Introduction
Pluripotent stem cells have the ability to
self-organize into three-dimensional aggregates termed cell
spheroids or organoids (1). The
three-dimensional cultures, such as stem cells spheroids are shown
to maintain cell survival and cell function (2). In a previous study, three-dimensional
cultures preserved the cell viability and three-dimensional
morphology, whilst exhibiting increased cellular function when
compared with two-dimensional monolayer culture (3). These multicellular spheroids from
induced pluripotent stem cells and three-dimensional culture
platforms have been used for testing cell-cell interactions, drug
sensitivity, anticancer activity, immune activation and organoid
generation (4,5).
Spheroid cultures are gaining increasing interest,
particularly in the field of tissue regeneration (6). Spheroid cultures exhibit increased
secretion of cytokines, including granulocyte colony stimulating
factor and vascular endothelial growth factor when compared with
two-dimensional cultures (7).
Various methods including spinner flasks, hanging drops,
non-adhesive surfaces, and microwells have been used for spheroid
production (5). Amongst these
methods, microwells with a three-dimensional concave geometry can
produce uniformly sized stem cell spheroids with reproducible
results (8). Various types of cells
from different anatomical regions have been used for
three-dimensional cultures (9-11).
Spheroids created from a co-culture of different cell types of stem
cells and endothelial cells exhibit enhanced functionality
(12). Similarly, the use of
multicellular spheroids composed of two types of cells results in
increased osteogenic potential (13). In light of the promising findings of
previous studies on co-culture techniques, the aim of the present
study was to evaluate the morphology, cellular viability and
expression of stem cell markers of three-dimensional cultures
established using bone marrow and/or gingiva-derived stem cells in
different ratios.
Materials and methods
Fabrication of cell spheroids using
human bone marrow and/or human gingiva-derived stem cells
The Institutional Review Board examined and approved
the present study (grant no. KC20SISE0703). Stem cell spheroids
were fabricated in silicone elastomer-based microwells that were
concave in shape with a 600 µm diameter (cat. no. H389600; StemFIT
3D; MicroFIT). A total of 1x106 gingiva-derived stem
cells and/or bone marrow-derived stem cells were seeded in per
well. Gingiva-derived stem cells were obtained as described
previously (14). Human bone
marrow-derived mesenchymal stem cells (Catholic MASTER Cells) were
obtained from the Catholic Institute of Cell Therapy (15), and informed consent was obtained from
all participants. The experiments were performed in accordance with
the relevant guidelines and regulations specified in the
Declaration of Helsinki (16). The
ratios between human bone marrow-derived mesenchymal stem cells and
gingiva-derived stem cells were: 6:0, Group 1; 4:2, Group 2; 3:3,
Group 3; 2:4, Group 4; and 0:6, Group 5(13). Cell aggregation and cell-spheroid
formation were observed using an inverted microscope.
Determination of cell viability
The viability of spheroids was qualitatively
analyzed using a Live/Dead kit assay (Molecular Probes) on day
1(17). Stem cell spheroids were
cultured in α-minimal essential medium (α-MEM; Gibco; Thermo Fisher
Scientific, Inc.) containing 15% FBS (Gibco; Thermo Fisher
Scientific, Inc.), 100 U/ml penicillin, 100 µg/ml streptomycin
(Sigma-Aldrich; Merck KGaA), 200 mM L-Glutamine (Sigma-Aldrich;
Merck KGaA) and 10 mM ascorbic acid 2-phosphate (Sigma-Aldrich;
Merck KGaA). These spheroids were washed twice with growth media. A
suspension containing calcein acetoxymethyl ester working solution
ethidium homodimer-1 was added and incubated at room temperature
for 30 min. The spheroids were observed under a fluorescence
microscope on days 3 and 5 (magnification, x100).
Qualitative cellular viability analysis was
performed on days 1, 3, 5 and 7 using a Cell Counting Kit-8 (CCK-8)
assay (Dojindo Molecular Technologies, Inc.) (18). The spheroids were incubated for 45
min at 37˚C and the spectrophotometric absorbance was measured at
450 nm.
Reverse transcription-quantitative
(RT-q)PCR
Cells were harvested on days 7 and 10. The total RNA
was isolated using a GeneJET RNA Purification kit according to the
manufacturer's protocol (Thermo Fisher Scientific, Inc.); 1 ng
total RNA was used as a template for reverse transcription using
SuperScript II Reverse Transcriptase (Invitrogen; Thermo Fisher
Scientific, Inc.), and quantities were determined by
spectrophotometry on a NanoDrop 2000 (Thermo Fisher Scientific,
Inc.) at 260 and 280 nm on days 7 and 10.
mRNA expression was detected using qPCR with a
SYBR-Green Real-Time PCR MasterMix (Enzynomics) according to the
manufacturer's protocol (19). The
sense and antisense primers were designed based on GenBank. The
primer sequences were as follows: Nanog (accession no.
NM_001297698.2) forward, 5'-AGTCCCAAAGGCAAACAACCCACTTC-3' and
reverse, 5'-TGCTGGAGGCTGAGGTATTTCTGTCTC-3'; and β-actin (accession
no. NM_001101.5) forward, 5'-TGGCACCCAGCACAATGAA-3' and reverse,
5'-CTAAGTCATAGTCCGCCTAGAAGCA-3'; the mRNA levels were normalized to
β-actin and expressed as the fold change (20). The mRNA expression was detected by
qPCR using SYBR Green Real-Time PCR MasterMix (Enzynomics, Daejeon,
South Korea) according to the manufacturer's protocol.
Statistical analysis
Statistical analysis was performed using SPSS
version 12 (SPSS, Inc.) Data are presented as the mean ± standard
deviation. A test of normality was performed, and a one-way ANOVA
with a post-hoc Tukey's test, or a Kruskal Wallis test with
Bonferroni corrected Mann-Whitney-U test was performed to determine
differences between the groups. P<0.05 was considered to
indicate a statistically significant difference.
Results
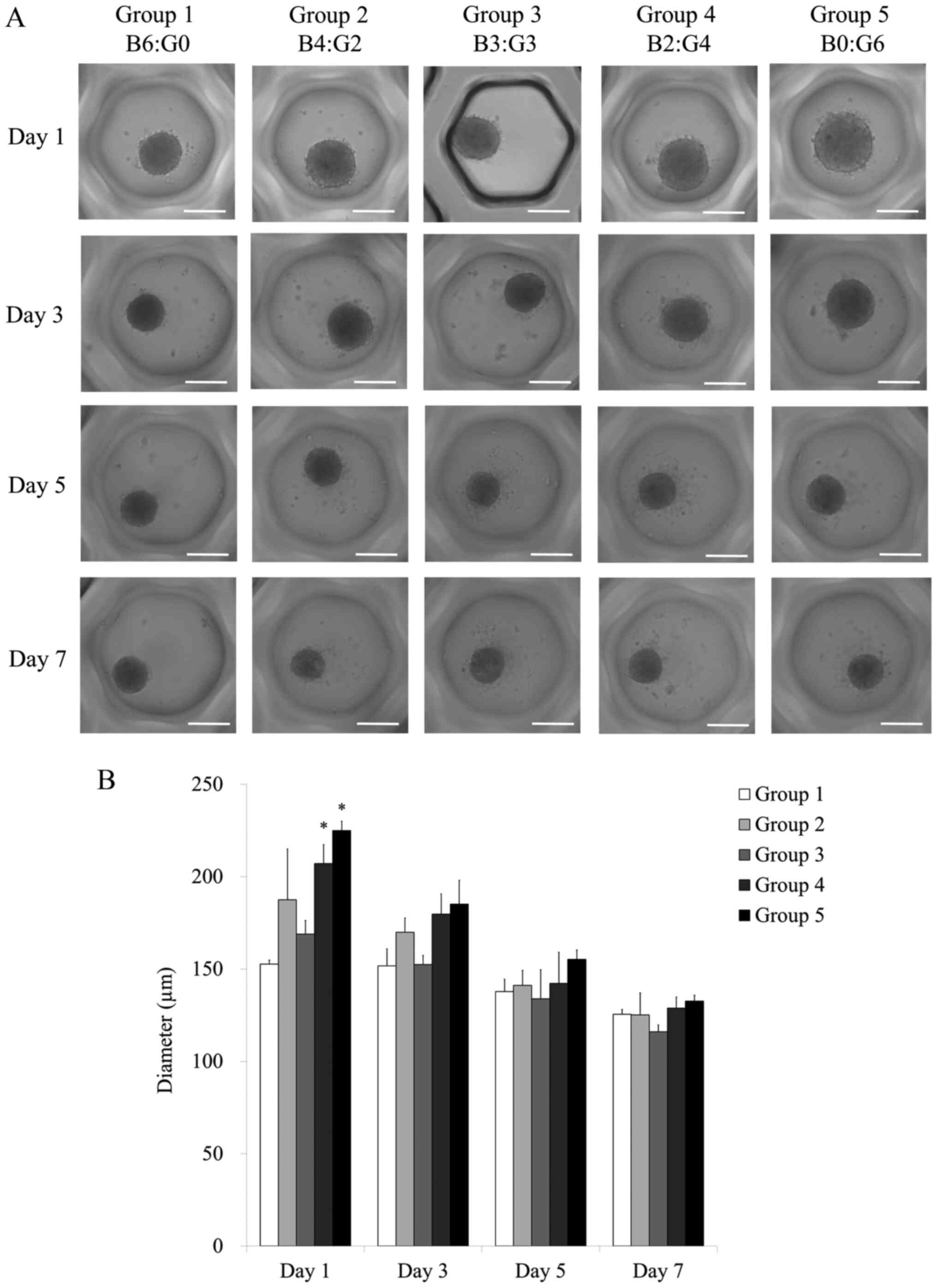
Evaluation of cell morphology
Stem cell spheroids were well formed in the silicone
elastomer-based concave microwells with only bone marrow-derived
stem cells on days 1, 3, 5 and 7 (Fig.
1A). There were no significant changes in the morphology with
the different ratios of bone marrow and gingiva-derived stem cells
on days 1 and 3. In general, the shapes of the cells on day 5 were
similar to the shapes in each group on day 1. There were no
significant changes in the morphology with the longer incubation
time of 7 days.
The average spheroid diameter in each group on days
1, 3, 5 and 7 are shown in Fig. 1B.
The average spheroid diameter for Groups 1, 2, 3, 4 and 5 on day 1
were 152.7±2.1, 187.4±27.6, 168.9±7.4, 207.1±10.2 and 224.9±5.1 µm,
respectively (P<0.05). There were statistically significant
increases in Groups 4 and 5 on day 1 when compared with Group 1 on
day 1. The average spheroid diameters for Groups 1, 2, 3, 4 and 5
on day 3 were 151.6±9.3, 169.9±7.7, 152.5±4.9, 179.7±10.9 and
185.1±12.9 µm, respectively. The diameters of the spheroids
appeared to increase the ratio of gingiva-derived stem cells
increased.
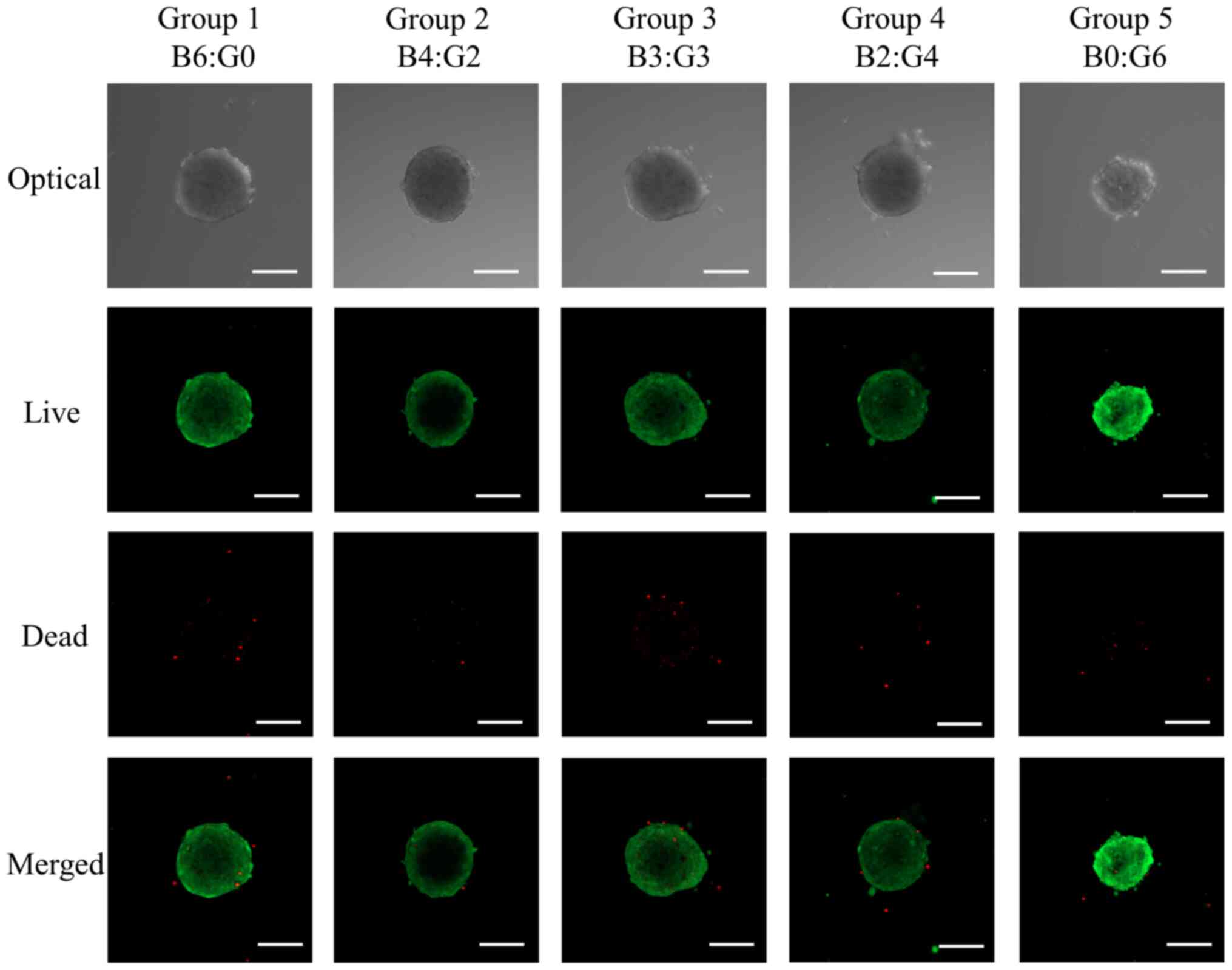
Determination of cell viability
The cellular viability was determined using a
Live/Dead kit assay using a fluorescent microscope and is shown in
Fig. 2. Most of the cells in the
spheroids emitted green fluorescence, and the morphology was round
without significant changes at day 1.
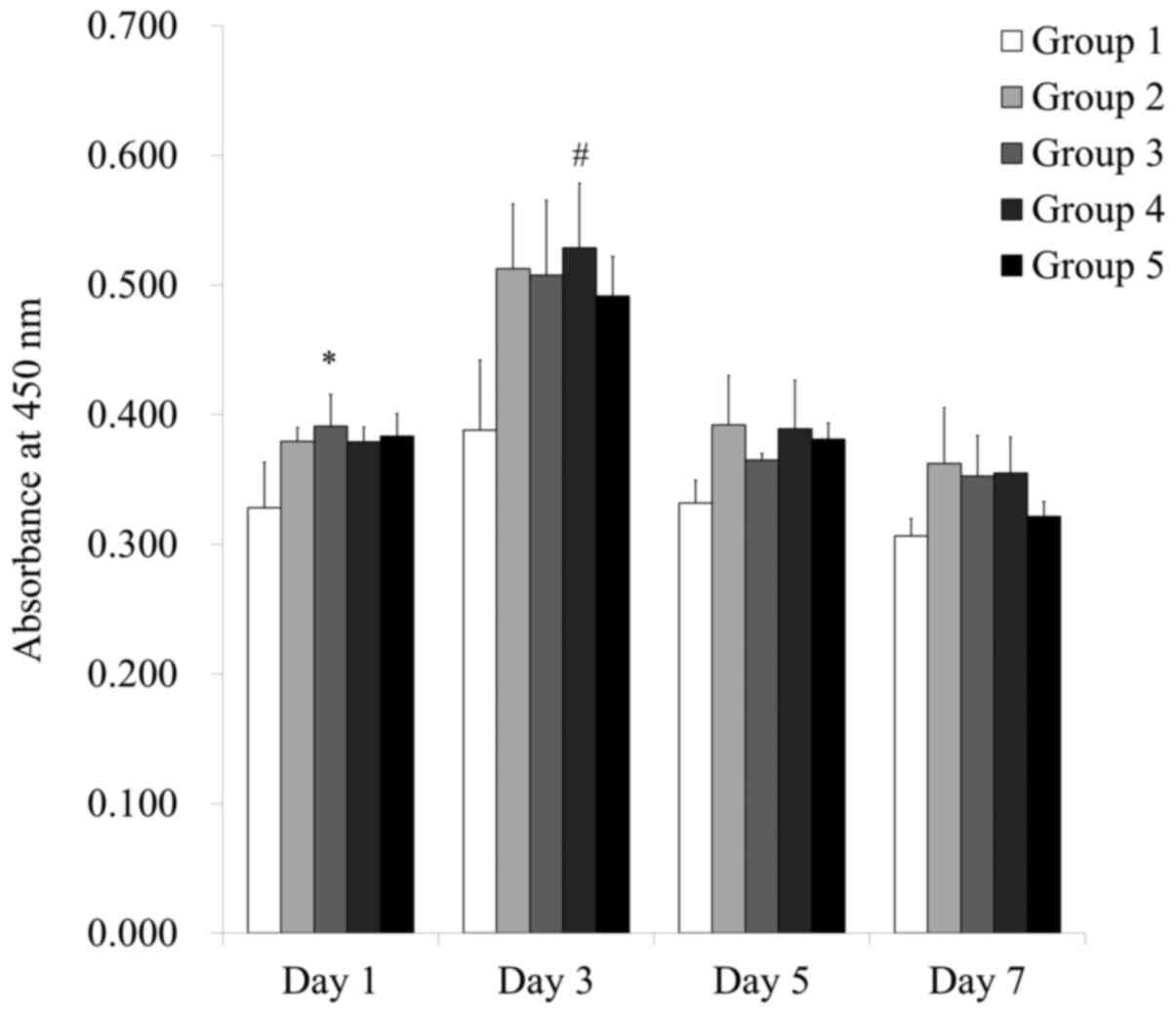
The results of cell viability on days 1, 3, 5 and 7
are shown in Fig. 3. The relative
viability value of Groups 1, 2, 3, 4 and 5 on day 1 were
100.0±10.7%, 115.5±3.3%, 119.1±7.5%, 115.4±3.4% and 116.8±5.4%,
respectively (P<0.05). There were significant increases in the
values seen in Groups 3 when compared with Group 1 on day 1.
Validation of mRNA expression using
RT-qPCR
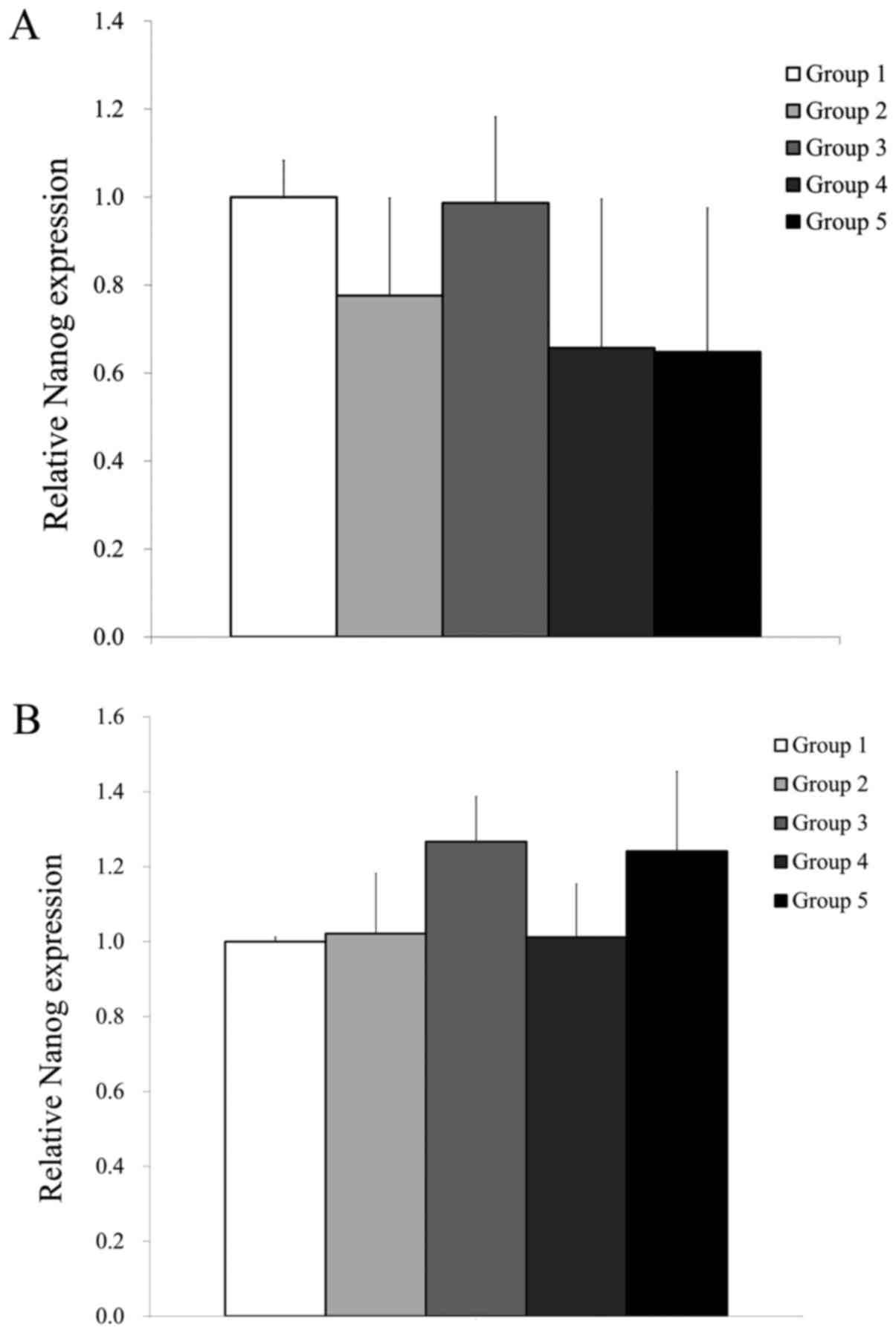
RT-qPCR was performed to assess the mRNA expression
levels of Nanog and β-actin on days 7 and 10 (Fig. 4). The mRNA levels were normalized to
β-actin levels and expressed as a fold change. The relative
expression of Nanog in Groups 1, 2, 3, 4 and 5 on day 7 were
100.0±8.3%, 77.6±22.1%, 98.6±19.6%, 65.7±33.9% and 64.8±32.7%,
respectively (Fig. 4A). The relative
expression of Nanog in Groups 1, 2, 3, 4 and 5 on day 10
were 100.0±1.2, 102.1±16.0, 126.7±12.0, 101.2±14.2 and 124.1±21.3%,
respectively, with the highest value observed in Group 3 (Fig. 4B). However, no significant
differences in Nanog expression were observed in Groups 2-5
when compared with Group 1 (P>0.05; Fig. 4A and B).
Discussion
In the present study, stem-cell spheroids were
formed with human bone marrow and gingiva-derived stem cells using
concave microwells. This study clearly showed that the shape of the
spheroids were maintained throughout the entirety of the
experimental procedure and increased cellular viability was
achieved when using a co-culture of bone marrow and gingiva-derived
stem cells.
A co-culture method has been used in previous
reports to generate spheroids (21,22). The
co-culture system of endothelial cells and adipose-derived stem
cells led to the promotion of vascular morphogenesis in microvessel
structures (21). Higher cell-cell
interactions were noted when bone marrow-derived stem cells were
co-cultured with glioblastoma multiform cell lines (22). In the present study, stem cells
derived from different anatomical regions including bone marrow and
gingival were used for analysis, and the results showed that this
approach can be useful when there is a limited number of cells of
each type.
Stem cells derived from different anatomical regions
have been used for various applications. Spheroids fabricated with
several types of cells, including bone marrow-derived stem cells
for Bio 3D printer-produced histological chondrogenesis and
vasculogenesis (10). Similarly,
adipose-derived stem cells can be used as a promising therapeutic
approach for soft tissue healing (11). A previous study showed that human
periodontal ligament-derived stem cells enhanced retinal ganglion
cell survival, and this approach can be used for protection against
optic neuropathy (9).
Gingiva-derived stem cells have various advantages, as the cells
can be obtained under local anesthesia without severe complications
(23,24). Applying ≥2 types of cells may
complement the limitations in each type.
RT-qPCR was performed to detect the mRNA expression
levels of Nanog and β-actin in each group. Nanog is a
transcription factor that is involved in self-renewal of
undifferentiated stem cells (25).
Nanog has been shown to be expressed in several types of
tumors, highlighting the potential presence of a population of
cancer stem cells (26). In the
present study, no significant changes in Nanog expression
were observed between the groups with different ratios of
cells.
There are several limitations in the present study
that should be considered when interpretating the results.
Nanog expression has been used as a stem cell marker.
However, there are various markers for evaluating the
characteristics of stem cells and CD73 and CD90 have been widely
served as positive markers of stem cells (27). Cellular viability was evaluated
qualitatively using a Live/Dead kit and quantitatively using a
CCK-8 assay. Caspase activity can be used to evaluate the apoptosis
of stem cells (28). The
differential behaviors between the groups can be explained based on
cell-cell interactions. These cell-cell interactions can be
visualized using photo excitation of fluorescent proteins (29). Evaluation of additional adipogenic
and chondrogenic differentiation potential may broaden the
applicability for various purposes.
In conclusion, the present study demonstrated that
stem cell spheroids could be formed with human bone marrow and
gingiva-derived stem cells using concave microwells. The shape of
the spheroids were maintained throughout the entirety of the
experimental procedure. The use of co-cultures with higher ratios
of gingiva-derived stem cells produced stem cell spheroids with
larger diameters. Highest cellular viability and highest levels of
Nanog expression was achieved with co-culture of bone marrow
and gingiva-derived stem cells. This co-culture technique may be
used for stem cell therapy with allogenic stem cell
transplantation. Further studies regarding cell-cell interactions
should be performed to analyze the underlying mechanisms.
Application of stem cell spheroids formed of bone marrow and
gingiva-derived stem cells using various ratios in in vivo
models are warranted to evaluate their therapeutic efficacy.
Acknowledgements
Not applicable.
Funding
This study was supported by the National Research
Foundation of Korea grant funded by the Korea government (MSIT)
(grant no. 2020R1A2C4001624), and supported by research funding
from Seoul St. Mary's Hospital, The Catholic University of
Korea.
Availability of data and materials
All data generated or analyzed during this study are
included in the published article.
Authors' contributions
J-YT, HyunjinL, HyunaL, YS and J-BP designed the
study, analyzed the data, performed the experiments as well as
wrote and reviewed the manuscript. All authors have read and
approved the final manuscript.
Ethics approval and consent to
participate
The present study was reviewed and approved by the
Institutional Review Board of Seoul St. Mary's Hospital, College of
Medicine, the Catholic University of Korea (approval no.
KC20SISE0703). Informed consent was obtained from all participants.
All experiments were performed in accordance with relevant
guidelines and regulations specified in the Declaration of
Helsinki.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Pasca SP: The rise of three-dimensional
human brain cultures. Nature. 553:437–445. 2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Mazza G, Al-Akkad W, Rombouts K and
Pinzani M: Liver tissue engineering: From implantable tissue to
whole organ engineering. Hepatol Commun. 2:131–141. 2017.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Ong SM, Zhang C, Toh YC, Kim SH, Foo HL,
Tan CH, van Noort D, Park S and Yu H: A gel-free 3D microfluidic
cell culture system. Biomaterials. 29:3237–3244. 2008.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Pamies D, Block K, Lau P, Gribaldo L,
Pardo CA, Barreras P, Smirnova L, Wiersma D, Zhao L, Harris G, et
al: Rotenone exerts developmental neurotoxicity in a human brain
spheroid model. Toxicol Appl Pharmacol. 354:101–114.
2018.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Lee GH, Suh Y and Park JY: A paired bead
and magnet array for molding microwells with variable concave
geometries. J Vis Exp. 28(55548)2018.PubMed/NCBI View
Article : Google Scholar
|
|
6
|
Bauman E, Feijao T, Carvalho DTO, Granja
PL and Barrias CC: Xeno-free pre-vascularized spheroids for
therapeutic applications. Sci Rep. 8(230)2018.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Redondo-Castro E, Cunningham CJ, Miller J,
Brown H, Allan SM and Pinteaux E: Changes in the secretome of
tri-dimensional spheroid-cultured human mesenchymal stem cells in
vitro by interleukin-1 priming. Stem Cell Res Ther.
9(11)2018.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Lee SI, Yeo SI, Kim BB, Ko Y and Park JB:
Formation of size-controllable spheroids using gingiva-derived stem
cells and concave microwells: Morphology and viability tests.
Biomed Rep. 4:97–101. 2016.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Cen LP, Ng TK, Liang JJ, Zhuang X, Yao X,
Yam GHF, Chen H, Cheung HS, Zhang M and Pang CP: Human periodontal
ligament-derived stem cells promote retinal ganglion cell survival
and axon regeneration after optic nerve injury. Stem Cells.
36:844–856. 2018.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Taniguchi D, Matsumoto K, Tsuchiya T,
Machino R, Takeoka Y, Elgalad A, Gunge K, Takagi K, Taura Y,
Hatachi G, et al: Scaffold-free trachea regeneration by tissue
engineering with bio-3D printing. Interact Cardiovasc Thorac Surg.
26:745–752. 2018.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Oberringer M, Bubel M, Jennewein M,
Guthörl S, Morsch T, Bachmann S, Metzger W and Pohlemann T: The
role of adipose-derived stem cells in a self-organizing 3D model
with regard to human soft tissue healing. Mol Cell Biochem.
445:195–210. 2018.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Tae JY, Lee SI, Ko Y and Park JB: Enhanced
osteogenic differentiation potential of stem-cell spheroids created
from a coculture of stem cells and endothelial cells. Implant Dent.
26:922–928. 2017.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Tae JY, Lee H, Lee H, Ko Y and Park JB:
Osteogenic potential of cell spheroids composed of varying ratios
of gingiva-derived and bone marrow stem cells using concave
microwells. Exp Ther Med. 16:2287–2294. 2018.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Jin SH, Lee JE, Yun JH, Kim I, Ko Y and
Park JB: Isolation and characterization of human mesenchymal stem
cells from gingival connective tissue. J Periodontal Res.
50:461–467. 2015.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Jeong CH, Kim SM, Lim JY, Ryu CH, Jun JA
and Jeun SS: Mesenchymal stem cells expressing brain-derived
neurotrophic factor enhance endogenous neurogenesis in an ischemic
stroke model. Biomed Res Int. 2014(129145)2014.PubMed/NCBI View Article : Google Scholar
|
|
16
|
World Medical Association. World medical
association declaration of helsinki: Ethical principles for medical
research involving human subjects. JAMA. 310:2191–2194.
2013.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Kang SH, Park JB, Kim I, Lee W and Kim H:
Assessment of stem cell viability in the initial healing period in
rabbits with a cranial bone defect according to the type and form
of scaffold. J Periodontal Implant Sci. 49:258–267. 2019.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Tae JY, Ko Y and Park JB: Evaluation of
fibroblast growth factor-2 on the proliferation of osteogenic
potential and protein expression of stem cell spheroids composed of
stem cells derived from bone marrow. Exp Ther Med. 18:326–331.
2019.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Lee H, Min SK, Song Y, Park YH and Park
JB: Bone morphogenetic protein-7 upregulates genes associated with
osteoblast differentiation, including collagen I, Sp7 and IBSP in
gingiva-derived stem cells. Exp Ther Med. 18:2867–2876.
2019.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Kook YM, Kim H, Kim S, Heo CY, Park MH,
Lee K and Koh WG: Promotion of vascular morphogenesis of
endothelial cells co-cultured with human adipose-derived
mesenchymal stem cells using polycaprolactone/gelatin nanofibrous
scaffolds. Nanomaterials (Basel). 8(117)2018.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Oliveira MN, Pillat MM, Motaln H, Ulrich H
and Lah TT: Kinin-B1 receptor stimulation promotes invasion and is
involved in cell-cell interaction of co-cultured glioblastoma and
mesenchymal stem cells. Sci Rep. 8(1299)2018.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Lee SI, Ko Y and Park JB: Evaluation of
the maintenance of stemness, viability, and differentiation
potential of gingiva-derived stem-cell spheroids. Exp Ther Med.
13:1757–1764. 2017.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Ha DH, Pathak S, Yong CS, Kim JO, Jeong JH
and Park JB: Potential differentiation ability of gingiva
originated human mesenchymal stem cell in the presence of
tacrolimus. Sci Rep. 6(34910)2016.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Takahashi K, Tanabe K, Ohnuki M, Narita M,
Ichisaka T, Tomoda K and Yamanaka S: Induction of pluripotent stem
cells from adult human fibroblasts by defined factors. Cell.
131:861–872. 2007.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Zhou X, Zhou YP, Huang GR, Gong BL, Yang
B, Zhang DX, Hu P and Xu SR: Expression of the stem cell marker,
Nanog, in human endometrial adenocarcinoma. Int J Gynecol Pathol.
30:262–270. 2011.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Lee H, Min SK and Park JB: Effects of
demographic factors on adipogenic and chondrogenic differentiation
in bone marrow-derived stem cells. Exp Ther Med. 17:3548–3554.
2019.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Khoshlahni N, Sagha M, Mirzapour T, Zarif
MN and Mohammadzadeh-Vardin M: Iron depletion with deferoxamine
protects bone marrow-derived mesenchymal stem cells against
oxidative stress-induced apoptosis. Cell Stress Chaperones.
25:1059–1069. 2020.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Dustin ML: Visualization of cell-cell
interaction contacts: Synapses and kinapses. Self Nonself. 2:85–97.
2011.PubMed/NCBI View Article : Google Scholar
|