Introduction
Alzheimer's disease (AD), one of the most common
forms of dementia, is caused by neuronal damage in the hippocampus
and other brain regions that results in a decline in learning and
memory (1,2). For millions of individuals worldwide,
AD treatment represents a large economic burden (3). Additionally, the number of cases is
estimated to increase dramatically due to increasing lifespans and
the lifestyle changes (3,4).
A previous study determined that AD severity is
associated with cholinergic neuron loss and markedly decreased
levels of acetylcholine (ACh) in the brain (5). The cholinergic system serves an
important role in the central and peripheral control of multiple
cognitive processes (6). ACh is
synthesized by choline acetyltransferase (ChAT) in cholinergic
neurons and is hydrolyzed by acetylcholinesterase (AChE) (6). The decreased release of ACh following
cholinergic neuron loss results in learning deficits. When AD
occurs, cholinergic neurons in the hippocampus and prefrontal
cortex are lost, resulting in the decreased synthesis, storage and
release of Ach (7). This leads to a
variety of clinical manifestations, primarily being memory and
cognitive impairment (7). In the
brains of patients with AD, ChAT activity, a rate-limiting enzyme
for ACh production in the cholinergic system of the frontal cortex
and hippocampus, decreases significantly (8). Additionally, AD is associated with
oxidative stress, inflammation and hyperhomocysteinemia (8).
It was reported that microglia and astrocytes
release large numbers of pro-inflammatory cytokines,
anti-inflammatory cytokines, chemokines and other related factors,
including interleukin (IL)-1β, IL-10 and monocyte chemoattractant
protein-1(9). IL-1β is a key
regulator of neuroinflammation and is considered to be a potential
genetic risk factor of dementia (10). It was observed that increased levels
of IL-1β occur in the serum and cerebrospinal fluid of patients
with AD and dementia (10). Elevated
IL-1β also activates the microglia and astrocytes to further
increase levels of cytokines and free radicals, leading to
neurotoxicity (10). In patients
with AD, levels of various oxidation products, including sugar,
protein, lipid, nucleic acid and other macromolecule substances,
are significantly increased in the cerebrospinal fluid and blood
(11). Furthermore, markers of
oxidative stress are increased in the urine of patients with AD
(11). Although the brain accounts
for 2% of total body mass, oxygen consumption accounts for 25%
(11). Therefore, the brain is more
sensitive to oxidative stress compared with other organs (8). A previous study revealed that oxidative
stress serves an important role in the pathogenesis of AD (12). Excessive oxidative stress can result
in lipid peroxidation on the membrane of nerve cells or organelles,
and the destruction of protein nitration and nucleic acid,
affecting the synaptic ability of nerve cells and leading to
neuronal apoptosis (11).
The aerial parts of Polygala tenuifolia Willd
(APT) is widely used in China as a traditional medicine for
expectorant, sedative, anti-aging and anti-inflammatory properties
(13). APT was first recorded in
Divine Farmer's Classic of Materia Medica for the treatment
of amnesia (13). The Clinical
Manual of Traditional Chinese Medicine indicated that the medicinal
sections of APT are the stem and leaves, which are prescribed using
the name ‘Xiaocao’ (13). APT
contains various active ingredients, including flavonoids and
phenolic glycosides, which were reported to serve anti-inflammatory
and anti-oxidative effects (14,15).
However, whether APT improves learning and memory in AD animal
models is yet to be elucidated. Therefore, the current study aimed
to investigate the ameliorating effects of APT extract on
scopolamine-induced learning and memory impairment in mice. Morris
water maze and step-down passive avoidance tests were implanted to
achieve aims and explore potential mechanisms.
Materials and methods
Animals
Adult male Kunming mice (20±2 g) 12 weeks old were
purchased from the Experimental Animal Center of Xi'an Jiaotong
University (Xi'an, China) and maintained in specific pathogen-free
conditions with free access to standardized feed and water. Animals
were additionally housed at 23±2˚C with a relative humidity of
40-60% under a 12-h light/dark cycle. Animal experiments were
approved by the Ethics Committee of Northwest University (Xi'an,
China) and all efforts were made to minimize animal suffering and
the number of rats used for experimentation. Ten mice were used in
each group and a total of 60 mice were used in the experiments.
Animal health and behavior were monitored daily.
Chemicals and drugs
Scopolamine hydrobromide (Scop; cat. no. 1601211)
was purchased from Suicheng Pharmaceutical Co., Ltd. ELISA kits for
ChAT (cat. no. 20171211), Ach (cat. no. 20170926) and AChE (cat.
no. 20171027), brain-derived neurotrophic factor (BDNF; cat. no.
20171106), IL-10 (cat. no. 20171113) and IL-1β (cat. no. 20171113)
were purchased from Yuduo Biological Technology Co., Ltd.
Malondialdehyde (MDA; cat. no. 20171201), superoxide dismutase
(SOD; cat. no. 20171201), glutathione (GSH; cat. no. 20171201) kits
were obtained from the Nanjing Jiancheng Bioengineering Institute.
Bicinchoninic acid protein assay kits (BCA; cat. no. 12G28C46) were
purchased from Wuhan Boster Biological Technology Co., Ltd.
Piracetam (cat. no. 2160402) were obtained from Renfu
Pharmaceutical Co., Ltd.
Extract preparation
APT were collected from Chengcheng County (Shaanxi,
China) in June of 2017 and authenticated by Professor MF Fang of
Northwest University (Xi'an, China). APT (500 g) was ultrasonically
extracted in triplicate with 50% (v/v) ethanol ten times. The
filtrate was subsequently mixed and concentrated in a vacuum at
50-60˚C using a rotary evaporator. The dried residue obtained a
yield of 125.5 g, which was suspended in saline and subjected to
in vivo experiments. The normal dose of aerial parts of
Polygala tenuifolia Willd for human adults is 3.00 g/day
(16). Equivalently, the calculated
dose of aerial parts of Polygala tenuifolia Willd based on
respective body surface areas for mice is 0.4 g/kg/day. The
extraction rate of Polygala tenuifolia Willd is 25%, and so
the dose of aerial parts extract of Polygala tenuifolia
Willd for mice is 100 mg/kg/day. Therefore, 100 mg/kg/day was
chosen as the high dose, 50 mg/kg/day as the middle dose, and 25
mg/kg/day as the low dose in the present study.
Experimental design
Piracetam, a derivative of γ-aminobutyric acid, is a
new type of memory promoting drug which is used to treat memory
impairment and moderate brain dysfunction (17) and is selected as positive drug in the
present study. After 7 days of acclimatization, mice were randomly
divided into the following six groups (n=10): The Scop group [2
mg/kg/day; intraperitoneal (ip) Scop] (18); the Piracetam (Pir) group [2 mg/kg/day
ip Scop and 750 mg/kg/day rally administered (ig) Pir] (19); the APT-25, APT-50 and APT-100 groups,
which were treated with Scop (2 mg/kg/day ip) and 25, 50 and 100
mg/kg/day (ig) APT, respectively; and the normal control (NC)
group, which was treated with 5 ml/kg saline. Apart from the NC
group, mice were injected ip with 2 mg/kg Scop for 1 h following
administration of APT. Behavioral testing started 30 min following
ip injection of Scop. The therapeutic drug of APT or Pir was
administered for 14 days and Scop was continuously administered ip
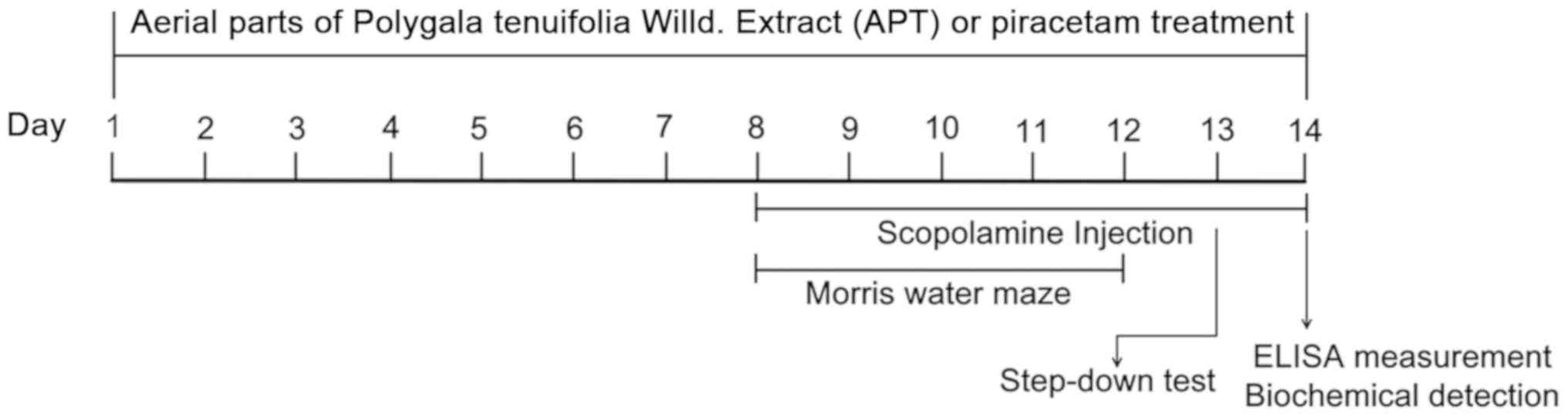
from days 8 to 14 (Fig. 1).
After 14 days of APT administration, the spatial
learning and memory of Scop-induced mice was assessed via Morris
water maze and step-down tests. Following behavioral assessment,
pentobarbital sodium (200 mg/kg body weight, ip) was used for
euthanasia, which was approved by the Ethical Committee of
Northwest University. When the animal has no breath and no
heartbeat, the brains were removed for biochemical analysis. The
hippocampus and prefrontal cortex were dissected and stored at
-80˚C until protein and ELISA analysis. The timeline of the present
study is presented in Fig. 1.
Morris water maze test
A Morris water maze test was performed 30 min
following Scop injection to evaluate memory-related behaviors. A
large circular tank (diameter, 150 cm; height, 35 cm) was filled
with water to a depth of 20 cm and maintained at a temperature of
25±2˚C. Ink was added into the water to prevent mice from seeing
the platform. The platform (10x10 cm) was fixed and hidden 2 cm
below the water surface. During the acquisition-testing phase, mice
completed four trials per day for four consecutive days during
which they were left to locate the submerged platform. During these
four consecutive days, starting positions were randomized for all
mice. The mouse was permitted to rest on the platform for 10 sec,
following which it was required to successfully locate the platform
within 120 sec. The time between the first placement in water to
platform location was recorded as the escape latency. If the mouse
failed to find the platform within the permitted time, escape
latency was recorded as 120 sec, after which the mouse was
physically placed on the platform for 10 sec. On day 5, the
retention of spatial reference memory was recorded by a probe trial
with the platform being removed from the pool. The number of
platform crossings in the target quadrant was recorded as indices
of spatial memory and the mice were allowed to swim freely for 120
sec.
Step-down test
After the Morris water maze test, the step-down test
was performed. Six plastic boxes (15x15x40 cm3) with a
high platform (diameter, 4.5 cm; height, 4.5 cm) were used for
experimentation. Parallel caliber stainless steel bars were
installed to produce a mild shock on the floor of the plastic box.
All mice received training before the test and were allowed to
acclimatize for the first 3 min. Mice were placed on the platform
and received continuous shocks (36 V) for 5 min as soon as they
stepped down on the grid and floor. Mice would then step up to the
platform. All animals underwent the same experimental procedure
after 24 h, during which the frequency of steps down to the grid
floor and the time from the first step down to the platform were
recorded. Results were obtained within 300 sec.
Determination of ACh, AChE, ChAT, BDNF
and IL-1β levels in the hippocampus and frontal cortex
Levels of ACh, AChE, ChAT, BDNF, IL-10 and IL-1β
were measured using ELISA. All ELISA experimental procedures were
performed in accordance with the manufacturer's protocol (Yuduo
Biotech). Absorbance was measured using a microtiter plate reader
(PerkinElmer, Inc.) at a wavelength of 450 nm. Levels of indicators
in the hippocampus and cortex were normalized to protein per mg,
and expressed as per mg protein.
Determination of MDA and GSH levels
and SOD activity in murine brains
Brain tissue was homogenized in ice-cold 0.9% saline
solution and centrifuged at 600 x g for 10 min at 4˚C. The
resulting supernatant was used to determine SOD, MDA and GSH levels
using biochemical kits (Nanjing Jiancheng Bioengineering Institute)
according to the manufacturer's protocol. The results of MDA, GSH
and SOD activity were normalized to protein content.
Statistical analysis
All results were expressed as the mean ± SD and data
were analyzed using SPSS 24.0 (IBM Corp.). Datasets with multiple
comparisons were evaluated using one-way ANOVA followed by Tukey's
post hoc test. P<0.05 was considered to indicate a statistically
significant difference.
Results
Morris water maze test
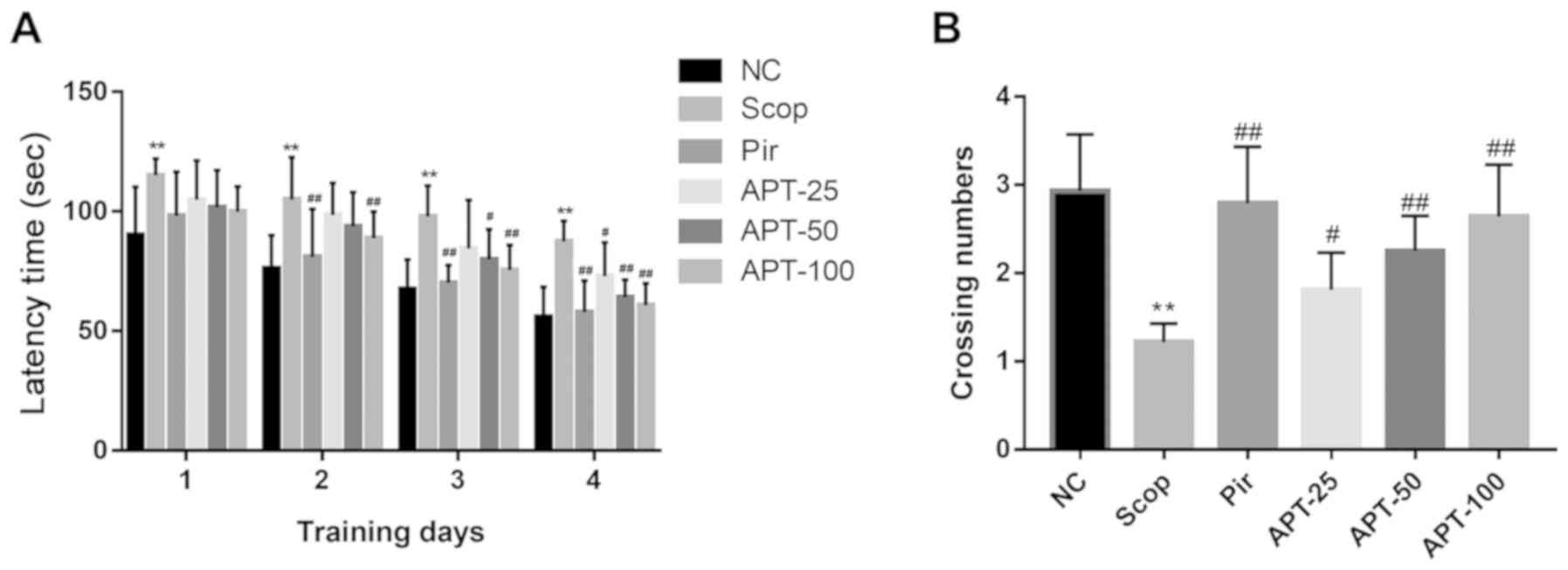
As shown in Fig. 2A,
the escape latencies of all groups gradually decreased over the
course of acquisition testing, indicating that all animals were
able to locate the platform. Compared with the NC group, the Scop
group demonstrated a significant increase in escape latency over
the four training days (P<0.01). On the second day of training,
when compared with the Scop group (105.08±17.58 sec), the Pir group
(81.12±19.99 sec) exhibited a significantly decreased escape
latency (P<0.01). When compared with the same group, the APT-100
group (80.80±11.08 sec) exhibited a significantly decreased escape
latency (P<0.01). On the third day of training, when compared
with the Scop group (97.89±12.80 sec), the escape latencies of mice
in the APT group were significantly decreased by 17.21% in the 50
mg/kg group (P<0.05) and 22.88% in the 100 mg/kg group
(P<0.01). On day 4, escape latencies in the 25, 50 and 100 mg/kg
APT groups were significantly decreased by 16.80 (P<0.05), 26.71
(P<0.01) and 30.57% (P<0.01), respectively when compared with
the Scop group (87.63±8.49 sec).
In the probe trial, the number of crossing target
quadrants was used to evaluate the retention of spatial memory. As
shown in Fig. 2B, compared with the
NC group, the Scop group exhibited a 58.36% decrease in the number
of crossing target quadrants (P<0.01). Furthermore, the 25, 50
and 100 mg/kg APT groups demonstrated a 48.36% (P<0.05), 84.43%
(P<0.01) and 116.39% (P<0.01) increase in the number of
crossing target quadrants when compared with the Scop group. Pir
groups demonstrated a significant increase in the number of
crossing target quadrants when compared with the Scop group
(P<0.01).
Step-down passive avoidance test
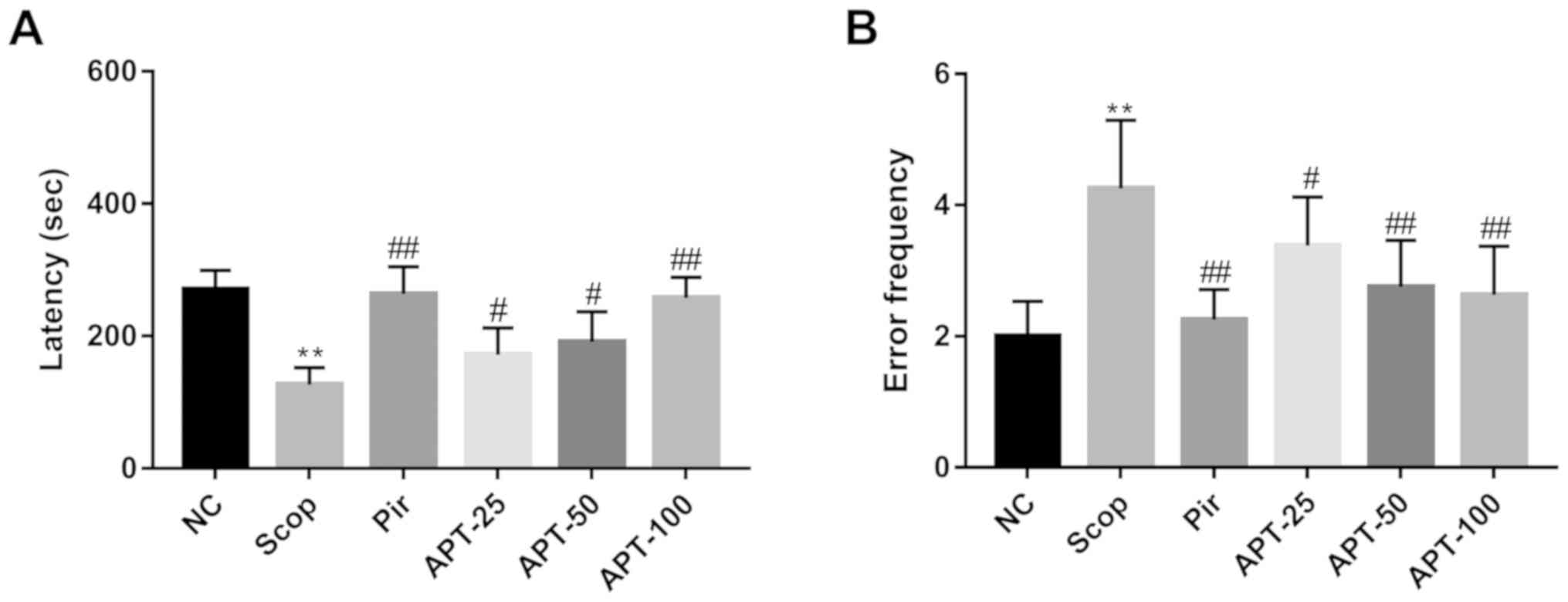
In the step-down test (Fig. 3), the Scop group demonstrated a
53.23% decrease in step-down latency (P<0.01) and a 112.50%
increase in error frequency (P<0.01) when compared with the NC
group. Treatment with 25 mg/kg (171.75±40.58 sec; P<0.05), 50
mg/kg (191.13±45.38 sec; P<0.05) and 100 mg/kg (257.88±30.77
sec; P<0.01) APT significantly increased the step-down latency
and decreased the error frequency (25 mg/kg, 3.38±0.74, P<0.05;
50 mg/kg, 2.75±0.71, P<0.01; 100 mg/kg, 2.63±0.74, P<0.01)
when compared with the Scop group (latency, 126.63±25.75 sec; error
frequency, 4.25± 104). The Pir group, in comparison to the Scop
group, also demonstrated significantly increased step-down latency
(263.63±41.12 sec; P<0.01) and significantly decreased error
frequency (2.25±0.46; P<0.01).
Effects of APT on ACh, AChE and ChAT
levels in brain tissue
As shown in Fig. 4A
and D, ACh levels in the hippocampus
and frontal cortex were significantly lower in the Scop group
compared with the NC group (P<0.01). Following APT
administration, ACh levels were significantly increased in the
hippocampus and frontal cortex compared with the Scop group
(P<0.05 and P<0.01). The Pir group also demonstrated a
significant increase in ACh levels compared with the Scop group
(P<0.01). There was no significant difference in ACh, AChE and
ChAT content between the Pir and APT groups in the hippocampus and
frontal cortex (Fig. 4).
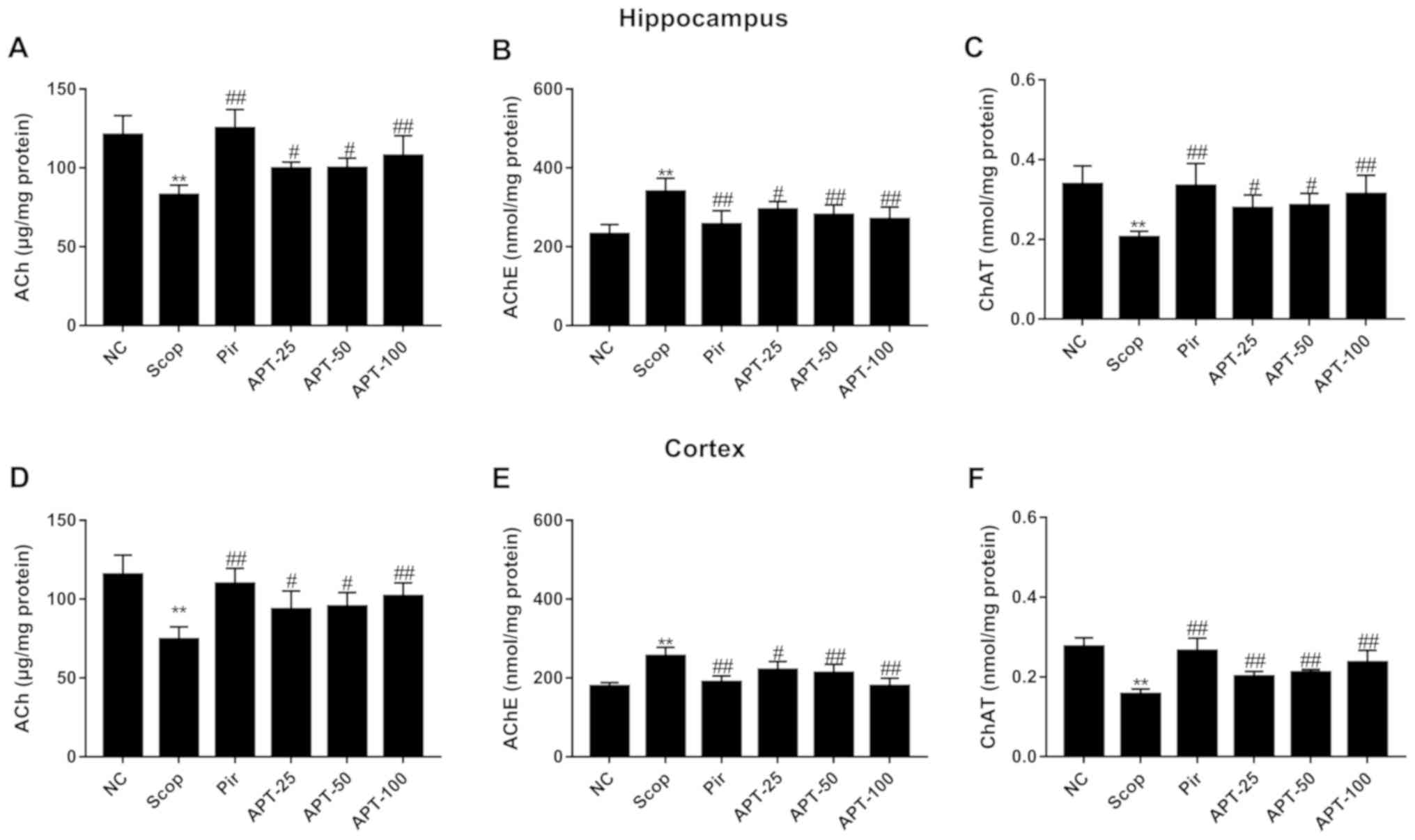 | Figure 4Effects of APT on Ach, AChE and ChAT
levels in the hippocampus and prefrontal cortex in the Scop-induced
model. (A) Ach, (B) AChE and (C) ChAT levels in the hippocampus.
(D) Ach, (E) AChE, (F) and ChAT levels in the prefrontal cortex.
Data are presented as the mean ± standard deviation.
**P<0.01 vs. NC group. #P<0.05 and
##P<0.01 vs. Scop. APT, aerial parts of Polygala
tenuifolia Willd; Scop, scopolamine; Pir, piracetam; APT-25, 25
mg/kg APT, APT-50, 50 mg/kg APT; APT-100, 100 mg/kg APT; Ach,
acetylcholine; AChE, acetylcholinesterase; ChAT, choline
acetyltransferase; NC, normal control. |
As presented in Fig.
4B and E, the Scop group
exhibited significantly higher levels of AChE compared with the NC
group (P<0.01). APT treatment significantly decreased AChE
activity compared with Scop-treated animals (P<0.05 and
P<0.01). A significant reduction was also observed in the Pir
group compared with the Scop group (P<0.01).
ChAT levels in the hippocampus and frontal cortex of
Scop-treated animals were significantly reduced compared with the
NC group (P<0.01). Additionally, following treatment with APT,
ChAT levels in the same areas of the brain were significantly
increased (P<0.05 and P<0.01) compared with the Scop group
(Fig. 4C).
Effects of APT on BDNF in brain
tissue
As shown in Fig. 5A,
BDNF levels in the hippocampus and frontal cortex of mice were
significantly decreased in the Scop group (P<0.01) compared with
the NC group. Furthermore, APT treatment significantly increased
BDNF levels compared with the Scop group (P<0.05). A significant
increase in BDNF levels was also observed in the Pir group compared
with the Scop group (P<0.01).
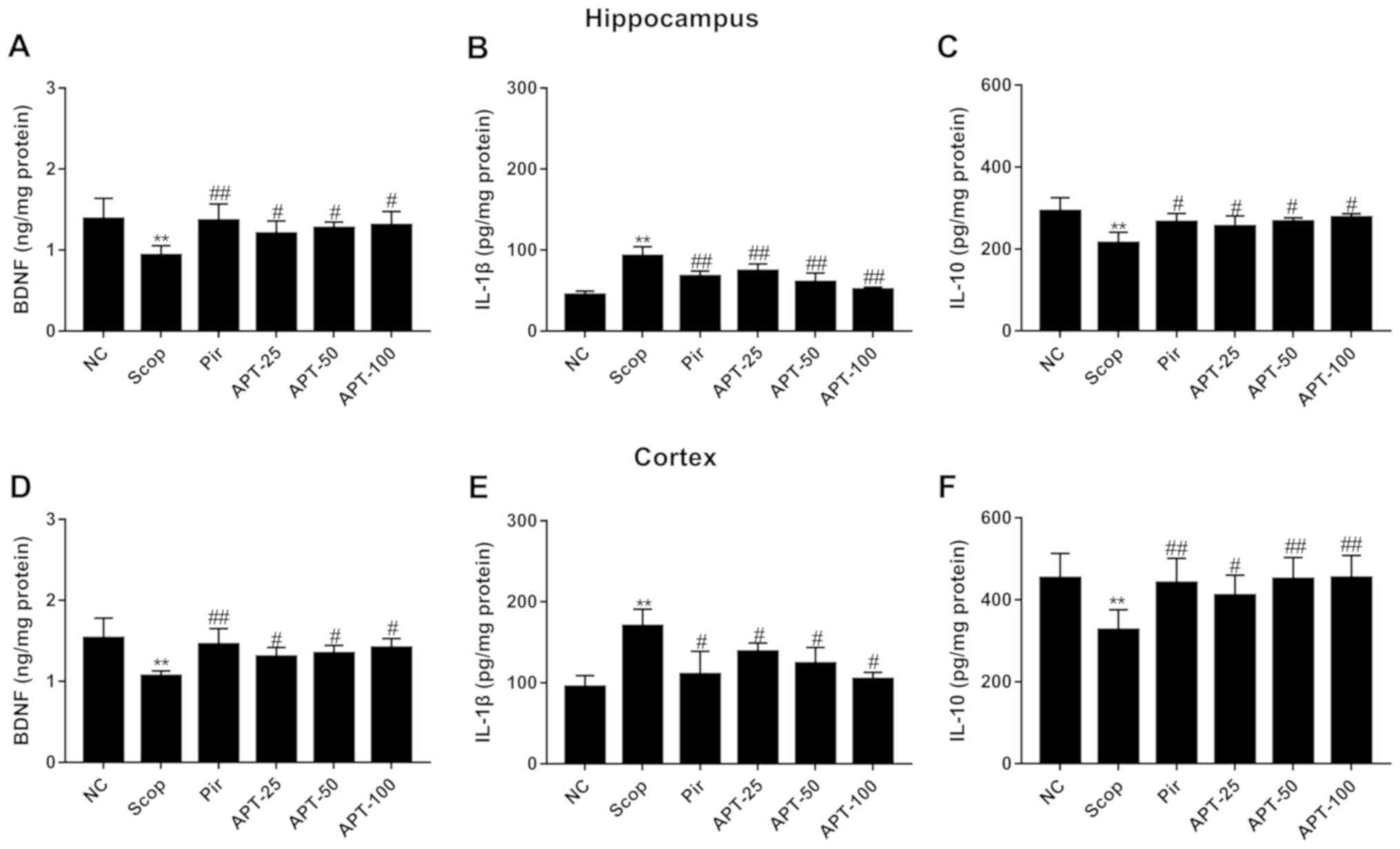 | Figure 5Effects of the aerial parts of APT on
BDNF, IL-1β and IL-10 levels in the hippocampus and prefrontal
cortex in the Scop-induced model. (A) BDNF, (B) IL-1β and (C) IL-10
levels in the hippocampus. (D) BDNF, (E) IL-1β and (F) IL-10 levels
in the prefrontal cortex. Data are presented as the mean ± standard
deviation. **P<0.01 vs. NC group.
#P<0.05 and ##P<0.01 vs. Scop. APT,
aerial parts of Polygala tenuifolia Willd; Scop,
scopolamine; Pir, piracetam; APT-25, 25 mg/kg APT, APT-50, 50 mg/kg
APT; APT-100, 100 mg/kg APT; NC, normal control; BDNF,
brain-derived neurotrophic factor; IL, interleukin. |
Effects of APT on IL-1β and IL-10 in
brain tissue
IL-1β levels were significantly increased in the
Scop group compared with the NC group (Fig. 5B; P<0.01). Additionally, treatment
with APT significantly decreased IL-1β activity compared with the
Scop group (P<0.01).
IL-10 levels in the hippocampus and frontal cortex
of mice were significantly lower in the Scop group compared with
the NC group (Fig. 5C and F; P<0.01). However, treatment with APT
significantly increased IL-10 levels when compared with the Scop
group (P<0.05 and P<0.01).
Effects of APT on SOD activity and MDA
and GSH levels in brain tissue
As shown in Fig. 6A,
SOD activity in the Scop group significantly decreased in the brain
tissue compared with the NC group (P<0.01). Treatment with 25
mg/kg (33.89%; P<0.05), 50 mg/kg (42.16%; P<0.05) and 100
mg/kg (56.78%; P<0.01) APT significantly increased SOD activity
compared with the Scop group. Furthermore, MDA levels in the
remaining brain tissue were significantly increased in the Scop
group compared with the NC group (P<0.01; Fig. 6B). However, APT treatment
demonstrated a significant dose-dependent decrease in MDA levels
compared with the Scop group (P<0.05 and P<0.01; Fig. 6B). As presented in Fig. 6C, GSH levels significantly decreased
in the Scop group compared with the NC group (P<0.01). However,
following treatment with 25 mg/kg (36.89%; P<0.05), 50 mg/kg
(42.21%; P<0.05) and 100 mg/kg (48.36%; P<0.01) APT, GSH
levels significantly increased compared with Scop-treated mice
(Fig. 6C).
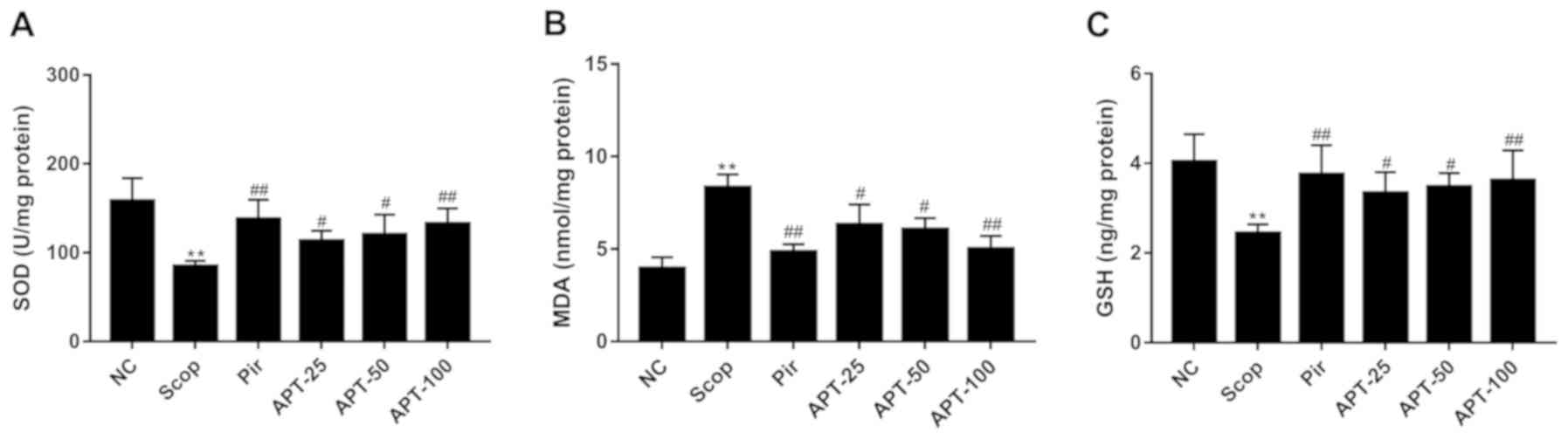 | Figure 6Effects of the aerial parts of APT on
SOD, MDA and GSH levels in the Scop-induced model. (A) SOD, (B) MDA
and (C) GSH levels. Data are presented as the mean ± standard
deviation. n=10. **P<0.01 vs. NC group.
#P<0.05 and ##P<0.01 vs. Scop. APT,
Polygala tenuifolia Willd extract; Scop, scopolamine; Pir,
piracetam; APT-25, 25 mg/kg APT, APT-50, 50 mg/kg APT; APT-100, 100
mg/kg APT; NC, normal control; SOD, superoxide dismutase; MDA,
malondialdehyde. |
Discussion
The present study assessed the effects of APT
treatment on learning and memory impairment in Scop-induced mice.
The results revealed that Scop administration induced spatial
learning and memory impairment, which was subsequently reversed
following APT treatment. It was also demonstrated that APT
treatment increased step-down latency and decreased error frequency
in Scop-induced mice. Additionally, APT treatment significantly
increased ACh and ChAT levels and decreased AChE content in the
hippocampus and prefrontal cortex of Scop-induced mice. The results
of the current study also demonstrated that APT significantly
increased BDNF and IL-10 levels, and decreased IL-1β levels in the
hippocampus and prefrontal cortex of Scop-induced mice.
Furthermore, APT significantly increased SOD activity and decreased
MDA and GSH levels. In the present study, piracetam was selected as
the positive drug, which has been originally used as medication for
AD and dementia (17). Its mechanism
of action bases on facilitating activity in neurotransmitter
systems such as cholinergic, dopaminergic, noradrenergic systems,
and maintaining neuron receptors (19). In the present study, piracetam or APT
can significantly ameliorate learning and memory impairment by
regulating cholinergic activity, promoting BDNF and inhibiting
neuro-inflammation and oxidative stress. The results indicated that
APT may be used as a neuroprotective drug for Scop-induced learning
and memory impairment.
The cholinergic system is considered to be the
epicenter of diseases characterized by cognitive deficits (7). Nicotinic and muscarinic receptors, the
cholinergic receptors of the brain, are involved in memory
formation (17). Scopolamine, a
muscarinic cholinergic receptor antagonist, impairs learning and
memory in humans and rodents, particularly during learning
acquisition and short-term memory (18). Furthermore, scopolamine significantly
increased AChE and MDA levels in the cortex and hippocampus,
increasing oxidative stress in the brain (20,21).
Scopolamine has been widely accepted as a pharmacological model to
study cognitive impairment (22).
Therefore, the present study utilized a murine scopolamine model to
evaluate the ameliorating amnesia effects of APT. The results
demonstrated that APT treatment not only reversed the
scopolamine-induced increase in escape latency but also increased
the number of crossings into the target quadrant. Furthermore, APT
increased step-down latency and decreased error frequency of
scopolamine-induced mice. The results indicated that APT improved
scopolamine-induced learning and memory impairment.
Cholinergic neurons are primarily distributed in the
basal nucleus, the diagonal band of Broca and the medial septal
nucleus of the brain (17). These
neurons transport large quantities of ACh to the cerebral cortex
and hippocampus via projecting fibers (16). Previous studies have demonstrated
that ACh, which is synthesized by ChAT in cholinergic neurons and
hydrolyzed by AChE after its release, is vital to learning and
memory processes (20-22).
In patients with AD, enzymes involved in the cholinergic system are
particularly involved and the activity of ChAT, the rate-limiting
enzyme for ACh production in frontal cortex and hippocampus, is
significantly decreased (7). Thus,
AChE inhibition may serve as a therapeutic target for the treatment
of AD (5). In the current study,
treatment with APT significantly inhibited AChE activity and
effectively increased the levels of ChAT and ACh, and ameliorated
learning and memory impairment.
BDNF is an important member of the neurotrophin
family and serves an important role in the differentiation,
proliferation, nutrition and maturation of neurons (23). BDNF can also enhance synaptic
connections, affect neuronal plasticity and influence
neurotransmitter synthesis (23,24). The
results of the present study indicated that treatment with APT
significantly increased BDNF levels in the hippocampus and frontal
cortex of mice. Previous studies have demonstrated that systemic
inflammation is closely associated with dementia (25,26).
IL-1β, a pro-inflammatory cytokine, is vital for the development of
a complex hormonal and cellular inflammatory cascade (9). It was reported that IL-1β can promote
the progression of neurodegenerative diseases by inducing nitric
oxide production and declining cholinergic function, in turn
improving AChE activity (10,27).
Furthermore, IL-10, an important anti-inflammatory cytokine, serves
an important role in recovery from brain injury and may reduce the
risk of AD (9,28). If cytokines in the brain are
dysregulated, they may cause corresponding pathologic changes,
including inflammatory effects and oxidative stress, affecting
neural homeostasis (27,29,30). In
the present study, APT treatment significantly decreased IL-1β
levels and increased IL-10 levels in the hippocampus and frontal
cortex of scopolamine-treated mice. The results indicated that APT
improved learning and memory via pro-inflammatory and
anti-inflammatory mediators in scopolamine-induced mice.
Scopolamine not only interrupts cholinergic
neurotransmitters, but also triggers oxidative stress in the brain,
which are associated with the pathogenesis of AD (18). Oxidative stress can cause damage to
brain cells and other neural tissues, leading to aging and untimely
cell apoptosis (11). The imbalance
between reactive oxygen species production and elimination by
antioxidant enzymes results in lipid peroxidation, cellular
signaling pathway and gene regulation alterations (31). MDA, produced via lipid peroxidation
in free radicals, causes the cross-linking polymerization of
protein, nucleic acid and other living macromolecules, and also
results in cytotoxicity (12). It is
one of the most important products of membrane lipid peroxidation
and serves as an indicator of oxidative stress (12,32). A
previous study measured antioxidant enzyme activity and peroxide
content in the brain tissue of patients with AD, the results of
which revealed that GSH and SOD activities were significantly lower
compared with healthy individuals, while MDA levels were
significantly increased (33). The
results further demonstrated that the production of free radicals
in patients with AD was increased, inducing serious damage and
decreasing the function of neurons (33). Lipid peroxide increases membrane
fluidity on the surface of the cell membrane and causes
neurodegenerative lesions (12). It
was reported that APT contains various active ingredients such as
flavonoids and phenolic glycosides, especially mangiferin, which is
able to protect the central nervous system from oxidative stress,
mitochondrial dysfunction and neuroinflammation and plays a role in
improving declined memory and cognition (34). In the present study, scopolamine
treatment significantly increased MDA levels and decreased SOD and
GSH levels. However, following treatment with APT, SOD activity and
GSH levels were significantly increased, and MDA levels were
significantly decreased, indicating that APT improved learning and
memory impairment through an anti-oxidant effect.
The administration of APT significantly decreased
escape latency, increased the number of crossings into the target
quadrant, increased step-down latency and decreased error frequency
in scopolamine-induced mice. APT also facilitated central
cholinergic activity by inhibiting AChE and increasing ACh and ChAT
levels in the brains of mice. Treatment with APT also significantly
increased BDNF and IL-10 levels, and decreased IL-1β levels in
murine brains. Furthermore, APT significantly increased SOD
activity and decreased levels of MDA and GSH. The results indicated
that APT treatment may ameliorate learning and memory impairment by
regulating cholinergic activity, promoting BDNF and inhibiting
neuroinflammation and oxidative stress.
Acknowledgements
Not applicable.
Funding
This study was supported by the Key Research and
Development Plan of Shaanxi Province (grant no. 2018ZDXM-SF-014),
the Shaanxi Provincial Education Department Serves Local Special
Projects (grant no. 2018JC032), the open funding of Key Laboratory
of Resource Biology and Biotechnology in Western China, Ministry of
Education, Northwest University (grant no. ZSK2018006), and the
Public Health Specialty of the Department of Traditional Chinese
Medicine (grant no. 2019-39).
Authors' contributions
MF designed the study and provided financial
support. XW, WS, CFC, ZZ and QF performed the experiments and
analyzed data. XW, DZ, MF and YC performed the experiments, wrote
and edited the manuscript. XY provided technical support and
performed the experiments. All authors read and approved the final
manuscript.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Ethics approval and consent to
participate
All experiments were performed according to the
protocol approved by the Ethics Committee of Northwest University
(Xi'an, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Zvěřová M: Clinical aspects of Alzheimer's
disease. Clin Biochem. 72:3–6. 2019.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Arranz AM and De Strooper B: The role of
astroglia in Alzheimer's disease: Pathophysiology and clinical
implications. Lancet Neurol. 18:406–414. 2019.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Cheng X, Wu J, Geng M and Xiong J: Role of
synaptic activity in the regulation of amyloid beta levels in
Alzheimer's disease. Neurobiol Aging. 35:1217–1232. 2014.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Ballard C, Gauthier S, Corbett A, Brayne
C, Aarsland D and Jones E: Alzheimer's disease. Lancet.
377:1019–1031. 2011.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Teipel S, Heinsen H, Amaro EJ, Grinberg
LT, Krause B and Grothe M: Alzheimer's disease neuroimaging
initiative. cholinergic basal forebrain atrophy predicts amyloid
burden in Alzheimer's disease. Neurobiol Aging. 35:482–491.
2014.PubMed/NCBI View Article : Google Scholar
|
|
6
|
McKeever PM, Kim T, Hesketh AR, MacNair L,
Miletic D, Favrin G, Oliver SG, Zhang Z, St George-Hyslop P and
Robertson J: Cholinergic neuron gene expression differences
captured by translational profiling in a mouse model of Alzheimer's
disease. Neurobiol Aging. 57:104–119. 2017.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Klaassens BL, van Gerven JMA, Klaassen ES,
van der Grond J and Rombouts S: Cholinergic and serotonergic
modulation of resting state functional brain connectivity in
Alzheimer's disease. NeuroImage. 199:143–152. 2019.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Ulep MG, Saraon SK and McLea S: Alzheimer
disease. J Nurse Pract. 14:129–135. 2018.
|
|
9
|
Ozben T and Ozben S: Neuro-inflammation
and anti-inflammatory treatment options for Alzheimer's disease.
Clin Biochem. 72:87–89. 2019.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Morgan AR, Touchard S, Leckey C, O'Hagan
C, Nevado-Holgado AJ, NIMA Consortium, Barkhof F, Bertram L, Blin
O, Bos I, et al: Inflammatory biomarkers in Alzheimer's disease
plasma. Alzheimers Dement. 15:776–787. 2019.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Jomova K, Vondrakova D, Lawson M and Valko
M: Metals, oxidative stress and neurodegenerative disorders. Mol
Cell Biochem. 345:91–104. 2010.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Pena-Bautista C, Baquero M, Vento M and
Chafer-Pericas C: Free radicals in Alzheimer's disease: Lipid
peroxidation biomarkers. Clin Chim Acta. 491:85–90. 2019.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Lin Z, Gu J, Xiu J, Mi T, Dong J and
Tiwari JK: Traditional chinese medicine for senile dementia. Evid
Based Complement Alternat Med. 2012(692621)2012.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Shi TX, Li Y and Jiang Y: Isolation of
flavonoids from the aerial parts of Polygala tenuifolia
Willd. and their antioxidant activities. J Chin Pharmaceut Sci.
22:36–39. 2013.
|
|
15
|
Yang XJ, Zou PP, Tu PF and Jiang Y: HPLC
determination of mangiferin in the leaves of Aquilaria sinensis and
the different aerial parts of Polygala tenuifolia. Chinese J
Pharmaceut Anal. 32:1175–1178. 2012.
|
|
16
|
Medicine NAoTC: Chinese Materia Medica.
Shanghai Scientific & Technical Publishers, 1999.
|
|
17
|
Wilms W, Woźniak-Karczewska M, Corvini PF
and Chrzanowski Ł: Nootropic drugs: Methylphenidate, modafinil and
piracetam-population use trends, occurrence in the environment,
ecotoxicity and removal methods-A review. Chemosphere. 233:771–785.
2019.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Pepeu G and Grazia Giovannini M: The fate
of the brain cholinergic neurons in neurodegenerative diseases.
Brain Res. 1670:173–184. 2017.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Krivinko JM, Koppel J, Savonenko A and
Sweet RA: Animal models of psychosis in Alzheimer disease. Am J
Geriatr Psychiatr. 28:1–19. 2020.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Tao L, Xie J, Wang Y, Wang S, Wu S, Wang Q
and Ding H: Protective effects of aloe-emodin on
scopolamine-induced memory impairment in mice and
H2O2-induced cytotoxicity in PC12 cells.
Bioorg Med Chem Lett. 24:5385–5389. 2014.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Jeong EJ, Lee KY, Kim SH, Sung SH and Kim
YC: Cognitive-enhancing and antioxidant activities of iridoid
glycosides from Scrophularia buergeriana in scopolamine-treated
mice. Eur J Pharmacol. 588:78–84. 2008.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Klinkenberg I and Blokland A: The validity
of scopolamine as a pharmacological model for cognitive impairment:
A review of animal behavioral studies. Neurosci Biobehav Rev.
34:1307–1350. 2010.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Hu W, Feng Z, Xu J, Jiang Z and Feng M:
Brain-derived neurotrophic factor modified human umbilical cord
mesenchymal stem cells-derived cholinergic-like neurons improve
spatial learning and memory ability in Alzheimer's disease rats.
Brain Res. 1710:61–73. 2019.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Bekinschtein P, Cammarota M and Medina JH:
BDNF and memory processing. Neuropharmacology. 76:677–683.
2014.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Tanila H: The role of BDNF in Alzheimer's
disease. Neurobiol Dis. 97:114–118. 2017.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Tapia-Arancibia L, Aliaga E, Silhol M and
Arancibia S: New insights into brain BDNF function in normal aging
and Alzheimer disease. Brain Res Rev. 59:201–220. 2008.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Kinney JW, Bemiller SM, Murtishaw AS,
Leisgang AM, Salazar AM and Lamb BT: Inflammation as a central
mechanism in Alzheimer's disease. Alzheimers Dement. 4:575–590.
2018.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Subhramanyam CS, Wang C, Hu Q and Dheen
ST: Microglia-mediated neuroinflammation in neurodegenerative
diseases. Semin Cell Dev Biol. 97:112–120. 2019.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Hampel H, Vergallo A, Aguilar LF, Benda N,
Broich K, Cuello AC, Cummings J, Dubois B, Federoff HJ, Fiandaca M,
et al: Precision pharmacology for Alzheimer's disease. Pharmacol
Res. 130:331–365. 2018.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Faria MC, Goncalves GS, Rocha NP, Moraes
EN, Bicalho MA, Gualberto Cintra MT, Jardim de Paula J, José Ravic
de Miranda LF, Clayton de Souza Ferreira A, Teixeira AL, et al:
Increased plasma levels of BDNF and inflammatory markers in
Alzheimer's disease. J Psychiatr Res. 53:166–172. 2014.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Schrag M, Mueller C, Zabel M, Crofton A,
Kirsch WM, Ghribi O, Squitti R and Perry G: Oxidative stress in
blood in Alzheimer's disease and mild cognitive impairment: A
meta-analysis. Neurobiol Dis. 59:100–110. 2013.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Tramutola A, Lanzillotta C, Perluigi M and
Butterfield DA: Oxidative stress, protein modification and
Alzheimer disease. Brain Res Bull. 133:88–96. 2017.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Jiang T, Sun Q and Chen S: Oxidative
stress: A major pathogenesis and potential therapeutic target of
antioxidative agents in Parkinson's disease and Alzheimer's
disease. Prog Neurobiol. 147:1–19. 2016.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Feng ST, Wang ZZ, Yuan YH, Sun HM, Chen NH
and Zhang Y: Mangiferin: A multipotent natural product preventing
neurodegeneration in Alzheimer's and Parkinson's disease models.
Pharmacol Res. 146(104336)2019.PubMed/NCBI View Article : Google Scholar
|