Introduction
Breast cancer is the most common type of invasive
malignancy in women worldwide, accounting for ~25% of all types of
cancer, and ranking second after lung cancer in cancer-related
deaths in women (1). Deaths from
breast cancer are typically lower in developed countries, but the
mortality is relatively higher in Turkey (2) and other developing countries (3). Despite substantial research efforts and
improvements in personalized treatment, including targeted
therapies, breast cancer remains a major health obstacle worldwide.
This is partly associated with the heterogeneous nature of breast
tumors, and hence a lack of appropriate and reliable biomarkers for
early detection, prediction of therapy outcomes and disease
recurrence (4).
Epigenetic changes are involved in the development
of cancer. Alterations in post-translational histone modification
pathways (PTHMs) are common in the development and progression of
several types of cancer, including breast cancer (5). The methylation of histone proteins is
vital for cells to perform their physiological functions; histone
proteins serve several regulatory functions in chromatin formation,
DNA damage repair, DNA replication and gene expression (6). Methylation of histones usually occurs
on the lysine residues in the tails of histone H3 and H4(7). Methylation of H4 lysine 20 (H4K20) is
evolutionarily conserved in mammalian cells, and occurs at three
different levels as mono-, di- and triple-methylation.
Triple-methylation of H4K20 (H4K20me3), catalyzed by the
methyltransferase enzyme SUV420H2, is enriched in the gene-poor
regions of the genome such as heterochromatin, telomeres, imprinted
regions and repetitive elements, and is involved in transcriptional
silencing of these regions (8,9). Loss of
H4K20me3 in tumor tissues has been described as a hallmark of
cancer (10-13).
Therefore, the study of PTHMs has become an essential part of
cancer research (14-18).
The loss of H4K20me3 in cultured breast cancer cells
was found to be accompanied by reduced expression of histone
methyltransferase SUV420H2(19).
Invasive breast cancer cells (such as MDA-MB-231 or BT-474) were
shown to express lower levels of SUV420H2 compared with less
invasive breast cancer cells (20).
Another study reported lower SUV420H2 expression in mesenchymal
breast cancer cells compared with epithelial breast cancer cells
(12). These data suggest that
reduced SUV420H2 expression may serve as a marker of cancer
progression. Accordingly, ectopic overexpression of SUV420H2 leads
to reduced breast cancer cell invasion in vitro (20). Data on the expression status of
SUV420H2 in breast cancer tissues are limited. Data retrieved from
databases showed that breast cancer tissues tended to express lower
levels of SUV420H2 compared with normal breast tissues (20).
In the present study, the influence of SUV420H2
suppression on the proliferation of breast cancer cells was first
determined. Subsequently, SUV420H2 expression in breast cancer was
assessed due to the limited availability of data on the expression
of SUV420H2 histone methyltransferase in breast cancer tissues. The
results showed that SUV420H2 expression in breast tumors relative
to non-cancerous regions was heterogeneous and tended to decrease
in more advanced tumors.
Materials and methods
Patients and cells
Patients with resectable breast cancer (n=102) with
no secondary malignancies, were enrolled in the present study.
Tissues were provided by Istanbul Training and Research Hospital
from cases between March 2012 and November 2013. The present study
was approved both by the Clinical Research Ethics Committee of
Istanbul University (approval no. 2017/887) and the Ethics
Committee of Istanbul Training and Research Hospital (approval no.
2012/1413-1201). Histological classification of breast tumors were
performed in accordance with the World Health Organization
guidelines (21). Tumor grading was
performed according to the Nottingham modification of the
Scarff-Bloom-Richardson grading system (22). Tumor staging was classified using the
Tumor-Node-Metastsasis system adopted by American Joint Committee
on Cancer (23). Tumor and adjacent
non-cancerous tissue samples were stored at -80˚C. The mean age of
the patients was 53 (range, 20-87) years. Table I depicts the clinical characteristics
of the patients with breast cancer from whom the tumors were
obtained.
 | Table IClinicopathological characteristics of
the breast cancer patients. |
Table I
Clinicopathological characteristics of
the breast cancer patients.
| Characteristics | N |
|---|
| Age, year | 52 |
|
≤53 | 50 |
|
>53 | |
| Menopausal
status |
|
Premenopausal | 36 |
|
Postmenopausal | 66 |
| Tumor size |
|
T1 | 37 |
|
T2 | 57 |
|
T3-T4 | 8 |
| Nodal status |
|
N=0 | 43 |
|
N≥1 | 59 |
| Stage |
|
I | 19 |
|
II | 53 |
|
III | 30 |
| Estrogen
receptor |
|
Positive | 81 |
|
Negative | 21 |
| Progesteron
receptor |
|
Positive | 73 |
|
Negative | 29 |
| HER2 expression |
|
Positive | 26 |
|
Negative | 76 |
| Molecular
classification |
|
Luminal
A | 35 |
|
Luminal
B | 42 |
|
Her2-like | 11 |
|
Basal-like | 14 |
| Histologic Grade |
|
1 | 8 |
|
2 | 67 |
|
3 | 27 |
| Nuclear Grade |
|
1 | 2 |
|
2 | 65 |
|
3 | 35 |
For the in vitro studies, the
hormone-sensitive breast cancer cell line, MCF-7, and the
triple-negative MDA-MB-231 breast cancer cell line were used for
silencing SUV420H2 expression. Cells with low passage numbers were
grown in standard conditions (37˚C with 5% CO2 in a
humidified incubator) in DMEM (Biochrom, Ltd.), containing 10% FBS
(Biochrom, Ltd.) and penicillin and streptomycin.
SUV420H2 knockdown in breast cancer
cells
The effect of SUV420H2 knockdown on the survival of
breast cancer cells was assessed. For silencing experiments,
commercially available small interfering (si)RNA oligonucleotides
(SMARTpool siRNA; GE Healthcare Dharmacon, Inc.) targeting four
different exons of the SUV420H2 gene (SUV420H2-siRNA) were used.
Scramble siRNA was used as the negative control (nc-siRNA). The
sequences of the siRNAs targeting SUV420H2 were:
5'-GUGAAGGUGCUCCGGGACA-3', 5'-GCGGUGAAGAGCUGUGACA-3',
5'-CGACAGAGUGACAGCACGA-3', and 5'-CUCAGCGCUGGAAACUUU-3'. The
sequence of the scrambled siRNA was
5'-GCACGCUCCUACGAAUGCUAGUAAA-3'. A total of 40 h after seeding,
~2x105 cells were transfected using the cationic
lipid-based commercial transfection agent Lipofectamine
2000® (Thermo Fisher Scientific, Inc.), according to the
manufacturer's protocol. Cells were incubated under standard
culture conditions for 48-72 h, after which, cells were harvested
and stored at -80˚C until further use. SUV420H2 gene expression in
transfected cells was analyzed as described below.
Real-time analysis of cell
proliferation
Real-time analysis of cell proliferation was
performed using an iCELLigence system (Roche Diagnostics GmbH). For
analysis, ~2x104 cells were seeded into each well of the
e-plate following baseline measurements using culture medium. siRNA
transfection was performed as described above. The proliferation
kinetics of the cells was monitored for up to 72 h after
transfection.
Colony formation assays
The colony forming capacity of breast cancer cells
was studied as a further measure of the effect of SUV420H2
knockdown. For this assay, 1x104 cells were seeded into
each well of 6-well culture plates. Transfection was performed as
described above, and cells were grown for 10 days under standard
culture conditions. At the end of incubation period, the medium was
removed, and cells were washed with PBS. Cells were fixed with
methanol (100%) for 20 min at room temperature and stained with
crystal violet for 30 min at room temperature. After washing, the
plates were allowed to dry overnight. Subsequently, the number of
colonies formed were counted under a light microscope
(magnification, x40; Jenaval, Carl Zeiss AG).
Measurement of SUV420H2 expression in
cultured cells and breast tissues
SUV420H2 expression was analyzed at the mRNA level.
Total RNA was isolated from cells or tissues using
TRIpure® RNA Isolation solution (Sigma-Aldrich; Merck
KGaA) according to the manufacturer's protocol. Extracted RNA was
diluted in a final volume of 30 µl using RNase-free water and
stored at -80˚C following assessment of integrity using agarose gel
electrophoresis on a 2% gel and spectrophotometric purity analysis
(Varioskan Flash; Thermo Fisher Scientific Inc.). cDNA synthesis
was performed using the commercially available RevertAid First
Strand cDNA Synthesis kit (Thermo Fisher Scientific Inc.) according
to the manufacturer's protocol. cDNA samples were stored at -20˚C
until further use.
SUV420H2 expression was determined
semi-quantitatively using GAPDH as the reference gene. SYBR-Green
(Thermo Fisher Scientific Inc.) was used as the fluorescent
molecule for quantitative PCR. The sequences of the primers used
were: SUV420H2 forward, 5'-GGCCCGCTACTTCCAGAG-3' and reverse,
5'-GCAGGATGGTAAAGCCACTT-3'; and GAPDH forward,
5'-GCTCTCTGCTCCTCCTGTTC-3' and reverse, 5'-ACGACCAAATCCGTTGACTC-3'.
Measurements were performed twice in a LightCycler 480 instrument
(Roche Diagnostics GmbH), and relative SUV420H2 expression was
calculated using the 2-ΔΔCq method (24).
Quantification of H4K20me3 levels in
tissue samples
For H4K20me3 measurement, histone proteins were
extracted from the tissues using the commercially available EpiQuik
Total Histone Extraction kit (Epigentek Group, Inc.). Briefly, 1 ml
1X lysis buffer (Epigentek Group, Inc.) was added to tissue lysates
and centrifuged at 9,500 x g for 1 min at 4˚C. Supernatant was
removed, and 200 µl lysis buffer was added to the pellet containing
the cell nuclei and incubated for 30 min on ice. This was followed
by a further centrifugation step (5 min, 12,000 rpm, 4˚C). The
supernatant containing histone proteins was transferred to fresh
tubes and 60 µl Balanced-DTT buffer (Epigentek Group, Inc.) was
added. Quantities of histone proteins were spectrophotometrically
measured using Varioskan (Thermo Fisher Scientific, Inc.) at 280 nm
using FBS as a standard. The purity of the samples was evaluated by
measuring the 260/280 nm ratio. Histones were aliquoted and stored
at -80˚C for subsequent use.
Extracted histones were used to measure H4K20me3
levels. An ELISA-like measurement of H4K20me3 was performed using
the EpiQuik Global Tri-Methylation Histone H4K20 Quantification kit
(cat. no. P-3068-98; Epigentek Group, Inc.) according to the
manufacturer's protocol. For measurement, 200 ng histone proteins
was applied, and measurements were performed twice at 450 nm.
H4K20me3 quantities were relatively calculated as the measured
absorbance values, and were directly proportional to the amount of
the modification.
Statistical analysis
The numerical data from the in vitro tests
were compared using a Student's t-test, and the data are presented
as the mean ± standard deviation. SUV420H2 and H4K20me3 levels in
tumor tissues and non-cancerous regions were compared using a
paired samples t-test, and the data are presented as box plots.
Statistical analysis of SUV420H2 and H4K20me3 in the patient
subgroups was performed using a Kruskal-Wallis test and a
χ2 test. Kaplan-Meier analysis and log rank tests were
used to assess the survival curves and prognostic value of SUV420H2
expression in breast cancer. P<0.05 was considered to indicate a
statistically significant difference. Statistical analysis was
performed using SPSS version 18 (SPSS, Inc.).
Results
SUV420H2 knockdown in breast cancer
cells results in stimulation of cell proliferation
Knockdown experiments in MCF-7 and MDA-MB-231 cells
were used to reduce the gene expression levels of SUV420H2.
Compared with cells transfected with nc-siRNA, SUV420H2 expression
was downregulated 45% in mean in SUV420H2-silenced MCF-7 cells
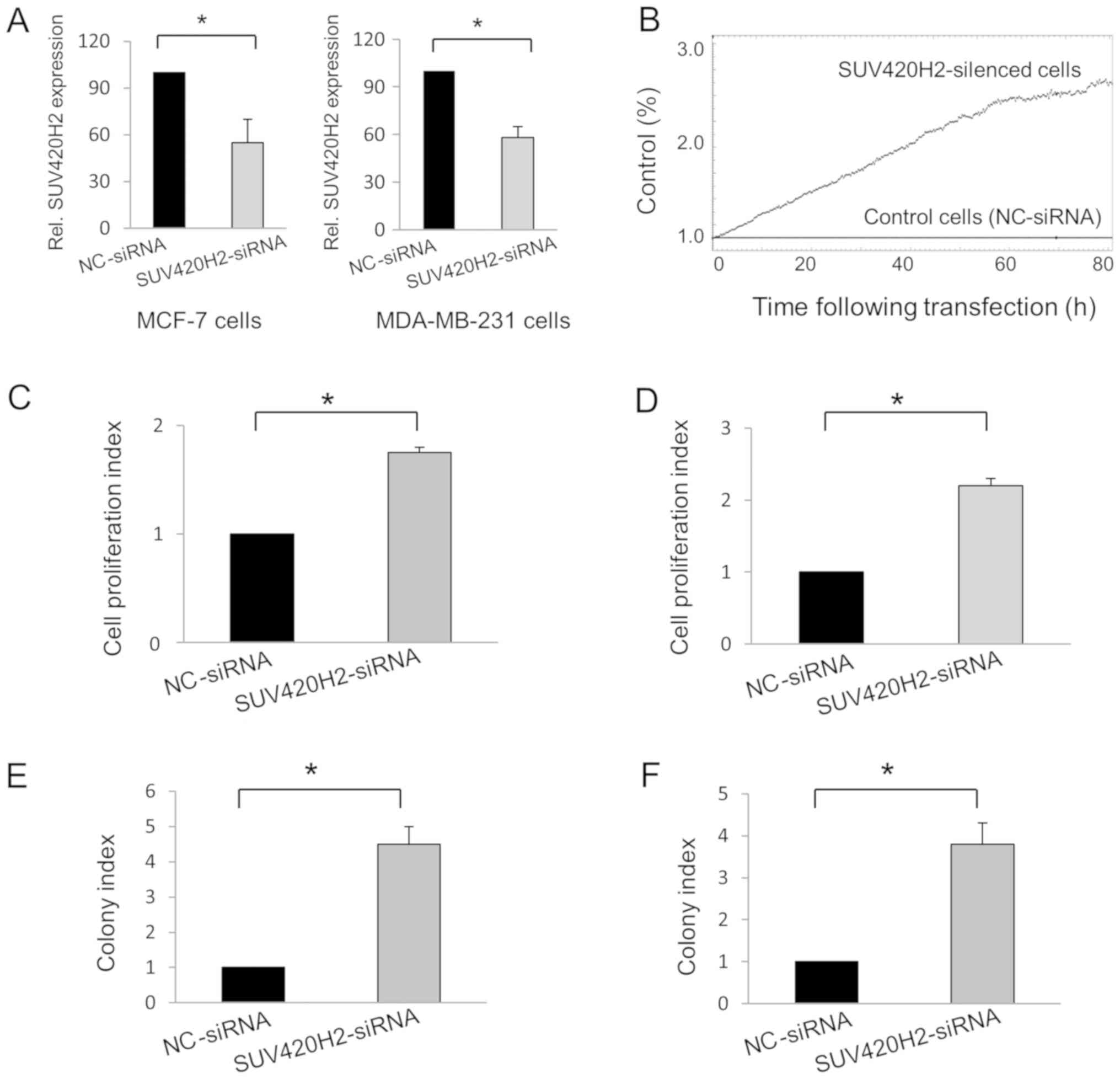
(P=0.048) and 42% in MDA-MB-231 cells (P=0.01; Fig. 1A). The effect of SUV420H2 knockdown
on cell proliferation relative to the control cells was assessed in
real-time using the iCELLigence instrument (Fig. 1B). Knockdown of the SUV420H2 gene in
both cell types resulted in increased cell proliferation compared
with nc-siRNA-transfected MCF-7 (Fig.
1C) and MDA-MB-231 cells (Fig.
1D). The colony formation assay also showed that SUV420H2
knockdown resulted in cell proliferation; the number of colonies
were increased in the SUV420H2-kncokdown cells compared with the
nc-siRNA-transfected cells (Fig. 1E
and F).
SUV420H2 gene expression status in
breast tumor relative to the non-cancerous tissues does differ
significantly
Compared with the reference gene, the expression
levels of SUV420H2 were relatively low in breast tissue (both
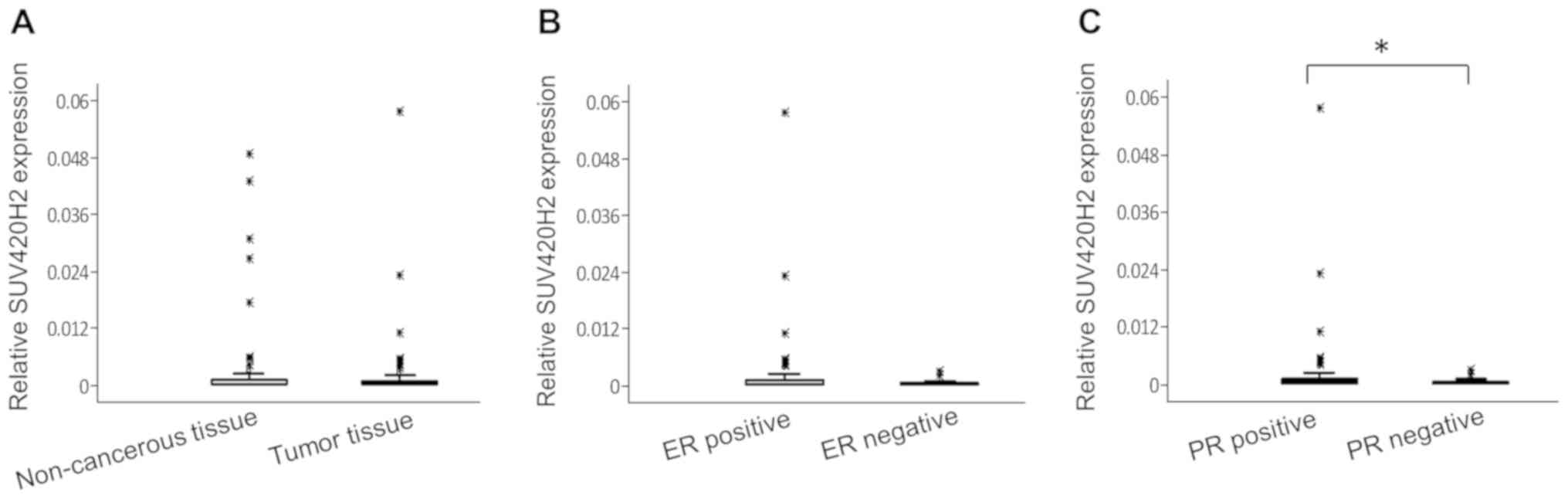
non-cancerous tissue and tumor tissue) (Fig. 2A). The relative median values of
SUV420H2 expression in non-cancerous regions and tumor tissues were
similar (0.0015 and 0.0022, respectively; P=0.46). There were no
association between SUV420H2 expression and clinical parameters
(data not shown) except for hormone receptor status; SUV420H2
expression was higher in hormone receptor-positive tumors. The
median SUV420H2 expression was 2.4-fold higher in ER+ tumors
(Fig. 2B; P=0.12) and 2.8-fold
higher in PR+ tumors (Fig. 2C;
P=0.047) compared with hormone receptor-negative tumors.
Although the median SUV420H2 expression was similar
in tumor tissues and non-cancer regions in the entire cohort, there
were notable differences between tumor tissue and adjacent
non-cancerous regions in the majority of individual patients.
SUV420H2 expression in tumor tissue significantly increased (at
least 2.5-fold) in 31 patients out of 102 (30%) compared with
non-cancerous regions, whereas a notable decrease of SUV420H2
expression (by at least 2.5-fold) in tumor tissue was observed in
30 patients (29%). Interestingly, the patients in whom SUV420H2
expression was higher in the tumor compared with the non-cancerous
tissue tended to have early-stage breast cancer, whereas in the
group with a decrease of SUV420H2 expression in tumors was
observed, the patients frequently presented with more advanced
staged cancer, with significant differences for both nodal status
and disease stage (Table II).
 | Table IIStatus of the SUV420H2 expression in
the breast tumors. |
Table II
Status of the SUV420H2 expression in
the breast tumors.
| Nodal status | | Stage | |
|---|
| Change in SUV420H2
expressiona | N0 | N≥1 | P-value | I-II | III | P-value |
|---|
| Increased,
n=31 | 20 | 11 | 0.001c | 27 | 4 | 0.02b |
| Decreased
(n=30) | 7 | 23 | | 18 | 12 | |
SUV420H2 gene expression is not
associated with H4K20me3 levels
H4K20me3 levels in breast tumor tissues and adjacent
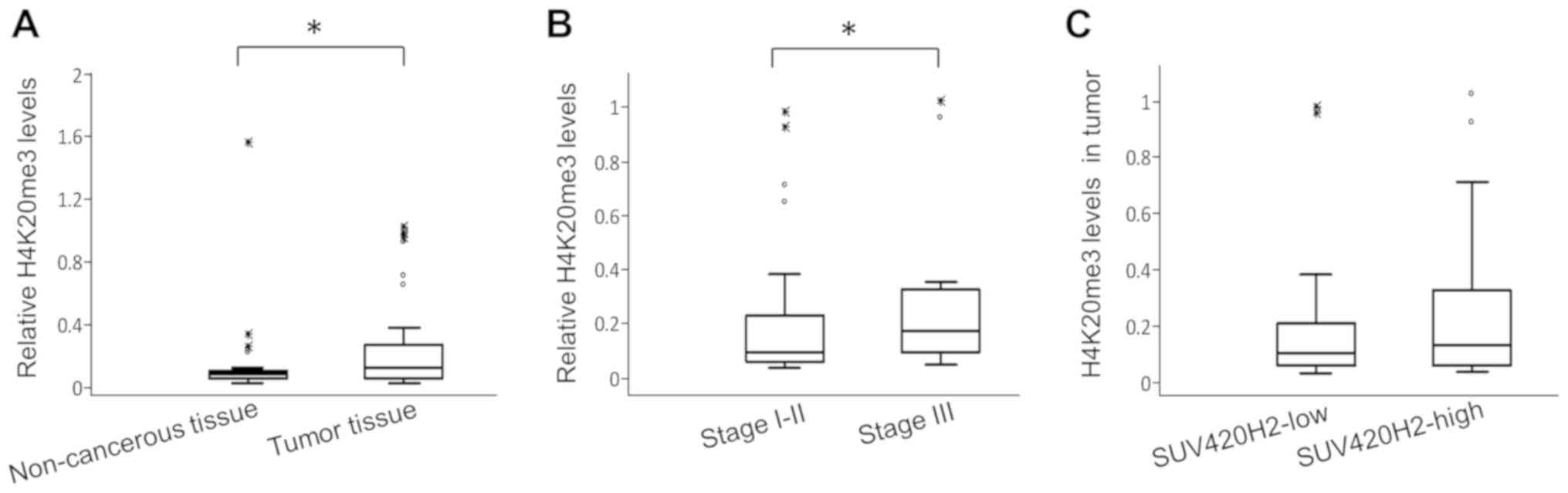
non-cancerous regions were also assessed. The relative quantities
of H4K20me3 in tumor tissues were significantly higher compared
with the adjacent non-cancerous regions (median values 0.1124 and
0.087, respectively; P=0.001; Fig.
3B). Patients with stage III breast cancer had 2-fold higher
H4K20me3 levels in their tumors compared with patients with stage
I-II (relative median levels 0.17 vs. 0.88, respectively; P=0.04;
Fig. 3B).
There was no association between SUV420H2 expression
and H4K20me3 levels in tumors. Accordingly, H4K20me3 levels were
similar (0.1 vs. 0.13, respectively; P=0.68) in patients with lower
SUV420H2 expression (below median) and in those with high
expression (above median) in tumors (Fig. 3C). Similarly, patients in whom
SUV420H2 expression was increased in tumors compared with adjacent
non-cancerous regions had similar H4K20me3 levels in their tumor
tissues to those in whom SUV420H2 expression was decreased in
tumors.
SUV420H2 gene expression does not
exhibit prognostic value in patients with breast cancer
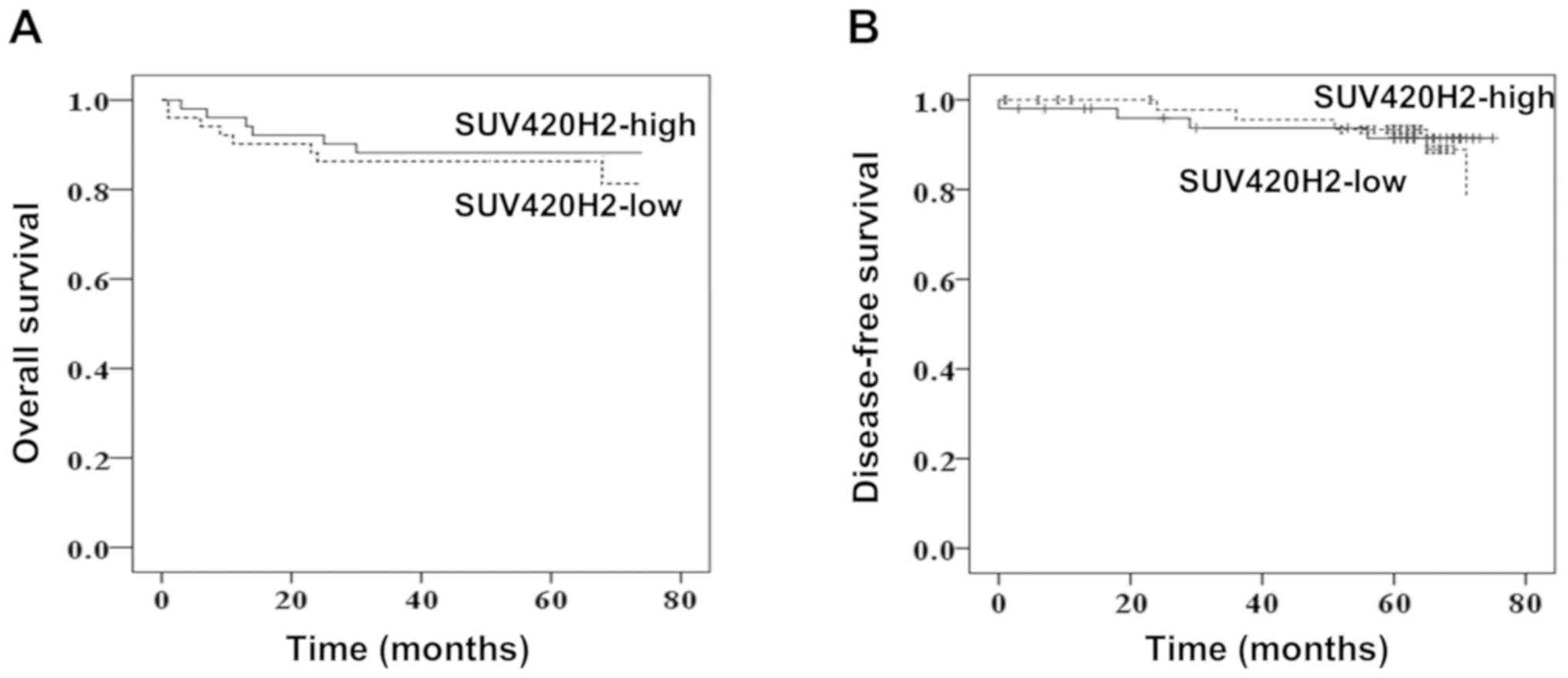
The patients were followed up for 75 months. The
overall survival (OS) and disease-free survival (DFS) were compared
between the low SUV420H2-expressing group and high
SUV420H2-expressing group (stratified by the median value). Median
OS times were 63 and 65 months for low and high expression groups,
respectively (P=0.734; Fig. 4A).
Similarly, DFS was very similar for both expression groups (P=0.86;
Fig. 4B) indicating that the
SUV420H2 expression in tumor tissues had no prognostic value.
Discussion
The experimental evidence in the present study and
previously published data suggest that SUV420H2 suppresses the
proliferation, migration and invasiveness of breast cancer cells
(20). However, SUV420H2 was found
to be an epigenetic regulator of epithelial-to-mesenchymal
transition in pancreatic cancer cells (25), suggesting that the role of SUV420H2
in tumor progression may be cell-type specific.
Data on the expression status of SUV420H2 in cancer
in general and in breast cancer are limited (11,20,25),
despite the well-defined tumor suppressive role of SUV420H2 in
breast cancer cells in vitro (11,20). In
the present study, SUV420H2 expression in breast cancer was
examined and found similar levels of SUV420H2 expression in the
entire cohort in paired samples comparison between tumor tissues
and adjacent non-cancerous tissues. In individual patients however,
a heterogeneous pattern of SUV420H2 expression status in tumors
relative to non-cancerous regions was observed. In patients with
early-stage breast tumors, SUV420H2 expression in tumor relative to
adjacent non-cancerous regions was frequently higher. In contrast,
in patients with larger tumors and/or lymphatic metastasis,
SUV420H2 expression in tumor tissues relative to non-cancerous
tissues tended to decrease. These results suggest that SUV420H2
expression decreases as breast cancer progresses. Data retrieved
from databases such as The Cancer Genome Atlas (TCGA), Gene
Expression Omnibus (GEO), and Methylation and Expression database
of Normal and Tumor tissues (MENT) revealed that SUV420H2
expression is lower in breast cancer tissues compared with normal
tissues (20). In contrast to the
present study, the previous study was not a paired samples
comparison of tumors with adjacent non-cancerous tissues in
individual patients. These data may thus be summarized by stating
that in a substantial portion of patients with breast cancer,
SUV420H2 expression is decreased in breast tumors compared with
normal tissue, and the loss of SUV420H2 expression may be an
indicator of breast cancer progression. A limitation of the present
study was that the protein expression levels of SUV420H2 were not
assessed, which may have provided additional information on its
role in tumor development and progression. Based on TCGA data,
SUV420H2 expression was found to be increased in tumor tissues in
pancreatic cancer compared with equivalent normal tissues, and high
levels of SUV420H2 were correlated with a loss of epithelial
characteristics in progressively invasive cancer (25). This contradictory state compared with
breast cancer implies a heterogeneous role of SUV420H2 in cancer
development and progression.
There was no significant association between
expression of SUV420H2 and the age, menopausal status, tumor stage,
lymphatic metastasis, histologic grade or nuclear grade of the
patients. However, there was an association between SUV420H2
expression and the hormone receptor status. SUV420H2 expression was
higher in patients with hormone receptor-positive tumors compared
with hormone receptor-negative tumors. The functional basis of this
association and its relationship with prognosis should be
investigated in future studies.
Based on the in vitro data and tissue
analysis, the loss of H4K20me3 in tumor tissues has been described
as a hallmark of cancer (10-13).
Similar to the heterogeneity in SUV420H2 expression in breast
cancer, the H4K20me3 expression pattern in breast tumors seems to
be heterogeneous. Elsheikh et al (26) reported in their immunohistochemical
study, which consisted of a large series of breast tumors, that
H4K20me3 expression was increased in breast tumors in 70% of
patients with invasive breast carcinoma compared with normal
tissue, whereas it was decreased in the remaining 30%. Yokoyama
et al (11) also described a
heterogeneous pattern of H4K20me3 levels in breast tumors using
immunohistochemistry, where the reduction of H4K20me3 in tumors
compared with non-cancerous regions was more frequently observed.
In the present study, the status of H4K20me3 in tumor tissue
relative to non-cancerous tissue among the patients was also
heterogeneous, although an increase was more frequently observed.
It is possible that the existing heterogeneity in H4K20me3
expression in tumors may be amplified by variations in
methodologies. In the present study, H4K20me3 levels were measured
on extracted histone proteins, whereas previous studies used
immunohistochemistry. The assessment of H4K20me3 by
immunohistochemistry may be a more informative method of assessing
distribution morphologically in tumor tissue.
There was no prognostic predictive role for SUV420H2
expression in breast cancer identified in the present study, based
on a 75 month follow-up period. However, longer follow-up times are
required to more accurately evaluate the prognostic role of
SUV420H2 expression in breast cancer.
In conclusion, the results of the present study
indicate that the methyltransferase SUV420H2, which exhibits
anti-tumor activity in vitro, is heterogeneously expressed
in breast tumors relative to matched non-cancerous regions of the
breast. The reduction of SUV420H2 in tumors relative to
non-cancerous regions was apparently more frequent in patients with
more advanced disease, which suggested that tumor cells which
downregulated SUV420H2 expression during progression were more
likely to survive or proliferate. These data may provide a basis
for further analysis of SUV420H2 in future studies examining the
potential SUV420H2 as a target for therapeutic interventions in
patients with breast cancer.
Acknowledgements
The present study forms part of a Ph.D. thesis of Ms
Hüsniye Isin (Istanbul University).
Funding
The present study was supported by the Scientific
Research Projects Coordination Unit of Istanbul University (grant
no. TDK-2017-27336).
Availability of data and materials
The datasets used and/or analyzed during the present
study and patient consent are available from the corresponding
author on reasonable request.
Authors' contributions
HI and UG were responsible for the conception and
design of the study. CKT, DCT and DK were involved in the provision
of study materials and clinical data, and critically revised the
manuscript. HI and EÖ analyzed and interpreted the data as well as
drafted the manuscript. All authors read and approved the final
version of the manuscript for publication.
Ethics approval and consent to
participate
The present study was approved both by the Clinical
Research Ethics Committee of Istanbul University (approval no.
2017/887) and the Ethics Committee of Istanbul Training and
Research Hospital (approval no. 2012/1413-1201), (Istanbul,
Turkey). All patients provided informed written consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Harbeck N, Penault-Llorca F, Cortes J,
Gnant M, Houssami N, Poortmans P, Ruddy K, Tsang J and Cardoso F:
Breast cancer. Nat Rev Dis Primers. 5(66)2019.
|
|
2
|
Dogan N and Toprak D: Female breast cancer
mortality rates in Turkey. Asian Pac J Cancer Prev. 15:7569–7573.
2014.
|
|
3
|
Torre LA, Siegel RL, Ward EM and Jemal A:
Global cancer incidence and mortality rates and trends-an update.
Cancer Epidemiol Biomarkers Prev. 25:16–27. 2016.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Sauter ER: Reliable biomarkers to identify
new and recurrent cancer. Eur J Breast Health. 13:162–167.
2017.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Zhao Z and Shilatifard A: Epigenetic
modifications of histones in cancer. Genome Biol. 20(245)2019.
View Article : Google Scholar
|
|
6
|
Miller JL and Grant PA: The role of DNA
methylation and histone modifications in transcriptional regulation
in humans. Subcell Biochem. 61:289–317. 2013.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Wang Y and Jia S: Degrees make all the
difference: The multifunctionality of histone H4 lysine 20
methylation. Epigenetics. 4:273–276. 2009.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Svobodová Kovaříková A, Legartová S,
Krejčí J and Bártová E: H3K9me3 and H4K20me3 represent the
epigenetic landscape for 53BP1 binding to DNA lesions. Aging
(Albany NY). 10:2585–2605. 2018.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Jørgensen S, Schotta G and Sørensen CS:
Histone H4 lysine 20 methylation: Key player in epigenetic
regulation of genomic integrity. Nucleic Acids Res. 41:2797–2806.
2013.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Tryndyak VP, Kovalchuk O and Pogribny IP:
Loss of DNA methylation and histone H4 lysine 20 trimethylation in
human breast cancer cells is associated with aberrant expression of
DNA methyltransferase 1, Suv4-20h2 histone methyltransferase and
methyl-binding proteins. Cancer Biol Ther. 5:65–70. 2006.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Yokoyama Y, Matsumoto A, Hieda M, Shinchi
Y, Ogihara E, Hamada M, Nishioka Y, Kimura H, Yoshidome K,
Tsujimoto M and Matsuura N: Loss of histone H4K20 trimethylation
predicts poor prognosis in breast cancer and is associated with
invasive activity. Breast Cancer Res. 16(R66)2014.PubMed/NCBI View
Article : Google Scholar
|
|
12
|
van Nuland R and Gozani O: Histone H4
lysine 20 (H4K20) methylation, expanding the signaling potential of
the proteome one methyl moiety at a time. Mol Cell Proteomics.
15:755–764. 2016.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Fraga MF, Ballestar E, Villar-Garea A,
Boix-Chornet M, Espada J, Schotta G, Bonaldi T, Haydon C, Ropero S,
Petrie K, et al: Loss of acetylation at Lys16 and trimethylation at
Lys20 of histone H4 is a common hallmark of human cancer. Nat
Genet. 37:391–400. 2005.PubMed/NCBI View
Article : Google Scholar
|
|
14
|
Füllgrabe J, Kavanagh E and Joseph B:
Histone onco-modifications. Oncogene. 30:3391–3403. 2011.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Sakabe K, Wang Z and Hart GW:
Beta-N-acetylglucosamine (O-GlcNAc) is part of the histone code.
Proc Natl Acad Sci USA. 107:19915–19920. 2010.PubMed/NCBI View Article : Google Scholar
|
|
16
|
West AC and Johnstone RW: New and emerging
HDAC inhibitors for cancer treatment. J Clin Investig. 124:30–39.
2014.PubMed/NCBI View
Article : Google Scholar
|
|
17
|
Shanmugam MK, Arfuso F, Arumugam S,
Chinnathambi A, Jinsong B, Warrier S, Wang LZ, Kumar AP, Ahn KS,
Sethi G and Lakshmanan M: Role of novel histone modifications in
cancer. Oncotarget. 19:11414–11426. 2017.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Noberini R, Osti D, Miccolo C, Richichi C,
Lupia M, Corleone G, Hong SP, Colombo P, Pollo B, Fornasari L, et
al: Extensive and systematic rewiring of histone post-translational
modifications in cancer model systems. Nucleic Acids Res.
46:3817–3832. 2018.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Simpson NE, Tryndyak VP, Beland FA and
Pogribny IP: An in vitro investigation of metabolically sensitive
biomarkers in breast cancer progression. Breast Cancer Res Treat.
133:959–968. 2012. View Article : Google Scholar
|
|
20
|
Shinchi Y, Hieda M, Nishioka Y, Matsumoto
A, Yokoyama Y, Kimura H, Matsuura S and Matsuura N: SUV420H2
suppresses breast cancer cell invasion through down regulation of
the SH2 domain-containing focal adhesion protein tensin-3. Exp Cell
Res. 334:90–99. 2015.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Lakhani SR, Ellis IO, Schnitt SJ, Tan PH
and van de Vijver MJ (eds): WHO classification of tumours of the
breast. In: WHO Classification of Tumours. Vol 4. 4th edition.
IARC, Lyon. 2012.
|
|
22
|
Elston CW and Ellis IO: Pathological
prognostic factors in breast cancer. I. The value of histological
grade in breast cancer: Experience from a large study with
long-term follow-up. Histopathology. 19:403–410. 1991.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Singletary SE, Allred C, Ashley P, Bassett
LW, Berry D, Bland KI, Borgen PI, Clark G, Edge SB, Hayes DF, et
al: Revision of the American Joint Committee on Cancer staging
system for breast cancer. J Clin Oncol. 20:3628–3636.
2002.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Viotti M, Wilson C, McCleland M, Koeppen
H, Haley B, Jhunjhunwala S, Klijn C, Modrusan Z, Arnott D, Classon
M, et al: SUV420H2 is an epigenetic regulator of
epithelial/mesenchymal states in pancreatic cancer. J Cell Biol.
217:763–777. 2018.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Elsheikh SE, Green AR, Rakha EA, Powe DG,
Ahmed RA, Collins HM, Soria D, Garibaldi JM, Paish CE, Ammar AA, et
al: Global histone modifications in breast cancer correlate with
tumor phenotypes, prognostic factors, and patient outcome. Cancer
Res. 69:3802–3809. 2009.PubMed/NCBI View Article : Google Scholar
|