Introduction
Hepatocellular carcinoma (HCC) is the fifth most
common type of cancer and the third leading cause of
cancer-associated death worldwide (1,2). Liver
transplantation (LT) is becoming an increasingly used treatment
strategy for patients with early stage unresectable HCC, as it
offers complete tumor excision along with the removal of the
carcinogenic liver. However, the incidence of tumor recurrence
following LT is reported to be between 15-24% and it is the primary
cause of death for patients with HCC who have undergone LT
(3-5).
To prevent rejection of the liver graft, patients require life-long
immunosuppression using appropriate drugs (3-5).
Unfortunately, immunosuppressants exhibit a range of side-effects,
with some immunosuppressive drugs possessing tumorigenic capacity
(6). It is therefore important to
develop drugs that demonstrate both immunosuppressive and
tumor-suppressive effects to effectively treat patients, prolonging
their survival and reducing the potential for tumor recurrence.
The choice of immunosuppressive regimen is
paramount, as several studies have shown that tumor progression is
more rapid and aggressive in immunosuppressed patients following
LT, and the degree of immunosuppression negatively affects post-LT
recurrence of HCC, and thus, long-term survival of these patients
(6). Traditionally, the most
commonly used immunosuppressive regimen following LT is a
calcineurin inhibitor (CNI) based regimen. CNIs, such as tacrolimus
and cyclosporine A, have been demonstrated to possess pro-oncogenic
effects both in experimental models, and in retrospective and
prospective clinical trials (7).
Mycophenolate mofetil (MMF) possesses anti-proliferative
properties, but has not been shown to prevent HCC recurrence
(7,8). However, mammalian target of rapamycin
inhibitors (mTORIs), such as sirolimus and everolimus, possess
anti-tumor properties (9) and are of
particular interest for use in LT patients with HCC. Studies have
shown promising results for both sirolimus and everolimus,
highlighting their beneficial effects on post transplantation
recurrence of HCC (10-12).
A high risk of recurrence may be associated with any
remaining cancer stem cells (CSCs) following therapy as well as
immunosuppression after LT (13-15).
The CSC theory provides novel insights into the formation of
tumors. Tumors are organized into a hierarchy of heterogeneous cell
populations, a small subset of which are termed CSCs or
tumor-initiating cells, and these cells exhibit the ability to
drive and sustain tumor growth (16). Cancer stem/progenitor cells have been
identified in several studies in patients with HCC (13-15).
Therefore, it is imperative to target and control the remaining or
circulating CSCs following LT to prevent recurrence. As such the
suitability of immunosuppression for targeting CSCs for prevention
of recurrence in LT patients should be considered.
In the present study, the role of potentially
suitable immunosuppressive candidates predicted to exhibit
beneficial immunosuppressive and tumor-suppressive effects in
patients with HCC were assessed utilizing a number of common HCC
cell lines and their CSC populations. The specific expression
levels of the CSC markers CD133, epithelial cell adhesion molecule
(EpCAM) and CD90 in multiple HCC cell lines was determined.
Additionally, the inhibition of proliferation on each of these cell
lines with the most commonly used LT immunosuppressant drugs
(sirolimus, tacrolimus, cyclosporin A and MMF) was evaluated. The
aim of the present study was to provide a clinically relevant
treatment model with optimal immunosuppressive effects for the best
potential outcome in patients undergoing LT for HCC.
Materials and methods
Cell lines and culture
Human HCC cell lines Huh7, Hep3B, SNU182, SNU387 and
SNU449 used in the present study were obtained from the American
Type Culture Collection. Huh7, Hep3B, SNU182, SNU387 and SNU449
cells were cultured in DMEM supplemented with 10% FBS (Gibco;
Thermo Fisher Scientific, Inc.), 1 mM sodium pyruvate, 100 U
penicillin and 100 mg streptomycin (Sigma-Aldrich; Merck KGaA) at
37˚C in a humidified incubator with 5% CO2.
Staining with stem cell markers using
fluorescence activated cell sorting (FACS)
As CSC markers, anti-CD133, anti-epithelial cell
adhesion molecule (EpCAM), anti-CD44 and anti-CD90 were used in the
present study. The expression levels of these CSC markers in each
of the four HCC cell lines were analyzed using FACS. Cells were
dissociated and re-suspended in PBS containing 0.5% BSA. Flow
cytometry was performed using phycoerythrin (PE)-conjugated
anti-human CD133 antibody (1:100; cat. no. 130-113-670; Miltenyi
Biotec) and fluorescein isothiocyanate (FITC) conjugated anti-human
EpCAM antibody (1:100; cat. no. ab8666; Abcam), PE-conjugated CD44
(1:50; cat. no. 130-113-336; Miltenyi Biotec) and FITC-conjugated
CD45 (1:20 cat. no. 103107; BioLegend, Inc.) or PE-conjugated CD90
(1:50; cat. no. 555596; BD Biosciences). Staining patterns were
visualized using flow cytometry [FACS Canto II (HTS); BD
Biosciences]. Data analysis was performed using FACSDiva software
(BD Biosciences).
Immunosuppressive agents
Sirolimus was obtained from Pfizer, Inc. and
tacrolimus, cyclosporin A and MMF were kindly supplied by Chong Kun
Dang Pharmaceutical Corp.
MTT assay
The anti-cancer activity of the drugs on Huh7,
HEP3B, SNU387 and SNU449 cells were determined using an MTT assay
(Sigma-Aldrich; Merck KGaA; cat. no. M2003) to assess their
cytotoxic effects, as previously described (5,17). For
each drug, two different doses were assessed; sirolimus (5 and 25
ng/ml), tacrolimus (5 and 25 ng/ml), cyclosporine A (100 and 500
ng/ml) and MMF (500 and 1,000 ng). Each dose used was the
clinically recommended trough level and clinically applicable
maximum trough level (9,18). Cell viability was calculated using
the following formula: [Cell viability (%)=Mean optical density
(OD)/Control OD x100%]. The proliferative index was shown as a
percentage relative to the control cells. All experiments were
performed in triplicate.
Cell cycle arrest
Huh7 cells were seeded at a density of
1.5X105 into 6-well plates. After 24 h, cells were
treated with sirolimus (5 or 25 ng/ml), tacrolimus (5 or 25 ng/ml),
MMF (500 or 1,000 ng/ml) or cyclosporine A (100 or 500 ng/ml).
After 24 or 48 h, cells were harvested by trypsinization, and
digestion was stopped by adding fresh media. Cells were washed
twice in cold PBS and fixed with 80% ethanol at 4˚C for 1 h. After
washing with PBS, cells were stained with propidium iodide (PI; 50
mg/ml) at room temperature for 5 min and treated with RNAse A (20
mg/ml). The DNA profile of cell populations was determined by flow
cytometry. All experiments were repeated at least three times.
Western blotting
Western blot was performed as described previously
(19). Total protein was extracted
from cells using RIPA lysis buffer supplemented with 1 mg/ml
aprotinin, 10 µl/ml leupeptin, 1 mM sodium vanadate and 1 mM
phenylmethylsulfonyl fluoride. The cell lysates were centrifuged at
10,000 x g at 4˚C for 30 min and 10 µg of each cell lysate were
quantified using a Bradford assay (Biosesang) and loaded per lane
on SDS-gels (7.5, 10 or 12%). Samples were resolved using SDS-PAGE
and transferred to PVDF membranes (EMD Millipore). The membranes
were blocked in TBS containing 0.1% (v/v) Tween-20 (TBST; Bio-Rad
Laboratories, Inc.) and 5% (w/v) skimmed milk for 1 h at room
temperature. Subsequently, the membranes were sequentially
incubated with the indicated primary and secondary antibodies
diluted in TBST containing 2% (w/v) BSA (Invitrogen; Thermo Fisher
Scientific, Inc.). Anti-phospho-(p-)mTOR (Ser2448) antibodies (Cell
Signaling Technology, Inc.; cat. no. 2971; 1:1,000) and anti-GAPDH
(Cell Signaling Technology, Inc.; cat. no. 2118; 1:1,000) were the
primary antibodies used, Samples were incubated with the primary
antibodies overnight at 4˚C. Subsequently, samples were incubated
with an anti-rabbit IgG, horseradish peroxidase-linked antibody
(Cell Signaling Technology, Inc.; cat. no. 7074; 1:5,000) was used
for 2 h at 4˚C. GAPDH was used as the loading control. Western
blots were repeated three times. Densitometry analysis was
performed using ImageJ (National Institutes of Health).
Statistical analysis
Statistical analysis was performed using GraphPad
Prism version 6 (GraphPad Software, Inc.). Data are presented as
the mean ± the standard error off the mean. Differences between
groups were compared using an ANOVA with a post-hoc and Tukey's
honest significant difference test.
Results
Selection of CSC markers and cell
lines in the various HCC cell lines
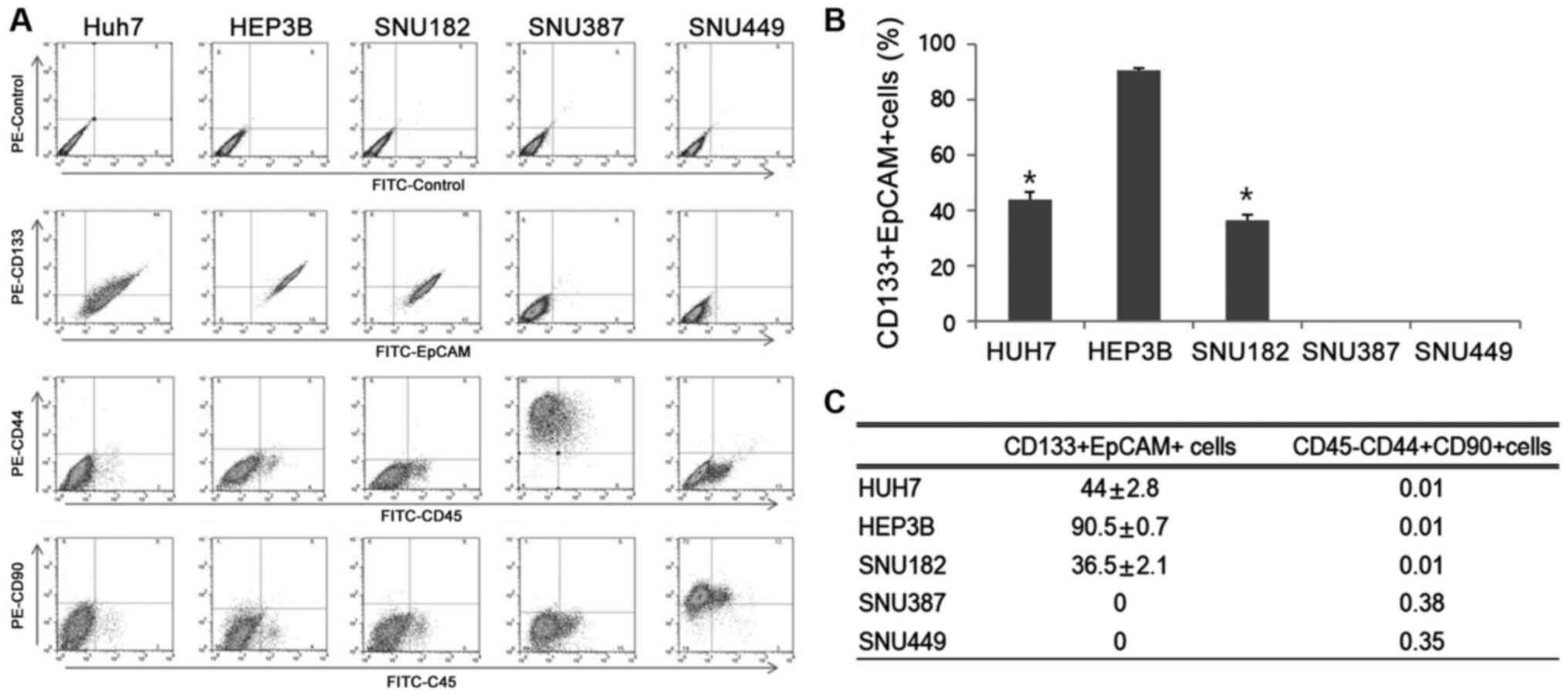
First, expression of four representative CSC markers
(CD133, EpCAM, CD44 and CD90) in four HCC cell lines were assessed
(Fig. 1A). The expression levels of
CD133+EpCAM+ were notably higher in Huh7, HEP3B and SNU182 cells
(44±2.8, 90.5±0.7 and 36.5±2.1%, respectively) compared with SNU387
and SNU449. SNUH387 and SNUH449 cell lines did not express either
of these markers (Fig. 1B and
C). Thus, SNUH387 and SNUH449 were
not used for subsequent experiments. CD44+CD90+ double positive
cells exhibit high degrees of proliferation in CSCs (20), but the proportion of cells attributed
to this population was <0.5% in all four cell lines (Fig. 1C). Due to the higher expression
levels of CD133+EpCAM+ observed in the HEP3B cell line and the
medium expression levels observed in the Huh7 cells, these cell
lines were chosen for use in subsequent experiments. Huh7 and
SNU182 cell lines showed roughly the same level of CD133+EpCAM+
expression, thus only the Huh7 cells were used for subsequent
experiments (Fig. 1B and C).
Effect of various immune suppressants
on proliferation and percentage of CSCs in HCC cell lines
Huh7 and HEP3B cell lines were used for the cell
viability assays in order to determine the effects of different
immunosuppressants including sirolimus, tacrolimus, cyclosporine A
and MMF on CSCs. For each drug, two different doses were assessed;
sirolimus (5 and 25 ng/ml), tacrolimus (5 and 25 ng/ml),
cyclosporine A (100 and 500 ng/ml) and MMF (500 and 1,000 ng). The
control cells were treated with saline without immunosuppressants
for each cell line. An MTT assay was used to measure cell
proliferation and survival. The proliferation index was calculated
by comparing the expression of the treated cells with the
respective control treated cells.
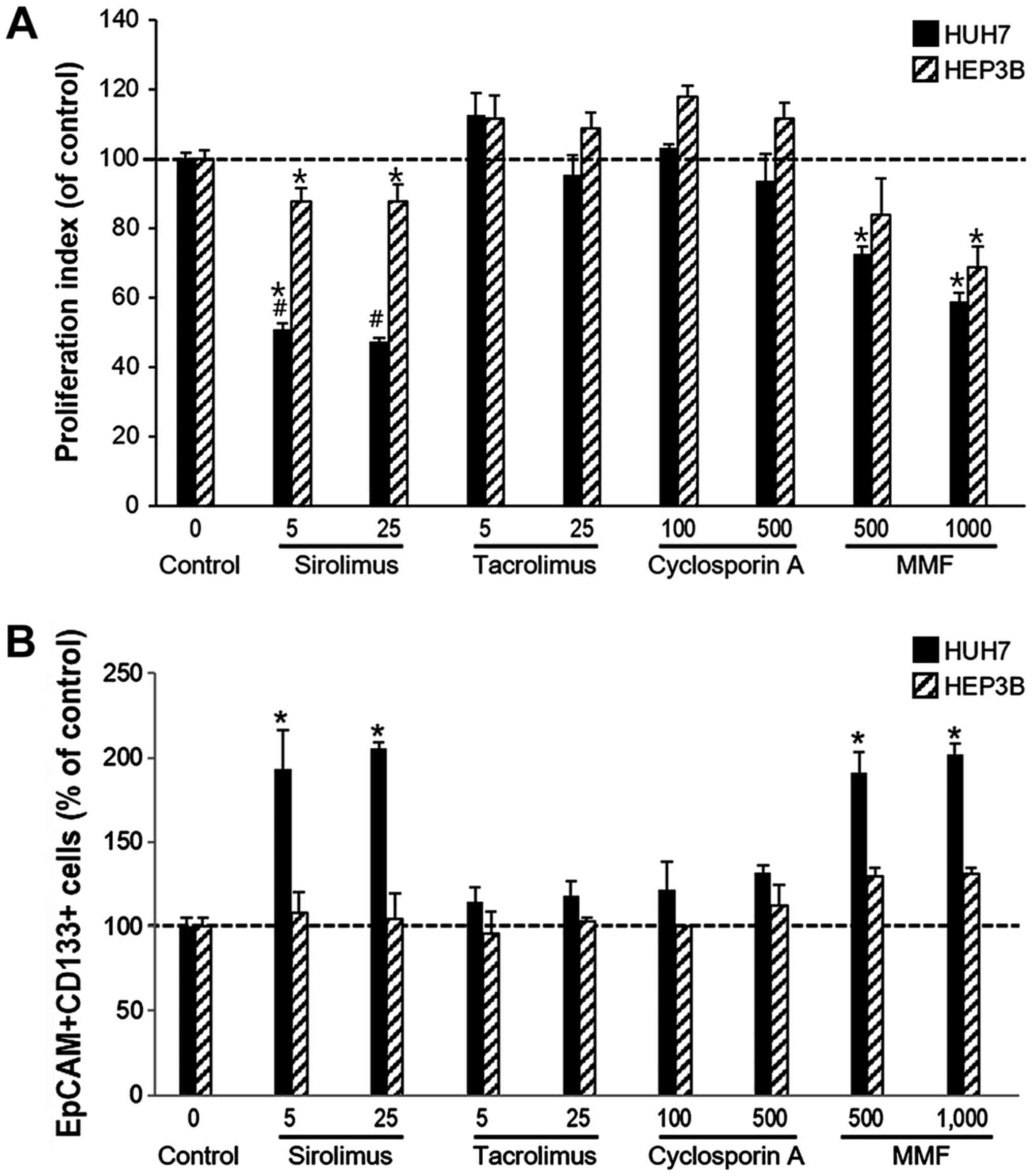
The proliferation rate of Huh7 cells was
significantly reduced by sirolimus (5 ng/ml, 50.70±1.86; 25 ng/ml,
47.50±0.96) and MMF (500 ng, 72.57±2.13; 1,000 ng, 58.88±2.54) when
compared with the control cells. However, neither cell line was
affected by tacrolimus or cyclosporine A. The Huh7 cell line was
considerably more sensitive than HEP3B cells to sirolimus. The
proliferation rate of HEP3B was also decreased by sirolimus (5
ng/ml, 87.62±3.96; 25 ng/ml, 87.65±5.04) and MMF (500 ng,
83.93±10.40; 1,000 ng, 68.74±5.84) but tacrolimus and cyclosporine
A did not affect the proliferation rate of HEP3B cells (Fig. 2A).
To determine any changes to the proportion of CSCs
following treatment with immunosuppressants in Huh7 and HEP3B
cells, the expression levels of CD133+EpCAM+ in each cell type
following treatment with immunosuppressants was measured. The
percentage of CD133+EpCAM+ expressing cells was significantly
increased in Huh7 cells when treated with either sirolimus (5
ng/ml, 192.86±23.57%; 25 ng/ml, 205.36±3.69%) and MMF (500 ng,
191.07±11.90%; 1,000 ng, 201.79±6.25%) treatment compared with the
respective control cells. However, the proliferation of
CD133+EpCAM+ expressing cells was significantly decreased by both
sirolimus and MMF. In the HEP3B cell line the percentage of
CD133+EpCAM+ cells was not significantly altered by sirolimus (5
ng/ml, 107.81±12.30%; 25 ng/ml, 104.69±14.78%), but proliferation
was increased by MMF (500 ng, 129.69±5.11%; 1,000 ng, 131.25±3.40%)
(Fig. 2B).
G1 or S phase arrest in Huh7 and HEP3B
cells treated with sirolimus or MMF
As shown in Fig. 2,
sirolimus and MMF both reduced the proliferation rate of Huh7
cells. To determine the mechanism of inhibition of growth mediated
by sirolimus and MMF treatment, cell cycle analyses were performed
in both Huh7 and HEP3B cells using PI staining. Sirolimus and MMF
are both known to induce cell cycle arrest, thus, their effects on
CD133+EpCAM+ or CD133-EpCAM- populations from Huh7 cells were
analyzed. The degree of cell cycle arrest of the total Huh7 cell
population or CD133+EpCAM+ or CD133-EpCAM- populations were
determined by gating for double positive or double negative events
following treatment with immunosuppressants. Cell cycle arrest in
HEP3B cells was not measured as >90% of the population were
CD133+EpCAM+ cells.
In HuH7 cells, sirolimus increased G0-G1 arrest (5
ng/ml, 63.44%; 25 ng/ml, 71.92%; control, 52.81%) and the
proportion of the CD133-EpCAM- population (5 ng/ml, 77.94%; 25
ng/ml, 84.01%; control, 68.48%). However, the CD133+EpCAM+
population only exhibited G1 arrest at the higher doses of
sirolimus (5 ng/ml, 46.63%; 25 ng/ml, 61.62%; control, 44.62%). In
contrast, arrest at the S-phase was induced by both doses of MMF in
Huh7 cells in the CD133-EpCAM- and CD133+EpCAM+ populations. Cell
cycle arrest was not induced by tacrolimus or cyclosporine A
treatment (data not shown). These results shows that the inhibitory
mechanism of sirolimus or MMF in Huh7 is mediated by cell cycle
arrest at the G1 or S phase (Fig.
3).
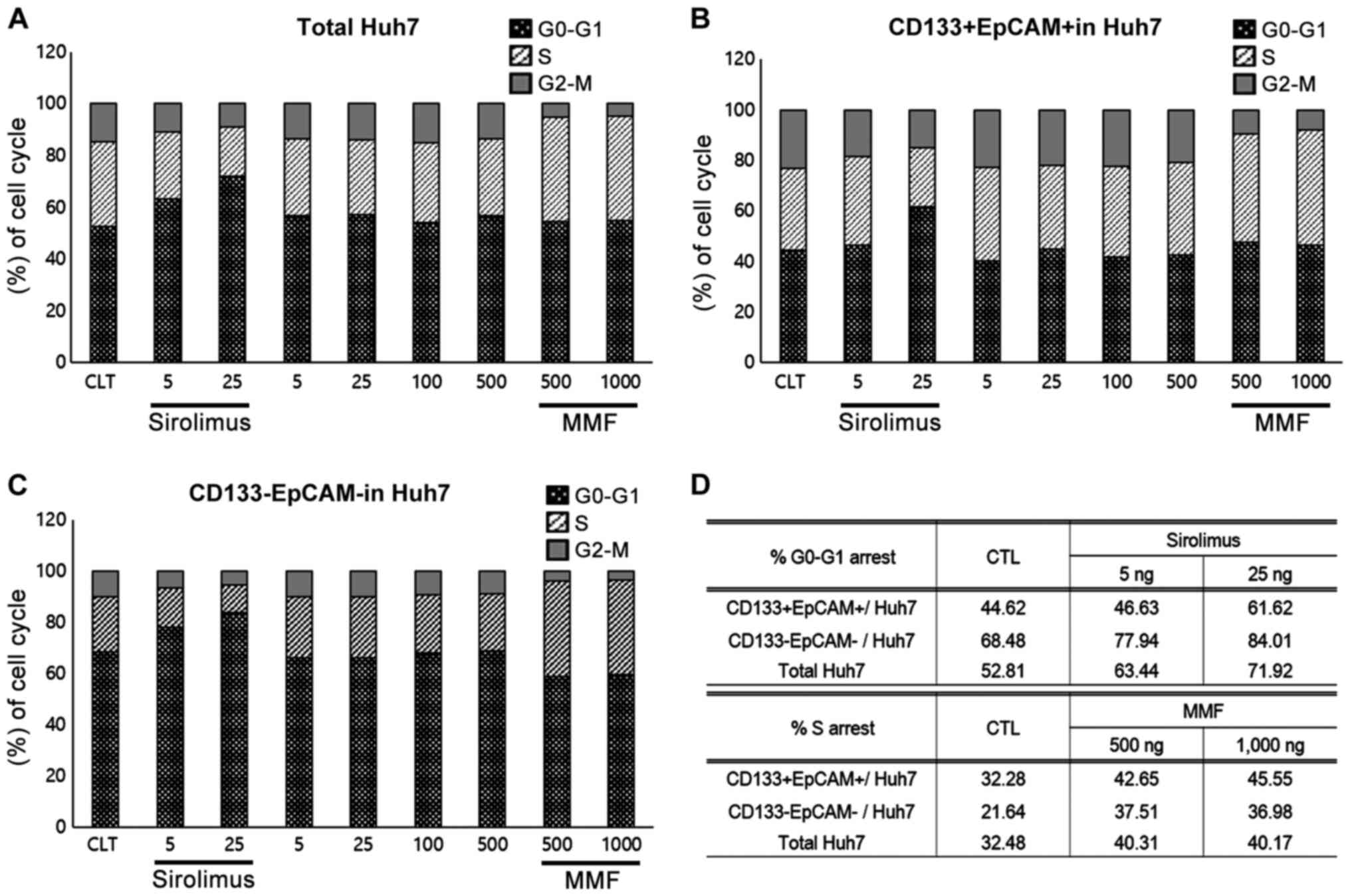 | Figure 3G1 or S phase cell-cycle arrest in
Huh7 cells treated with sirolimus or MMF. (A) In HuH7 cells,
sirolimus increased G0-G1 arrest (5 ng/ml, 63.44%; 25 ng/ml,
71.92%; control, 52.81%). (B) G1 arrest was only observed in the
CD133+EpCAM+ population at higher doses of sirolimus (5 ng/ml,
46.63%; 25 ng/ml, 61.62%; control, 44.62%). (C) Sirolimus increased
G0-G1 arrest in the CD133-EpCAM- population of HuH7 cells (5 ng/ml,
77.94%; 25 ng/ml, 84.01%; control, 68.48%). (A-D) In contrast, S
phase arrest by MMF was induced at all doses in the total Huh7,
CD133-EpCAM- and CD133+EpCAM+ populations, and no cell cycle arrest
was observed in the tacrolimus or cyclosporine A treated cells.
These results demonstrated that the inhibitory mechanism of
sirolimus or MMF on Huh7 proliferation was associated with cell
cycle arrest at the G1 and/or S phase. MMF, mycophenolate mofetil;
EpCAM, epithelial cell adhesion molecule. |
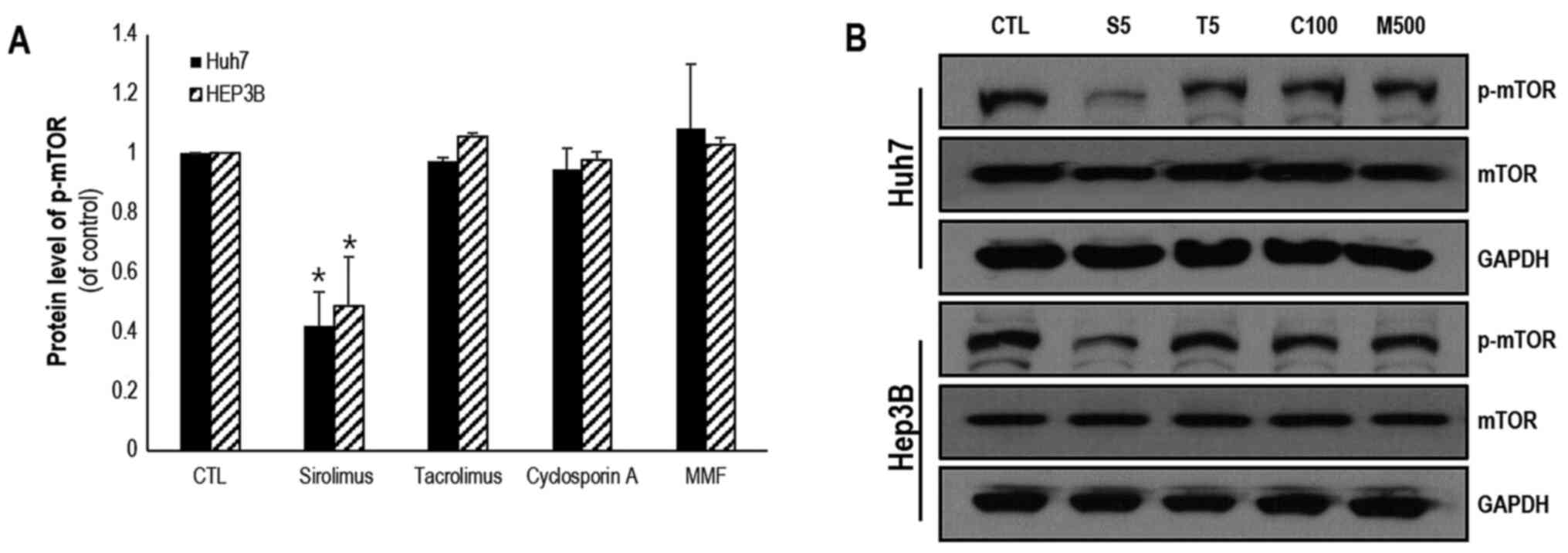
mTOR pathway is regulated by
immunosuppressants
mTOR has been shown to be a key molecule involved in
the PTEN/PI3K/mTOR signaling pathway and serves a critical role in
controlling cell proliferation and survival. The protein expression
levels of mTOR and p-mTOR in Huh7 and HEP3B cells were measured
following treatment with various immunosuppressants. In both Huh7
and HEP3B cells, the protein expression levels of mTOR were
significantly decreased by treatment with sirolimus (0.42±0.11 and
0.49±0.17, respectively) when compared with the respective control
treated cell lines. However, the protein expression levels of mTOR
were not affected notably by treatment with tacrolimus, cyclosporin
A and MMF. In HEP3B cells, the protein expression levels of mTOR
were reduced by sirolimus (0.44±0.08), MMF (0.79±0.14) and
cyclosporin A (0.73±0.002; Fig. 4A
and B). However, tacrolimus did not
affect the expression levels of mTOR. These results are summarized
in Table I.
 | Table ISummary of effects of MMF and
sirolimus on progression of hepatocellular cell lines. |
Table I
Summary of effects of MMF and
sirolimus on progression of hepatocellular cell lines.
| | Huh7 | HEP3B |
|---|
| Cellular process | Sirolimus | MMF | Sirolimus | MMF |
|---|
| Proliferation | | | | |
|
Overall | Inhibition | Inhibition | Inhibition | Inhibition |
| CD133+/EpCAM+
population | Increased | Increased | No change | Slightly
increased |
| Cell cycle
arrest | | | | |
|
Overall | G1 arrest | S arrest | ND | ND |
|
CD133+/EpCAM+ | Minimal G1 arrest
at highest dose | S arrest | ND | ND |
|
CD133-/EpCAM- | G1 arrest | S arrest | ND | ND |
| p-mTOR protein
expression | Reduction | No change | Reduction | No change |
Discussion
Tumor recurrence following LT is the leading cause
of death in patients with HCC, and the incidence of recurrence is
15-24% (4,5). Additionally, tumor progression may be
more rapid and aggressive in patients administered
immunosuppressants when they have received a LT (6). Therefore, the role of immunosuppressive
therapy in HCC recurrence remains a challenging issue, as a balance
between graft survival and HCC recurrence has to be taken into
consideration (5).
CNIs dose-dependently increase the risk of HCC
recurrence, despite the fact that these are the primary
immunosuppressive agents administered to LT recipients (21,22).
Although several centers use mTORIs in patients with advanced HCC,
there is a lack of prospective, randomized control trials to
support any recommendation for their use for this purpose. At the
same time, as the patient will require life-long use of
immunosuppressants following transplantation, careful selection of
appropriate drugs is required. It is therefore important to
determine which treatment regimens provide the optimal combination
of both immunosuppressive and tumor-suppressive effects.
Whether mTORIs can reduce HCC recurrence following
LT is still controversial. Even though some retrospective and
prospective studies have reported the positive results of mTORIs in
combination with a CNI (10,11,23,24), it
is still unclear whether this benefit is from the direct effect of
mTORIs or the indirect effect of reducing CNI doses. Furthermore,
one prospective randomized direct comparative study (SiLVER study)
of a rapamycin inhibitor demonstrated negative outcomes (25). The present study provides a
theoretical background with regard to this controversial issue.
Based on the results of the present study, mTORIs can reduce the
rate of recurrence in patients with HCC through reducing cell
proliferation; however, it cannot reduce the absolute rate of
recurrence, as it does not inhibit the actions of CSCs
(CD133+EpCAM+ cells). To prevent recurrence following LT, the
targeted control of the remnant or circulating CSCs should be
considered in balance with the appropriate immunosuppressant to
protect the graft.
Several CSC biomarkers (for example, CD133, EpCAM,
CD90, CD24 and Nanog) have been identified in HCC (26,27).
CD133, was originally classified as a hematopoietic stem cell
marker and CD133 has also been used to isolate stem-like cells from
HCC cell lines (25,26). Interestingly, analysis using flow
cytometry has shown that the percentage of CD133+ cells differs
significantly amongst several HCC cell lines (from 1 to >90%)
(28). EpCAM is also considered a
CSC marker (29) and >35% of HCC
tissues exhibit positive EpCAM expression (30-32).
Luo et al (33) showed that
CD90+ cells not only possess a high affinity to form tumors, but
also other features of CSCs, such as extensive proliferation,
differentiation, chemo-resistance, and invasive and metastatic
capacity. As such CD133, EpCAM and CD90 are ideal candidates for
investigation as CSC markers in HCC cell lines. Therefore, in the
present study the expression of these markers were used to
determine CSCs in the HCC cell lines.
The aim of the present study was to identify an
improved immunosuppression regimen for LT patients with HCC in
vitro using several HCC cell lines and their respective CSC
populations. Huh7 and HEP3B cell lines were used as they were shown
to possess high levels of CD133+EpCAM+ cell populations, which were
considered CSCs. Interestingly, sirolimus and MMF effectively
reduced the proliferation of Huh7 and HEP3B cells, but the
percentage of CD133+EpCAM+ expressing cells were significantly
increased in Huh7 following treatment in both cell lines. This
result suggested that sirolimus and MMF did not have an inhibitory
effect on the CD133+EpCAM+ subpopulation of cells in the Huh7 cell
line. The percentage of CD133+EpCAM+ expressing cells was not
significantly increased following treatment with sirolimus, but was
significantly increased by MMF in HEP3B cells. Over 90% of HEP3B
cells were shown to be CD133+EpCAM+; as the majority of the HEP3B
cells were CD133+EpCAM+, the proportion of CD133+EpCAM+ cells in
HEP3B was unlikely to be notably affected by sirolimus or MMF.
However, these results do show that sirolimus and MMF reduced the
proliferation of cancer cells, and by doing so may contribute to
increasing survival times in LT patients with HCC. However,
sirolimus and MMF may not be sufficient to reduce the recurrence
rate, as there remain CSCs following LT. As such even with the use
of sirolimus or MMF, which exhibit anti-proliferative effects in
HHC, these drugs may not be able to suppress the properties of the
CSC population.
The results from Yang et al (34) were in agreement with the results of
the present study. Luo et al (33) demonstrated that rapamycin
significantly increased both the proportion of CD133+ cells in
vitro and in vivo, but also increased the expression of
stem cell-like genes (34,35). However, there are no studies showing
the effects of MMF on the levels of CD133 cell populations in
cancer cells. As such, the present study is the first to provide
this data. The results of the present study suggest that the
inhibitory mechanism of sirolimus and MMF on Huh7 and HEP3B cells
were largely associated with cell cycle arrest at the G1 or S
phase. It was also confirmed that the protein expression levels of
mTOR, a key molecule in the PTEN/PI3K/mTOR signaling pathway, which
serves a critical role in controlling cell proliferation and
survival, was significantly decreased following sirolimus treatment
in Huh7 and HEP3B cells compared with the control cells.
Higher doses of mTORIs may provide an enhanced
anticancer effect; however, there are no clinical studies
investigating the minimal effective concentration for use following
LT for HCC to provide maximal anti-tumor effect. Furthermore, the
trough level of most clinical studies using mTORIs was 3-8 ng/ml,
as the adverse events associated with mTORIs appear to be
dose-related (35). Therefore, the
3-8 ng/ml range has been demonstrated to offer the optimal
risk-benefit profile, even in patients with HCC. In summary, the
present study showed that HCC cells express different levels of CSC
markers, such as CD133 or EpCAM, and they have different
sensitivities to immunosuppressants. Sirolimus effectively reduced
the proliferation of various cancer cell lines, but failed to
affect the proportion of the CD133+EpCAM+ cells specifically.
Therefore, these immunosuppressant agents should be further
assessed in vivo for potential use in patients, to help
regulate CSC populations, and thus reduce the risk of recurrence
HCC.
Acknowledgements
We deeply appreciate the contributions from Ms
Kwang-Sook Shin and Ms Yang-Hee Kim (Department of Surgery, Seoul
National University College of Medicine) for their assistance with
the molecular work performed in the present study.
Funding
This study was supported by funding from the Seoul
National University Hospital research fund by (grant no.
0320110450) and Basic Science Research Program through the National
Research Foundation of Korea funded by the Ministry of Science, ICT
and Future Planning (grant no. NRF-2016R1A2B4010665).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
HK, KWL and KSS designed the study, performed the
experiments, analyzed the data and wrote the manuscript. SCO, MYP,
SS and XLJ performed the experiments. SKH and KCY analyzed the
data. NJY designed the study and analyzed the data. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Parkin DM, Bray F, Ferlay J and Pisani P:
Global cancer statistics, 2002. CA Cancer J Clin. 55:74–108.
2005.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Park MS, Lee KW, Yi NJ, Choi YR, Kim H,
Hong G, Suh KS, Kwon CH, Joh JW and Lee SK: Optimal tailored
screening protocol after living donor liver transplantation for
hepatocellular carcinoma. J Korean Med Sci. 29:1360–1366.
2014.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Mukherjee S and Mukherjee U: A
comprehensive review of immunosuppression used for liver
transplantation. J Transplant. 2009(701464)2009.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Moini M, Schilsky ML and Tichy EM: Review
on immunosuppression in liver transplantation. World J Hepatol.
7:1355–1368. 2015.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Liu Z, Chen Y, Tao R, Xv J, Meng J and
Yong X: Tacrolimus-based versus cyclosporine-based
immunosuppression in hepatitis C virus-infected patients after
liver transplantation: A meta-analysis and systematic review. PLoS
One. 9(e107057)2014.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Rodríguez-Perálvarez M, De la Mata M and
Burroughs AK: Liver transplantation: Immunosuppression and
oncology. Curr Opin Organ Transplant. 19:253–260. 2014.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Chen K, Man K, Metselaar HJ, Janssen HL,
Peppelenbosch MP and Pan Q: Rationale of personalized
immunosuppressive medication for hepatocellular carcinoma patients
after liver transplantation. Liver Transpl. 20:261–269.
2014.PubMed/NCBI View
Article : Google Scholar
|
|
9
|
Lee KW, Seo YD, Oh SC, Suh SW, Jeong J,
Kim H, Yi NJ and Suh KS: What is the best immunosuppressant
combination in terms of antitumor effect in hepatocellular
carcinoma? Hepatol Res. 46:593–600. 2016.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Menon KV, Hakeem AR and Heaton ND:
Meta-analysis: Recurrence and survival following the use of
sirolimus in liver transplantation for hepatocellular carcinoma.
Aliment Pharm Ther. 37:411–419. 2013.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Jeng LB, Thorat A, Hsieh YW, Yang HR, Yeh
CC, Chen TH, Hsu SC and Hsu CH: Experience of using everolimus in
the early stage of living donor liver transplantation. Transplant
Proc. 46:744–748. 2014.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Finn RS: Current and future treatment
strategies for patients with advanced hepatocellular carcinoma:
Role of mTOR inhibition. Liver Cancer. 1:247–256. 2012.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Yin S, Li J, Hu C, Chen X, Yao M, Yan M,
Jiang G, Ge C, Xie H, Wan D, et al: CD133 positive hepatocellular
carcinoma cells possess high capacity for tumorigenicity. Int J
Cancer. 120:1444–1450. 2007.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Suetsugu A, Osawa Y, Nagaki M, Moriwaki H,
Saji S, Bouvet M and Hoffman RM: Simultaneous color-coded imaging
to distinguish cancer ‘stem-like’ and non-stem cells in the same
tumor. J Cell Biochem. 111:1035–1041. 2010.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Ma S, Chan KW, Hu L, Lee TK, Wo JY, Ng IO,
Zheng BJ and Guan XY: Identification and characterization of
tumorigenic liver cancer stem/progenitor cells. Gastroenterology.
132:2542–2556. 2007.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Reya T, Morrison SJ, Clarke MF and
Weissman IL: Stem cells, cancer, and cancer stem cells. Nature.
414:105–111. 2001.PubMed/NCBI View
Article : Google Scholar
|
|
17
|
Kumar P, Nagarajan A and Uchil PD:
Analysis of cell viability by the MTT assay. Cold Spring Harb
Protoc 2018, 2018.
|
|
18
|
Mussin N, Oh SC, Lee KW, Park MY, Seo S,
Yi NJ, Kim H, Yoon KC, Ahn SW, Kim HS, et al: Sirolimus and
metformin synergistically inhibits colon cancer in vitro and in
vivo. J Korean Med Sci. 32:1385–1395. 2017.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Hui IC, Tung EK, Sze KM, Ching YP and Ng
IO: Rapamycin and CCI-779 inhibit the mammalian target of rapamycin
signalling in hepatocellular carcinoma. Liver International.
30:65–75. 2010.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Ji J and Wang XW: Clinical implications of
cancer stem cell biology in hepatocellular carcinoma. Semin Oncol.
39:461–472. 2012.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Sgourakis G and Dedemadi G:
Corticosteroid-free immunosuppression in liver transplantation: An
evidence-based review. World J Gastroenterol. 20:10703–10714.
2014.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Turner AP and Knechtle SJ: Induction
immunosuppression in liver transplantation: A review. Transpl Int.
26:673–683. 2013.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Vivarelli M, Dazzi A, Cucchetti A,
Gasbarrini A, Zanello M, Di Gioia P, Bianchi G, Tamè MR, Gaudio MD,
Ravaioli M, et al: Sirolimus in liver transplant recipients: A
large single-center experience. Transplant Proc. 42:2579–2584.
2010.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Thorat A, Jeng LB, Yang HR, Yeh CC, Hsu
SC, Chen TH and Poon KS: Assessing the role of everolimus in
reducing hepatocellular carcinoma recurrence after living donor
liver transplantation for patients within the UCSF criteria:
Re-inventing the role of mammalian target of rapamycin inhibitors.
Ann Hepatobiliary Pancreat Surg. 21:205–211. 2017.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Geissler EK, Schnitzbauer AA, Schlitt HJ
and Si LSG: Lack of benefits of mammalian target of rapamycin
inhibitor in patients transplanted for hepatocellular carcinoma: Is
this the end of the story? Liver Transpl. 22:1162–1163.
2016.PubMed/NCBI View
Article : Google Scholar
|
|
26
|
Yamashita T, Ji J, Budhu A, Forgues M,
Yang W, Wang HY, Jia H, Ye Q, Qin LX, Wauthier E, et al:
EpCAM-positive hepatocellular carcinoma cells are tumor-initiating
cells with stem/progenitor cell features. Gastroenterology.
136:1012–1024. 2009.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Na DC, Lee JE, Yoo JE, Oh BK, Choi GH and
Park YN: Invasion and EMT-associated genes are up-regulated in B
viral hepatocellular carcinoma with high expression of CD133-human
and cell culture study. Exp Mol Pathol. 90:66–73. 2011.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Zhu Z, Hao X, Yan M, Yao M, Ge C, Gu J and
Li J: Cancer stem/progenitor cells are highly enriched in
CD133+CD44+ population in hepatocellular carcinoma. Int J Cancer.
126:2067–2078. 2010.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Winter MJ, Nagelkerken B, Mertens AE,
Rees-Bakker HA, Briaire-de Bruijn IH and Litvinov SV: Expression of
Ep-CAM shifts the state of cadherin-mediated adhesions from strong
to weak. Exp Cell Res. 285:50–58. 2003.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Kim JW, Ye QH, Forgues M, Chen YD, Budhu
A, Sime J, Hofseth LJ, Kaul R and Wang XW: Cancer-associated
molecular signature in the tissue samples of patients with
cirrhosis. Hepatology. 39:518–527. 2004.PubMed/NCBI View Article : Google Scholar
|
|
31
|
de Boer CJ, Van Krieken JH, Janssen-Van
Rhijn CM and Litvinov SV: Expression of Ep-CAM in normal,
regenerating, metaplastic, and neoplastic liver. J Pathol.
188:201–206. 1999.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Ruck P, Wichert G, Handgretinger R and
Kaiserling E: Ep-CAM in malignant liver tumours. Journal of
Pathology. 191:102–103. 2000.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Luo J, Wang P, Wang R, Wang J, Liu M,
Xiong S, Li Y and Cheng B: The Notch pathway promotes the cancer
stem cell characteristics of CD90+ cells in hepatocellular
carcinoma. Oncotarget. 7:9525–9537. 2016.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Yang Z, Zhang L, Ma A, Liu L, Li J, Gu J
and Liu Y: Transient mTOR inhibition facilitates continuous growth
of liver tumors by modulating the maintenance of CD133+ cell
populations. PLoS One. 6(e28405)2011.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Matsumoto K, Arao T, Tanaka K, Kaneda H,
Kudo K, Fujita Y, Tamura D, Aomatsu K, Tamura T, Yamada Y, et al:
mTOR signal and hypoxia-inducible factor-1 alpha regulate CD133
expression in cancer cells. Cancer Res. 69:7160–7164.
2009.PubMed/NCBI View Article : Google Scholar
|