Introduction
Stress serves a primary role in the pathogenesis of
psychiatric disorders (1). Previous
studies have demonstrated that stress may eventually trigger or
exacerbate mood disorders (2-4).
Exposure to stress has profound consequences on physiological,
biochemical and neurobehavioral function (5,6).
Environmentally induced depression, such as chronic
social isolation, has long been implicated as a risk factor for
depression in humans and also induces anxiety and depression-like
behavior in rodents. To date, environmental models are most
commonly used for studying depression (7,8).
Although depression and anxiety are highly prevalent
serious stress-related psychiatric disorders, they are poorly
understood (9,10). In Saudi Arabia, the overall
prevalence of depression has been reported to be ~12% (11). Current treatment strategies have
several major limitations, demonstrating the need to investigate
pathological mechanisms and thus determine the most effective
treatment strategy.
The blood-brain-barrier (BBB) is composed of
endothelial cellular units and astrocytes interconnected by tight
junction proteins. The integrity of BBB vascularity relies on the
function of these tight junctions. The tight junction unit consists
of various proteins, including claudin-5 (Cldn5) and certain tight
junction proteins, such as tight junction protein 1 (TJP1). These
are critical components that modulate BBB permeability (12). They are affected in multiple
psychiatric and neurological diseases such as depression,
Alzheimer’s and other neurodegenerative disorders, brain trauma,
stroke and multiple sclerosis (13).
A previous study demonstrated that cldn5
levels were decreased in the nucleus accumbens in depressed model
rats. Furthermore, the introduction of a Cldn5 adeno-associated
virus adeno-associated virus delivering shRNA against cldn5
in different brain regions increased the passage of peripheral IL-6
into the central nervous system (CNS), leading to depression-like
behavior (14).
In a pharmacological model of depression
(lipopolysaccharide-injected mice), tight junction proteins,
including cldn5 and tip, were significantly reduced. These results
indicated that BBB dysfunction is associated with the dysregulation
of ion transport, homeostasis and the passage of immune cells into
the CNS (13). A widely recognized
hypothesis is that of inflammation and depression. This theory
explains the relationship between immune system function and its
contribution to the neurobiology of depression (15,16). It
was previously reported that chronically isolated rats exhibited
depressive-like behavior and, at a molecular level, multiple
members of the Toll-like receptor (TLR) family were increased in
the hippocampus (17).
Accumulating evidence has uncovered an association
between mood disorders, particularly depression, and
neuroinflammation (18). Clinical
and preclinical studies have suggested that alterations in IL-6
levels are fundamental in the provocation of depression (19-21).
The present study aimed to: i) Examine the impact of
stressful chronic social isolation on IL-6 levels in the
hippocampal region of the brain; ii) investigate the mRNA
expression of BBB markers in the hippocampus; iii) analyze the
effect of acute fluoxetine treatment, a standard antidepressant;
and iv) analyze the mRNA expression of BBB markers in different
environmental conditions, such as an enriched environment and
short-term isolation. To address these aims, the current study
utilized different environmental conditions and pharmacological
treatments.
Materials and methods
Animals
A total of 46 adult, 5-7 weeks of age, male Wistar
rats (150-175 g) were obtained from the Animal Care Centre at the
College of Pharmacy, King Saud University (Riyadh, Saudi Arabia).
Animals were housed under a 12-h light/dark cycle at a temperature
of 25±1˚C, with ad libitum access to food and water. Rats
were left to adapt to the laboratory environment for 1 week prior
to experimentation and were randomly divided into three main
experiments. All experiments were carried out in accordance with
the recommendations of the Experimental Animals Ethics Committee
Acts of King Saud University, The Research Ethics Committee
(approval no. KSU-SE-18-20).
Experiment one
For a 6-week period, rats were divided into four
groups as follows (n=10): i) Paired ii) isolated; iii) isolated
with fluoxetine treatment; and iv) paired with fluoxetine
treatment. Fluoxetine (25 mg/kg p.o.) was administered to isolated
and paired rats on the 6th week (22) and to minimize stress during the
experimental procedure, fluoxetine was administered in drinking
water (17). The acutely treated
group was used to examine the effects of short-term treatment. The
paired-treated group served as a control. Antidepressants are known
to significantly alter synaptic plasticity as these agents
massively modulate multiple pathways and physiological mechanisms.
They influence mood-related circuits, adult neurogenesis, neuronal
survival, resiliency and adaptability (23).
After euthanizing the animals by CO2,
using up to 30% displacement rate (approximately 5 l/min), and the
absence of reflexes verified death, trunk body blood was collected
and brains were rapidly removed, snap-frozen in liquid nitrogen and
stored at -80˚C until further use. Molecular changes in levels of
BBB inflammatory markers (cldn5 and tjp1) and the
central inflammatory marker (IL-6) were examined via reverse
transcription-quantitative PCR (RT-qPCR) analysis. Levels of
corticosterone, a peripherally stress-related marker, were
additionally determined using ELISA.
Experiment two: Enriched environment
(EE) housing as described previously (17)
Parallel with experiment one, experiment two,
involving EE conditions, was conducted. EE housing criteria was
selected to further our understanding of the effect of
environmental conditions on the molecular expression of BBB
parameters at the mRNA level. A total of 10 rats were housed in a
cage with dimensions of 1.5x0.5x0.7 m. Bedding was changed every
day for a 6-week period. The animals were also provided with 8-10
toys, which were removed and washed three times a week, at which
point half were then changed (22).
Experiment three: Short-term isolation
as described previously (24)
A total of 6 rats were divided into two groups
(each, n=3). The first group was housed in standard conditions with
three animals per cage. The second group had one rat per cage,
where animals were housed for a total of 5 days. Rats were then
sacrificed.
Serum corticosterone level
determination
A trunk blood sample from each sacrificed rat was
collected in regular tubes. Samples were then centrifuged at 30,588
x g at 4˚C for 30 min to obtain serum. After serum was collected in
Eppendorf tubes, samples were stored at -80˚C. Serum corticosterone
levels were analyzed using an ELISA kit in accordance with the
manufacturer's protocol (Abcam; cat. no. ab108821). The absorbance
of the standards and samples was measured using a
BioTek® Synergy™ HT microplate reader (Bio-Tek
Instruments, Inc.) at a wavelength of 450 nm.
Quantification of mRNA using
RT-qPCR
RT-qPCR was conducted as described previously
(24). Isolated hippocampal RNA was
purified and converted to cDNA using a High-Capacity cDNA Reverse
Transcription kit in accordance with the manufacturer's protocol.
The following primers were utilized: IL-6 forward,
5'-CTTCCTAAAGATGGCTGCACTA-3' and reverse,
5'-CTGACTTGGCAGAGGACAAA-3'; cldn5 forward,
5'-AGCCCGCGTTCGGAAA-3' and reverse, 5'-ATTCAGCGGTGGTCGTCATC-3';
tjp1 forward, 5'-CGAGGCATCGTTCCTAATAAGAA-3' and reverse,
5'-ATCGCCACCTGCTGTCTTTG-3'; GAPDH forward,
5'-GACATGCCGCCTGGAGAAAC-3' and reverse, 5'-AGCCCAGGATGCCCTTTAGT-3'.
RT-qPCR analysis was conducted using SYBR Green based detection
(Applied Biosystems 7500 QPCR detection system) with 7500 software
(version 2.0.1) in accordance with the supplier's recommendations
(each, Applied Biosystems; Thermo Fisher Scientific, Inc.). The
relative expression of target mRNA was computed from the target
cycle threshold (CT) value and the GAPDH CT value using the
quantitative comparative CT (∆∆CT) method. These normalized values
were then used to calculate a value expressing the fold change of
the gene relative to the control according to the Livak method. The
RT-qPCR was set up as follows: 50˚C for 2 min, 95˚C for 10 min,
then 40 cycles: 95˚C for 15 sec, 60˚C for 1 min (25).
Statistical analysis
All statistical analyses were conducted using
GraphPad Prism version 8 (GraphPad Software, Inc.). Differences
between two groups were determined using an unpaired Student's
t-test and a Mann-Whitney U-test. Differences between paired
isolated, isolated + fluoxetine and paired + fluoxetine groups were
determined using two-way ANOVA followed by Tukey's multiple
comparisons post hoc tests (α level, 0.05). Data are presented as
the mean ± SEM and P<0.05 was considered to indicate a
statistically significant difference.
Results
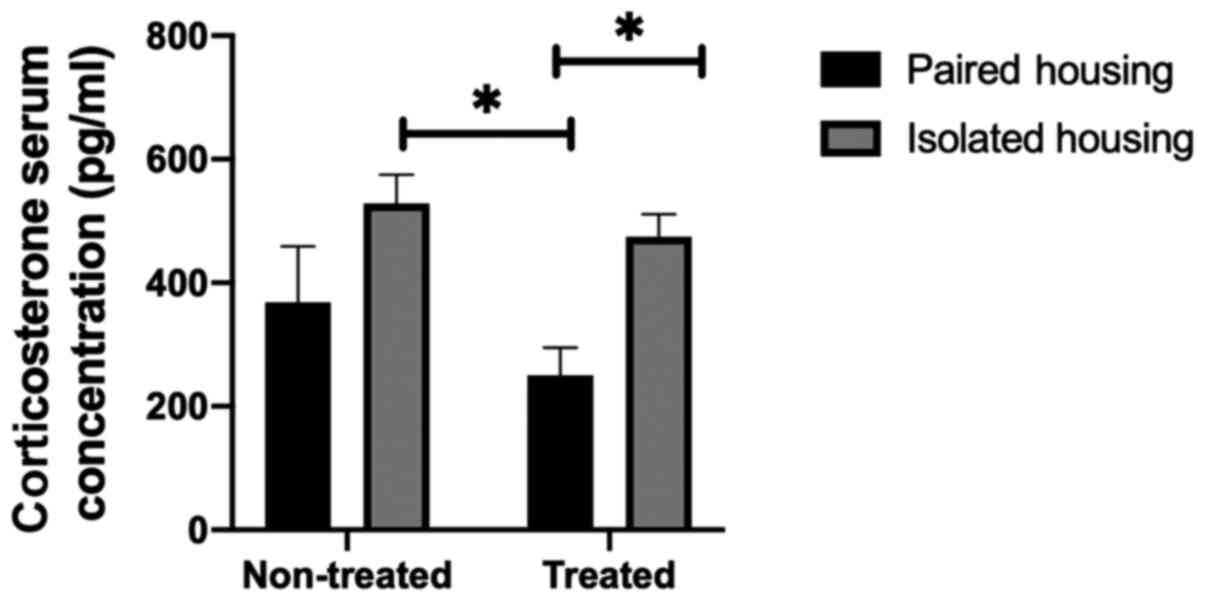
Corticosterone levels in the
periphery
As corticosterone is an indicator of stress, the
present study examined serum levels in paired, chronically
isolated, treated paired and isolated rats using two-way ANOVA
followed by a Tukey post hoc test. Treatment by housing condition
interaction was not significant (F1, 17=4.689;
P=0.5809); however the overall effect of housing conditions was
significant (F1, 17=11.37; P=0.0036). Serum
corticosterone levels in the chronically isolated group were
increased when compared with the paired group. Furthermore, a
significant difference between the levels of serum corticosterone
in chronically isolated rats and paired rats treated with
fluoxetine was determined (P=0.0168; determined using a Tukey's
post-hoc multiple comparisons test). Similarly, the level of serum
corticosterone was significantly higher in isolated treated rats
compared with paired treated rats (P=0.0498). The results indicated
that the 1-week treatment with fluoxetine did not have a
significant effect on serum corticosterone levels in the isolated
groups compared with non-treated isolated groups (P=0.9000;
Fig. 1).
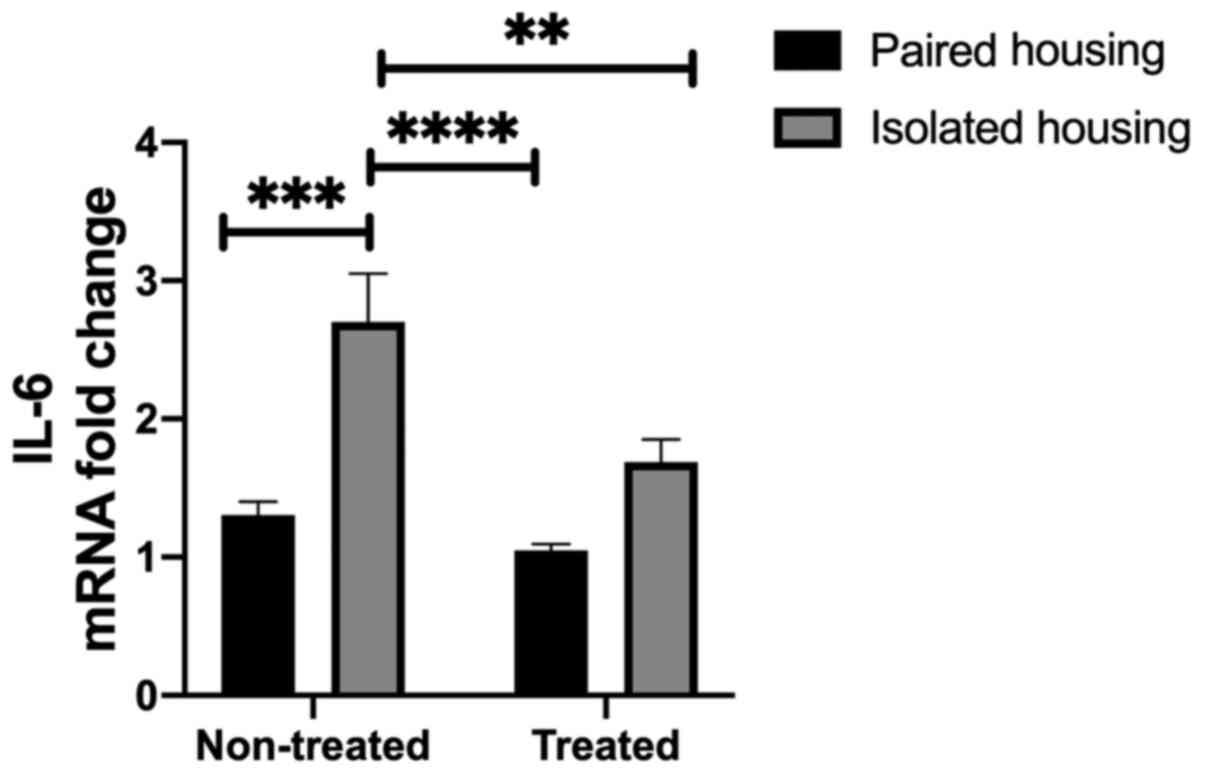
Inflammatory mediator levels in the
hippocampus of chronically isolated rats, isolated rats treated
with fluoxetine and paired rats treated with fluoxetine
Levels of IL-6 in the hippocampus were assessed in
the four tested groups. Two-way ANOVA indicated significant effects
following housing (F1, 24=25.69; P<0.0001) and
treatment (F1, 24=9.960; P=0.0043); however, the
interaction between the two was not significant (F1,
24=3.529; P=0.0725). A significant increase in IL-6 mRNA
levels were demonstrated in the chronic social isolation-induced
group compared with the paired housing group (P=0.0003), as
determined using a Tukey's post-hoc multiple comparisons test.
Additionally, IL-6 mRNA was significantly increased in the
hippocampi of isolated rats when compared with paired rats treated
with fluoxetine (P<0.0001). Treatment with fluoxetine
significantly reduced IL-6 levels in the isolated group compared
with non-treated isolated rats (P=0.0081; Fig. 2).
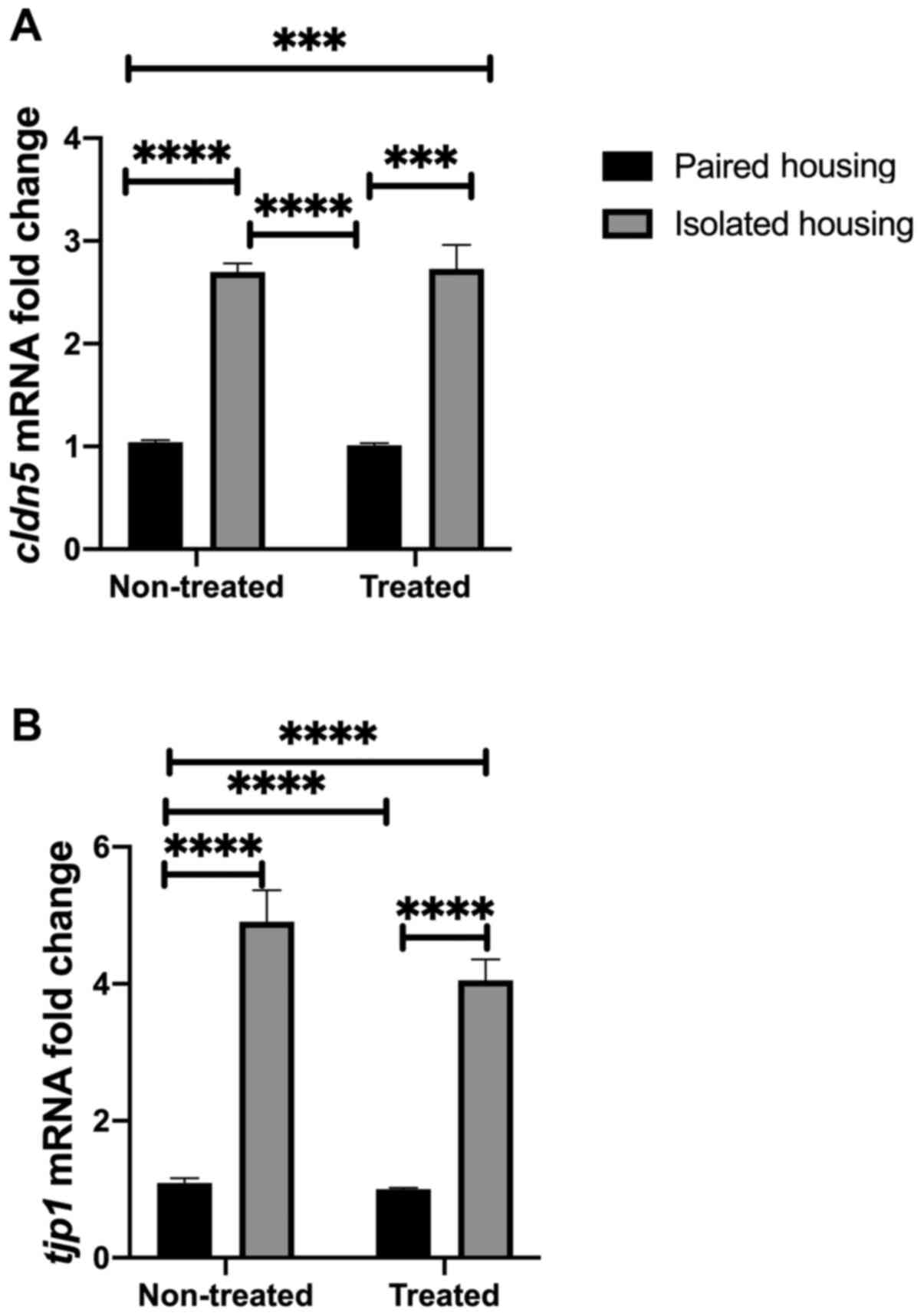
Neurovascular integrity at the
molecular level
The current study investigated whether alterations
in tight junction proteins were associated with changes in BBB
integrity at the mRNA level. To achieve this, the mRNA levels of
cldn5 and tjp1 were assessed in the four tested
groups (paired, isolated, isolated + fluoxetine and paired
+fluoxetine). Two-way ANOVA was utilized to quantify cldn5
mRNA expression. The results indicated no significance between
tight junction proteins and BBB integrity. However, the effect of
housing conditions was substantial (F1, 27=170.4;
P<0.0001). Tukey's post hoc analysis indicated that cldn5
mRNA expression was increased in both isolated and
fluoxetine-treated isolated groups compared to the paired housed
group (P<0.0001). Additionally, the expression of cldn5
was significantly higher in the isolated group compared with the
paired fluoxetine-treated group (P<0.0001). In each treated
group, post hoc analysis revealed that Cldn5 expression was
significantly increased in the isolated group compared with the
paired group (P<0.0001; Fig. 3A).
BBB in conditions of stress was further assessed by measuring
tjp1, an additional BBB-related gene. tjp1 acts as a
tight junction adaptor protein that also regulates adherence
junctions (26). Two-way ANOVA
analysis indicated that the interaction between tjp1 and
treatment was not significant, while the effect of housing
conditions was (F1, 27=170.4; P<0.0001). Tukey's post
hoc analysis indicated that tjp1 mRNA expression was
increased in the isolated and fluoxetine-treated isolated groups
compared to the paired housed group (P<0.0001). Additionally,
TJP1 expression was significantly higher in the isolated group
compared with the paired fluoxetine-treated group (P<0.0001). In
each treated group, post hoc analysis demonstrated that TJP1
expression was significantly increased in the isolated group
compared with the paired group (P<0.0001; Fig. 3B).
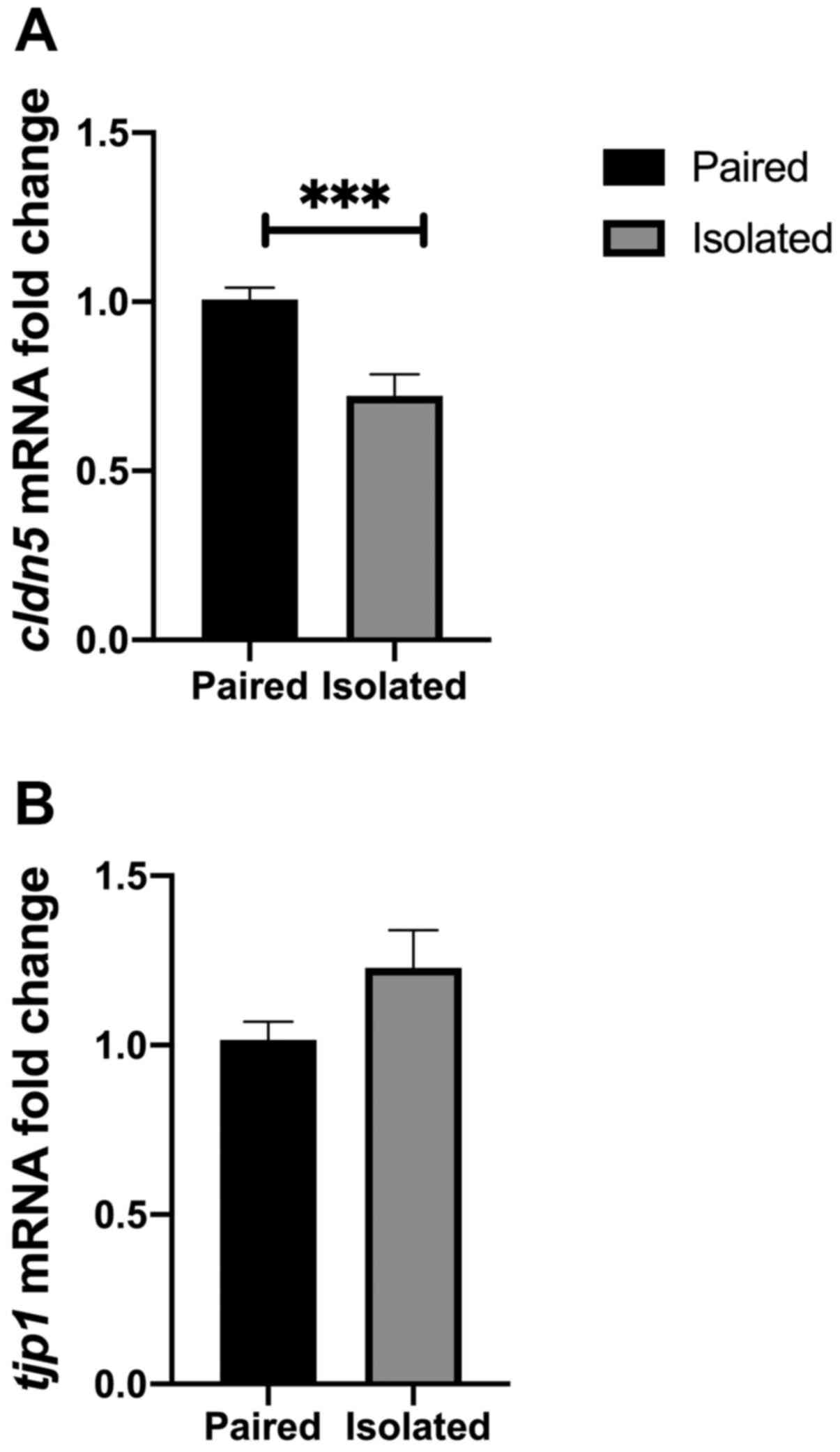
BBB marker mRNA levels in different
environmental conditions
The current study investigated whether BBB markers
could be affected by two different environmental conditions.
Short-term isolation was conducted using two main groups, a
short-term isolated group and a standard paired housing group.
Short-term isolation is considered a stressful condition that has
been employed to address questions associated with mood disorders
(27). The results of the present
study indicated that cldn5 mRNA was decreased in the
short-term isolation conditions compared with standard paired
housed rats (P=0.0008; Fig. 4A).
Although tjp1 mRNA levels were not affected by short term
isolation, increased levels were observed in rats of the isolated
housing group (P>0.05; Fig.
4B).
The second experimental setup comprised an EE
approach. This was employed for 6 weeks, along with 6 weeks of
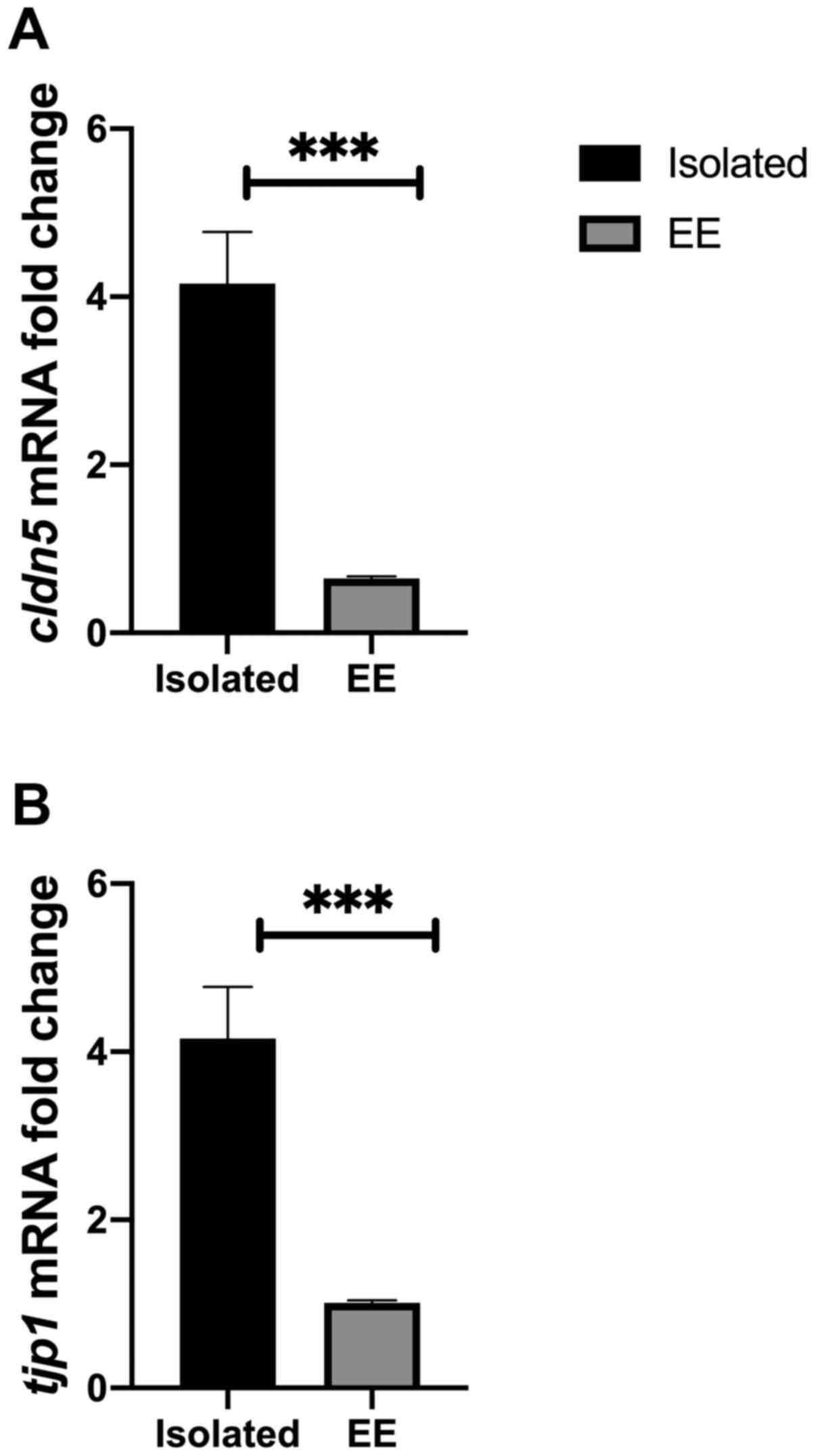
social isolation. The results indicated that cldn5 mRNA
levels were significantly reduced in EE compared with chronically
isolated rats (P=0.0004; Fig. 5A).
The results suggested that there was a significant reduction in the
level of tjp1 mRNA in the EE group compared with the
chronically isolated group (P=0.0006; Fig. 5B).
Discussion
The present study determined that hippocampal IL-6
levels were increased in rats experiencing chronic social
isolation. Additionally, the mRNA levels of certain
blood-brain-barrier (BBB) markers, including claudin-5
(cldn5) and tight junction protein (tjp1), were
increased in the isolated groups. The vulnerability of BBB markers
was determined at the mRNA level in short-term isolated rats and in
those under enriched environmental (EE) conditions. The results of
the present study supports existing research (14,28),
which furthers our understanding regarding the complexity of
pathological changes affecting BBB integrity and the level of
inflammation in stress-related disorders.
Social isolation has been linked to the development
of anxiety and depressive-like behaviors in rodents and depression
in humans (17,24,29).
Different environmental approaches were utilized in the current
study. EE housing is commonly used to investigate mechanisms
associated with stress-related disorders and resilience (2,30,31),
while acute stress is a valid tool for the etiological examination
of stress and stress coping mechanisms (24,32-34).
Under physiological conditions, the BBB serves a
pivotal role in regulating molecular exchange between peripheral
blood and the central nervous system (CNS). Proper maintenance of
this perfusion homeostasis is essential. Aberrant structures and
functions in endothelial subunits that constitute the BBB result in
an inability to maintain adequate CNS perfusion. However, the
pathological relevance of neurovascular dysfunction is poorly
understood (35-37).
Changes in BBB integrity have been reported in
various conditions such as poisoning, disruptions to the immune
system and diabetes (38).
Furthermore, in the context of neurological disorders, BBB
permeability is altered in cases of traumatic brain and spinal cord
injury, and in stroke patients (39). Furthermore, in a model of major
depressive disorder, Menard et al (14) reported that Cldn5, a standard BBB
marker, was altered. Li et al (40) also reported that connexin 40, which
is a gap junction protein and an indicator of astrocytic population
general health, was altered in chronic mildly stressed rats. It was
additionally determined that oral administration of fluoxetine
reversed these changes. Similarly, postmortem studies have revealed
that various members of the connexin family were altered in the
brains of depressed, suicidal patients. These tight junction
proteins were dysregulated at the mRNA level in brain regions such
as the cerebellar cortex, thalamus and caudate nucleus (41). Taken together, this evidence supports
the hypothesis of BBB dysfunction in depression.
The results of the current study demonstrated that
chronic social isolation decreases BBB permeability, as determined
by an increase in cldn5 and tjp1 mRNA expression.
Conditions of acute social isolation reduced the expression of
cldn5 compared with the controls. However, the results also
indicated a non-significant elevation in the mRNA levels of
tjp1 in the hippocampus. This result suggested that
cldn5 may be more sensitive and vulnerable to stressful
social conditions. This could be a compensation mechanism that
occurs in response to prolonged stressful isolation.
In contrast to the results of the present study,
Menard et al (14)
demonstrated that a model of chronic social defeat stress reduced
the mRNA expression of cldn5 in the nucleus accumbens.
Conversely, cldn5 expression was elevated in the hippocampus
of socially defeated rats at the protein level, suggesting a
potential compensatory mechanism at the nucleic acid and protein
level, as well as region-specific compensation. The discrepancy
between these results may be attributed to the fact that the
increased cldn5 expression found in the current study was
detected in the hippocampus, and different brain regions may have
different responses. Another explanation is that different animal
models might affect this mechanism in different ways. For example,
the present study used an environmental isolation model, whereas
Menard et al (14) used a
social defeat model. Another marker is tjp1, which is an
indispensable protein of the BBB. It is required for the
appropriate assembly of tight junctions, which are pivotal to the
interendothelial integrity of the BBB (42).
In contrast to previous research (14), the current study demonstrated that
tjp1 mRNA expression was increased in chronically isolated
rats. This may be due to inflammatory mediators, such as TLR7,
residing in close proximity to these elements of the BBB, leading
to overall inflammation, swelling and junction closing. Previous
studies have demonstrated that tjp1 expression is reduced in
patients with depression (14,43).
A reduction in BBB integrity leads to the
infiltration of peripheral cytokines, including IL-6, into the
brain, which affects neuronal populations and leads to observable
depression-like behavior (14).
Furthermore, tight junction proteins control the passage of
macromolecules and ionic components in and out of the BBB and, as a
consequence, regulate homeostasis (44,45). The
current study demonstrated that the administration of fluoxetine
altered the permeability of the BBB, indicating that social
isolation alters BBB permeability and that acute pharmacological
treatment with fluoxetine normalizes this effect.
A study by Fiorentino et al (46) demonstrated that, in the postmortem
tissue of patients diagnosed with autism spectrum disorders, the
BBB was disrupted. CLDN5 expression was elevated in
different brain regions, including the cortex and cerebellum.
Furthermore, these alterations were associated with a 66% increase
in tight junction proteins, including claudin, in the intestines of
these patients. This change in BBB integrity was coupled with
peripheral inflammation. However, an elevation in BBB markers does
not necessarily indicate that the protein produced is functional;
in fact, these data could suggest that the CLDN5 protein produced
in patients with autism may be disrupted or truncated, and that the
body then provides additional mRNA to compensate.
Previous research revealed that patients with
depression exhibit all the cardinal features of an inflammatory
response. Peripheral blood gene expression profiles are consistent
with an over-production of IL-6 and IL-8. Furthermore, the
increased expression of a variety of innate immune genes and
proteins, including IL-1β, IL-6, TNF, TLR3 and TLR4, has been found
in post-mortem brain samples from suicide victims with depression
(16). IL-6 has been linked to
stress-related disorders such as depression and anxiety. Many
patients with major depressive disorder also have higher levels of
IL-6 (14,47,48). In
rodents, both peripheral and hippocampal levels of IL-6 are
increased. For example, 4 weeks of constant darkness used as a
model of seasonal affective disorder, has been revealed to cause
depression-like behavior in rodents. Moreover, levels of IL-6 were
also altered (49). In line with
this result, IL-6 knockout mice have been found to be resistant to
the development of a depression-like phenotype following exposure
to constant darkness. This suggests a functional role for IL-6 in
stress susceptibility (47,49).
The results of the present study are similar to
those of previous reports, with data indicating that exposure to a
stressful environment leads to changes in the serum levels of
corticosterone and inflammatory mediators, along with an increase
in IL-6 levels of the brain. The current study provides evidence
that, following exposure to stressful events (chronically and
acutely isolated housing conditions), rats exhibited alterations in
the levels of BBB mRNA expression in the hippocampus. Some of these
changes were reversed by acute pharmacological treatment with
fluoxetine. The present study emphasized the role of the BBB in the
pathology of stress-related mood disorders. Future studies should
examine the functional kinetics of BBB integrity and fully
characterize the architecture of the BBB unit, which would aid in
addressing whether elevations in mRNA levels reflect an increase in
the expression of fully functional tight junction proteins. Future
studies should also describe the expression and structure of other
BBB components.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on request.
Authors' contributions
TKA designed the current study and contributed to
the acquisition, analysis and interpretation of data. HMA conducted
RT-qPCR and biochemical experiments and contributed to the analysis
and interpretation of data. HEA wrote the manuscript and
contributed to the analysis of the data. NMA, MAA and MFS provided
intellectual support and contributed to the study design and
drafting of the manuscript. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
All experiments were carried out in accordance with
the recommendations of the Experimental Animals Ethics Committee
Acts of King Saud University, The Research Ethics Committee
(approval no. KSU-SE-18-20).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Abelaira HM, Réus GZ and Quevedo J: Animal
models as tools to study the pathophysiology of depression. Braz J
Psychiatry. 35 (Suppl 2):S112–S120. 2013.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Ilin Y and Richter-Levin G: Enriched
environment experience overcomes learning deficits and
depressive-like behavior induced by juvenile stress. PLoS One.
4(e4329)2009.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Fernández-Guasti A, Fiedler JL, Herrera L
and Handa RJ: Sex, stress, and mood disorders: At the intersection
of adrenal and gonadal hormones. Horm Metab Res. 44:607–618.
2012.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Calabrese F, Molteni R, Racagni G and Riva
MA: Neuronal plasticity: A link between stress and mood disorders.
Psychoneuroendocrinology. 34 (Suppl 1):S208–S216. 2009.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Segerstrom SC and Miller GE: Psychological
stress and the human immune system: A meta-analytic study of 30
years of inquiry. Psychol Bull. 130:601–630. 2004.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Lupien SJ, McEwen BS, Gunnar MR and Heim
C: Effects of stress throughout the lifespan on the brain,
behaviour and cognition. Nat Rev Neurosci. 10:434–445.
2009.PubMed/NCBI View
Article : Google Scholar
|
|
7
|
Cacioppo JT, Cacioppo S, Capitanio JP and
Cole SW: The neuroendocrinology of social isolation. Annu Rev
Psychol. 66:733–767. 2015.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Coleman K, Weed JL and Schapiro SJ:
Environmental enrichment for animals used in research. In: Animal
Models for the Study of Human Disease. Conn PM (ed). Academic
Press, London, pp74-94, 2013.
|
|
9
|
Jia J and Le W: Molecular network of
neuronal autophagy in the pathophysiology and treatment of
depression. Neurosci Bull. 31:427–434. 2015.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Mehta-Raghavan NS, Wert SL, Morley C, Graf
EN and Redei EE: Nature and nurture: Environmental influences on a
genetic rat model of depression. Transl Psychiatry.
6(e770)2016.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Al-Qadhi W, Ur Rahman S, Ferwana MS and
Abdulmajeed IA: Adult depression screening in Saudi primary care:
Prevalence, instrument and cost. BMC Psychiatry.
14(190)2014.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Jiao H, Wang Z, Liu Y, Wang P and Xue Y:
Specific role of tight junction proteins claudin-5, occludin, and
ZO-1 of the blood-brain barrier in a focal cerebral ischemic
insult. J Mol Neurosci. 44:130–139. 2011.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Daneman R and Prat A: The blood-brain
barrier. Cold Spring Harb Perspect Biol. 7(a020412)2015.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Menard C, Pfau ML, Hodes GE, Kana V, Wang
VX, Bouchard S, Takahashi A, Flanigan ME, Aleyasin H, LeClair KB,
et al: Social stress induces neurovascular pathology promoting
depression. Nat Neurosci. 20:1752–1760. 2017.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Haapakoski R, Ebmeier KP, Alenius H and
Kivimäki M: Innate and adaptive immunity in the development of
depression: An update on current knowledge and technological
advances. Prog Neuropsychopharmacol Biol Psychiatry. 66:63–72.
2016.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Miller AH and Raison CL: The role of
inflammation in depression: From evolutionary imperative to modern
treatment target. Nat Rev Immunol. 16:22–34. 2016.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Alshammari TK, Alghamdi H, Green TA, Niazy
A, Alkahdar L, Alrasheed N, Alhosaini K, Alswayyed M, Elango R,
Laezza F, et al: Assessing the role of toll-like receptor in
isolated, standard and enriched housing conditions. PLoS One.
14(e0222818)2019.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Liu YZ, Wang YX and Jiang CL:
Inflammation: The common pathway of stress-related diseases. Front
Hum Neurosci. 11(316)2017.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Hodes GE, Ménard C and Russo SJ:
Integrating Interleukin-6 into depression diagnosis and treatment.
Neurobiol Stress. 4:15–22. 2016.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Ting EY, Yang AC and Tsai SJ: Role of
interleukin-6 in depressive disorder. Int J Mol Sci.
21(2194)2020.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Himmerich H, Patsalos O, Lichtblau N,
Ibrahim MAA and Dalton B: Cytokine research in depression:
principles, challenges, and open questions. Front Psychiatry.
10(30)2019.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Moncek F, Duncko R, Johansson BB and
Jezova D: Effect of environmental enrichment on stress related
systems in rats. J Neuroendocrinol. 16:423–431. 2004.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Castrén E and Hen R: Neuronal plasticity
and antidepressant actions. Trends Neurosci. 36:259–267.
2013.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Alshammari TK, Alghamdi H, Alkhader LF,
Alqahtani Q, Alrasheed NM, Yacoub H, Alnaem N, AlNakiyah M and
Alshammari MA: Analysis of the molecular and behavioral effects of
acute social isolation on rats. Behav Brain Res.
377(112191)2020.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Bauer HC, Krizbai IA, Bauer H and Traweger
A: ‘You Shall Not Pass’-tight junctions of the blood brain barrier.
Front Neurosci. 8(392)2014.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Holt-Lunstad J: The potential public
health relevance of social isolation and loneliness: Prevalence,
epidemiology, and risk factors. Public Policy Aging Rep.
27:127–130. 2017.
|
|
28
|
Dudek KA, Dion-Albert L, Lebel M, LeClair
K, Labrecque S, Tuck E, Ferrer Perez C, Golden SA, Tamminga C,
Turecki G, et al: Molecular adaptations of the blood-brain barrier
promote stress resilience vs depression. Proc Natl Acad Sci USA.
117:3326–3336. 2020.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Sargin D, Oliver DK and Lambe EK: Chronic
social isolation reduces 5-HT neuronal activity via upregulated SK3
calcium-activated potassium channels. Elife.
5(e21416)2016.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Lehmann ML and Herkenham M: Environmental
enrichment confers stress resiliency to social defeat through an
infralimbic cortex-dependent neuroanatomical pathway. J Neurosci.
31:6159–6173. 2011.PubMed/NCBI View Article : Google Scholar
|
|
31
|
McCreary JK and Metz GAS: Environmental
enrichment as an intervention for adverse health outcomes of
prenatal stress. Environ Epigenet. 2(dvw013)2016.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Takatsu-Coleman AL, Patti CL, Zanin KA,
Zager A, Carvalho RC, Borçoi AR, Ceccon LM, Berro LF, Tufik S,
Andersen ML and Frussa-Filho R: Short-term social isolation induces
depressive-like behaviour and reinstates the retrieval of an
aversive task: Mood-congruent memory in male mice? J Psychiatry
Neurosci. 38:259–268. 2013.PubMed/NCBI View Article : Google Scholar
|
|
33
|
de Kloet ER, Joëls M and Holsboer F:
Stress and the brain: From adaptation to disease. Nat Rev Neurosci.
6:463–475. 2005.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Kim JW, Ko MJ, Gonzales EL, Kang RJ, Kim
DG, Kim Y, Seung H, Oh HA, Eun PH and Shin CY: Social support
rescues acute stress-induced cognitive impairments by modulating
ERK1/2 phosphorylation in adolescent mice. Sci Rep.
8(12003)2018.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Quaegebeur A, Lange C and Carmeliet P: The
neurovascular link in health and disease: Molecular mechanisms and
therapeutic implications. Neuron. 71:406–424. 2011.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Rubio-Araiz A, Porcu F, Pérez-Hernández M,
García-Gutiérrez MS, Aracil-Fernández MA, Gutierrez-López MD,
Guerri C, Manzanares J, O'Shea E and Colado MI: Disruption of
blood-brain barrier integrity in postmortem alcoholic brain:
Preclinical evidence of TLR4 involvement from a binge-like drinking
model. Addict Biol. 22:1103–1116. 2017.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Wolburg H, Noell S, Mack A,
Wolburg-Buchholz K and Fallier-Becker P: Brain endothelial cells
and the glio-vascular complex. Cell Tissue Res. 335:75–96.
2009.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Venkat P, Chopp M and Chen J: Blood-brain
barrier disruption, vascular impairment, and ischemia/reperfusion
damage in diabetic stroke. J Am Heart Assoc.
6(e005819)2017.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Chodobski A, Zink BJ and
Szmydynger-Chodobska J: Blood-brain barrier pathophysiology in
traumatic brain injury. Transl Stroke Res. 2:492–516.
2011.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Li DQ, Li XJ, Duan JF and Cai W: Wuling
Capsule promotes hippocampal neurogenesis by improving expression
of connexin 43 in rats exposed to chronic unpredictable mild
stress. Zhong Xi Yi Jie He Xue Bao. 8:662–669. 2010.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Nagy C, Torres-Platas SG, Mechawar N and
Turecki G: Repression of astrocytic connexins in cortical and
subcortical brain regions and prefrontal enrichment of H3K9me3 in
depression and suicide. Int J Neuropsychopharmacol. 20:50–57.
2017.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Bauer H, Zweimueller-Mayer J, Steinbacher
P, Lametschwandtner A and Bauer HC: The dual role of zonula
occludens (ZO) proteins. J Biomed Biotechnol.
2010(402593)2010.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Esposito P, Gheorghe D, Kandere K, Pang X,
Connolly R, Jacobson S and Theoharides TC: Acute stress increases
permeability of the blood-brain-barrier through activation of brain
mast cells. Brain Res. 888:117–127. 2001.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Greene C and Campbell M: Tight junction
modulation of the blood brain barrier: CNS delivery of small
molecules. Tissue Barriers. 4(e1138017)2016.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Stamatovic SM, Keep RF and Andjelkovic AV:
Brain endothelial cell-cell junctions: how to ‘open’ the blood
brain barrier. Curr Neuropharmacol. 6:179–192. 2008.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Fiorentino M, Sapone A, Senger S, Camhi
SS, Kadzielski SM, Buie TM, Kelly DL, Cascella N and Fasano A:
Blood-brain barrier and intestinal epithelial barrier alterations
in autism spectrum disorders. Mol Autism. 7(49)2016.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Hodes GE, Kana V, Menard C, Merad M and
Russo SJ: Neuroimmune mechanisms of depression. Nat Neurosci.
18:1386–1393. 2015.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Ménard C, Hodes GE and Russo SJ:
Pathogenesis of depression: Insights from human and rodent studies.
Neuroscience. 321:138–162. 2016.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Monje FJ, Cabatic M, Divisch I, Kim EJ,
Herkner KR, Binder BR and Pollak DD: Constant darkness induces
IL-6-dependent depression-like behavior through the NF-κB signaling
pathway. J Neurosci. 31:9075–9083. 2011.PubMed/NCBI View Article : Google Scholar
|