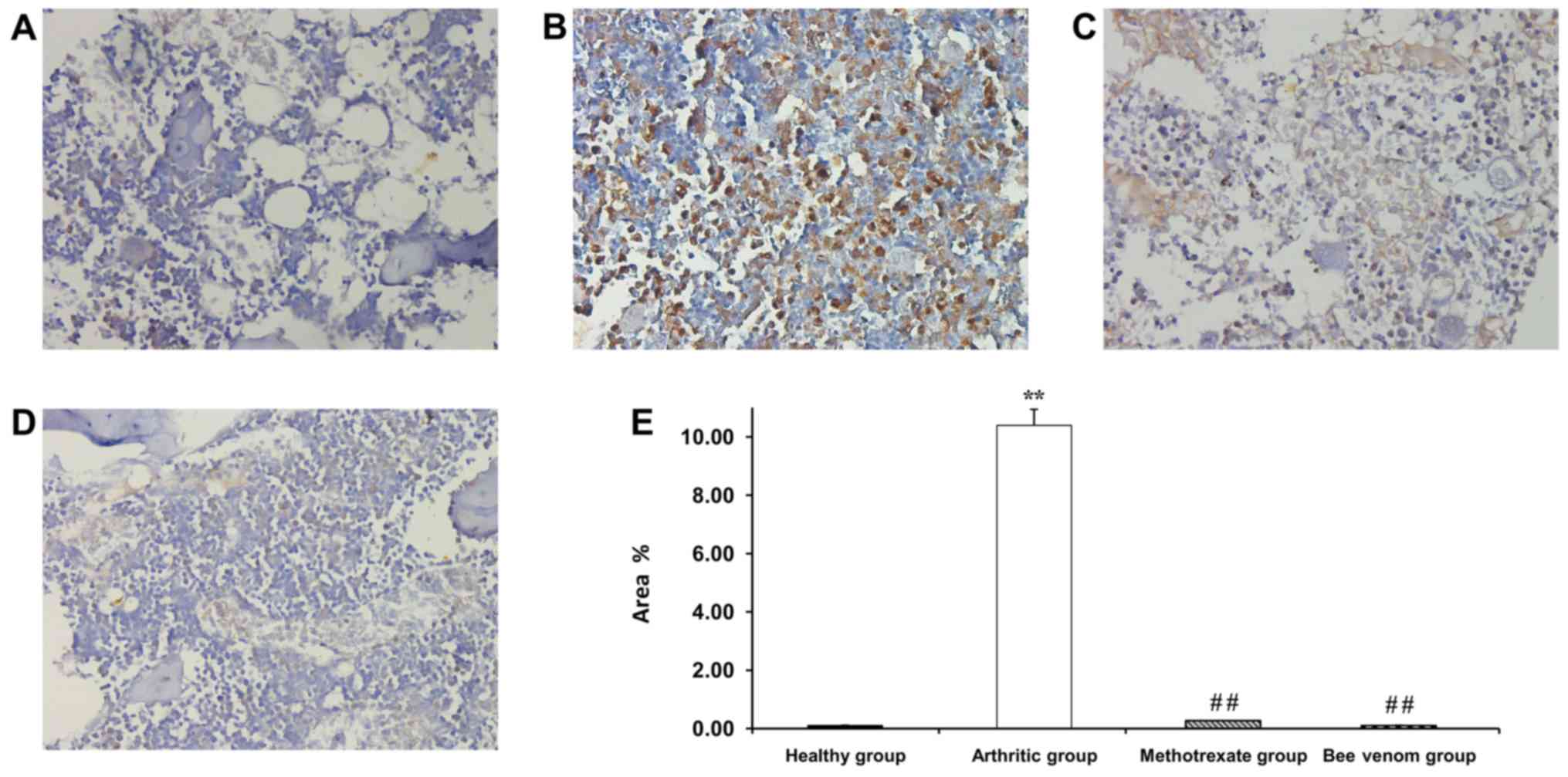
Immunostaining for NF-κB (p65)
expression in arthritic rats
Healthy normal rats showed nearly no staining for
NF-κB p65, whereas the arthritic control knee joint sections
exhibited the highest intensity of immunostaining and was
significantly greater compared with the normal, methotrexate and BV
treated groups (P<0.01). Methotrexate treatment resulted in
moderate levels of staining, and treatment with BV resulted in a
mild density of NF-κB immunostaining in the affected knee joints.
The percentage of the area stained was scored to validate the
difference in the immunostaining intensity (Fig. 4). A greater intensity of the color
represented an increase in NF-κB(p65) expression.
In vitro COX inhibition,
IC50 and COX-2 selectivity index
BV inhibited COX-2 at relatively lower
IC50 doses compared with indomethacin and diclofenac
sodium. The COX-2 selectivity index of BV was higher compared with
both indomethacin and diclofenac sodium (Table I).
 | Table IAssessment of in vitro COX
inhibition IC50 values of bee venom. |
Table I
Assessment of in vitro COX
inhibition IC50 values of bee venom.
| Treatment | COX-1
IC50, µM | COX-2
IC50, µM | COX-2 selectivity
index |
|---|
| Celecoxib | 15.1 | 0.049 | 308 |
| Indomethacin | 0.041 | 0.51 | 0.08 |
| Diclofenac
sodium | 3.8 | 0.84 | 4.5 |
| Bee venom | 9.41 | 0.15 | 63 |
In vivo anti-inflammatory and
analgesic activity of BV
BV exhibited systemic anti-inflammatory activity
that was shown by the 40.74% reduction in hind paw
carrageenan-induced edema compared with diclofenac sodium (37.03%)
at 4 h. BV also demonstrated notable analgesic activity as shown by
the comparatively lower number of abdominal writhes when compared
with the control or diclofenac sodium groups (Table II).
 | Table IIEffect of BV on acetic acid writhing
test and carrageenan induced paw edema. |
Table II
Effect of BV on acetic acid writhing
test and carrageenan induced paw edema.
| | Anti-inflammatory
activity | Analgesic
activity |
|---|
| Groups | Number of writhes
in 20 min | Percentage
inhibition | Percentage change
in paw volume, mean ± SEM | Percentage of
inhibition of edema after 4 h |
|---|
| Normal control | 0 | 0 | 1 h | 3 h | 4 h | |
| Arthritic
control |
58±0.58a | 0 | 0.32±0.02 | 0.44±0.04 | 0.54±0.02 | 0 |
| Diclofenac
sodium |
22±0.58a,b | 62.06 | 0.22±0.02 | 0.3±0.0 |
0.34±0.02b | 37.03 |
| BV |
16±0.51a,b | 72.41 | 0.22±0.02 | 0.28±0.02 |
0.32±0.02b | 40.74 |
Acute toxicity study
Administration of BV in the albino mice did not
exhibit any toxic effects up to and including a dose of 1,200
mg/kg. The mice did not demonstrate any signs of toxicity and there
were no mortalities.
Liver function was assessed by performing an alanine
aminotransferase (ALT) test. ALT levels in the normal control group
were 21.8±0.8 U/l; in the mice treated with 60 mg/kg BV, 27±0.89
U/l; mice treated with 600 mg/kg BV, 36.6±0.5 U/l; and in the mice
treated with 1,200 mg/kg, 71.6±1.96 U/l.
Kidney function was preliminarily assessed based on
the concentration of serum creatinine. In the normal control group,
serum creatinine levels were 0.27±0.01 mg/dl; in the mice treated
with 60 mg/kg BV, 0.31±0.008 mg/dl; mice treated with 600 mg/kg BV,
0.39±0.007 mg/dl; and in the mice treated with 1,200 mg/kg BV
0.47±0.009 mg/dl.
Discussion
RA is one of the most common autoimmune diseases.
The overall age-standardized prevalence and incidence rates have
been increasing globally (40) to
almost affect ~1% of the worldwide population (2). The pathophysiology of RA involves the
contribution of several complex and connected inflammatory pathways
(4).
BV has been widely used in traditional Chinese
medicine to alleviate pain and inflammation during chronic
inflammatory conditions such as RA (13,41).
Several studies have assessed the effects of
administration of different doses of BV via different routes of
administration to determine a suitable dosing regimen for
management of arthritis. For example, BV treatment was
administrated subcutaneously at zusanli acupoint, and it exhibited
potent anti-arthritic activity, although it was not compared with
methotrexate (42). In addition,
another study demonstrated that concurrent treatment of BV at
zusanli acupoint with methotrexate in adjuvant-induced arthritis
was more effective than methotrexate alone (29). Additionally, BV administered i.p. 20
µl/100 g/day in collagen induced arthritis (14), and up to 20 mg/kg intraperitoneally
for adjuvant-induced arthritis (43)
both showed good anti-arthritic properties, although these were not
compared with a standard treatment.
The primary purpose of the present study was to
evaluate the activity of i.p. BV, and the results of BV treatment
showed it may exhibit potential for treatment of RA.
In the present study, induction of arthritis was
performed using unilateral intra-articular injection of CFA.
Previous studies reported the competence of CFA to induce systemic
arthritis in rats (22,44,45).
CFA-induced arthritis models in rats were found to result in
histological and immunological manifestations of RA. This provided
a practical model for investigating systemic BV as a potential
anti-arthritic agent (46).
Injection of CFA in the knee joint was shown to
produce significant edema in the affected joint and a corresponding
increase in the arthritis scoring index within 2 days when compared
with the healthy control. Histopathological examination
demonstrated the presence of a high density of cell infiltration,
alterations in joint integrity and overexpression of NF-κB in the
affected joint. Additionally, the arthritic group also showed a
significant increase in ESR values, serum TNFα and IL-1β levels.
All these changes showed successful induction of arthritis by
CFA.
BV was administered by i.p. injection of 60 mg/kg.
The dose was selected according to preliminary testing of a range
of doses. BV 60 mg/kg was selected as it showed the most prominent
anti-arthritic activity based on biochemical and histopathological
examination (data not shown). Systemic injection of BV was
previously reported to exhibit potent anti-arthritic activity at
different doses (14,43,47).
Previous studies proposed the use of BV at different doses which
varied between 1-50 mg. Doses ≤20 mg/kg, i.p, have been evaluated
for their anti-arthritic potential (13,43), and
another study used 50 mg whole BV in dogs to determine the effect
of BV on plasma cortisol levels (48).
The results of the present study showed that
individual treatment of BV (60 mg/kg) and methotrexate (a
widely-used standard treatment for RA) exhibited potent
anti-arthritic activity, as demonstrated through a significant
reduction in knee joint swelling circumferences, and a
corresponding low arthritic index score. These findings agree with
previous studies examining the use of methotrexate and BV (21,43).
Systemic BV was found to significantly reduce ESR
values compared with the arthritic group to a similar degree as the
methotrexate group. Methotrexate was previously shown to be an
effective agent in reducing ESR associated with RA (21). This highlights the potential of BV to
reduce RA-associated inflammation.
BV significantly reduced TNF-α and IL-1β serum
levels compared with the arthritic and methotrexate groups. This
may explain the effectiveness of BV in management and alleviation
of RA, as both TNF-α and IL-1β are key pro-inflammatory cytokines
in the pathogenesis of RA (3).
The results of the present study agree with a
previous study which showed that subcutaneous BV treatment reduced
serum TNF-α and IL-1β levels in a dose-dependent manner, although
they did not compare the effects of BV with a standard treatment
(43). BV at a dose of 60 mg/kg
reduced serum TNF-α levels to levels similar level as that observed
in the healthy group.
Previous studies suggested that methotrexate reduced
NF-κB expression with limited effect on TNF-α and IL-1β levels
(49-51).
BV and methotrexate treatment reduced NF-κB levels to a similar
degree, which was upregulated in the joints of the arthritic rats.
This result was consistent with a study by Darwish et al
(29), where methotrexate and BV
were administered at the zusanli point (an acupuncture point
located below the knee). These findings confirmed the effect of
systemic BV on the inhibition of a key inflammatory pathway which
contributes to the aggravation of RA manifestations.
The anti-inflammatory activity of BV was evaluated
using an in vitro assay of COX inhibition and confirmed in
the in vivo model, carrageenan-induced paw edema. Both
results suggested that BV is a more potent anti-inflammatory agent
for use of inhibition of COX-2 than the previously well-established
anti-inflammatory standards (diclofenac sodium and indomethacin)
(52,53), and supports the hypothesis that BV is
pivotal in suppressing the pro-inflammatory COX-2 levels. COX-2
inhibition was found to result in subsequent inhibition of
pro-inflammatory PGs, particularly PGE2, which are partly involved
in the progression of the inflammatory cascade. Previous studies
showed in vitro inhibition of COX-2 activity using BV and
its sub-fractions (54,55).
COX-2 selectivity index of BV was higher compared
with diclofenac sodium and indomethacin, which may also explain the
increased anti-inflammatory properties, whilst exhibiting
acceptable relative COX-1/COX-2 inhibition (56,57).
The results of the acetic acid writhing test
demonstrated that systemic BV had potent analgesic activity, which
was evident by the lower number of abdominal writhes compared with
diclofenac sodium. These findings agree with a previous study that
suggested that a high dose of BV treatment by acupuncture produced
potent anti-nociceptive effects, regardless of the site of BV
injection in an abdominal stretch assay (42).
BV as an anti-nociceptive agent may explain the
collective properties of BV on the inhibition of TNF-α levels,
which results in desensitizing nociceptive primary afferents
(58,59). In addition, BV-mediated inhibition of
NF-κB, results in subsequent inhibition of pro-inflammatory COX-2
and iNOS expression, and thus inhibition of PGE2 and NO as
demonstrated previously in vitro (55). BV inhibited the COX-2 signaling
pathway in both the in vitro assay and in the carrageenan
induced paw edema, and NF-κB and COX-2 pathways have been studied
previously for their role in the induction of pain and hyperalgesia
(60).
Regarding the safety of BV, doses of 60-1,200 mg/kg,
i.p. showed no apparent toxicological manifestations or
mortalities. Previous studies also showed that there were no
adverse toxic or fatal outcomes detected using a single 1,500 mg/kg
dermal dose of BV (61,62). However, a dose of 1,200 mg/kg
(20-fold higher than the selected dose) may exert certain
pathological effects on the liver and kidneys as shown by the liver
and kidney function tests. Previous studies proposed similar
concerns, particularly regarding the kidneys (29,63).
The findings of the present study highlight the
potential anti-arthritic, anti-inflammatory and anti-nociceptive
mechanisms of action of BV (60 mg/kg/day, i.p.) for treatment of
RA, BV exerted its effects through inhibition of basic inflammatory
axes, including the combined reduction of serum TNF-α, IL-1β and
NF-κB expression levels, and inhibition of the COX-2 signaling
pathway, all of which are considered cornerstones in the
pathophysiology of RA.
Supplementary Material
Preliminary screening of bee venom
doses.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analysed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
All authors contributed to the study conception and
design. DMET wrote the original draft of the manuscript and
performed the experiments. MMAA reviewed the manuscript. MWH
performed the data analysis. AIG supervised the study. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
Ethical approval for all the procedures was granted
by the Ethics Committee of the Faculty of Pharmacy, Damanhour
University, (Damanhour, Egypt) (approval no. 717PO5).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests and all authors confirm its accuracy.
References
|
1
|
Arnett FC, Edworthy SM, Bloch DA, Bloch
DA, McShane DJ, Fries JF, Cooper NS, Healey LA, Kaplan SR, Liang
MH, Luthra HS, et al: The American rheumatism association 1987
revised criteria for the classification of rheumatoid arthritis.
Arthritis Rheum. 31:315–324. 1988.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Uhlig T, Moe RH and Kvien TK: The burden
of disease in rheumatoid arthritis. Pharmacoeconomics. 32:841–851.
2014.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Bingham CO III: The pathogenesis of
rheumatoid arthritis: Pivotal cytokines involved in bone
degradation and inflammation. J Rheumatol Suppl. 65:3–9.
2002.PubMed/NCBI
|
|
4
|
Choy EH and Panayi GS: Cytokine pathways
and joint inflammation in rheumatoid arthritis. N Engl J Med.
344:907–916. 2001.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Mateen S, Zafar A, Moin S, Khan AQ and
Zubair S: Understanding the role of cytokines in the pathogenesis
of rheumatoid arthritis. Clin Chim Acta. 455:161–171.
2016.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Matsuno H, Yudoh K, Katayama R, Nakazawa
F, Uzuki M, Sawai T, Yonezawa T, Saeki Y, Panayi GS, Pitzalis C and
Kimura T: The role of TNF-alpha in the pathogenesis of inflammation
and joint destruction in rheumatoid arthritis (RA): A study using a
human RA/SCID mouse chimera. Rheumatology (Oxford). 41:329–337.
2002.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Iwakura Y: Roles of IL-1 in the
development of rheumatoid arthritis: Consideration from mouse
models. Cytokine Growth Factor Rev. 13:341–355. 2002.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Roman-Blas JA and Jimenez SA: NF-kappaB as
a potential therapeutic target in osteoarthritis and rheumatoid
arthritis. Osteoarthritis Cartilage. 14:839–848. 2006.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Crofford LJ, Tan B, McCarthy CJ and Hla T:
Involvement of nuclear factor kappa B in the regulation of
cyclooxygenase-2 expression by interleukin-1 in rheumatoid
synoviocytes. Arthritis Rheum. 40:226–236. 1997.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Poligone B and Baldwin AS: Positive and
negative regulation of NF-kappaB by COX-2: roles of different
prostaglandins. J Biol Chem. 276:38658–38664. 2001.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Yamamoto Y and Gaynor RB: Role of the
NF-kappaB pathway in the pathogenesis of human disease states. Curr
Mol Med. 1:287–296. 2001.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Rader K, Wildfeuer A, Wintersberger F,
Bossinger P and Mucke HW: Characterization of bee venom and its
main components by high-performance liquid chromatography. J
Chromatogr. 408:341–348. 1987.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Kwon YB, Lee JD, Lee HJ, Han HJ, Mar WC,
Kang SK, Beitz AJ and Lee JH: Bee venom injection into an
acupuncture point reduces arthritis associated edema and
nociceptive responses. Pain. 90:271–280. 2001.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Kim KW, Shin YS, Kim KS, Chang YC, Park
KK, Park JB, Choe JY, Lee KG, Kang MS, Park YG and Kim CH:
Suppressive effects of bee venom on the immune responses in
collagen-induced arthritis in rats. Phytomedicine. 15:1099–1107.
2008.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Hwang DS, Kim SK and Bae H: Therapeutic
effects of bee venom on immunological and neurological diseases.
Toxins (Basel). 7:2413–2421. 2015.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Khamis AA, Ali EM, El-Moneim MA,
Abd-Alhaseeb MM, El-Magd MA and Salim EI: Hesperidin, piperine and
bee venom synergistically potentiate the anticancer effect of
tamoxifen against breast cancer cells. Biomed Pharmacother.
105:1335–1343. 2018.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Lee JD, Kim SY, Kim TW, Lee SH, Yang HI,
Lee DI and Lee YH: Anti-inflammatory effect of bee venom on type II
collagen-induced arthritis. Am J Chin Med. 32:361–367.
2004.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Zatroch KK, Knight CG, Reimer JN and Pang
DS: Refinement of intraperitoneal injection of sodium pentobarbital
for euthanasia in laboratory rats (Rattus norvegicus). BMC
Vet Res. 13(60)2017.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Liu YL, Lin HM, Zou R, Wu JC, Han R,
Raymond LN, Reid PF and Qin ZH: Suppression of complete Freund's
adjuvant-induced adjuvant arthritis by cobratoxin. Acta Pharmacol
Sin. 30:219–227. 2009.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Lewis A and Levy A: Anti-inflammatory
activities of Cassia alata leaf extract in complete Freund's
adjuvant arthritis in rats. West Indian Med J. 60:615–621.
2011.PubMed/NCBI
|
|
21
|
Banji D, Pinnapureddy J, Banji OJ, Kumar
AR and Reddy KN: Evaluation of the concomitant use of methotrexate
and curcumin on Freund's complete adjuvant-induced arthritis and
hematological indices in rats. Indian J Pharmacol. 43:546–550.
2011.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Chung JI, Barua S, Choi BH, Min BH, Han HC
and Baik EJ: Anti-inflammatory effect of low intensity ultrasound
(LIUS) on complete Freund's adjuvant-induced arthritis synovium.
Osteoarthritis Cartilage. 20:314–322. 2012.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Larsson P, Kleinau S, Holmdahl R and
Klareskog L: Homologous type II collagen-induced arthritis in rats.
Characterization of the disease and demonstration of clinically
distinct forms of arthritis in two strains of rats after
immunization with the same collagen preparation. Arthritis Rheum.
33:693–701. 1990.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Mossiat C, Laroche D, Prati C, Pozzo T,
Demougeot C and Marie C: Association between arthritis score at the
onset of the disease and long-term locomotor outcome in
adjuvant-induced arthritis in rats. Arthritis Res Ther.
17(184)2015.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Jou JM, Lewis SM, Briggs C, Lee SH, De La
Salle B and McFadden S: International Council for Standardization
in Haematology. ICSH review of the measurement of the erythocyte
sedimentation rate. Int J Lab Hematol. 33:125–132. 2011.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Kyei S, Koffuor GA and Boampong JN:
Antiarthritic effect of aqueous and ethanolic leaf extracts of
Pistia stratiotes in adjuvant-induced arthritis in
Sprague-Dawley rats. J Exp Pharmacol. 4:41–51. 2012.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Petrovic-Rackov L and Pejnovic N: Clinical
significance of IL-18, IL-15, IL-12 and TNF-alpha measurement in
rheumatoid arthritis. Clin Rheumatol. 25:448–452. 2006.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Klopfleisch R: Multiparametric and
semiquantitative scoring systems for the evaluation of mouse model
histopathology-a systematic review. BMC Vet Res.
9(123)2013.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Darwish SF, El-Bakly WM, Arafa HM and
El-Demerdash E: Targeting TNF-α and NF-κB activation by bee venom:
Role in suppressing adjuvant induced arthritis and methotrexate
hepatotoxicity in rats. PLoS One. 8(e79284)2013.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Blobaum AL and Marnett LJ: Structural and
functional basis of cyclooxygenase inhibition. J Med Chem.
50:1425–1441. 2007.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Maclouf J, Grassi J and Pradelles P:
Development of enzyme-immunoassay techniques for measurement of
eicosanoids In: Prostaglandin and Lipid Metabolism in Radiation
Injury. Springer. pp355–364. 1987.
|
|
32
|
Vyas S, Agrawal RP, Solanki P and Trivedi
P: Analgesic and anti-inflammatory activities of Trigonella
foenum-graecum (seed) extract. Acta Pol Pharm. 65:473–476.
2008.PubMed/NCBI
|
|
33
|
Morris CJ: Carrageenan-induced paw edema
in the rat and mouse. Methods Mol Biol. 225:115–121.
2003.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Domiati S, El-Mallah A, Ghoneim A, Bekhit
A and El Razik HA: Evaluation of anti-inflammatory, analgesic
activities, and side effects of some pyrazole derivatives.
Inflammopharmacology. 24:163–172. 2016.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Taber RI, Greenhouse DD, Rendell JK and
Irwin S: Agonist and antagonist interactions of opioids on acetic
acid-induced abdominal stretching in mice. J Pharmacol Exp Ther.
169:29–38. 1969.PubMed/NCBI
|
|
36
|
Young HY, Luo YL, Cheng HY, Hsieh WC, Liao
JC and Peng WH: Analgesic and anti-inflammatory activities of
(6)-gingerol. J Ethnopharmacol. 96:207–210. 2005.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Ghosh MN: Fundamentals of experimental
pharmacology. Indian J Pharmacol. 39(216)2007.
|
|
38
|
Yang RZ, Park S, Reagan WJ, Goldstein R,
Zhong S, Lawton M, Rajamohan F, Qian K, Liu L and Gong DW: Alanine
aminotransferase isoenzymes: Molecular cloning and quantitative
analysis of tissue expression in rats and serum elevation in liver
toxicity. Hepatology. 49:598–607. 2009.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Husdan H and Rapoport A: Estimation of
creatinine by the Jaffe reaction A comparison of three methods.
Clin Chem. 14:222–238. 1968.PubMed/NCBI
|
|
40
|
Safiri S, Kolahi AA, Hoy D, Smith E,
Bettampadi D, Mansournia MA, Almasi-Hashiani A, Ashrafi-Asgarabad
A, Moradi-Lakeh M, Qorbani M, et al: Global, regional and national
burden of rheumatoid arthritis 1990-2017: A systematic analysis of
the global burden of disease study 2017. Ann Rheum Dis.
78:1463–1471. 2019.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Luo H, Zuo XX, Li T and Zhang J: Effect of
bee venom on adjuvant induced arthritis in rats. Zhong Nan Da Xue
Xue Bao Yi Xue Ban. 31:948–951. 2006.PubMed/NCBI(In Chinese).
|
|
42
|
Kwon YB, Kang MS, Kim HW, Ham TW, Yim YK,
Jeong SH, Park DS, Choi DY, Han HJ, Beitz AJ and Lee JH:
Antinociceptive effects of bee venom acupuncture (apipuncture) in
rodent animal models: A comparative study of acupoint versus
non-acupoint stimulation. Acupunct Electrother Res. 26:59–68.
2001.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Kocyigit A, Guler EM and Kaleli S:
Anti-inflammatory and antioxidative properties of honey bee venom
on freund's complete adjuvant-induced arthritis model in rats.
Toxicon. 161:4–11. 2019.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Gohil P, Patel V, Deshpande S, Chorawala M
and Shah G: Anti-arthritic activity of cell wall content of
Lactobacillus plantarum in freund's adjuvant-induced
arthritic rats: Involvement of cellular inflammatory mediators and
other biomarkers. Inflammopharmacology. 26:171–181. 2018.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Silva JC, Rocha MF, Lima AA, Brito GA, de
Menezes DB and Rao VS: Effects of pentoxifylline and nabumetone on
the serum levels of IL-1beta and TNFalpha in rats with adjuvant
arthritis. Inflamm Res. 49:14–19. 2000.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Ahmed O, Fahim H, Mahmoud A and Eman Ahmed
EA: Bee venom and hesperidin effectively mitigate complete freund's
adjuvant-induced arthritis via immunomodulation and enhancement of
antioxidant defense system. Arch Rheumatol. 33:198–212.
2017.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Kim HW, Kwon YB, Ham TW, Roh DH, Yoon SY,
Kang SY, Yang IS, Han HJ, Lee HJ, Beitz AJ and Lee JH: General
pharmacological profiles of bee venom and its water soluble
fractions in rodent models. J Vet Sci. 5:309–318. 2004.PubMed/NCBI
|
|
48
|
Vick JA and Shipman WH: Effects of whole
bee venom and its fractions (apamin and melittin) on plasma
cortisol levels in the dog. Toxicon. 10:377–380. 1972.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Chang DM, Baptiste P and Schur PH: The
effect of antirheumatic drugs on interleukin 1 (IL-1) activity and
IL-1 and IL-1 inhibitor production by human monocytes. J Rheumatol.
17:1148–1157. 1990.PubMed/NCBI
|
|
50
|
Kane D, Gogarty M, O'Leary J, Silva I,
Bermingham N, Bresnihan B and Fitzgerald O: Reduction of synovial
sublining layer inflammation and proinflammatory cytokine
expression in psoriatic arthritis treated with methotrexate.
Arthritis Rheum. 50:3286–3295. 2004.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Segal R, Mozes E, Yaron M and Tartakovsky
B: The effects of methotrexate on the production and activity of
interleukin-1. Arthritis Rheum. 32:370–377. 1989.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Lucas S: The pharmacology of indomethacin.
Headache. 56:436–446. 2016.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Todd PA and Sorkin EM: Diclofenac sodium A
reappraisal of its pharmacodynamic and pharmacokinetic properties,
and therapeutic efficacy. Drugs. 35:244–285. 1988.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Nam KW, Je KH and Lee JH: Inhibition of
COX-2 activity and proinflammatory cytokines (TNF-alpha and
IL-1beta) production by water-soluble sub-fractionated parts from
bee (Apis mellifera) venom. Arch Pharm Res. 26:383–388.
2003.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Park HJ, Lee SH, Son DJ, Oh KW, Kim KH,
Song HS, Kim GJ, Oh GT, Yoon DY and Hong JT: Antiarthritic effect
of bee venom: Inhibition of inflammation mediator generation by
suppression of NF-kappaB through interaction with the p50 subunit.
Arthritis Rheum. 50:3504–3515. 2004.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Amin AR, Dave M, Attur M and Abramson SB:
COX-2, NO, and cartilage damage and repair. Curr Rheumatol Rep.
2:447–453. 2000.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Ku EC, Lee W, Kothari HV and Scholer DW:
Effect of diclofenac sodium on the arachidonic acid cascade. Am J
Med. 80:18–23. 1986.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Junger H and Sorkin LS: Nociceptive and
inflammatory effects of subcutaneous TNFalpha. Pain. 85:145–151.
2000.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Leung L and Cahill CM: TNF-alpha and
neuropathic pain-a review. J Neuroinflammation.
7(27)2010.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Dirig DM, Isakson PC and Yaksh TL: Effect
of COX-1 and COX-2 inhibition on induction and maintenance of
carrageenan-evoked thermal hyperalgesia in rats. J Pharmacol Exp
Ther. 285:1031–1038. 1998.PubMed/NCBI
|
|
61
|
Han SM, Lee GG and Park KK: Acute dermal
toxicity study of bee venom (Apis mellifera L.) in Rats.
Toxicol Res. 28:99–102. 2012.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Han SM, Lee KG, Yeo JH and Pak SC: Dermal
and ocular irritation studies of honeybee (Apis mellifera
L.) venom. Am J Chin Med. 40:795–800. 2012.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Grisotto LS, Mendes GE, Castro I, Baptista
MA, Alves VA, Yu L and Burdmann EA: Mechanisms of bee venom-induced
acute renal failure. Toxicon. 48:44–54. 2006.PubMed/NCBI View Article : Google Scholar
|