Introduction
Platelet-derived growth factor-BB (PDGF-BB) is a
potent mitogenic and angiogenic agent, and chemoattractant
(1) that is involved in tissue
repair and stimulates tissue regeneration following injury
(2,3). PDGF-BB can be used for wound healing as
it enhances the formation of granulation tissue (2). Furthermore, PDGF-BB induces cell
migration of preosteoblast cells (4), and increases osteoclast formation and
chemotaxis of osteoclast precursor cells (5). Moreover, preosteoclasts secrete PDGF,
which leads to enhanced angiogenesis and osteogenesis (6). Recently, it has been shown that PDGF-BB
significantly promotes stem cell proliferation and increases stem
cell marker expression (7). PDGF-BB
has been confirmed to stimulate mesenchymal stem cell recruitment
(8), and to significantly increase
the migration of adipose tissue-derived stem cells in a
dose-dependent manner (9). Moreover,
PDGF-BB alters gene targeting related to the differentiation of
stem cells (9). Additionally,
PDGF-BB facilitates bone-marrow stem-cell-based bone regeneration
by enhancing the osteogenic and angiogenic capabilities of stem
cells (10). However, PDGF-BB has
been shown to suppress adipogenic differentiation in vitro
(11).
The use of 3D cultures for assessing the effects of
agents is increasing (12,13). 3D cultures can interact well with
their surroundings and more accurately simulate in vivo
conditions compared with 2D cultures (14). Various methods can be used to produce
3D cultures, including the hanging-drop method and bioreactors
(15). The use of silicone
elastomer-based concave microwells is suitable for producing
spheroids (16).
To the best of our knowledge, there are no previous
studies evaluating the effects of PDGF on the cell spheroids
composed of bone-marrow-derived stem cells using microwells. In
light of the promising findings in previous studies on PDGF-BB, the
aim of the present study was to examine the effects of PDGF-BB on
cellular morphology and cellular viability using 3D cultures of
stem cells.
Materials and methods
Generation of cell spheroids using
bone marrow mesenchymal stem cells
The Institutional Review Board of Seoul St Mary's
Hospital, College of Medicine, The Catholic University of Korea
reviewed and approved the present study (approval no.
KC19SESI0234). Human bone marrow mesenchymal stem cells (BMSCs;
Catholic MASTER cells) were obtained from the Catholic Institute of
Cell Therapy (CIC) (17). CIC
verified that all samples showed >90% positive CD-73 and CD-90
expression. The Catholic MASTER Cells supplied by CIC were derived
from human bone marrow donated by healthy donors who provided
informed consent.
Fig. 1 shows an
overview of the study design. The cells were plated on a culture
dish, and any cells which did not adhere to the dish after 2 days
were removed. The culture medium was replaced every 2 or 3 days,
and the BMSCs were grown in a humidified incubator with an
atmosphere of 95% air and 5% CO2 at 37˚C. Commercially
available concave microwells (cat. no. H389600; StemFIT 3D;
MicroFIT) was used to establish the stem cell spheroids. A total of
1x106 cells was added to each well, and 1 ml medium was
added to each microwell. The cell spheroids of BMSCs were treated
with 0, 10 or 100 ng/ml PDGF-BB, based on previous studies
(7,18). Their morphological characteristics
were evaluated using an inverted microscope (Leica DM IRM, Leica
Microsystems GmbH). The morphology of the spheroids was evaluated
on days 1, 3 and 7.
Determination of cellular
viability
The stem cell spheroids were cultured in growth
medium and a commercially available two-color assay based on plasma
membrane integrity and esterase activity (Live/Dead kit assay;
Molecular Probes; Thermo Fisher Scientific, Inc.) was used for
qualitative analysis of the stem cell spheroids on days 1 and 3
according to the manufacturer's protocol. A Cell Counting Kit-8
(CCK-8) assay was also used to assess cell viability according to
the manufacturer's protocol (Dojindo Molecular Technologies, Inc.).
Absorbance was measured at 450 n using a microplate reader (BioTek
Instruments, Inc.).
Statistical analysis
Statistical analysis was performed using SPSS
version 12 (SPSS, Inc.). Data are presented as the mean ± the
standard deviation. A normality and equal variance test was
performed. Subsequently, a two-way ANOVA was used to evaluate the
effects of concentration and time points, with a post hoc test
Tukey's to compare the differences amongst groups. P<0.05 was
considered to indicate a statistically significant difference.
Results
Morphological characteristics of stem
cell spheroids with human bone marrow-derived stem cells
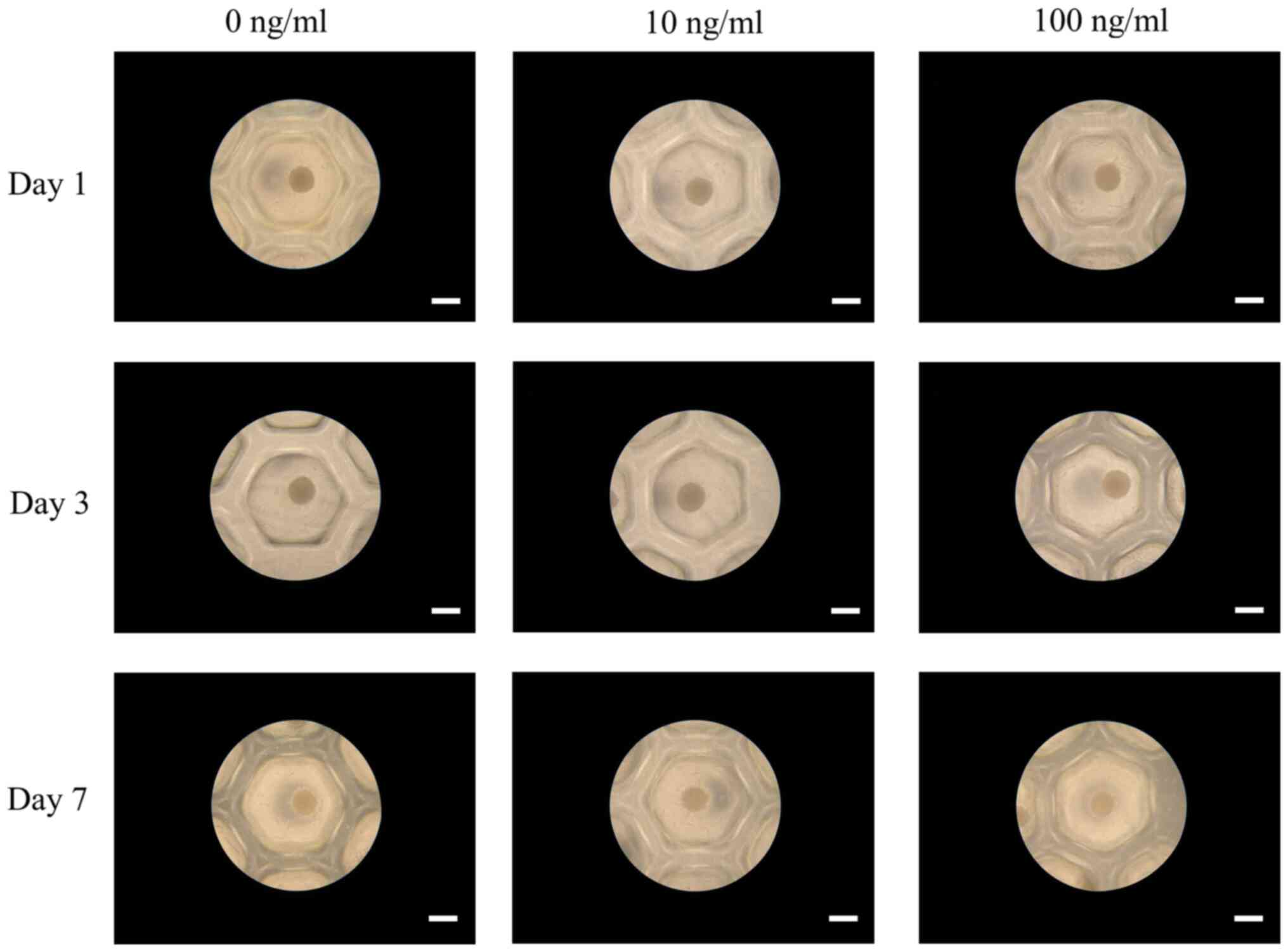
Intact stem cell spheroids were established in
concave microwells made of a silicone elastomer on day 1 (Fig. 2). The addition of 10 or 100 ng/ml
PDGF-BB did not affect cell spheroid morphology after 3 days
(Fig. 2). Following longer periods
of incubation of 7 days, the cell spheroids maintained their shape,
and no noticeable alterations were observed.
Determination of cellular
viability
Figs.
3-5 show the results of qualitative cell spheroid viability
analyzed using a live/dead assay on days 1, 3 and 7, respectively.
In all cases, the majority of cells in the spheroids presented
green fluorescence when analyzed using the live/dead assay,
indicating that the majority of cells were alive.
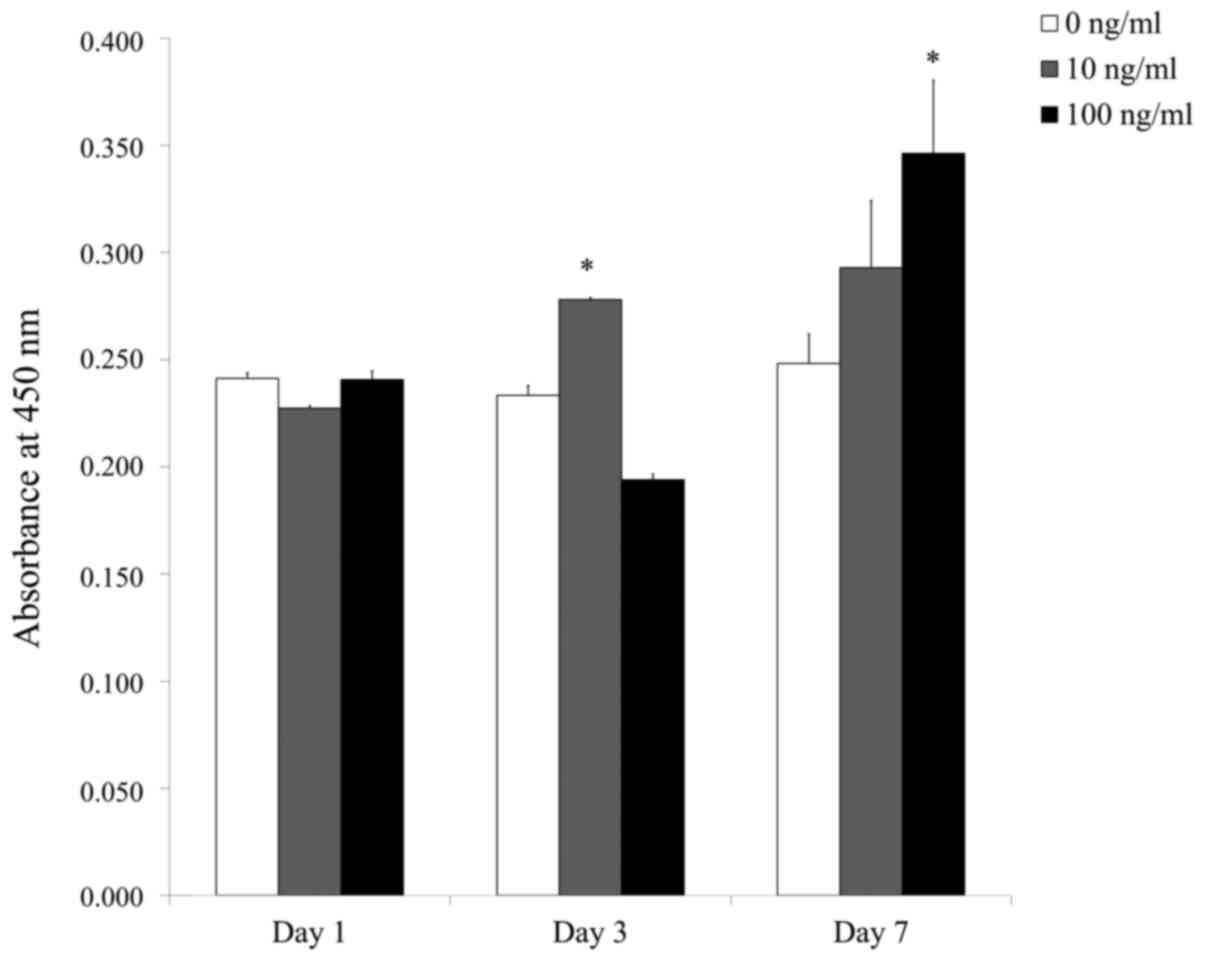
Fig. 6 shows
quantitative results of cellular viability on days 1, 3 and 7. On
day 1, the CCK-8 assay values for PDGF-BB at 0, 10 and 100 ng/ml
were 0.241±0.003, 0.227±0.001 and 0.241±0.004, respectively; on day
3, the CCK-8 assay values for 0, 10 and 100 ng/ml PDGF-BB were
0.233±0.005, 0.278±0.001 and 0.194±0.003, respectively; and on day
7, they were 0.248±0.014, 0.293±0.031 and 0.346±0.034,
respectively. On day 3, the 10 ng/ml group showed significantly
higher viability compared with the control group
(P<0.05), and on day 7 the 100 ng/ml group showed
significantly increased viability compared with the control group
(P<0.05).
Discussion
The aim of the present study was to assess the
effects of PDGF-BB on cellular morphology and cellular viability of
stem cell spheroids produced using 3D culturing methods. Treatment
with 10 and 100 ng/ml PDGF-BB increased cellular viability.
PDGF-BB is applied with bone matrix for the clinical
treatment of intraosseous periodontal defects (19). PDGF-BB applied with an
osteoconductive bone matrix exhibits similar or superior efficacy
to autogenous bone grafts in terms of bone regeneration (20). Histological analysis has shown that
the addition of recombinant human PDGF-BB (rhPDGF-BB) to bone
marrow stem cells and β-tricalcium phosphate yields superior
performance when compared with a β-tricalcium phosphate group
(18). When PDGF-BB was delivered to
tooth-supporting osseous defects, an increased release of
pyridinoline cross-linked carboxyterminal telopeptide of type I
collagen (a biomarker of bone turnover) was observed. This increase
promoted periodontal regeneration (21). Furthermore, rhPDGF-BB can be applied
to ridge augmentation, which is necessary for implant installation
(22). The effects of PDGF-BB, a
potent osteoinductive factor, are modulated by pro-inflammatory
cytokines (23). PDGF-BB enhances
the chemotaxis of osteoclast precursor cells and the formation of
osteoclasts (5).
The effects of different concentrations of PDGF-BB
have been evaluated in previous studies (7,18,24-26).
rhPDGF-BB concentrations of 0, 10, and 50 ng/ml were evaluated for
stem cell proliferation and differentiation, and it was shown that
50 ng/ml increased osteogenic differentiation, as exhibited by
increased alkaline phosphatase activity and elevated mRNA
expression levels of osteogenic genes (18). Similarly, PDGF-BB stimulated the
proliferation of human periodontal ligament cells with maximal
effects observed with 50 ng/ml (7).
In the present study, 10 and 100 ng/ml PDGF-BB increased cellular
viability. Moreover, 100 ng/ml appeared to be more effective when
cells were incubated for a longer period of 7 days. However, it
should be noted that this was based on qualitative evaluation of
trends in the data rather than a statistically significant
difference in the effects of the two concentrations. Moreover, the
use of only two concentrations of 10 and 100 ng/ml may be
considered a limitation of the present study. In a previous study,
application of PDGF-BB at the physiologically relevant
concentration of 20 ng/ml promoted osteogenic differentiation and
vascular network stability (24). In
an in vivo experiment, mice were treated with 0.25 or 1
mg/ml/day PDGF-BB, and PDGF-BB treatment yielded a range of
favorable results (25). A
meta-analysis found that 0.3 mg/ml PDGF-BB exhibited greater
capacity for clinical periodontal regeneration than other
concentrations (26). However, when
using 3D cultures, several considerations should be taken into
account. The cells in the center of a spheroid may receive
insufficient oxygen and nutrients and it is difficult to evaluate
the actual conditions of the cells in the central area (27,28).
Discrepancies in cellular viability between groups and times may
result from culture conditions, interactions between the cells and
the distribution of nutrients and waste (29,30).
Several studies have explored the molecular
mechanisms modulated by PDGF-BB (20,23,31-33),
and PDGF-BB is known to initiate the repair and regeneration of
bone and surrounding soft tissue (20). The effects of PDGF-BB on mesenchymal
stem cells and endothelial cells may be explained by notch
signaling, the PI3K pathway, ERK pathway and the protein kinase B
pathway (10,32,33).
PDGF-BB was also involved in multiple signaling pathways which
strongly stimulate migration and reduce sensitivity to inhibitory
signals (31). PDGF-BB accelerates
the maturation of collagen chains through increased lysyl oxidase
activity and expression of secreted protein, acidic and rich in
cysteine (7). PDGF-BB also
stimulates cell proliferation and osteogenic differentiation of
stem cells through the ERK pathway (34). PDGF-BB-induced ERK signaling, which
has been reported to be involved in parallel stimulatory and
inhibitory pathways, promotes Smad1/5/8 signaling (35).
A steady-rate of growth factor release (without
burst release) is required to achieve stable results (36,37).
Various scaffolds, including composite scaffolds, have been applied
for clinically relevant sustained release of PDGF-BB (4). Using a partition-type tubular scaffold
resulted in a steady cumulative release of 52% of PDGF-BB for 4
weeks (37). In a previous report,
PDGF-BB was loaded in chitosan nanoparticles incorporated into
electrospun nanofibers; this led to increased fibroblast migration,
showing possible applications for wound dressing (38). Polycaprolactone electrospun fibers
containing PDGF-BB-loaded chitosan nanoparticles enhanced the
chemotaxic effects of PDGF-BB (38).
PDGF-BB-encapsulated poly(lactic-co-glycolic acid) microspheres
showed increased responses in terms of cell attachment, cell
viability and release of osteogenic differentiation markers
(39). Dual delivery of PDGF-BB and
vascular endothelial growth factor was achieved by electrospinning
chitosan and poly(ethylene oxide) into nanofibrous meshes, which
led to short-term release of vascular endothelial growth factor and
sustained release of PDGF-BB (40).
Dual delivery of PDGF-BB and fibroblast growth factor-2 showed
synergistic effects on cell proliferation, migration and secretion
of vascular endothelial growth factor by endothelial progenitor
cells (41).
Another means of achieving sustained growth factor
release is gene therapy (42).
Sustained PDGF nonviral gene delivery was attained through a
gene-activated matrix delivering polyplexes of
polyethylenimine-plasmid DNA encoding PDGF (42). PDGF-BB gene-modified stem cells
through lentiviral delivery resulted in enhanced dentin-pulp tissue
regeneration (1). Dual delivery of
PDGF-BB and stem-cell-expressing bone morphogenetic protein-2
resulted in enhanced bone formation in critical-sized defects with
a higher-quality od regenerated bone (43). Similarly, co-expression of PDGF-BB
and vascular endothelial growth factor led to increased
angiogenesis (44), as well as
stable angiogenesis with long-term safety outcomes (45).
Nonetheless, some opposing results have been
reported regarding the effects of PDGF-BB (7,46,47). In
one study, PDGF-BB enhanced osteogenesis in adipose-derived stem
cells, but not in bone marrow-derived mesenchymal stem cells
(46). Conversely, PDGF-BB was found
to inhibit the production of collagen, but to accelerate the
maturation of collagen chains (7).
Additional studies are also required before PDGF-BB can be applied
confidently in socket augmentation (47).
In conclusion, the present study showed that
application of 10 and 100 ng/ml PDGF-BB increased cellular
viability, highlighting its potential for use in cell therapy.
Further studies are required to elucidate the underlying mechanisms
by which PDGF-BB exerts its beneficial effects.
Acknowledgements
Not applicable.
Funding
This study was supported by a National Research
Foundation of Korea grant funded by the Korean government (MSIT)
(grant no. 2020R1A2C4001624) and by the Research Fund of Seoul St.
Mary's Hospital, The Catholic University of Korea.
Availability of data and materials
All data generated or analyzed during this study are
included in the published article.
Authors' contributions
S-CP, SKM and J-BP designed the study, performed the
experiments and data analysis, and wrote the manuscript. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Institutional
Review Board at Seoul St Mary's Hospital, College of Medicine and
The Catholic University of Korea (approval no. KC19SESI0234).
Informed consent was obtained from all participants as specified in
the Declaration of Helsinki, and all of the experiments were
performed in accordance with the guidelines set out in the
Declaration of Helsinki.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Zhang M, Jiang F, Zhang X, Wang S, Jin Y,
Zhang W and Jiang X: The effects of platelet-derived growth
factor-BB on human dental pulp stem cells mediated dentin-pulp
complex regeneration. Stem Cells Transl Med. 6:2126–2134.
2017.PubMed/NCBI View Article : Google Scholar
|
|
2
|
LeGrand EK: Preclinical promise of
becaplermin (rhPDGF-BB) in wound healing. Am J Surg. 176 (Suppl
2A):S48–S54. 1998.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Wang C, Liu Y and He D: Diverse effects of
platelet-derived growth factor-BB on cell signaling pathways.
Cytokine. 113:13–20. 2019.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Zhao X and Hadjiargyrou M: Induction of
cell migration in vitro by an electrospun PDGF-BB/PLGA/PEG-PLA
nanofibrous scaffold. J Biomed Nanotechnol. 7:823–829.
2011.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Li DQ, Wan QL, Pathak JL and Li ZB:
Platelet-derived growth factor BB enhances osteoclast formation and
osteoclast precursor cell chemotaxis. J Bone Miner Metab.
35:355–365. 2017.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Gao SY, Zheng GS, Wang L, Liang YJ, Zhang
SE, Lao XM, Li K and Liao GQ: Zoledronate suppressed angiogenesis
and osteogenesis by inhibiting osteoclasts formation and secretion
of PDGF-BB. PLoS One. 12(e0179248)2017.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Mihaylova Z, Tsikandelova R, Sanimirov P,
Gateva N, Mitev V and Ishkitiev N: Role of PDGF-BB in
proliferation, differentiation and maintaining stem cell properties
of PDL cells in vitro. Arch Oral Biol. 85:1–9. 2018.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Phipps MC, Xu Y and Bellis SL: Delivery of
platelet-derived growth factor as a chemotactic factor for
mesenchymal stem cells by bone-mimetic electrospun scaffolds. PLoS
One. 7(e40831)2012.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Gehmert S, Gehmert S, Hidayat M, Sultan M,
Berner A, Klein S, Zellner J, Müller M and Prantl L: Angiogenesis:
The role of PDGF-BB on adipose-tissue derived stem cells (ASCs).
Clin Hemorheol Microcirc. 48:5–13. 2011.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Zhang M, Yu W, Niibe K, Zhang W, Egusa H,
Tang T and Jiang X: The effects of platelet-derived growth
factor-BB on bone marrow stromal cell-mediated vascularized bone
regeneration. Stem Cells Int. 2018(3272098)2018.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Jin Y, Zhang W, Liu Y, Zhang M, Xu L, Wu
Q, Zhang X, Zhu Z, Huang Q and Jiang X: rhPDGF-BB via ERK pathway
osteogenesis and adipogenesis balancing in ADSCs for critical-sized
calvarial defect repair. Tissue Eng Part A. 20:3303–3313.
2014.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Edmondson R, Broglie JJ, Adcock AF and
Yang L: Three-dimensional cell culture systems and their
applications in drug discovery and cell-based biosensors. Assay
Drug Dev Technol. 12:207–218. 2014.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Kim BB, Tae JY, Ko Y and Park JB:
Lovastatin increases the proliferation and osteoblastic
differentiation of human gingiva-derived stem cells in
three-dimensional cultures. Exp Ther Med. 18:3425–3430.
2019.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Tae JY, Ko Y and Park JB: Evaluation of
fibroblast growth factor-2 on the proliferation of osteogenic
potential and protein expression of stem cell spheroids composed of
stem cells derived from bone marrow. Exp Ther Med. 18:326–331.
2019.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Chaicharoenaudomrung N, Kunhorm P and
Noisa P: Three-dimensional cell culture systems as an in vitro
platform for cancer and stem cell modeling. World J Stem Cells.
11:1065–1083. 2019.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Tae JY, Lee H, Lee H, Ko Y and Park JB:
Osteogenic potential of cell spheroids composed of varying ratios
of gingiva-derived and bone marrow stem cells using concave
microwells. Exp Ther Med. 16:2287–2294. 2018.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Jeong CH, Kim SM, Lim JY, Ryu CH, Jun JA
and Jeun SS: Mesenchymal stem cells expressing brain-derived
neurotrophic factor enhance endogenous neurogenesis in an ischemic
stroke model. Biomed Res Int. 2014(129145)2014.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Xu L, Lv K, Zhang W, Zhang X, Jiang X and
Zhang F: The healing of critical-size calvarial bone defects in rat
with rhPDGF-BB, BMSCs, and β-TCP scaffolds. J Mater Sci Mater Med.
23:1073–1084. 2012.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Lee JY, Na HJ, Kim HM, Lee SC, Lee JY,
Chung CP, Seol YJ and Park YJ: Comparative study of rhPDGF-BB plus
equine-derived bone matrix versus rhPDGF-BB plus β-TCP in the
treatment of periodontal defects. Int J Periodontics Restorative
Dent. 37:825–832. 2017.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Friedlaender GE, Lin S, Solchaga LA, Snel
LB and Lynch SE: The role of recombinant human platelet-derived
growth factor-BB (rhPDGF-BB) in orthopaedic bone repair and
regeneration. Curr Pharm Des. 19:3384–3390. 2013.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Sarment DP, Cooke JW, Miller SE, Jin Q,
McGuire MK, Kao RT, McClain PK, McAllister BS, Lynch SE and
Giannobile WV: Effect of rhPDGF-BB on bone turnover during
periodontal repair. J Clin Periodontol. 33:135–140. 2006.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Scheines C, Hokett SD and Katancik JA:
Recombinant human platelet-derived growth factor-BB in human
alveolar ridge augmentation: A review of the literature. Int J Oral
Maxillofac Implants. 33:1047–1056. 2018.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Davies OG, Grover LM, Lewis MP and Liu Y:
PDGF is a potent initiator of bone formation in a tissue engineered
model of pathological ossification. J Tissue Eng Regen Med.
12:e355–e367. 2018.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Hutton DL, Moore EM, Gimble JM and Grayson
WL: Platelet-derived growth factor and spatiotemporal cues induce
development of vascularized bone tissue by adipose-derived stem
cells. Tissue Eng Part A. 19:2076–2086. 2013.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Ye C, Zhang W, Jiang S, Yu Y, Zhou X, Zhu
L, Xue D and He R: Platelet-derived growth factor-BB attenuates
titanium-particle-induced osteolysis in vivo. Growth Factors.
34:177–186. 2016.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Li F, Yu F, Xu X, Li C, Huang D, Zhou X,
Ye L and Zheng L: Evaluation of recombinant human FGF-2 and PDGF-BB
in periodontal regeneration: A systematic review and meta-analysis.
Sci Rep. 7(65)2017.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Sirenko O, Mitlo T, Hesley J, Luke S,
Owens W and Cromwell EF: High-content assays for characterizing the
viability and morphology of 3D cancer spheroid cultures. Assay Drug
Dev Technol. 13:402–414. 2015.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Park JB, Jeong JH, Lee M, Lee DY and Byun
Y: Xenotransplantation of exendin-4 gene transduced pancreatic
islets using multi-component (alginate, poly-L-lysine, and
polyethylene glycol) microcapsules for the treatment of type 1
diabetes mellitus. J Biomater Sci Polym Ed. 24:2045–2057.
2013.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Barisam M, Saidi MS, Kashaninejad N and
Nguyen NT: Prediction of necrotic core and hypoxic zone of
multicellular spheroids in a microbioreactor with a U-shaped
barrier. Micromachines (Basel). 9(94)2018.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Kang SH, Park JB, Kim I, Lee W and Kim H:
Assessment of stem cell viability in the initial healing period in
rabbits with a cranial bone defect according to the type and form
of scaffold. J Periodontal Implant Sci. 49:258–267. 2019.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Pukac L, Huangpu J and Karnovsky MJ:
Platelet-derived growth factor-BB, insulin-like growth factor-I,
and phorbol ester activate different signaling pathways for
stimulation of vascular smooth muscle cell migration. Exp Cell Res.
242:548–560. 1998.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Liang T, Zhu L, Gao W, Gong M, Ren J, Yao
H, Wang K and Shi D: Coculture of endothelial progenitor cells and
mesenchymal stem cells enhanced their proliferation and
angiogenesis through PDGF and Notch signaling. FEBS Open Bio.
7:1722–1736. 2017.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Salha S and Gehmert S, Brebant V, Anker A,
Loibl M, Prantl L and Gehmert S: PDGF regulated migration of
mesenchymal stem cells towards malignancy acts via the PI3K
signaling pathway. Clin Hemorheol Microcirc. 70:543–551.
2018.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Zhao Y, Zhang S, Zeng D, Xia L, Lamichhane
A, Jiang X and Zhang F: rhPDGF-BB promotes proliferation and
osteogenic differentiation of bone marrow stromal cells from
streptozotocin-induced diabetic rats through ERK pathway. Biomed
Res Int. 2014(637415)2014.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Tsioumpekou M, Papadopoulos N, Burovic F,
Heldin CH and Lennartsson J: Platelet-derived growth factor
(PDGF)-induced activation of Erk5 MAP-kinase is dependent on Mekk2,
Mek1/2, PKC and PI3-kinase, and affects BMP signaling. Cell Signal.
28:1422–1431. 2016.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Park JB, Lee JY, Park HN, Seol YJ, Park
YJ, Rhee SH, Lee SC, Kim KH, Kim TI, Lee YM, et al: Osteopromotion
with synthetic oligopeptide-coated bovine bone mineral in vivo. J
Periodontol. 78:157–163. 2007.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Chen X, Xu ML, Wang CN, Zhang LZ, Zhao YH,
Zhu CL, Chen Y, Wu J, Yang YM and Wang XD: A partition-type tubular
scaffold loaded with PDGF-releasing microspheres for spinal cord
repair facilitates the directional migration and growth of cells.
Neural Regen Res. 13:1231–1240. 2018.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Piran M, Vakilian S, Piran M,
Mohammadi-Sangcheshmeh A, Hosseinzadeh S and Ardeshirylajimi A: In
vitro fibroblast migration by sustained release of PDGF-BB loaded
in chitosan nanoparticles incorporated in electrospun nanofibers
for wound dressing applications. Artif Cells Nanomed Biotechnol. 46
(Suppl 1):S511–S520. 2018.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Mohan S, Raghavendran HB, Karunanithi P,
Murali MR, Naveen SV, Talebian S, Mehrali M, Mehrali M, Natarajan
E, Chan CK and Kamarul T: Incorporation of human-platelet-derived
growth factor-BB encapsulated Poly(lactic-co-glycolic acid)
microspheres into 3D CORAGRAF enhances osteogenic differentiation
of mesenchymal stromal cells. ACS Appl Mater Interfaces.
9:9291–9303. 2017.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Xie Z, Paras CB, Weng H, Punnakitikashem
P, Su LC, Vu K, Tang L, Yang J and Nguyen KT: Dual growth factor
releasing multi-functional nanofibers for wound healing. Acta
Biomater. 9:9351–9359. 2013.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Sufen G, Xianghong Y, Yongxia C and Qian
P: bFGF and PDGF-BB have a synergistic effect on the proliferation,
migration and VEGF release of endothelial progenitor cells. Cell
Biol Int. 35:545–551. 2011.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Plonka AB, Khorsand B, Yu N, Sugai JV,
Salem AK, Giannobile WV and Elangovan S: Effect of sustained PDGF
nonviral gene delivery on repair of tooth-supporting bone defects.
Gene Ther. 24:31–39. 2017.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Park SY, Kim KH, Shin SY, Koo KT, Lee YM
and Seol YJ: Dual delivery of rhPDGF-BB and bone marrow mesenchymal
stromal cells expressing the BMP2 gene enhance bone formation in a
critical-sized defect model. Tissue Eng Part A. 19:2495–2505.
2013.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Banfi A, von Degenfeld G, Gianni-Barrera
R, Reginato S, Merchant MJ, McDonald DM and Blau HM: Therapeutic
angiogenesis due to balanced single-vector delivery of VEGF and
PDGF-BB. FASEB J. 26:2486–2497. 2012.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Gianni-Barrera R, Burger M, Wolff T,
Heberer M, Schaefer DJ, Gürke L, Mujagic E and Banfi A: Long-term
safety and stability of angiogenesis induced by balanced
single-vector co-expression of PDGF-BB and VEGF164 in skeletal
muscle. Sci Rep. 6(21546)2016.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Hung BP, Hutton DL, Kozielski KL, Bishop
CJ, Naved B, Green JJ, Caplan AI, Gimble JM, Dorafshar AH and
Grayson WL: Platelet-derived growth factor BB enhances osteogenesis
of adipose-derived but not bone marrow-derived mesenchymal
stromal/stem cells. Stem Cells. 33:2773–2784. 2015.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Yao W, Shah B, Chan HL, Wang HL and Lin
GH: Bone quality and quantity alterations after socket augmentation
with rhPDGF-BB or BMPs: A systematic review. Int J Oral Maxillofac
Implants. 33:1255–1265. 2018.PubMed/NCBI View Article : Google Scholar
|