Genes for 122 protein kinases have been identified
in yeast cells, 540 in mice and 518 genes in the human genome
(1). One of these protein kinases is
protein kinase CK2, formerly known as casein kinase 2, which is a
ubiquitously expressed, constitutively active serine/threonine and
tyrosine kinase (2). In total, more
than 500 protein substrates have been identified and CK2 is
estimated to be responsible for up to 10% of the human
phosphoproteome (3,4). CK2 is a soluble, readily extractable
form in all eukaryotic cells. Moreover, Burnett and Kennedy
(5) purified the soluble kinase
activity from rat liver and named the enzyme according to ‘casein’,
which was used as a substrate to analyse the kinase activity.
The CK2 holoenzyme is a tetramer, comprised of two
catalytic α- or α'- and two non-catalytic β-subunits (6). The α-subunits are encoded by two
distinct homologous genes, CSNK2A1 which encodes CK2α (7) and CSNK2A2 which encodes CK2α' (8). The β-subunit is encoded by CSNK2B
(9). CK2β is not a simple on-off
regulator of the catalytic activity of CK2α. It regulates
thermostability, substrate specificity and the ability to attach
and penetrate cell membranes (10-13).
In addition to the tetramer, higher molecular weight forms of CK2
have been identified (14,15). Although the CK2 tetramer has a
dissociation constant of around 4 nM (16,17)
suggesting a permanent or a strong transient hetero complex, there
is increasing evidence that the catalytic CK2α subunits exist in
the absence of CK2β (18) and that
CK2β exists in the absence of CK2α and CK2α' (19,20).
CK2α and CK2β are essential for embryonic
development. For instance, mortality occurs in
CK2α-/- embryos in mid-gestation,
with defects in heart and neural tube (26).
CK2β-/- mice die shortly after
implantation with no signs of apoptosis but reduced cell
proliferation. Furthermore,
CK2β-/- blastocysts cannot develop
an inner cell mass in vitro (27). It has also been revealed that CK2α'
knockout mice are viable but the male knockout mice exhibit
globozoospermia (28). A recent
review summarizes the knowledge about the role of CK2 in
development and differentiation (29).
There are two major classes of sodium channels in
mammals known as the voltage gated sodium channels (VGSCs,
Nav) and the epithelial sodium channels (ESCs) (60). A major physiological role for VGSCs
is the generation of action potentials at the axonal initial
segments (AIS) and in myelinated axons (61,62). The
generation and propagation of action potentials requires the
precise accumulation of the voltage-gated sodium channels, such as
Nav1.1, Nav1.2 and Nav1.6 at the
AIS and in the nodes of Ranvier, which is achieved via ankyrin G
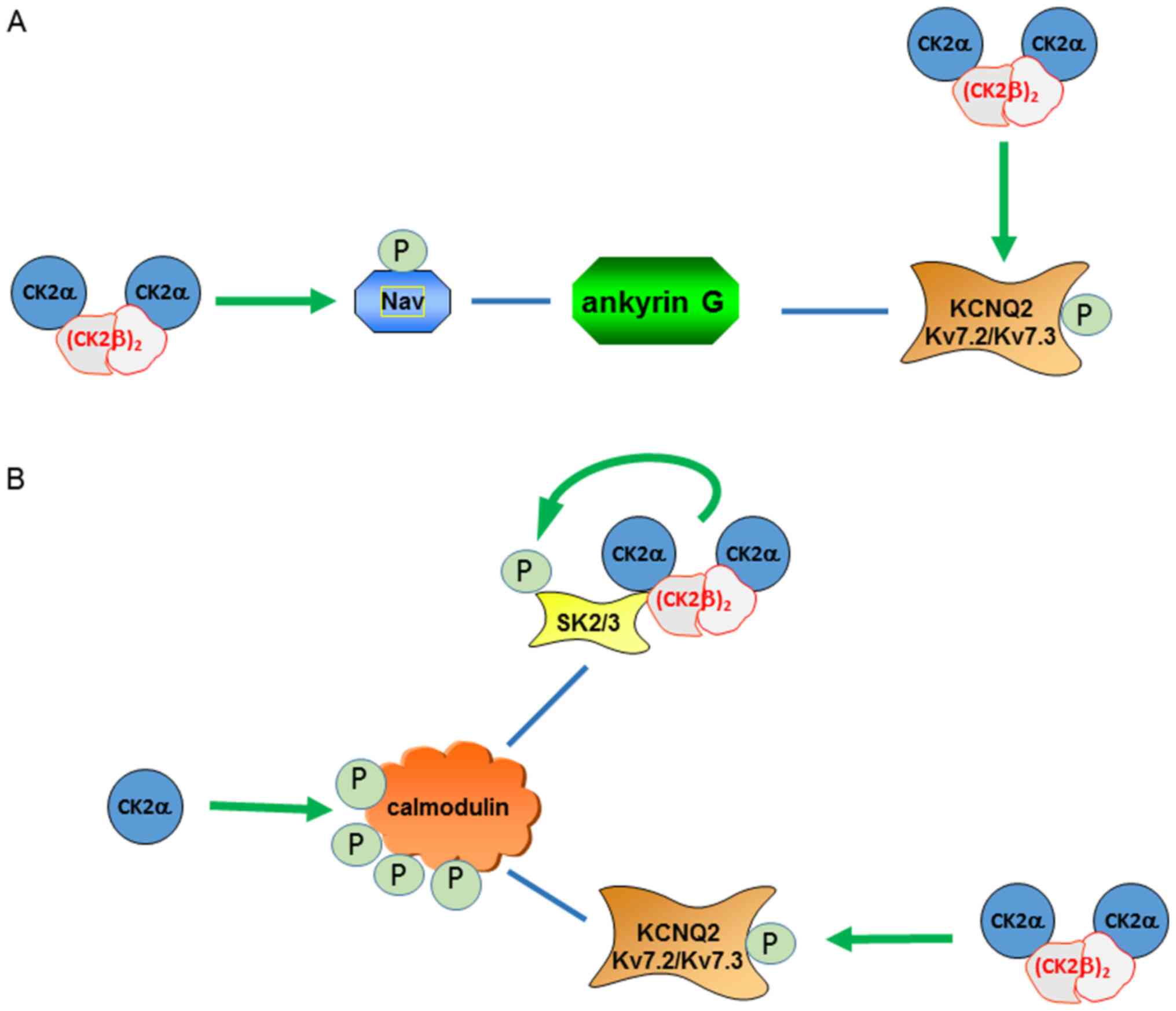
scaffolding (Fig. 1A). It has been
observed that the large intracellular domain of the VGSCs contains
a highly conserved ankyrin G binding motif. However, the binding
motif for the Navs is also highly conserved on the
polypeptide chain of ankyrin G (63,64).
Brachet et al (62) reported
that CK2 phosphorylates the ankyrin G binding motif on the
polypeptide chain of Nav1 (Fig. 1A). Moreover, mutation of the CK2
phosphorylation site on Nav1 to a non-phosphorylatable
alanine abrogated the Nav1/ankyrin G interaction. This
mutation, as well as the use of the CK2 kinase inhibitor DMAT,
leads to a decrease of Nav1 at AIS (62). Thus, CK2 may be involved in the
modulation of Nav1 binding to ankyrin G as well as the
accumulation of Nav1 at AIS at least in young neurons.
In agreement with these observations, CK2 is enriched in AIS and
nodes of Ranvier (65).
Amiloride-sensitive epithelial sodium channels
(ENaCs) mediate the transport of Na+ ions across
membranes of epithelial cells and are composed of α, β and γ
subunits or δ, β and γ subunits (66). Alterations in the composition of the
ENaCs are responsible for differences in conductance, open
probability, sensitivity to amiloride, and sensitivity to
extra-cellular protons (66). The
activity of ENaC is regulated by various protein kinases such as
protein kinase A (PKA), PKC, ERK1/2 and CK2(67). CK2 phosphorylates the ENaC β subunit
at serine 631 and the γ subunit at threonine 599(68). Inhibition of the CK2 kinase activity
as well as the use of ENaC subunits, in which both CK2 sites were
mutated, demonstrates a reduced amiloride sensitive Na+
transport (69). Furthermore, it was
shown that CK2 directly binds to ENaC (68) and CK2 is transported to the cell
membrane by wild-type ENaC, but not by ENaC, in which both CK2
phosphorylation sites are mutated (69). Regulation of ENaC by signalling
molecules including hormones is critical for the regulation of
electrolyte and water excretion and consequently for the regulation
of blood pressure (70). Recently,
the influence of CK2 on ENaC and sodium excretion was analysed in
living organisms. For instance, Berman et al found that
inhibition of CK2 kinase activity leads to a significant decrease
in ENaC activity and natriuresis in mice. These results demonstrate
that an appropriate regulation of ENaC by CK2 is necessary for fine
regulation of the sodium concentration (71).
The largest group of potassium channels are the
voltage-gated channels known as Kv channels (72). While ligand activated potassium
channels also exist, their interaction with CK2 is yet to be
elucidated. Similar to Navs, Kvs are located
in different parts of the AIS and carry an ankyrin G binding site
(73). Pharmacological inhibition of
the CK2 kinase activity using TBB or tetrabromocinnamide acid
(TBCA) prevents the distal redistribution of Kv7.3
channels along the AIS (74).
Although not directly analysed by Lezmy et al (74), according to their results, it was
suggested, that CK2 phosphorylates Kv7.2/3 to increase
their affinity to ankyrin G (Fig.
1A). A possible explanation for these results is that
inhibition of CK2 kinase activity may prevent the insertion of new
Kv7.2/3 into the AIS. Alternatively, or in addition, CK2
may phosphorylate calmodulin, which increases its interaction with
the Kv7.2 subunit, and is crucial for the aforementioned
redistribution (Fig. 1B) (75).
SK2 channel phosphorylation by CK2 results in a
deactivation of the channel, while dephosphorylation has the
reverse effect (83). Allen et
al (84) and Bildl et al
(83) reported, that both CK2α and
CK2β and PP2A bind to the cytoplasmic N- and C-termini of SK
channels to form a multiprotein complex at the plasma membrane of
rat brains. Furthermore, PP2A binds to a region on the polypeptide
chain of SK, which was previously identified as the PP2A binding
site on the polypeptide chain of SV40 small T antigen and CK2α
(18,85). Positively charged compounds, such as
spermine or poly-L-lysine, are known to stimulate the kinase
activity of CK2(79). The N-terminal
domain of SK2 contains a cluster of positively charged residues
close to the site of interaction with CK2α and it was revealed that
this region stimulated CK2 similar to poly-L-lysine (84). Within this complex, CK2
phosphorylates calmodulin, thereby reducing the Ca2+
sensitivity and accelerating channel deactivation (86).
Neurotransmitters, such as noradrenalin, inhibit SK2
channels independently of changes in the activity of the priming
Ca2+ channels (82). In
total, there are three homologous SK channels, namely SK1-3,
expressed in the mammalian brain (87). Inhibition of CK2 by TBB or the use of
a dominant-negative CK2α K68M mutant strongly reduces the effect of
noradrenalin on SK channels (82).
However, the signalling pathway from the activated receptor to CK2
awaits further analysis.
The influence of CK2 and potassium channels in
disease is yet to be fully elucidated. However, it has been shown
that increased expression of CK2 in the infarct border is
associated with reduced SK1/Kir2.1 protein levels (88). Furthermore, overexpression of CK2
suppressed the KCNJ2/Kir2.1 expression and inhibition of CK2 kinase
activity enhanced KCNJ2/Kir2.1 expression (89). It has been shown that hypoxia leads
to increased CK2 expression in the heart of male Wistar rats, and
the CK2/Kir2.1 pathway may be a potential therapeutic target for
ventricular arrhythmias (vAs) after myocardial infarction (89). CK2 phosphorylates the transcription
factor SP1, which regulates the expression of the potassium
inwardly rectifying channel subfamily J member 2 gene, encoding
Kir2.1. The angiotensin 1 receptor antagonist valsartan reduces CK2
activation at the infarct border and increases Kir2.1 expression
(89). These findings provide an
insight into the pathophysiological molecular mechanisms which
occur following myocardial infarction, and in particular, into the
role of CK2 in this process.
The L-type calcium current is critical for the
development, function and regulation of many different cell types
including physiologic functions of nerve and muscle cells (103). L-type calcium channels are
implicated in the excitation-contraction coupling in cardiac,
skeletal and smooth muscle, in the regulation of Ca2+
homeostasis and secretion, tissue development, neuron excitability,
excitation-transcription coupling and in learning and memory in the
brain, reviewed in (104).
Furthermore, L-type Ca2+ channel activation results in
uterine contraction of mice, the activation of which is suppressed
by inhibition of CK2(58).
Cav1.1 is the L-type Ca2+ channel present in
the skeletal muscle and Cav1.2 is the L-type channel
present in the heart. Both of these channels are regulated via
phosphorylation by a number of different protein kinases, such as
PKA, Akt, PKC and CK2(103).
Multiple regulatory sites are located in the large C-terminal
domain of Cav1.1 and Cav1.2 channels
(105-107).
For instance, the PKA phosphorylation site at serine 1700 was
required for the stimulation of channel activity (108), while threonine 1704 phosphorylation
by CK2 is necessary for the regulation of basal channel activity.
Mice with mutations at these two phosphorylation sites have a
significantly reduced basal L-type calcium current and a reduced
response to β-adrenergic stimulation (109,110).
In addition these mutant mice have an impaired contractile
function, decreased exercise capacity and cardiac hypertrophy
(109,110).
In total, at least two other proteins, including
syntaxin-1 and synaptotagmin-1, specifically interact with
Cav2.1 channels by binding to a synaptic protein
interaction site within an intracellular loop of the channel
(121,122). CK2 is present in the membrane
micro-domains from rat brain nerve endings and it phosphorylates
syntaxin-1 at serine 14 as assessed using phospho-specific
antibodies (59). This N-terminal
segment of syntaxin-1 including the CK2 phosphorylation site is
involved in direct protein-protein interactions and leads to
alterations in the neurotransmitter release (59). Furthermore, it has been demonstrated
that the CK2 phosphorylation of syntaxin-1 may play a role in the
pathophysiology of schizophrenia (123). Therefore, these data might suggest
a differential regulation of Cav2.1 by CK2, where
syntaxin-1 and synaptotagmin-1 are phosphorylated by the CK2
holoenzyme while calmodulin is phosphorylated by CK2α alone.
Chloride or bicarbonate are transported across
membranes by complex membrane proteins called anion channels. The
transport of chloride and bicarbonate ions results in alterations
of the pH within cells and also in alterations in the transport of
water (124). A reduction in
chloride and bicarbonate concentrations leads to a disease called
cystic fibrosis. The cystic fibrosis transmembrane conductance
regulator (CFTR) is an example of an anion channel present in
epithelial cells and is a member of the family of ATP binding
cassette (ABC) proteins (125,126).
The activity of CFTR is, in part regulated by the cAMP-dependent
protein kinase PKA (126). In
addition, CK2 is implicated in the regulation of CFTR (127-133).
It has been reported that TBB treatment of Calu-3 cells resulted in
a significant inhibition of the basolateral
Cl-/HCO3- exchanger. Treatment
with the more efficient and specific inhibitor CX-4945 completely
abolishes Cl-/HCO3- exchanger
activity.
Recently, it has been revealed that CK2 is required
for the physiological expression of the Ca2+ activated
Cl- channel anoctamin 1 (ANO1), previously known as
TMEM16A, in the plasma membrane. ANO1 is stimulated via G-protein
coupled receptors (134). Small
interfering RNA knockdown of CK2α' or inhibition of the kinase
activity by TBB or CX-4945 leads to a reduced expression in the
plasma membrane and an inhibition of the whole cell current in
airways epithelial cells (134).
Furthermore, these treatments result in an inhibition of cell
proliferation. However, it remains to be analysed whether CK2α'
directly phosphorylates ANO1 alone or as a CKα'/CK2β holoenzyme and
whether CK2α might have the same effect.
CK2 is not only stimulatory for the functions of
channels. It inhibits the lipid flippase ABCA1, which is a CFTR
related protein (135). A total of
three residues, threonine 1,242, threonine 1,243 and serine 1,255
in the cytoplasmic part of ABCA1 have been identified as CK2
phosphorylation sites (135).
Moreover, mutation analysis and the use of CK2 specific inhibitors
has revealed that CK2 phosphorylation affects flippase activity,
apolipoprotein AI and AII binding and phospholipid and cholesterol
efflux (80,135).
The cellular uptake of a wide range of endogenous
and exogenous molecules including many clinically used drugs is
mediated by solute carrier transporters (SLC), which are
transmembrane proteins (136).
SLC4A2 is another member of the
Cl-/HCO3- exchanger in human
airway epithelia cells, which is phosphorylated by CK2 and whose
activity is reduced by inhibition of CK2 by TBB or CX-4945 or by
knockdown experiments, suggesting that CK2 may be a key regulator
of trans-epithelial transport in human airways (137). However, it remains unknown whether
CK2 regulates SLC4A2 directly or indirectly by regulating
calmodulin. CK2 has also been shown to influence the activity of
the nucleoside transporters SLC29A1 and SLC29A2, previously known
as ENT1 and ENT2, respectively (138).
In conclusion, protein kinase CK2 is implicated in
central cellular processes, such as regulation of cell
proliferation, differentiation, RNA splicing, DNA repair and
angiogenesis. The present review has summarized the knowledge
regarding the regulation of cation and anion channels. This
regulation is achieved either by direct phosphorylation of proteins
building the channels (Table I) or
via phosphorylation of platform proteins such as calmodulin and
ankyrin G (Fig. 1), which are
responsible for binding, transport and physiological orientation of
channel proteins into the plasma membrane. In addition, CK2
subunits bind to certain proteins which compose the channels
(Table II), which might reflect an
enzyme /substrate interaction or a currently unknown function.
Regulation of the intracellular ion concentration contributes to an
altered membrane potential, which influences cellular excitability
of a variety of different cell systems including neuronal and
muscle cells. Moreover, the intracellular ion concentrations plays
an important role in a variety of different conditions such as
heart failure, epilepsy, cystic fibrosis and diabetes. These
effects have been considered when CK2 inhibitors are used for the
treatment of cancer. Furthermore, the knowledge of the role of CK2
in the regulation of ion channels in the plasma membrane may
facilitate the targeting CK2 for the regulation of intracellular
ion concentrations and ultimately cellular signalling.
Not applicable.
This work was supported by the Rolf M. Schwiete
Stiftung, Mannheim, Germany (grant nos. 06/2015 and 2020-006).
Not applicable.
MM and CG performed literature research, wrote the
paper, and read and approved the final manuscript.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Manning G: Genomic overview of protein
kinases. WormBook. 1–19. 2005.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Litchfield DW: Protein kinase CK2:
Structure, regulation and role in cellular decisions of life and
death. Biochem J. 369:1–15. 2003.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Salvi M, Sarno S, Cesaro L, Nakamura H and
Pinna LA: Extraordinary pleiotropy of protein kinase CK2 revealed
by weblogo phosphoproteome analysis. Biochim Biophys Acta.
1793:847–859. 2009.PubMed/NCBI View Article : Google Scholar
|
|
4
|
de Villavicencio-Diaz T, Rabalski AJ and
Litchfield DW: Protein kinase CK2: Intricate relationships within
regulatory cellular networks. Pharmaceuticals (Basel).
10(27)2017.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Burnett G and Kennedy EP: The enzymatic
phosphorylation of proteins. J Biol Chem. 211:969–980.
1954.PubMed/NCBI
|
|
6
|
Boldyreff B, Meggio F, Pinna LA and
Issinger OG: Protein kinase CK2 structure-function relationship:
Effects of the β subunit on reconstitution and activity. Cell Mol
Biol Res. 40:391–399. 1994.PubMed/NCBI
|
|
7
|
Wirkner U, Voss H, Lichter P, Ansorge W
and Pyerin W: The human gene (CSNK2A1) coding for the casein kinase
II subunit alpha is located on chromosome 20 and contains tandemly
arranged Alu repeats. Genomics. 19:257–265. 1994.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Ackermann K, Neidhart T, Gerber J, Waxmann
A and Pyerin W: The catalytic subunit alpha' gene of human protein
kinase CK2 (CSNK2A2): Genomic organization, promoter identification
and determination of Ets1 as a key regulator. Mol Cell Biochem.
274:91–101. 2005.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Albertella MR, Jones H, Thomson W,
Olavesen MG and Campbell RD: Localization of eight additional genes
in the human major histocompatibility complex, including the gene
encoding the casein kinase II beta subunit (CSNK2B). Genomics.
36:240–251. 1996.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Raaf J, Brunstein E, Issinger OG and
Niefind K: The interaction of CK2alpha and CK2beta, the subunits of
protein kinase CK2, requires CK2beta in a preformed conformation
and is enthalpically driven. Protein Sci. 17:2180–2186.
2008.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Meggio F, Boldyreff BS, Marin O, Pinna LA
and Issinger OG: CK2: Role of the beta-subunit on the stability and
specificity of the recombinant reconstituted holoenzyme. Eur J
Biochem. 204:293–297. 1992.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Boldyreff BS, Meggio F, Pinna LA and
Issinger O-G: Casein kinase-2 structure-function relationship:
Creation of a set of mutants of the β subunit that variably
surrogate the wildtype β subunit function. Biochem Biophys Res
Commun. 188:228–234. 1992.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Rodriguez FA, Contreras C, Bolanos-Garcia
V and Allende JE: Protein kinase CK2 as an ectokinase: The role of
the regulatory CK2beta subunit. Proc Natl Acad Sci USA.
105:5693–5698. 2008.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Lolli G, Pinna LA and Battistutta R:
Structural determinants of protein kinase CK2 regulation by
autoinhibitory polymerization. ACS Chem Biol. 7:1158–1163.
2012.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Lolli G, Naressi D, Sarno S and
Battistutta R: Characterization of the oligomeric states of the CK2
alpha2beta2 holoenzyme in solution. Biochem J. 474:2405–2416.
2017.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Raaf J, Guerra B, Neundorf I, Bopp B,
Issinger OG, Jose J, Pietsch M and Niefind K: First structure of
protein kinase CK2 catalytic subunit with an effective
CK2β-competitive ligand. ACS Chem Biol. 8:901–907. 2013.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Raaf J, Bischoff N, Klopffleisch K,
Brunstein E, Olsen BB, Vilk G, Litchfield DW, Issinger OG and
Niefind K: Interaction between CK2α and CK2β, the subunits of
protein kinase CK2: Thermodynamic contributions of key residues on
the CK2α surface. Biochemistry. 50:512–522. 2011.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Heriche JK, Lebrin F, Rabilloud T, LeRoy
D, Chambaz EM and Goldberg Y: Regulation of protein phosphatase 2A
by direct interaction with casein kinase 2alpha. Science.
276:952–955. 1997.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Lüscher B and Litchfield DW: Biosynthesis
of casein kinase II in lymphoid cell lines. Eur J Biochem.
220:521–526. 1994.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Guerra B, Siemer S, Boldyreff B and
Issinger OG: Protein kinase CK2: Evidence for a protein kinase CK2β
subunit fraction, devoid of the catalytic CK2α subunit, in mouse
brain and testicles. FEBS Lett. 462:353–357. 1999.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Trembley JH, Wang G, Unger G, Slaton J and
Ahmed K: CK2: A key player in cancer biology. Cell Mol Life Sci.
66:1858–1867. 2009.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Benveniste EN, Gray GK and McFarland BC:
Protein kinase CK2 and dysregulated oncogenic inflammatory
signaling pathways Protein kinase CK2 cellular function in normal
and disease states Springer e-book, 2015.
|
|
23
|
Okur V, Cho MT, Henderson L, Retterer K,
Schneider M, Sattler S, Niyazov D, Azage M, Smith S, Picker J, et
al: De novo mutations in CSNK2A1 are associated with
neurodevelopmental abnormalities and dysmorphic features. Hum
Genet. 135:699–705. 2016.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Owen CI, Bowden R, Parker MJ, Patterson J,
Patterson J, Price S, Sarkar A, Castle B, Deshpande C, Splitt M, et
al: Extending the phenotype associated with the CSNK2A1-related
Okur-Chung syndrome-A clinical study of 11 individuals. Am J Med
Genet A. 176:1108–1114. 2018.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Trinh J, Huning I, Budler N, Hingst V,
Lohmann K and Gillessen-Kaesbach G: A novel de novo mutation in
CSNK2A1: Reinforcing the link to neurodevelopmental abnormalities
and dysmorphic features. J Hum Genet. 62:1005–1006. 2017.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Lou DY, Dominguez I, Toselli P,
Landesman-Bollag E, O'Brien C and Seldin DC: The alpha catalytic
subunit of protein kinase CK2 is required for mouse embryonic
development. Mol Cell Biol. 28:131–139. 2008.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Buchou T, Vernet M, Blond O, Jensen HH,
Pointu H, Olsen BB, Cochet C, Issinger OG and Boldyreff B:
Disruption of the regulatory b subunit of protein kinase CK2 in
mice leads to a cell-autonomous defect and early embryonic
lethality. Mol Cell Biol. 23:908–915. 2003.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Xu X, Toselli PA, Russell LD and Seldin
DC: Globozoospermia in mice lacking the casein kinase II a'
catalytic subunit. Nat Genet. 23:118–121. 1999.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Götz C and Montenarh M: Protein kinase CK2
in development and differentiation. Biomed Rep. 6:127–133.
2016.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Niefind K, Pütter M, Guerra B, Issinger OG
and Schomburg D: CTP plus water mimic ATP in the active site of
protein kinase CK2. Nat Struct Biol. 6:1100–1103. 1999.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Lin WJ, Tuazon PT and Traugh JA:
Characterization of the catalytic subunit of casein kinase II
expressed in Escherichia coli and regulation of activity. J Biol
Chem. 266:5664–5669. 1991.PubMed/NCBI
|
|
32
|
Guerra B: Protein kinase CK2 subunits are
positive regulators of AKT kinase. Int J Oncol. 28:685–693.
2006.PubMed/NCBI
|
|
33
|
Shehata M, Schnabl S, Demirtas D, Hilgarth
M, Hubmann R, Ponath E, Badrnya S, Lehner C, Hoelbl A, Duechler M,
et al: Reconstitution of PTEN activity by CK2 inhibitors and
interference with the PI3-K/Akt cascade counteract the
antiapoptotic effect of human stromal cells in chronic lymphocytic
leukemia. Blood. 116:2513–2521. 2010.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Wang S and Jones KA: CK2 controls the
recruitment of Wnt regulators to target genes in vivo. Curr Biol.
16:2239–2244. 2006.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Gao Y and Wang HY: Casein kinase 2 Is
activated and essential for Wnt/beta-catenin signaling. J Biol
Chem. 281:18394–18400. 2006.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Ponce DP, Yefi R, Cabello P, Maturana JL,
Niechi I, Silva E, Galindo M, Antonelli M, Marcelain K, Armisen R
and Tapia JC: CK2 functionally interacts with AKT/PKB to promote
the β-catenin-dependent expression of survivin and enhance cell
survival. Mol Cell Biochem. 356:127–132. 2011.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Ponce DP, Maturana JL, Cabello P, Yefi R,
Niechi I, Silva E, Armisen R, Galindo M, Antonelli M and Tapia JC:
Phosphorylation of AKT/PKB by CK2 is necessary for the
AKT-dependent up-regulation of β-catenin transcriptional activity.
J Cell Physiol. 226:1953–1959. 2011.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Götz C and Montenarh M: Protein kinase CK2
in the ER stress response. Ad Biological Chemistry. 3A:1–5.
2013.
|
|
39
|
Montenarh M: Protein kinase CK2 in DNA
damage and repair. Transl Cancer Res. 5:49–63. 2016.
|
|
40
|
Cozza G, Pinna LA and Moro S: Protein
kinase CK2 inhibitors: A patent review. Expert Opin Ther Pat.
22:1081–1097. 2012.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Cozza G: The development of CK2
inhibitors: From traditional pharmacology to in silico rational
drug design. Pharmaceuticals (Basel). 10(26)2017.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Prudent R and Cochet C: New protein kinase
CK2 inhibitors: Jumping out of the catalytic box. Chem Biol.
16:112–120. 2009.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Bollacke A, Nienberg C, Borgne ML and Jose
J: Toward selective CK2alpha and CK2alpha' inhibitors: Development
of a novel whole-cell kinase assay by Autodisplay of catalytic
CK2alpha'. J Pharm Biomed Anal. 121:253–260. 2016.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Battistutta R, Sarno S, De Moliner E,
Papinutto E, Zanotti G and Pinna LA: The replacement of ATP by the
competitive inhibitor emodin induces conformational modifications
in the catalytic site of protein kinase CK2. J Biol Chem.
275:29618–29622. 2000.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Battistutta R, De Moliner E, Sarno S,
Zanotti G and Pinna LA: Structural features underlying selective
inhibition of protein kinase CK2 by ATP site-directed
tetrabromo-2-benzotriazole. Protein Sci. 10:2200–2206.
2001.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Pagano MA, Bain J, Kazimierczuk Z, Sarno
S, Ruzzene M, Di Maira G, Elliott M, Orzeszko A, Cozza G, Meggio F
and Pinna LA: The selectivity of inhibitors of protein kinase CK2.
An update. Biochem J. 415:353–365. 2008.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Sarno S, De Moliner E, Ruzzene M, Pagano
MA, Battistutta R, Bain J, Fabbro D, Schoepfer J, Elliott M, Furet
P, et al: Biochemical and three-dimensional-structural study of the
specific inhibition of protein kinase CK2 by
[5-oxo-5,6-dihydroindolo-(1,2-a)quinazolin-7-yl]acetic acid (IQA).
Biochem J. 374:639–646. 2003.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Sarno S, Reddy H, Meggio F, Ruzzene M,
Davies SP, Donella-Deana A, Shugar D and Pinna LA: Selectivity of
4,5,6,7-tetrabromobenzotriazole, an ATP site-directed inhibitor of
protein kinase CK2 (‘casein kinase-2’). FEBS Lett. 496:44–48.
2001.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Pierre F, Chua PC, O'Brien SE,
Siddiqui-Jain A, Bourbon P, Haddach M, Michaux J, Nagasawa J,
Schwaebe MK, Stefan E, et al: Discovery and SAR of
5-(3-chlorophenylamino)benzo[c][2,6]naphthyridine-8-carboxylic acid
(CX-4945), the first clinical stage inhibitor of protein kinase CK2
for the treatment of cancer. J Med Chem. 54:635–654.
2011.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Siddiqui-Jain A, Drygin D, Streiner N,
Chua P, Pierre F, O'Brien SE, Bliesath J, Omori M, Huser N, Ho C,
et al: CX-4945, an orally bioavailable selective inhibitor of
protein kinase CK2, inhibits prosurvival and angiogenic signaling
and exhibits antitumor efficacy. Cancer Res. 70:10288–10298.
2010.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Lee JY, Yun JS, Kim WK, Chun HS, Jin H,
Cho S and Chang JH: Structural basis for the selective inhibition
of Cdc2-like kinases by CX-4945. Biomed Res Int.
2019(6125068)2019.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Chua MM, Ortega CE, Sheikh A, Lee M,
Abdul-Rassoul H, Hartshorn KL and Dominguez I: CK2 in cancer:
Cellular and biochemical mechanisms and potential therapeutic
target. Pharmaceuticals (Basel). 10(18)2017.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Faust M, Jung M, Günther J, Zimmermann R
and Montenarh M: Localization of individual subunits of protein
kinase CK2 to the endoplasmic reticulum and to the Golgi apparatus.
Mol Cell Biochem. 227:73–80. 2001.PubMed/NCBI
|
|
54
|
Faust M, Schuster N and Montenarh M:
Specific binding of protein kinase CK2 catalytic subunits to
tubulin. FEBS Letters. 462:51–56. 1999.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Faust M, Günther J, Morgenstern E,
Montenarh M and Götz C: Specific localization of the catalytic
subunits of protein kinase CK2 at the centrosomes. Cell Mol Life
Sci. 59:2155–2164. 2002.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Faust M and Montenarh M: Subcellular
localization of protein kinase CK2: A key to its function? Cell
Tissue Res. 301:329–340. 2000.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Montenarh M and Götz C: Ecto-protein
kinase CK2, the neglected form of CK2 (review). Biomed Rep.
8:307–313. 2018.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Suhas KS, Parida S, Gokul C, Srivastava V,
Prakash E, Chauhan S, Singh TU, Panigrahi M, Telang AG and Mishra
SK: Casein kinase 2 inhibition impairs spontaneous and
oxytocin-induced contractions in late pregnant mouse uterus. Exp
Physiol. 103:621–628. 2018.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Gil C, Falques A, Sarro E, Cubi R, Blasi
J, Aguilera J and Itarte E: Protein kinase CK2 associates to lipid
rafts and its pharmacological inhibition enhances neurotransmitter
release. FEBS Lett. 585:414–420. 2010.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Hernandez CM and Richards JR: Physiology,
sodium channels. StatPearls Publishing 2020.
|
|
61
|
Savio-Galimberti E, Gollob MH and Darbar
D: Voltage-gated sodium channels: Biophysics, pharmacology, and
related channelopathies. Front Pharmacol. 3(124)2012.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Brachet A, Leterrier C, Irondelle M, Fache
MP, Racine V, Sibarita JB, Choquet D and Dargent B: Ankyrin G
restricts ion channel diffusion at the axonal initial segment
before the establishment of the diffusion barrier. J Cell Biol.
191:383–395. 2010.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Grubb MS and Burrone J: Building and
maintaining the axon initial segment. Curr Opin Neurobiol.
20:481–488. 2010.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Xu M and Cooper EC: An Ankyrin-G
N-terminal gate and protein kinase CK2 dually regulate binding of
voltage-gated sodium and KCNQ2/3 potassium channels. J Biol Chem.
290:16619–16632. 2015.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Bréchet A, Fache MP, Brachet A, Ferracci
G, Baude A, Irondelle M, Pereira S, Leterrier C and Dargent B:
Protein kinase CK2 contributes to the organization of sodium
channels in axonal membranes by regulating their interactions with
ankyrin G. J Cell Biol. 183:1101–1114. 2008.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Giraldez T, Rojas P, Jou J, Flores C and
Alvarez de la Rosa D: The epithelial sodium channel delta-subunit:
New notes for an old song. Am J Physiol Renal Physiol.
303:F328–F338. 2012.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Baines D: Kinases as targets for ENaC
regulation. Curr Mol Pharmacol. 6:50–64. 2013.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Shi HK, Asher C, Yung YV, Kligman L,
Reuveny E, Seger R and Garty H: Casein kinase 2 specifically binds
to and phosphorylates the carboxy termini of ENaC subunits. Eur J
Biochem. 269:4551–4558. 2002.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Bachhuber T, Almaca J, Aldehni F, Mehta A,
Amaral MD, Schreiber R and Kunzelmann K: Regulation of the
epithelial Na+ channel by protein kinase CK2. J Biol Chem.
283:13225–13232. 2008.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Hanukoglu I and Hanukoglu A: Epithelial
sodium channel (ENaC) family: Phylogeny, structure-function, tissue
distribution, and associated inherited diseases. Gene. 579:95–132.
2016.PubMed/NCBI View Article : Google Scholar
|
|
71
|
Berman JM, Mironova E and Stockand JD:
Physiological regulation of the epithelial Na+ channel by casein
kinase II. Am J Physiol Renal Physiol. 314:F367–F372.
2017.PubMed/NCBI View Article : Google Scholar
|
|
72
|
Wulff H, Castle NA and Pardo LA:
Voltage-gated potassium channels as therapeutic targets. Nat Rev
Drug Discov. 8:982–1001. 2009.PubMed/NCBI View Article : Google Scholar
|
|
73
|
Misonou H: Precise localizations of
voltage-gated sodium and potassium channels in neurons. Dev
Neurobiol. 78:271–282. 2018.PubMed/NCBI View Article : Google Scholar
|
|
74
|
Lezmy J, Lipinsky M, Khrapunsky Y, Patrich
E, Shalom L, Peretz A, Fleidervish IA and Attali B: M-current
inhibition rapidly induces a unique CK2-dependent plasticity of the
axon initial segment. Proc Natl Acad Sci USA. 114:E10234–E10243.
2017.PubMed/NCBI View Article : Google Scholar
|
|
75
|
Kang S, Xu M, Cooper EC and Hoshi N:
Channel anchored protein kinase CK2 and protein phosphatase 1
reciprocally regulate KCNQ2-containing M-channels via
phosphorylation of calmodulin. J Biol Chem. 289:11536–11544.
2014.PubMed/NCBI View Article : Google Scholar
|
|
76
|
Jentsch TJ: Neuronal KCNQ potassium
channels: Physiology and role in disease. Nat Rev Neurosci.
1:21–30. 2000.PubMed/NCBI View Article : Google Scholar
|
|
77
|
Greene DL and Hoshi N: Modulation of Kv7
channels and excitability in the brain. Cell Mol Life Sci.
74:495–508. 2017.PubMed/NCBI View Article : Google Scholar
|
|
78
|
Kshatri AS, Gonzalez-Hernandez A and
Giraldez T: Physiological roles and therapeutic potential of
Ca2+ activated potassium channels in the nervous system.
Front Mol Neurosci. 11(258)2018.PubMed/NCBI View Article : Google Scholar
|
|
79
|
Meggio F, Boldyreff BS, Marin O, Marchiori
F, Perich JW, Issinger OG and Pinna LA: The effect of polylysine on
CK-2 activity is influenced by both the structure of the
protein/peptide substrates and subunit composition of the enzyme.
Eur J Biochem. 205:939–945. 1992.PubMed/NCBI View Article : Google Scholar
|
|
80
|
Meggio F, Brunati AM and Pinna LA:
Polycation-dependent, Ca2+-antagonized phosphorylation of
calmodulin by casein kinase-2 and a spleen tyrosine protein kinase.
FEBS Lett. 215:241–246. 1987.PubMed/NCBI View Article : Google Scholar
|
|
81
|
Sacks DB, Davis HW, Crimmins DL and
McDonald JM: Insulin-stimulated phosphorylation of calmodulin.
Biochem J. 286:211–216. 1992.PubMed/NCBI View Article : Google Scholar
|
|
82
|
Maingret F, Coste B, Hao J, Giamarchi A,
Allen D, Crest M, Litchfield DW, Adelman JP and Delmas P:
Neurotransmitter modulation of small-conductance Ca2+-activated K+
channels by regulation of Ca2+ gating. Neuron. 59:439–449.
2008.PubMed/NCBI View Article : Google Scholar
|
|
83
|
Bildl W, Strassmaier T, Thurm H, Andersen
J, Eble S, Oliver D, Knipper M, Mann M, Schulte U, Adelman JP and
Fakler B: Protein kinase CK2 is coassembled with small conductance
Ca2+-activated K+ channels and regulates
channel gating. Neuron. 43:847–858. 2004.PubMed/NCBI View Article : Google Scholar
|
|
84
|
Allen D, Fakler B, Maylie J and Adelman
JP: Organization and regulation of small conductance Ca2+-activated
K+ channel multiprotein complexes. J Neurosci. 27:2369–2376.
2007.PubMed/NCBI View Article : Google Scholar
|
|
85
|
Pallas DC, Shahrik LK, Martin BL, Jaspers
S, Miller TB, Brautigan DL and Roberts TM: Polyoma small and middle
T antigens and SV40 small t antigen form stable complexes with
protein phosphatase 2A. Cell. 60:167–176. 1990.PubMed/NCBI View Article : Google Scholar
|
|
86
|
Zhang M, Meng XY, Cui M, Pascal JM,
Logothetis DE and Zhang JF: Selective phosphorylation modulates the
PIP2 sensitivity of the CaM-SK channel complex. Nat Chem Biol.
10:753–759. 2014.PubMed/NCBI View Article : Google Scholar
|
|
87
|
Stocker M, Krause M and Pedarzani P: An
apamin-sensitive Ca2+-activated K+ current in hippocampal pyramidal
neurons. Proc Natl Acad Sci USA. 96:4662–4667. 1999.PubMed/NCBI View Article : Google Scholar
|
|
88
|
Jiang ZS, Srisakuldee w, Soulet F, Bouche
G and Kardami E: Non-angiogenic FGF-2 protects the ischemic heart
from injury, in the presence or absence of reperfusion. Cardiovasc
Res. 62:154–166. 2004.PubMed/NCBI View Article : Google Scholar
|
|
89
|
Li X, Hu H, Wang Y, Xue M, Li X, Cheng W,
Xuan Y, Yin J, Yang N and Yan S: Valsartan Upregulates Kir2.1 in
Rats Suffering from Myocardial Infarction via Casein Kinase 2.
Cardiovasc Drugs Ther. 29:209–218. 2015.PubMed/NCBI View Article : Google Scholar
|
|
90
|
Stocker M and Pedarzani P: Differential
distribution of three Ca(2+)-activated K(+) channel subunits, SK1,
SK2, and SK3, in the adult rat central nervous system. Mol Cell
Neurosci. 15:476–493. 2000.PubMed/NCBI View Article : Google Scholar
|
|
91
|
Xia XM, Fakler B, Rivard A, Wayman G,
Johnson-Pais T, Keen JE, Ishii T, Hirschberg B, Bond CT, Lutsenko
S, et al: Mechanism of calcium gating in small-conductance
calcium-activated potassium channels. Nature. 395:503–507.
1998.PubMed/NCBI View
Article : Google Scholar
|
|
92
|
Brehme H, Kirschstein T, Schulz R and
Kohling R: In vivo treatment with the casein kinase 2 inhibitor
4,5,6,7-tetrabromotriazole augments the slow afterhyperpolarizing
potential and prevents acute epileptiform activity. Epilepsia.
55:175–183. 2013.PubMed/NCBI View Article : Google Scholar
|
|
93
|
Bajorat R, Porath K, Kuhn J, Gossla E,
Goerss D, Sellmann T, Köhling R and Kirschstein T: Oral
administration of the casein kinase 2 inhibitor TBB leads to
persistent KCa2.2 channel up-regulation in the epileptic CA1 area
and cortex, but lacks anti-seizure efficacy in the pilocarpine
epilepsy model. Epilepsy Res. 147:42–50. 2018.PubMed/NCBI View Article : Google Scholar
|
|
94
|
Schulze F, Muller S, Guli X, Schumann L,
Brehme H, Riffert T, Rohde M, Goerss D, Rackow S, Einsle A,
Kirschstein T and Kohling R: CK2 inhibition prior to status
epilepticus persistently enhances KCa 2 function in CA1
which slows down disease progression. Front Cell Neurosci.
14(33)2020.PubMed/NCBI View Article : Google Scholar
|
|
95
|
Clapham DE: Calcium signaling. Cell.
131:1047–1058. 2007.PubMed/NCBI View Article : Google Scholar
|
|
96
|
Afzal M, Kren BT, Naveed AK, Trembley JH
and Ahmed K: Protein kinase CK2 impact on intracellular calcium
homeostasis in prostate cancer. Mol Cell Biochem. 470:131–143.
2020.PubMed/NCBI View Article : Google Scholar
|
|
97
|
Pankratov Y and Lalo U: Calcium
permeability of ligand-gated Ca2+ channels. Eur J Pharmacol.
739:60–73. 2014.PubMed/NCBI View Article : Google Scholar
|
|
98
|
Prakriya M and Lewis RS: Store-operated
calcium channels. Physiol Rev. 95:1383–1436. 2015.PubMed/NCBI View Article : Google Scholar
|
|
99
|
Zamponi GW, Striessnig J, Koschak A and
Dolphin AC: The physiology, pathology, and pharmacology of
voltage-gated calcium channels and their future therapeutic
potential. Pharmacol Rev. 67:821–870. 2015.PubMed/NCBI View Article : Google Scholar
|
|
100
|
Zamponi GW: A crash course in calcium
channels. ACS Chem Neurosci. 8:2583–2585. 2017.PubMed/NCBI View Article : Google Scholar
|
|
101
|
Catterall WA: Structure and regulation of
voltage-gated Ca2+ channels. Annu Rev Cell Dev Biol. 16:521–555.
2000.PubMed/NCBI View Article : Google Scholar
|
|
102
|
Christel C and Lee A: Ca2+-dependent
modulation of voltage-gated Ca2+ channels. Biochim Biophys Acta.
1820:1243–1252. 2012.PubMed/NCBI View Article : Google Scholar
|
|
103
|
Xu L, Sun L, Xie L, Mou S, Zhang D, Zhu J
and Xu P: Advance in L-type calcium channel structures, functions
and molecular modeling. Curr Med Chem: Jul 14, 2020, Doi:
10.2174/0929867327666200714154059 Online ahead of print.
|
|
104
|
Weiss S, Oz S, Benmocha A and Dascal N:
Regulation of cardiac L-type Ca2+ channel CaV1.2 via the
β-adrenergic-cAMP-protein kinase A pathway: Old dogmas, advances,
and new uncertainties. Circ Res. 113:617–631. 2013.PubMed/NCBI View Article : Google Scholar
|
|
105
|
Hulme JT, Lin TW, Westenbroek RE, Scheuer
T and Catterall WA: Beta-adrenergic regulation requires direct
anchoring of PKA to cardiac CaV1.2 channels via a leucine zipper
interaction with A kinase-anchoring protein 15. Proc Natl Acad Sci
USA. 100:13093–13098. 2003.PubMed/NCBI View Article : Google Scholar
|
|
106
|
De Jongh KS, Murphy BJ, Colvin AA, Hell
JW, Takahashi M and Catterall WA: Specific phosphorylation of a
site in the full-length form of the alpha 1 subunit of the cardiac
L-type calcium channel by adenosine 3',5'-cyclic
monophosphate-dependent protein kinase. Biochemistry.
35:10392–10402. 1996.PubMed/NCBI View Article : Google Scholar
|
|
107
|
Peterson BZ, DeMaria CD, Adelman JP and
Yue DT: Calmodulin is the Ca2+ sensor for Ca2+ -dependent
inactivation of L-type calcium channels. Neuron. 22:549–558.
1999.PubMed/NCBI View Article : Google Scholar
|
|
108
|
Fuller MD, Emrick MA, Sadilek M, Scheuer T
and Catterall WA: Molecular mechanism of calcium channel regulation
in the fight-or-flight response. Sci Signal. 3(ra70)2010.PubMed/NCBI View Article : Google Scholar
|
|
109
|
Fu Y, Westenbroek RE, Scheuer T and
Catterall WA: Basal and β-adrenergic regulation of the cardiac
calcium channel CaV1.2 requires phosphorylation of serine 1700.
Proc Natl Acad Sci USA. 111:16598–16603. 2014.PubMed/NCBI View Article : Google Scholar
|
|
110
|
Fu Y, Westenbroek RE, Scheuer T and
Catterall WA: Phosphorylation sites required for regulation of
cardiac calcium channels in the fight-or-flight response. Proc Natl
Acad Sci USA. 110:19621–19626. 2013.PubMed/NCBI View Article : Google Scholar
|
|
111
|
Kashihara T, Nakada T, Kojima K, Takeshita
T and Yamada M: Angiotensin II activates CaV 1.2 Ca2+
channels through β-arrestin2 and casein kinase 2 in mouse immature
cardiomyocytes. J Physiol. 595:4207–4225. 2017.PubMed/NCBI View Article : Google Scholar
|
|
112
|
Hauck L, Harms C, Rohne J, Gertz K, Dietz
R, Endres M and von HR: Protein kinase CK2 links extracellular
growth factor signaling with the control of p27(Kip1) stability in
the heart. Nat Med. 14:315–324. 2008.PubMed/NCBI View
Article : Google Scholar
|
|
113
|
Gomez-Ospina N, Tsuruta F, Barreto-Chang
O, Hu L and Dolmetsch R: The C terminus of the L-type voltage-gated
calcium channel Ca(V)1.2 encodes a transcription factor. Cell.
127:591–606. 2006.PubMed/NCBI View Article : Google Scholar
|
|
114
|
Feng R, Xu J, Minobe E, Kameyama A, Yang
L, Yu L, Hao L and Kameyama M: Adenosine triphosphate regulates the
activity of guinea pig Cav1.2 channel by direct binding to the
channel in a dose-dependent manner. Am J Physiol Cell Physiol.
306:C856–C863. 2014.PubMed/NCBI View Article : Google Scholar
|
|
115
|
Braun M, Ramracheya R, Bengtsson M, Zhang
Q, Karanauskaite J, Partridge C, Johnson PR and Rorsman P:
Voltage-gated ion channels in human pancreatic beta-cells:
Electrophysiological characterization and role in insulin
secretion. Diabetes. 57:1618–1628. 2008.PubMed/NCBI View Article : Google Scholar
|
|
116
|
Yang SN and Berggren PO: The role of
voltage-gated calcium channels in pancreatic beta-cell physiology
and pathophysiology. Endocr Rev. 27:621–676. 2006.PubMed/NCBI View Article : Google Scholar
|
|
117
|
Scheuer R, Philipp SE, Becker A, Nalbach
L, Ampofo E, Montenarh M and Götz C: Protein kinase CK2 controls
CaV2.1-dependent calcium currents and insulin release in pancreatic
β-cells. Int J Mol Sci. 21(4668)2020.PubMed/NCBI View Article : Google Scholar
|
|
118
|
Lolli G, Cozza G, Mazzorana M, Tibaldi E,
Cesaro L, Donella-Deana A, Meggio F, Venerando A, Franchin C, Sarno
S, et al: Inhibition of protein kinase CK2 by flavonoids and
tyrphostins. A structural insight. Biochemistry. 51:6097–6107.
2012.PubMed/NCBI View Article : Google Scholar
|
|
119
|
Catterall WA: Voltage-gated calcium
channels. Cold Spring Harb Perspect Biol. 3(a003947)2011.PubMed/NCBI View Article : Google Scholar
|
|
120
|
Kahle JJ, Gulbahce N, Shaw CA, Lim J, Hill
DE, Barabási AL and Zoghbi HY: Comparison of an expanded ataxia
interactome with patient medical records reveals a relationship
between macular degeneration and ataxia. Hum Mol Genet. 20:510–527.
2011.PubMed/NCBI View Article : Google Scholar
|
|
121
|
Rettig J, Sheng ZH, Kim DK, Hodson CD,
Snutch TP and Catterall WA: Isoform-specific interaction of the
alpha1A subunits of brain Ca2+ channels with the presynaptic
proteins syntaxin and SNAP-25. Proc Natl Acad Sci USA.
93:7363–7368. 1996.PubMed/NCBI View Article : Google Scholar
|
|
122
|
Hilfiker S, Pieribone VA, Nordstedt C,
Greengard P and Czernik AJ: Regulation of synaptotagmin I
phosphorylation by multiple protein kinases. J Neurochem.
73:921–932. 1999.PubMed/NCBI View Article : Google Scholar
|
|
123
|
Castillo MA, Ghose S, Tamminga CA and
Ulery-Reynolds PG: Deficits in syntaxin 1 phosphorylation in
schizophrenia prefrontal cortex. Biol Psychiatry. 67:208–216.
2010.PubMed/NCBI View Article : Google Scholar
|
|
124
|
Hwang TC, Yeh JT, Zhang J, Yu YC, Yeh HI
and Destefano S: Structural mechanisms of CFTR function and
dysfunction. J Gen Physiol. 150:539–570. 2018.PubMed/NCBI View Article : Google Scholar
|
|
125
|
Fajac I and De BK: New horizons for cystic
fibrosis treatment. Pharmacol Ther. 170:205–211. 2017.PubMed/NCBI View Article : Google Scholar
|
|
126
|
Csanady L, Vergani P and Gadsby DC:
Structure, gating, and regulation of the CFTR anion channel.
Physiol Rev. 99:707–738. 2019.PubMed/NCBI View Article : Google Scholar
|
|
127
|
Cesaro L, Marin O, Venerando A,
Donella-Deana A and Pinna LA: Phosphorylation of cystic fibrosis
transmembrane conductance regulator (CFTR) serine-511 by the
combined action of tyrosine kinases and CK2: The implication of
tyrosine-512 and phenylalanine-508. Amino Acids. 45:1423–1429.
2013.PubMed/NCBI View Article : Google Scholar
|
|
128
|
Luz S, Kongsuphol P, Mendes AI, Romeiras
F, Sousa M, Schreiber R, Matos P, Jordan P, Mehta A, Amaral MD, et
al: Contribution of casein kinase 2 and spleen tyrosine kinase to
CFTR trafficking and protein kinase A-induced activity. Mol Cell
Biol. 31:4392–4404. 2011.PubMed/NCBI View Article : Google Scholar
|
|
129
|
Mehta A: Cystic fibrosis as a bowel cancer
syndrome and the potential role of CK2. Mol Cell Biochem.
316:169–175. 2008.PubMed/NCBI View Article : Google Scholar
|
|
130
|
Pagano MA, Arrigoni G, Marin O, Sarno S,
Meggio F, Treharne KJ, Mehta A and Pinna LA: Modulation of protein
kinase CK2 activity by fragments of CFTR encompassing F508 may
reflect functional links with cystic fibrosis pathogenesis.
Biochemistry. 47:7925–7936. 2008.PubMed/NCBI View Article : Google Scholar
|
|
131
|
Treharne KJ, Xu Z, Chen JH, Best OG,
Cassidy DM, Gruenert DC, Hegyi P, Gray MA, Sheppard DN, Kunzelmann
K and Mehta A: Inhibition of protein kinase CK2 closes the CFTR Cl
channel, but has no effect on the cystic fibrosis mutant
deltaF508-CFTR. Cell Physiol Biochem. 24:347–360. 2009.PubMed/NCBI View Article : Google Scholar
|
|
132
|
Venerando A, Pagano MA, Tosoni K, Meggio
F, Cassidy D, Stobbart M, Pinna LA and Mehta A: Understanding
protein kinase CK2 mis-regulation upon F508del CFTR expression.
Naunyn Schmiedebergs Arch Pharmacol. 384:473–488. 2011.PubMed/NCBI View Article : Google Scholar
|
|
133
|
Pagano MA, Marin O, Cozza G, Sarno S,
Meggio F, Treharne KJ, Mehta A and Pinna LA: Cystic fibrosis
transmembrane regulator fragments with the Phe508 deletion exert a
dual allosteric control over the master kinase CK2. Biochem J.
426:19–29. 2010.PubMed/NCBI View Article : Google Scholar
|
|
134
|
Pinto MC, Schreiber R, Lerias J,
Ousingsawat J, Duarte A, Amaral M and Kunzelmann K: Regulation of
TMEM16A by CK2 and its role in cellular proliferation. Cells.
9(1138)2020.PubMed/NCBI View Article : Google Scholar
|
|
135
|
Roosbeek S, Peelman F, Verhee A, Labeur C,
Caster H, Lensink MF, Cirulli C, Grooten J, Cochet C,
Vandekerckhove JL, et al: Phosphorylation by protein kinase CK2
modulates the activity of the ATP binding cassette A1 transporter.
J Biol Chem. 279:37779–37788. 2004.PubMed/NCBI View Article : Google Scholar
|
|
136
|
Bai X, Moraes TF and Reithmeier RAF:
Structural biology of solute carrier (SLC) membrane transport
proteins. Mol Membr Biol. 34:1–32. 2017.PubMed/NCBI View Article : Google Scholar
|
|
137
|
Ibrahim SH, Turner MJ, Saint-Criq V,
Garnett J, Haq IJ, Brodlie M, Ward C, Borgo C, Salvi M, Venerando A
and Gray MA: CK2 is a key regulator of SLC4A2-mediated
Cl-/HCO3-exchange in human airway epithelia.
Pflugers Arch. 469:1073–1091. 2017.PubMed/NCBI View Article : Google Scholar
|
|
138
|
Stolk M, Cooper E, Vilk G, Litchfield DW
and Hammond JR: Subtype-specific regulation of equilibrative
nucleoside transporters by protein kinase CK2. Biochem J.
386:281–289. 2005.PubMed/NCBI View Article : Google Scholar
|