Introduction
Ketamine has been used clinically for >40 years
(1). By disconnecting thalamic and
limbic brain functions through antagonism of the NMDAR, ketamine
induces dissociative anesthesia while preserving spontaneous
respiration and cardiovascular stability (2,3). With a
low risk profile for severe adverse effects, ketamine is widely
used in pediatric anesthesia (4,5).
However, the potential neurotoxic effects of ketamine on the
developing brain have raised concern amongst healthcare
professionals for >20 years (6,7).
Numerous animal studies have reported apoptotic neurodegeneration
and impaired neurological outcomes due to ketamine treatment,
casting doubt on the safety of ketamine use in neonatal and
pediatric patients (7-15).
Ikonomidou et al (7) showed
that exposure to ketamine resulted in widespread neuronal apoptosis
in rat pups. These findings were supported by subsequent studies on
developing rat brains, which linked ketamine exposure to
upregulated expression of NMDA receptor subunits and pro-apoptotic
genes and proteins, such as p53 and cleaved caspase-3 (10,12).
Similarly, increased expression of NMDA receptors and apoptosis was
observed in the frontal cortex of ketamine-treated perinatal rhesus
monkeys (8). Furthermore, rhesus
monkeys underperformed in cognitive behavior tests for several
years following exposure to ketamine early in life (9).
In contrast to these findings, neuroprotective
effects of ketamine on the developing brain have also been
demonstrated. Anand et al (16) reported that ketamine may ameliorate
pain-induced neurotoxicity in newborn rats, likely, but not solely
through the inhibition of inflammatory pathways (6,16).
Perinatal hypoxia-ischemia may lead to a variety of
types of severe brain damage in term and preterm neonates, such as
hypoxic-ischemic encephalopathy and periventricular leukomalacia
(17,18). Several in vitro and in
vivo studies have provided evidence of the neuroprotective
effects of ketamine under hypoxic conditions (19-21).
In 1987, Rothman et al (19)
showed that ketamine attenuated the neurotoxic effects of hypoxia
on hippocampal neurons in vitro. NMDA receptor antagonism is
considered a key factor in the protection of neurons and glial
cells from glutamate-induced excitotoxicity, providing improved
outcomes in hypoxic-ischemic rat pups (22,23).
Chang et al (21) showed that
ketamine ameliorated the inflammatory response due to hypoxia in
the cortex of fetal sheep via a Toll-like receptor mediated
pathway. To the best of our knowledge, there are no studies
published to date on the interaction between ketamine and hypoxia
in primates. Similarly, there are no prospective studies examining
the safety of ketamine in paediatric patients to the best of our
knowledge; however, retrospective studies on the use of ketamine
and other anesthetic drugs in childhood have not found convincing
evidence of an association with impaired neurocognitive function in
later life (24,25).
In summary, there is conflicting evidence regarding
the neurotoxic and neuroprotective effects of ketamine on the
developing brain. Ketamine has been shown to protect neurons and
glial cells by attenuating glutamate-induced excitotoxicity and
inhibiting an inflammatory response (19-21,26).
However, the number of studies available on the relevant
neurocellular mechanisms are limited (14,21,27-30),
and hypoxia-induced neurotoxicity and potential amelioration
through ketamine remains poorly understood.
In the present study, it was hypothesized that
ketamine attenuated the neurotoxic effects of hypoxia, and this was
assessed by measuring the expression of cellular markers of
proliferation, neurogenesis and extracellular matrix homeostasis.
The results of the present study may contribute to an improved
understanding of how hypoxia-induced neurotoxicity is affected by
the presence of ketamine.
In vitro experiments were used as they allow
for identification of relevant cellular pathways under standardized
and simplified conditions (31).
Experiments were performed on HT22 murine hippocampal cells.
Studies investigating pathways in the central nervous system often
focus on hippocampal formation, which is known to serve a key role
in long-term potentiation and memory consolidation, emotional
perception as well as endocrinological responses (32-34).
An improved understanding of the hippocampal cellular pathways
activated under hypoxic conditions and potential alterations due to
ketamine is therefore crucial for the development of potential new
anesthetic techniques with the aim of reducing the effects of
hypoxia-induced brain damage in neonates.
Materials and methods
Cell culture
Murine hippocampal HT22 cells were generously
provided by Professor Axel Methner, Department of Neurology,
Düsseldorf University Hospital. Cells were cultured at 37̊C with 5%
CO2 volume fraction in DMEM, high glucose (Thermo
Scientific Fisher, Inc.) supplemented with 10% FBS (Sigma-Aldrich;
Merck KGaA) and 1% penicillin-streptomycin solution (Sigma-Aldrich;
Merck KGaA). A total of 1x105-1x106 HT22
cells were plated in TC Schale 100 cell culture dishes (Sarstedt,
Inc.) and cultured for 48 h in supplemented DMEM.
For differentiation, the cells were treated with
Neurobasal™ medium (Thermo Fisher Scientific, Inc.) for 24 h,
supplemented with 1% 100x N2 supplement (Thermo Fisher
Scientific, Inc.) and 10% FBS and 1% penicillin-streptomycin
solution.
Hypoxia and ketamine model
HT22 cells have long been used as a model cell line
for examining oxidative glutamate toxicity (31,35,36), but
to the best of our knowledge, have not been previously used to
study the effects of hypoxia and ketamine treatment on the
developing brain.
After 24 h of differentiation, Neurobasal™ medium
was replaced with supplemented DMEM. Ketamine (10 mg/ml;
Ketamin-Actavis, Injektionslösung, Actavis Group PTC EHF) was added
to culture dishes to final ketamine concentrations of 1, 10 or 20
µM. Control cell culture dishes were handled in the same manner,
but no ketamine or vehicle was added. Ketamine-incubated HT22 cells
and controls were cultured for 24 h either under hypoxic (1%
O2, 5% CO2) or normoxic (21% O2,
5% CO2) conditions. Ketamine doses of 1, 10 or 20 µM
(equivalent to 0.238, 2.38 or 5.76 µg/ml, respectively, in culture
medium) were chosen in accordance with in vivo measurements
of brain and plasma ketamine concentration as reported by Liu et
al (10).
Reverse transcription-quantitative
(q)PCR
mRNA was isolated from harvested HT22 cells using
TriReagent® RNA Isolation Reagent (Sigma-Aldrich; Merck
KGaA) according to the manufacturer's protocol. mRNA concentrations
were measured using a Nano Quant infinite M200 Pro (Tecan Group,
Ltd.). Moloney Murine Leukemia Virus Reverse Transcriptase was used
to synthesize first strand cDNA according to the manufacturer's
protocol (Promega Corporation).
For TaqMan™ qPCR (7500 Real-Time PCR system, Applied
Biosystems; Thermo Fisher Scientific, Inc.), 0.5 µl each of the
forward and reverse primers, and the probe (all purchased from
Eurofins Genomics), 2.5 µl cDNA template and 12.5 µl Platinum™
Quantitative PCR SuperMix-UDG (Thermo Fisher Scientific, Inc.) were
added per well in a 96-well plate and amplified for 40 cycles
(annealing temperature, 60̊C) according to the manufacturer's
protocol. The sequences of the primers used and the respective
probes are presented in Table SI.
qPCR data of mRNA expression levels were normalized using the
2-ΔΔCq method in Microsoft Excel
2010 (Microsoft Corporation) using GAPDH as the reference gene
(37).
Statistical analysis
To evaluate the effects of ketamine treatment,
comparisons between multiple groups were performed using a
Kruskal-Wallis one-way ANOVA with a post-hoc Dunn's test. All
statistical tests were performed using GraphPad Prism version 8
(GraphPad Software, Inc.). Results are presented as the means of
mRNA expression. Error bars represent the standard deviations.
Results
The aim of the present study was to improve our
understanding of the effects of ketamine on developing neurons
under hypoxic conditions. Differentiated murine HT22 cells were
incubated with different concentrations of ketamine (1, 10 or 20
µM) for 24 h under hypoxic or normoxic conditions. qPCR analysis of
cellular markers associated with neurogenesis, proliferation and
extracellular matrix homeostasis was performed to determine the
effects of ketamine.
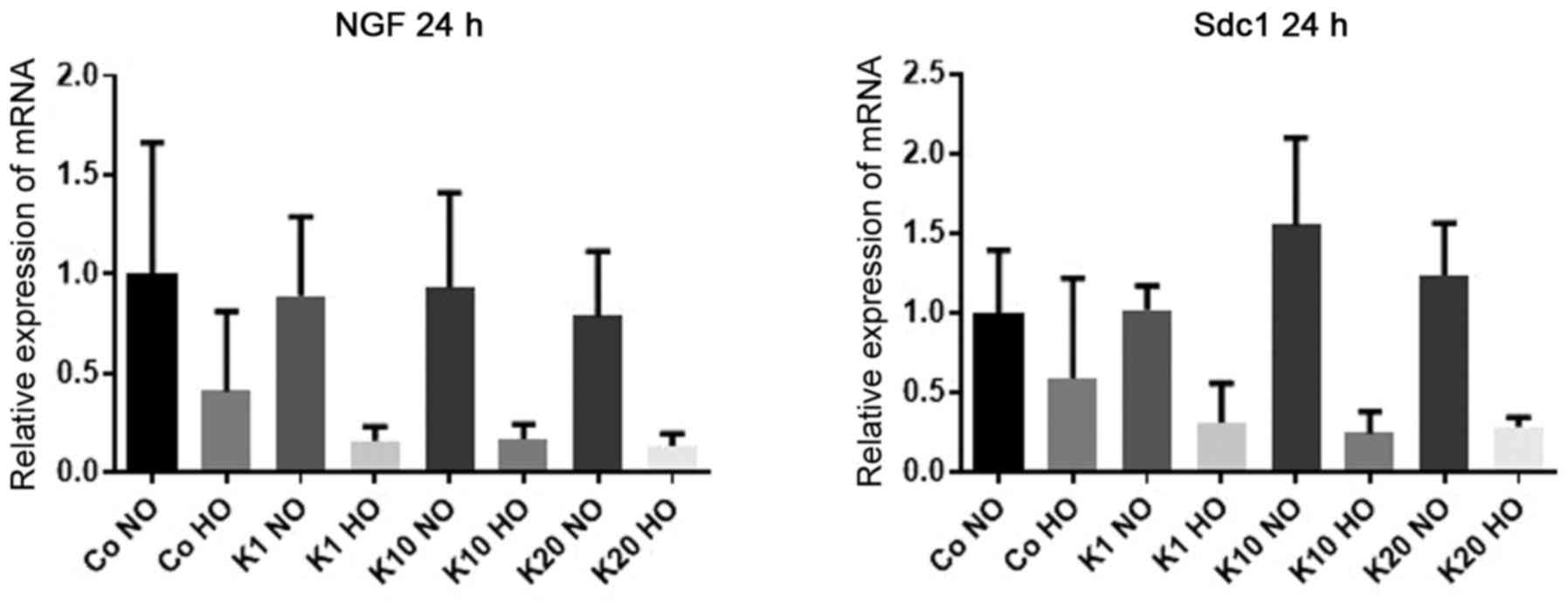
mRNA expression levels of NGF
To investigate how the mRNA expression levels of
NGF, an important neurotrophin for migration and maturation
of neurons in the developing brain (38), were affected by hypoxia and ketamine
treatment, the mRNA expression levels of NGF in
differentiated HT22 cells were determined after 24 h of treatment
with various ketamine concentrations under hypoxic and normoxic
conditions.
There was no evidence of differences in the mRNA
expression levels of NGF following ketamine treatment
compared with the hypoxic and normoxic control groups (P>0.99
for all comparisons). However, there was a decrease in NGF
expression levels in cells cultured under hypoxic conditions
compared with the normoxic cultured cells treated with the same
concentration of ketamine to 16% (K1; P=0.25), 17% (K10; P=0.38)
and 13% (K20; P=0.19). All mRNA expression levels stated are
relative to the expression levels in the control (Co NO) group.
There was no evidence of differences in the mRNA expression levels
in hypoxic and normoxic cultured cells in the control groups
(P>0.99; Fig. 1).
mRNA expression levels of Sdc1
To investigate how the mRNA expression levels of
Sdc1, which is expressed by neuronal progenitor cells
(39), were affected by hypoxia and
ketamine treatment, the mRNA expression levels of Sdc1 in
differentiated HT22 cells after 24 h of treatment with various
ketamine concentrations were determined under hypoxic and normoxic
conditions.
There was no evidence of differences in the mRNA
expression levels of Sdc1 following ketamine treatment
compared with the hypoxic and normoxic control groups (P>0.99
for all comparisons). However, there was a decrease in the
expression levels in cells cultured under hypoxic conditions
respectively compared with the normoxic cultured cells treated with
the same concentration of ketamine to 24% (K10; P=0.11) and 28%
(K20; P=0.41). All mRNA expression levels stated are relative to
the expression levels in the Co NO. There was no evidence of a
difference in mRNA expression levels between hypoxia and normoxia
for K1 (P>0.99) or in the control groups (P>0.99; Fig. 1).
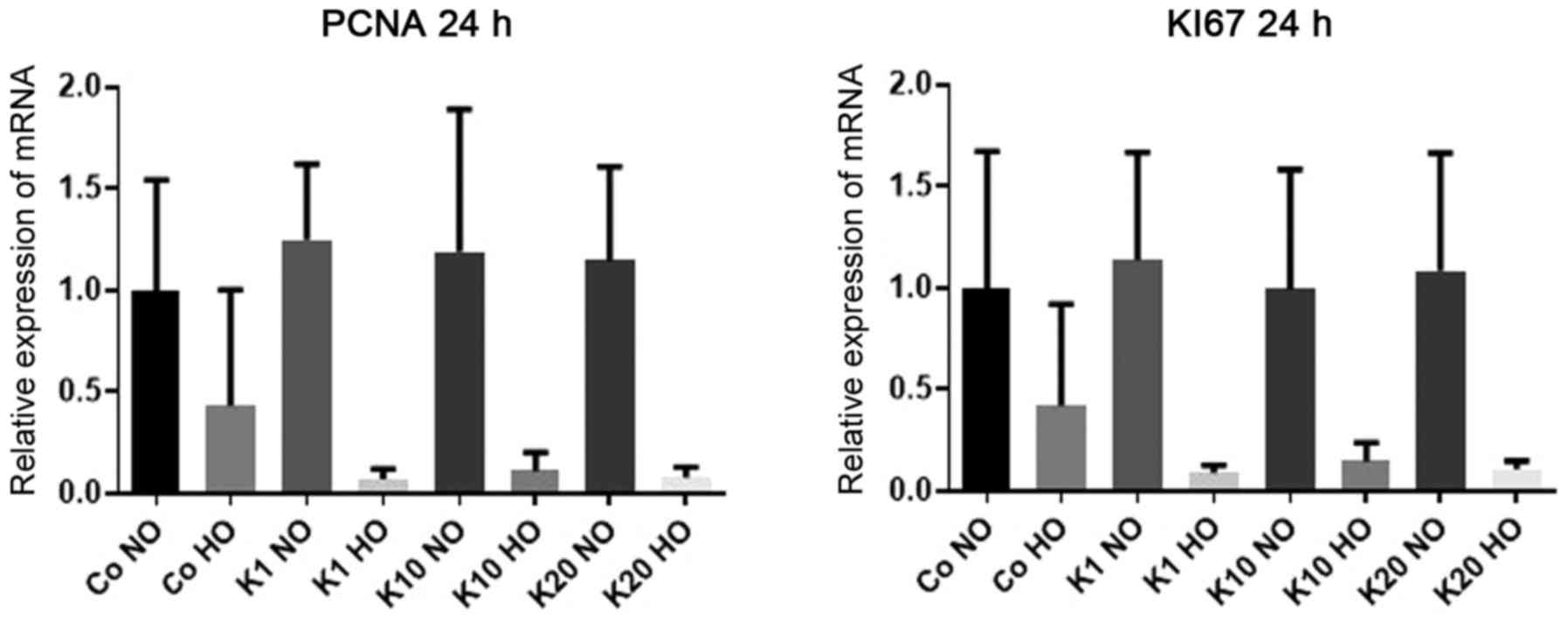
mRNA expression levels of PCNA
To investigate how the mRNA expression levels of
PCNA, a widely used proliferation marker (40,41),
were affected by hypoxia and ketamine treatment, the mRNA
expression levels of PCNA were determined in the
differentiated HT22 cells after 24 h of treatment with various
concentrations of ketamine under hypoxic and normoxic
conditions.
There was no evidence of differences in the mRNA
expression levels of PCNA following ketamine treatment
compared with the hypoxic and normoxic control groups (P>0.99
for all comparisons). However, there was a downregulation of
PCNA expression in cells cultured under hypoxic conditions
respectively compared with normoxic cultured cells treated with the
same concentration of ketamine to 7% (K1; P=0.09), 11% (K10;
P=0.35) and 8% (K20; P=0.25). All mRNA expression levels stated are
relative to the expression levels in the Co NO group. There was no
evidence of a difference in mRNA expression levels between hypoxic
and normoxic cultured cells in the control groups (P>0.99;
Fig. 2).
mRNA expression levels of Ki67
To investigate how the mRNA expression of
Ki67, which is expressed in all phases of the cell cycle of
proliferating cells (42), was
affected by hypoxia and ketamine treatment, the mRNA expression
levels of Ki67 were determined in the differentiated HT22
cells after 24 h of treatment with various concentrations of
ketamine under hypoxic and normoxic conditions.
There was no evidence of a difference in the mRNA
expression levels of Ki67 following treatment with ketamine
compared with the hypoxic and normoxic control groups (P>0.99
for all comparisons). However, there was a downregulation in the
Ki67 expression levels cultured under hypoxic conditions
respectively compared with the normoxic cultured cells treated with
the same concentration of ketamine to 9% (K1; P=0.04) and 11% (K20;
P=0.27). All mRNA expression levels stated are relative to the
expression levels in the Co NO group. There was no evidence of a
difference in the mRNA expression levels between the hypoxic and
normoxic cultured cells in K10 (P=0.63) or in the control group
(P>0.99; Fig. 2).
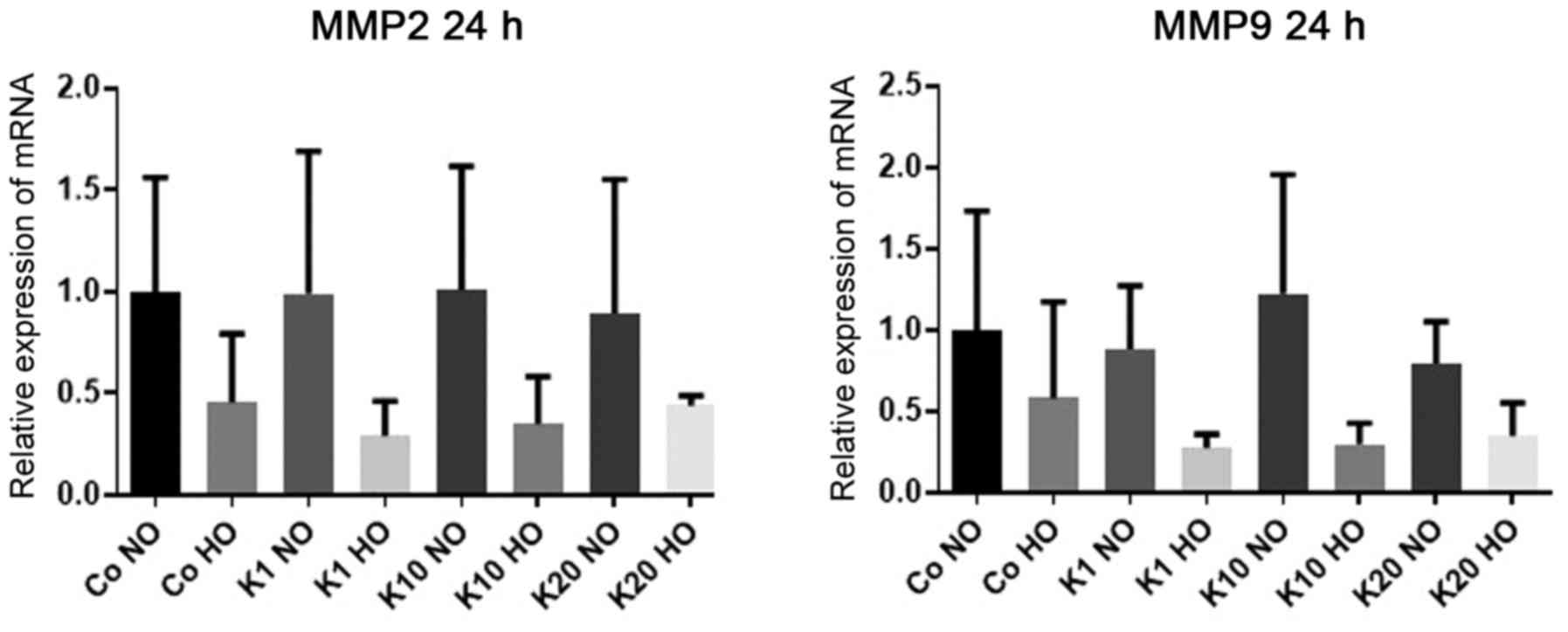
mRNA expression levels of MMP2
To investigate how the mRNA expression levels of
MMP2, an enzyme in the MMP group of proteins which serve
important roles in the proteolysis of the extracellular matrix
following brain injury (43), were
affected by hypoxia and ketamine exposure, the mRNA expression
levels of MMP2 in differentiated HT22 cells after 24 h of treatment
with various concentrations of ketamine were determined under
hypoxic and normoxic conditions. There was no evidence of
differences in the mRNA expression levels of MMP2 following
ketamine treatment or hypoxia (P>0.99 for all between-group
comparisons) (Fig. 3).
mRNA expression levels of MMP9
To investigate how the mRNA expression levels of
MMP9 were affected by hypoxia and ketamine exposure, the
mRNA expression levels of MMP9 in differentiated HT22 cells
were determined after 24 h of treatment with various concentrations
of ketamine under hypoxic and normoxic conditions.
There was no evidence of differences in the mRNA
expression of MMP9 following ketamine treatment compared
with the hypoxic and normoxic control groups (P>0.99 for all
comparisons). However, there was a downregulation of MMP9
expression in cells cultured under hypoxic conditions respectively
compared with the normoxic cultured cells treated with the same
concentration of ketamine to 28% (K1; P=0.42) and 30% (K10;
P=0.23). All mRNA expression levels stated are relative to the
expression levels in the Co NO group. There was no significant
difference between hypoxia and normoxia for K20 (P>0.99) or in
the control groups (P>0.99; Fig.
3).
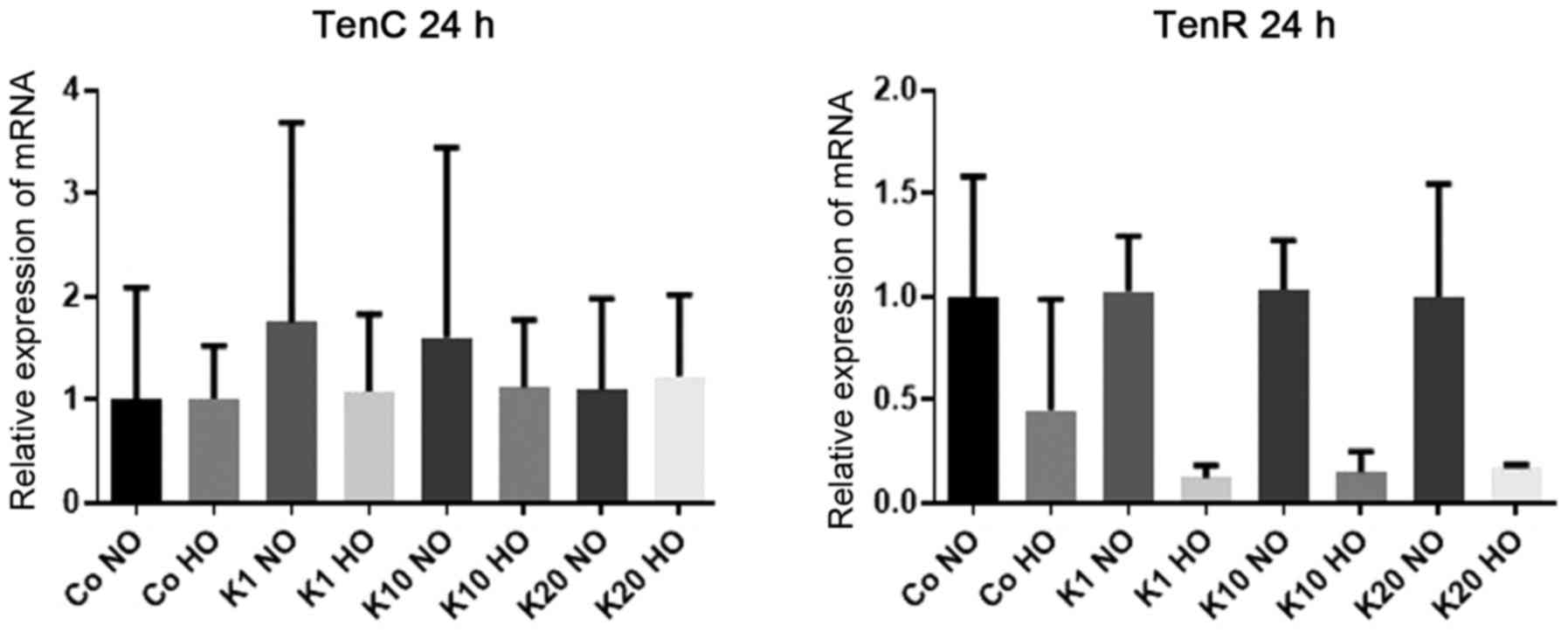
mRNA expression levels of TenC
To investigate how the mRNA expression of
TenC, which is an extracellular matrix protein expressed
following tissue injury (44), was
affected by hypoxia and ketamine exposure, the mRNA expression
levels of TenC in differentiated HT22 cells were determined
after 24 h of treatment with various concentrations of ketamine
under hypoxic and normoxic conditions.
There was no evidence of differences in the mRNA
expression of TenC following ketamine treatment or hypoxia
(P>0.99 for all between-group comparisons) (Fig. 4).
mRNA expression levels of TenR
To investigate how the mRNA expression of
TenR, an extracellular matrix protein exclusively expressed
in the nervous system (45), was
affected by hypoxia and ketamine exposure, the mRNA expression
levels of TenR in differentiated HT22 cells were determined
after 24 h of treatment with various concentrations of ketamine
under hypoxic and normoxic conditions.
There was no evidence of differences in the mRNA
expression levels of TenR following ketamine treatment
compared with the hypoxic and normoxic control groups. However,
there was a downregulation in the expression of TenR in
cells cultured under hypoxic conditions respectively compared with
the normoxic cultured cells treated with the same concentration of
ketamine to 12% (K1, P=0.14) and 15% (K10, P=0.25). All mRNA
expression levels stated are relative to the expression levels in
the Co NO group. There was no significant difference in the
expression of TenR between cells grown under hypoxic and
normoxic conditions for K20 (P=0.89) or between the normoxic and
hypoxic controls groups (P>0.99; (Fig. 4).
Discussion
The aim of the present study was to investigate the
effects of ketamine on the expression of markers involved in
cellular pathways associated with proliferation, neurogenesis and
extracellular matrix homeostasis in hypoxia-exposed neurons.
The results showed that ketamine treatment alone in
HT22 cells did not significantly affect the expression of the
assessed markers of proliferation, neurogenesis or extracellular
matrix homeostasis compared with their respective untreated
controls. Furthermore, untreated control groups did not exhibit any
significant differences in expression of the markers assessed when
compared with hypoxia cultured cells.
However, there was a tendency towards downregulation
of markers of proliferation, neurogenesis and, to a lesser extent,
extracellular matrix homeostasis under hypoxic conditions combined
with ketamine treatment compared with their respective normoxic
control group. No dose-dependent association was observed among the
ketamine treated groups. Taken together, these results suggest
increased vulnerability of hippocampal neurons in vitro to
hypoxia in the presence of ketamine, independent of the dose of
ketamine.
Several tested markers allow for specific
interpretations. Sdc1 is a transmembrane heparan sulfate
proteoglycan that is found in abundance in mammalian brains prior
to neurogenesis (39). NGF
stimulates migration and maturation of neurons in the developing
brain and has neuroprotective functions in the adult brain
(38). Ki67 and PCNA
are expressed by replicating cells only and are widely used as
markers to evaluate cellular proliferation (40,42).
Thus, ketamine treatment may not be neurotoxic itself but may
aggravate the effects of hypoxia, which thus results in an earlier
onset of neurotoxic effects in the ketamine-treated groups compared
with the untreated control groups. This would support findings from
previous studies which demonstrated the potential neurotoxicity of
ketamine treatment (7-15).
The results of expression of markers of
extracellular matrix homeostasis were less conclusive. Only
MMP-9 and TenR exhibited a similar pattern to that
described above; whereas there was no evidence of differences in
the expression of MMP2 and TenC. Although not
significant, MMP2 mRNA expression levels still exhibited a
tendency similar to that of MMP9. The downregulation in
MMP2 and MMP9 expression under hypoxic conditions is
a potentially interesting find, as matrix-metalloproteinases
degrade the extracellular matrix, activate TNF-α and their
expression is known to be elevated in cerebral ischemia (43,46).
However, MMPs are also known for their dual involvement in
cellular processes of early tissue damage and late tissue repair
during brain injury (47). Future
studies investigating the mRNA expression patterns of MMPs
following hypoxia exposure for various time periods may provide an
improved understanding of this complex matter.
A possible explanation for the observations of the
present study lies in the limitation of the cell culture model.
Zhao et al (31) showed that
HT22 cells differentiated using N2 supplement expressed
NMDA receptors which were not expressed by undifferentiated HT22
cells. It is possible that the HT22 cells may have returned to
their undifferentiated state as a result of prolonged exposure to
hypoxia with a subsequent downregulation of all the examined genes.
However, the fact that no significant effects of hypoxia alone were
detected in the untreated groups suggests the possibility of a
specific ketamine-dependent effect in the present study.
Ketamine concentrations of 1, 10 and 20 µM were
chosen based on a previous study (10). As neurotoxicity due to ketamine was
only observed when cells were treated with high doses in various
studies (8,10,13), the
ketamine concentrations used in the present study were likely too
low to produce a direct neurotoxic effect.
Several previous studies have demonstrated the
significant effects of ketamine treatment on neuronal tissue when
the drug is applied as a bolus (6,10,13-15).
In the present study, hippocampal cells were incubated for 24 h to
simulate extended ketamine treatment in neonates, which may partly
explain the inconsistencies in the data when compared with previous
studies. There are certain studies showing significant ketamine
neurotoxicity following continuous infusion (8,9,21), thus further investigation that
directly compares the effects of short and long term treatment is
required to elucidate the effects of the length of treatment with
ketamine on neurotoxicity.
Although there are several studies demonstrating the
potential neurotoxic and neuroapoptotic effects of ketamine
(7-15),
comparatively less research has been performed on its effects in
hypoxic conditions. Animal studies showed that NMDA receptor
blockade and ketamine treatment may result in neuroprotective
outcomes in hypoxia (20,22,23).
However, Ulbrich et al (48)
did not observe any significant effects of ketamine incubation in a
study performed on a SH-SY5Y neuronal cell line. The latter is
consistent with the results of the present study and is in contrast
to the findings of Rothman et al (19), who described preserved action
potentials and higher ATP levels in hypoxic primary hippocampal
neurons when treated with ketamine. Similarly, ketamine ameliorated
the neuroinflammatory response to transient hypoxia in fetal sheep
(21). A possible explanation for
these contradictory findings is that previously described
neuroprotective findings may not be directly caused by the effects
of ketamine on neurons, but rather through more complex
interactions between the drug and its metabolites, glial cells and
neurons. Glial cells are naturally present in animal studies and in
primary neuronal cell cultures, but not in immortalized cell lines
consisting of a sole cell type such as HT22 or SH-SY5Y (49). This supports the previously suggested
hypothesis of glial cells serving a key role in neuroprotection
following acute brain injury (50-52).
Furthermore, ketamine is metabolized in complex pathways in
vitro, which results in the circulation of various ketamine
metabolites (53). This may be
crucial regarding the neurotoxic and neuroprotective ketamine
effects described in animal studies and will be omitted by default
in a cell culture model.
Another limitation of the present study is that the
experiments were performed on a single cell line only. Further
studies, particularly animal and primary cell culture models are
required to obtain a more definite answer regarding the potential
aggravation of hypoxia-induced damage due to ketamine
treatment.
The present study is a modest contribution to the
ongoing discussion of the potential neurotoxic and neuroprotective
effects of the commonly used anesthetic and analgesic pediatric
drug ketamine. Overall, the data obtained from the present study
are not conclusive and thus should not be used in clinical decision
making. However, the findings do broaden our understanding of
neuronal gene expression in hypoxia and may thus serve as a
starting point for future investigations.
In conclusion, the results of the present study
suggest potential aggravation of hypoxia-induced neurotoxicity in
hippocampal neurons in the presence of ketamine, as observed in the
patterns of downregulation of genetic markers of proliferation,
neurogenesis and extracellular matrix homeostasis. However, there
was no dose-response association for ketamine treatment observed,
which may be due to the ketamine concentrations used and the
limitations of the cell culture model used.
Supplementary Material
Table SI. Sequences of the primers and
probes used in the present study.
Acknowledgements
We would like to thank Professor Axel Methner at the
Johannes Gutenberg University Mainz for providing us with the HT22
cell line. Furthermore, we would like to thank Miss Christina
Vohlen, Miss Maria Wohlfarth and Mr. Gregor Fink (Department of
Pediatrics and Adolescent Medicine, University of Cologne) for
their excellent technical assistance.
Funding
This study was supported by a Köln Fortune grant
(grant no. 69/2010).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
BR, CH, TK and EH-R. participated in the design of
the study. Molecular analyses and cell culture experiments were
performed by TP and SA. TP, SA, RJ and EH-R. analyzed and
interpreted the data. TP and EH-R drafted and revised the
manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Domino EF: Taming the ketamine tiger.
Anesthesiology. 113:678–686. 2010.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Arora S: Combining ketamine and propofol
(‘ketofol’) for emergency department procedural sedation and
analgesia: A review. West J Emerg Med. 9:20–23. 2008.PubMed/NCBI
|
|
3
|
Green SM, Roback MG, Kennedy RM and Krauss
B: Clinical practice guideline for emergency department ketamine
dissociative sedation: 2011 update. Ann Emerg Med. 57:449–461.
2011.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Green SM, Roback MG, Krauss B, Brown L,
McGlone RG, Agrawal D, McKee M, Weiss M, Pitetti RD, Hostetler MA,
et al: Predictors of emesis and recovery agitation with emergency
department ketamine sedation: An individual-patient data
meta-analysis of 8,282 children. Ann Emerg Med. 54:171–180.e4.
2009.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Street MH and Gerard JM: A fixed-dose
ketamine protocol for adolescent sedations in a pediatric emergency
department. J Pediatr. 165:453–458. 2014.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Yan J and Jiang H: Dual effects of
ketamine: Neurotoxicity versus neuroprotection in anesthesia for
the developing brain. J Neurosurg Anesthesiol. 26:155–160.
2014.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Ikonomidou C, Bosch F, Miksa M, Bittigau
P, Vöckler J, Dikranian K, Tenkova TI, Stefovska V, Turski L and
Olney JW: Blockade of NMDA receptors and apoptotic
neurodegeneration in the developing brain. Science. 283:70–74.
1999.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Slikker W Jr, Zou X, Hotchkiss CE, Divine
RL, Sadovova N, Twaddle NC, Doerge DR, Scallet AC, Patterson TA,
Hanig JP, et al: Ketamine-induced neuronal cell death in the
perinatal rhesus monkey. Toxicol Sci. 98:145–158. 2007.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Paule MG, Li M, Allen RR, Liu F, Zou X,
Hotchkiss C, Hanig JP, Patterson TA, Slikker W Jr and Wang C:
Ketamine anesthesia during the first week of life can cause
long-lasting cognitive deficits in rhesus monkeys. Neurotoxicol
Teratol. 33:220–230. 2011.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Liu F, Paule MG, Ali S and Wang C:
Ketamine-induced neurotoxicity and changes in gene expression in
the developing rat brain. Curr Neuropharmacol. 9:256–261.
2011.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Ye Z, Li Q, Guo Q, Xiong Y, Guo D, Yang H
and Shu Y: Ketamine induces hippocampal apoptosis through a
mechanism associated with the caspase-1 dependent pyroptosis.
Neuropharmacology. 128:63–75. 2018.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Yan J, Huang Y, Lu Y, Chen J and Jiang H:
Repeated administration of ketamine can induce hippocampal
neurodegeneration and long-term cognitive impairment via the
ROS/HIF-1α pathway in developing rats. Cell Physiol Biochem.
33:1715–1732. 2014.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Huang L, Liu Y, Jin W, Ji X and Dong Z:
Ketamine potentiates hippocampal neurodegeneration and persistent
learning and memory impairment through the PKCγ-ERK signaling
pathway in the developing brain. Brain Res. 1476:164–171.
2012.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Hayashi H, Dikkes P and Soriano S:
Repeated administration of ketamine may lead to neuronal
degeneration in the developing rat brain. Pediatr Anesth.
12:770–774. 2002.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Zou X, Patterson TA, Sadovova N, Twaddle
NC, Doerge DR, Zhang X, Fu X, Hanig JP, Paule MG, Slikker W and
Wang C: Potential neurotoxicity of ketamine in the developing rat
brain. Toxicol Sci. 108:149–158. 2009.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Anand KJ, Garg S, Rovnaghi CR, Narsinghani
U, Bhutta AT and Hall RW: Ketamine reduces the cell death following
inflammatory pain in newborn rat brain. Pediatr Res. 62:283–290.
2007.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Khwaja O and Volpe JJ: Pathogenesis of
cerebral white matter injury of prematurity. Arch Dis Child Fetal
Neonatal Ed. 93:F153–F161. 2008.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Douglas-Escobar M and Weiss MD:
Hypoxic-ischemic encephalopathy. JAMA Pediatr. 169:397–403.
2015.
|
|
19
|
Rothman SM, Thurston JH, Hauhart RE, Clark
GD and Solomon JS: Ketamine protects hippocampal neurons from
anoxia in vitro. Neuroscience. 21:673–678. 1987.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Spandou E, Karkavelas G, Soubasi V,
Avgovstides-Savvopoulou P, Loizidis T and Guiba-Tziampiri O: Effect
of ketamine on hypoxic-ischemic brain damage in newborn rats. Brain
Res. 819:1–7. 1999.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Chang EI, Zárate MA, Rabaglino MB,
Richards EM, Arndt TJ, Keller-Wood M and Wood CE: Ketamine
decreases inflammatory and immune pathways after transient hypoxia
in late gestation fetal cerebral cortex. Physiol Rep.
4(e12741)2016.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Chen HS, Wang Y, Rayudu P, Edgecomb P,
Neill J, Segal M, Lipton SA and Jensen FE: Neuroprotective
concentrations of the N-methyl-D-aspartate open-channel blocker
memantine are effective without cytoplasmic vacuolation following
post-ischemic administration and do not block maze learning or
long-term potentiation. Neuroscience. 86:1121–1132. 1998.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Manning SM, Talos DM, Zhou C, Selip DB,
Park HK, Park CJ, Volpe JJ and Jensen FE: NMDA receptor blockade
with memantine attenuates white matter injury in a rat model of
periventricular leukomalacia. J Neurosci. 28:6670–6678.
2008.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Guerra GG, Robertson CMT, Alton GY, Joffe
AR, Cave DA, Dinu IA, Creighton DE, Ross DB and Rebeyka IM: Western
Canadian Complex Pediatric Therapies Follow-up Group:
Neurodevelopmental outcome following exposure to sedative and
analgesic drugs for complex cardiac surgery in infancy. Paediatr
Anaesth. 21:932–941. 2011.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Hansen TG, Pedersen JK, Henneberg SW,
Pedersen DA, Murray JC, Morton NS and Christensen K: Academic
performance in adolescence after inguinal hernia repair in infancy:
A nationwide cohort study. Anesthesiology. 114:1076–1085.
2011.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Loss CM, Córdova SD and De Oliveira DL:
Ketamine reduces neuronal degeneration and anxiety levels when
administered during early life-induced status epilepticus in rats.
Brain Res. 1474:110–117. 2012.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Turner CP, Gutierrez S, Liu C, Miller L,
Chou J, Finucane B, Carnes A, Kim J, Shing E, Haddad T and Phillips
A: Strategies to defeat ketamine-induced neonatal brain injury.
Neuroscience. 210:384–392. 2012.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Zhang X, Zhao J, Chang T, Wang Q, Liu W
and Gao L: Ketamine exerts neurotoxic effects on the offspring of
pregnant rats via the Wnt/β-catenin pathway. Environ Sci Pollut
Res. 27:305–314. 2020.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Slikker W Jr, Liu F, Rainosek SW,
Patterson TA, Sadovova N, Hanig JP, Paule MG and Wang C:
Ketamine-induced toxicity in neurons differentiated from neural
stem cells. Mol Neurobiol. 52:959–969. 2015.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Jiang S, Li X, Jin W, Duan X, Bo L, Wu J,
Zhang R, Wang Y, Kang R and Huang L: Ketamine-induced neurotoxicity
blocked by N-Methyl-D-aspartate is mediated through activation of
PKC/ERK pathway in developing hippocampal neurons. Neurosci Lett.
673:122–131. 2018.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Zhao Z, Lu R, Zhang B, Shen J, Yang L,
Xiao S, Liu J and Suo W: Differentiation of HT22 neurons induces
expression of NMDA receptor that mediates homocysteine
cytotoxicity. Neurol Res. 34:38–43. 2012.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Lathe R: Hormones and the hippocampus. J
Endocrinol. 169:205–231. 2001.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Neves G, Cooke SF and Bliss TV: Synaptic
plasticity, memory and the hippocampus: A neural network approach
to causality. Nat Rev Neurosci. 9:65–75. 2008.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Femenía T, Gómez-Galán M, Lindskog M and
Magara S: Dysfunctional hippocampal activity affects emotion and
cognition in mood disorders. Brain Res. 1476:58–70. 2012.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Lee Y, Park HW, Park SG, Cho S, Myung PK,
Park BC and Lee DH: Proteomic analysis of glutamate-induced
toxicity in HT22 cells. Proteomics. 7:185–193. 2007.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Zhang J, Feng J, Ma D, Wang F, Wang Y, Li
C, Wang X, Yin X, Zhang M, Dagda RK and Zhang Y: Neuroprotective
mitochondrial remodeling by AKAP121/PKA protects HT22 cell from
glutamate-induced oxidative stress. Mol Neurobiol. 56:5586–5607.
2019.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Manni L, Rocco ML, Bianchi P, Soligo M,
Guaragna M, Barbaro SP and Aloe L: Nerve growth factor: Basic
studies and possible therapeutic applications. Growth Factors.
31:115–122. 2013.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Wang Q, Yang L, Alexander C and Temple S:
The niche factor syndecan-1 regulates the maintenance and
proliferation of neural progenitor cells during mammalian cortical
development. PLoS One. 7(e42883)2012.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Bologna-Molina R, Mosqueda-Taylor A,
Molina-Frechero N, Mori-Estevez AD and Sánchez-Acuña G: Comparison
of the value of PCNA and Ki-67 as markers of cell proliferation in
ameloblastic tumors. Med Oral Patol Oral Cir Bucal. 18:e174–e179.
2013.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Boehm EM, Gildenberg MS and Washington MT:
The many roles of PCNA in eukaryotic DNA replication. Enzymes.
39:231–254. 2016.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Juríková M, Danihel Ľ, Polák Š and Varga
I: Ki67, PCNA, and MCM proteins: Markers of proliferation in the
diagnosis of breast cancer. Acta Histochem. 118:544–552.
2016.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Leonardo CC, Eakin AK, Ajmo JM, Collier
LA, Pennypacker KR, Strongin AY and Gottschall PE: Delayed
administration of a matrix metalloproteinase inhibitor limits
progressive brain injury after hypoxia-ischemia in the neonatal
rat. J Neuroinflammation. 5(34)2008.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Midwood KS, Hussenet T, Langlois B and
Orend G: Advances in tenascin-C biology. Cell Mol Life Sci.
68:3175–3199. 2011.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Anlar B and Gunel-Ozcan A: Tenascin-R:
Role in the central nervous system. Int J Biochem Cell Biol.
44:1385–1389. 2012.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Cunningham LA, Wetzel M and Rosenberg GA:
Multiple roles for MMPs and TIMPs in cerebral ischemia. Glia.
50:329–339. 2005.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Yang Y and Rosenberg GA: Matrix
metalloproteinases as therapeutic targets for stroke. Brain Res.
1623:30–38. 2015.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Ulbrich F, Eisert L, Buerkle H, Goebel U
and Schallner N: Propofol, but not ketamine or midazolam, exerts
neuroprotection after ischaemic injury by inhibition of Toll-like
receptor 4 and nuclear factor kappa-light-chain-enhancer of
activated B-cell signalling: A combined in vitro and animal study.
Eur J Anaesthesiol. 33:670–680. 2016.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Gordon J, Amini S and White MK: General
overview of neuronal cell culture. Methods Mol Biol. 1078:1–8.
2013.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Huang R, Sochocka E and Hertz L: Cell
culture studies of the role of elevated extracellular glutamate and
K+ in neuronal cell death during and after anoxia/ischemia.
Neurosci Biobehav Rev. 21:129–134. 1997.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Montero M, González B and Zimmer J:
Immunotoxic depletion of microglia in mouse hippocampal slice
cultures enhances ischemia-like neurodegeneration. Brain Res.
1291:140–152. 2009.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Neumann J, Gunzer M, Gutzeit HO, Ullrich
O, Reymann KG and Dinkel K: Microglia provide neuroprotection after
ischemia. FASEB J. 20:714–716. 2006.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Zanos P, Moaddel R, Morris PJ, Riggs LM,
Highland JN, Georgiou P, Pereira EFR, Albuquerque EX, Thomas CJ,
Zarate CA Jr and Gould TD: Ketamine and ketamine metabolite
pharmacology: Insights into therapeutic mechanisms. Pharmacol Rev.
70:621–660. 2018.PubMed/NCBI View Article : Google Scholar
|