Introduction
Hepatitis B virus (HBV) infection is a worldwide
health problem, and patients who are chronically infected with HBV
are at greater risk of developing liver cirrhosis and liver cancer.
Worldwide, ~292 million individuals are estimated to be chronically
infected with HBV (1). Once HBV
infects hepatocytes, its genome translocates to the nucleus and
covalently closed circular DNA (cccDNA) is formed (2). The stability of cccDNA is one of the
primary reasons why it is difficult to eliminate HBV completely.
The serum levels of hepatitis B surface antigen (HBsAg) are
considered to be associated with cccDNA levels in the liver
(3), and the removal of HBsAg is
regarded as the optimal treatment endpoint, termed ‘functional
cure’ (4,5).
Nucleos(t)ide analogues (NAs), including entecavir
(ETV), tenofovir disoproxil fumarate (TDF) and tenofovir
alafenamide fumarate (TAF), as well as interferons (IFNs), are
widely used for treatment of chronic HBV infection worldwide
(1,2,4,5). These treatments inhibit the reverse
transcription of the HBV genome and HBV DNA in the serum can be
reduced rapidly. Although IFNs were reported to reduce serum HBsAg
levels more efficiently when used appropriately in combination with
NAs (6) or sequentially after NA
discontinuation (7,8), NA monotherapies are still beneficial
for most patients as they can be taken orally and have fewer side
effects than IFNs (4,5). However, long-term administration of NA
is required, as the discontinuation can lead to frequent hepatitis
exacerbations (5). Generally, it is
hypothesized that NA does not reduce cccDNA efficiently and HBsAg
is released into the blood continuously in most cases during NA
treatments (9). A previous report
showed that high serum levels of HBsAg increases the risk of
developing hepatocellular carcinoma in patients with low levels of
HBV DNA (10). Additionally, low
levels of HBsAg are reported to be a surrogate marker for the safe
discontinuation of NA (11,12). Therefore, NAs that can reduce HBsAg
efficiently are required in clinical settings.
A phase 3 clinical trial of TDF in Japan showed a
significantly greater decrease in HBsAg levels in TDF-treated
individuals compared with ETV-treated patients amongst
treatment-naïve patients (13).
Additionally, TDF is effective in patients with ETV-resistant HBV,
even if TDF is administered as a monotherapy (14). However, it is unclear whether such an
effect of TDF can be obtained in NA-treated patients in whom the
hepatitis is stable. In the present study, a pilot prospective
randomized control study was performed to evaluate the efficacy of
switching to TDF in ETV-treated patients.
Patients and methods
Patients
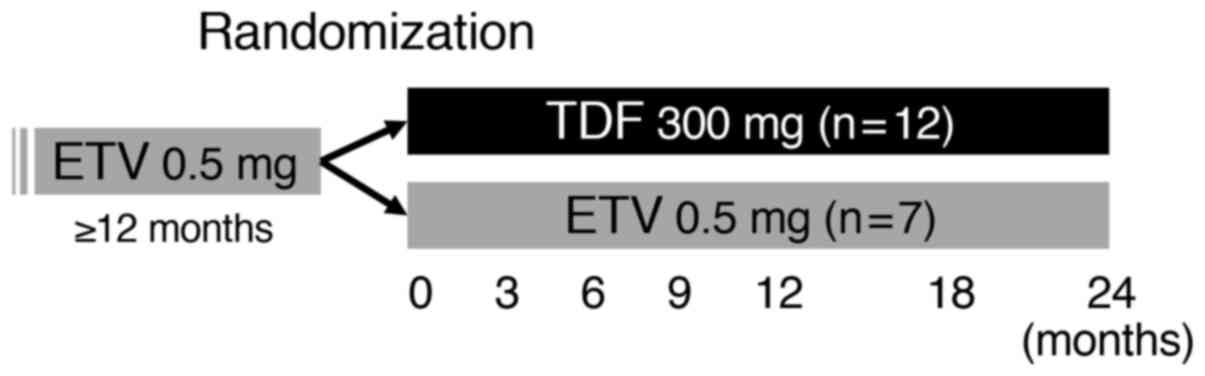
A total of 20 patients were enrolled from 4
hospitals. Inclusion criteria were as follows: i) ETV had been
administered for >1 year continuously without drug resistance;
ii) HBsAg in the serum had been continuously positive; iii)
patients ≥20 years old; and iv) and they had no history of
decompensated liver cirrhosis or liver cancer. The definition of
drug resistance is a 1-log (10-fold) increase in HBV DNA from the
nadir in a patient who had an initial virological response
(5). The exclusion criteria were as
follows: i) Patients receiving immunosuppressive therapies; ii)
estimated glomerular filtration rate (eGFR) <50 ml/min/1.73
m2; iii) presence of hypophosphatemia (<2.5 mg/dl);
iv) pregnant women and women suspected of being pregnant; v)
breast-feeding women and vi) coinfection with hepatitis C virus or
human immunodeficiency virus. The patients were randomized into 2
groups, a TDF-switching group or ETV-continuing group (Fig. 1). Randomization was performed using a
random number table. Among the 20 patients enrolled in the present
study, 1 patient was excluded due to a low eGFR and a total of 19
patients were randomized. The median age of the randomized patients
was 62 years old (range, 32-79); 13 patients (68%) were male, and 6
patients (32%) were female. After randomization, 12 patients
(median age, 63; range, 32-79; 9 males and 3 females) were assigned
to the TDF switching group and 7 patients (median age, 48; range,
37-72; 4 males and 3 females) were assigned to the ETV continuing
group. ETV at 0.5 mg/day was administered orally while fasting, and
TDF at 300 mg/day was administered orally after a meal. The
patients were observed every 3 months for 24 months and the
clinical data were collected at 3, 6, 9, 12, 18 and 24 months after
enrollment. The primary efficacy endpoint was the change of serum
HBsAg at 24 months, and the secondary endpoints were the changes of
alanine aminotransferase (ALT), eGFR and inorganic phosphorus (IP).
Imaging tests including abdominal ultrasonography were performed
for the screening of liver cancer. This study was registered on
University Hospital Medical Information Network Clinical Trials
Registry (UMIN-CTR, ID: UMIN000021948). The study enrollment was
started at April 2016 and the observation was performed until March
2019. The study protocol conformed to the guidelines described in
the Declaration of Helsinki (15),
and was approved by the Medical Ethics Committee of Tohoku
University (approval no. 2016-2-11-1). Written informed consent was
obtained from each patient.
Determination of serological markers
and HBV genotype
The serum levels of HBsAg were quantified using a
chemiluminescent enzyme immunoassay (CLEIA) with LUMIPULSE HBsAg-HQ
(Fujirebio; cat. no. 296851). Hepatitis B e antigen (HBeAg) was
assessed using a CLEIA by ARCHITECT (Abbott Pharmaceutical Co.
Ltd.; cat. no. G06241R03). The HBV DNA levels were quantified using
reverse transcription-quantitative (RT-q)PCR assays using Cobas
TaqMan HBV Auto, according to the manufacturer's protocol (Roche
Diagnostics). HBcrAg was tested using a CLEIA with LUMIPULSE
(Fujirebio; cat. no. 294109). HBV genotypes were determined using
the IMMUNIS HBV genotype EIA kit (Institute of Immunology; cat. no.
1A65).
Statistical analysis
Statistical analysis was performed using JMP version
14.2 (SAS Institute Inc.). Statistical comparisons were performed
using a χ2 test for comparison of frequencies between
the two groups or a Wilcoxon rank sum test for comparison of
continuous variables between two groups. P<0.05 was considered
to indicate a statistically significant difference.
Results
Clinical characteristics of the
enrolled patients
Among the 19 patients randomized in the present
study, a total of 5 (26%) and 14 (74%) patients were infected with
HBV genotype B and C, respectively. The clinical characteristics of
the chronic hepatitis B patients in the TDF switching group (n=12)
and the ETV continuing group (n=7) are shown in Table I. There were no statistically
significant differences in the clinical characteristics between
these groups.
 | Table IClinicopathological characteristics of
the patients randomized in the two groups. |
Table I
Clinicopathological characteristics of
the patients randomized in the two groups.
| Characteristics | TDF
groupa, n=12 | ETV
groupa, n=7 | P-value |
|---|
| Age, years | 63 (49-70) | 48 (40-67) | 0.421 |
| Sex, male/female | 9/3 | 4/3 | 0.423 |
| T-Bil, mg/dl | 0.8 (0.6-0.8) | 0.5 (0.5-0.8) | 0.198 |
| AST, U/l | 20 (19-25) | 17 (16-21) | 0.098 |
| ALT, U/l | 17 (15-31) | 16 (11-21) | 0.289 |
| g-GTP, U/l | 25 (18-28) | 18 (17-27) | 0.928 |
| Alb, g/dl | 4.5 (3.8-5.1) | 4.2 (3.9-5.2) | 0.442 |
| Cr, mg/dl | 0.73
(0.70-0.77) | 0.73
(0.653-0.76) | 0.735 |
| eGFR, ml/min/1.73
m2 | 79.1
(74.8-90.3) | 78.2
(71.7-86.3) | 0.899 |
| IP, mg/dl | 3.2 (2.8-3.3) | 2.8 (2.5-3.0) | 0.071 |
| PLT,
x104/ml | 19.3
(16.4-23.5) | 18.7
(15.4-23.2) | 0.966 |
| FIB-4 index | 1.50
(1.06-1.92) | 1.24
(0.63-2.28) | 0.899 |
| AFP, ng/ml | 2.3 (2.0-2.3) | 2.7 (1.4-2.8) | 1.000 |
| HBV DNA, log
IU/ml | BDL (BDL-BDL) | BDL (BDL-1.0) | 0.258 |
| HBsAg, IU/ml | 1,006
(391-9,011) | 2,500
(483-4,085) | 1.000 |
| HBeAg, +/- | 4/8 | 3/4 | 0.679 |
| HBcrAg, log
U/ml | 4.3 (BDL-4.9) | 3.7 (3.1-4.8) | 0.719 |
| HBV genotype,
B/C | 4/8 | 1/6 | 0.348 |
| ETV duration,
months | 62 (34-93) | 40 (31-49) | 0.206 |
| NA prior to ETV,
LAM/LAM+ADV/none | 1/0/11 | 0/1/6 | 0.231 |
Comparison of the antiviral effects
between the TDF and ETV group
At randomization, the number of patients whose HBV
DNA levels in the serum were lower than the detection limits were
11/12 (92%) and 5/7 (71%) in the TDF and ETV groups, respectively.
At 12 months after enrollment, they were 11/12 (92%) and 6/7 (86%),
and at 24 months, 12/12 (100%) and 6/7 (86%) in the TDF and ETV
groups, respectively.
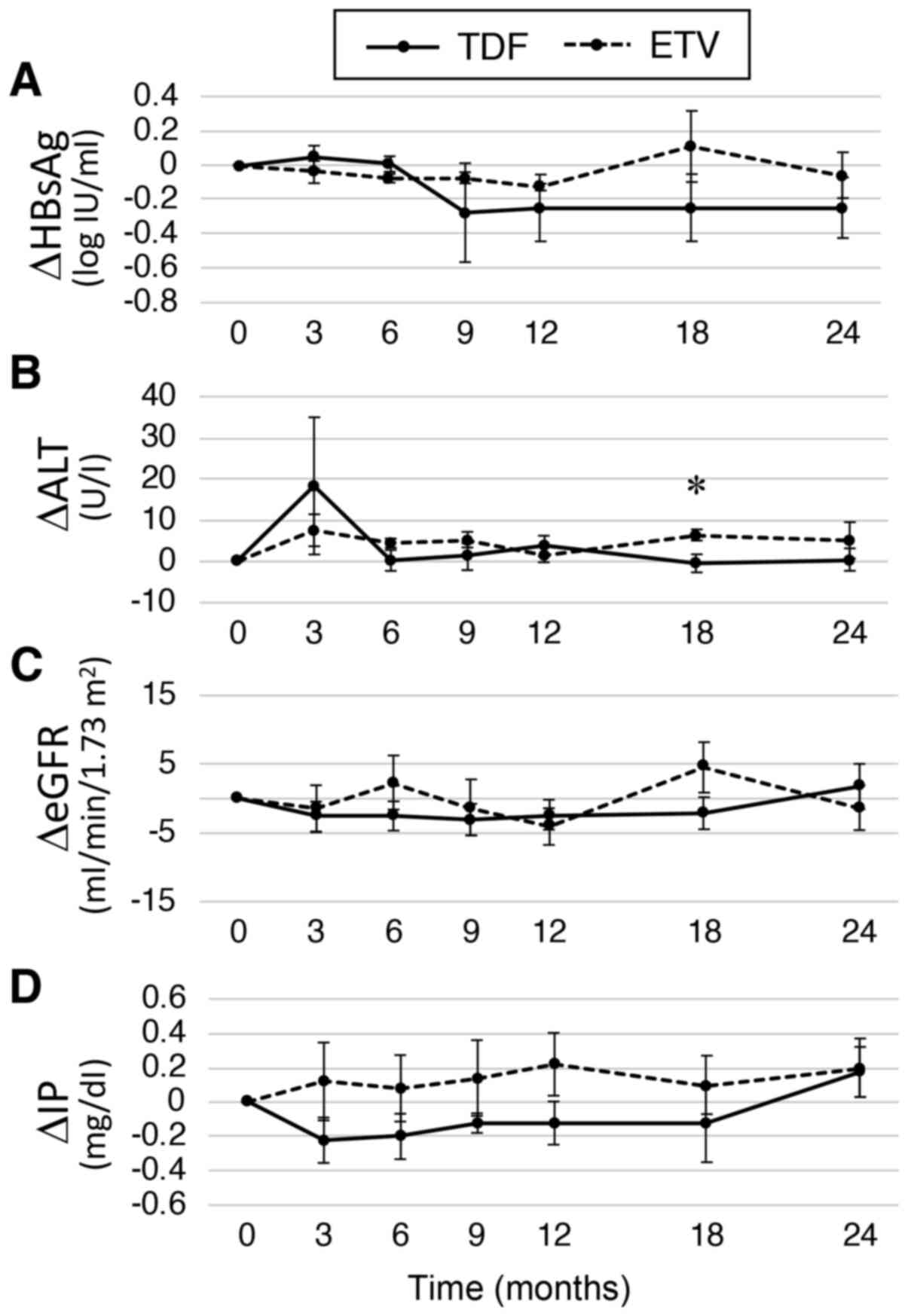
In the overall analysis, the mean change of HBsAg
was -0.20 and -0.17 log IU/ml at 12 and 24 months, respectively.
When comparing the TDF and ETV groups, HBsAg changes were greater
in the TDF group at 12 months (-0.25 vs. -0.13 log IU/ml) and at 24
months (-0.25 vs. -0.06 log IU/ml), but the differences were not
statistically significant (Fig. 2A).
The results showed that HBsAg tended to decrease more in the first
12 months in the TDF group, whereas the changes of HBsAg were lower
in the ETV group during the 24 months. The changes in ALT were
similar other than at 18 months when it was significantly lower in
the TDF group (Fig. 2B). There were
7 patients who were positive for HBeAg at randomization, and HBeAg
sero-clearance was achieved in 2/4 (50%) patients in the TDF group
and 0/3 (0%) patients in the ETV group. No patients developed liver
cancer during the observation period.
Comparison of safety profiles between
the TDF and ETV groups
As renal toxicity has been reported as a major
adverse effect of TDF (16), the
changes in the eGFR and serum IP levels were compared. However, no
differences in eGFR changes were observed (Fig. 2C). The IP levels seemed to be reduced
in the TDF group, but the differences were not significant at any
time points (Fig. 2D). Of note, a
62-year-old male patient in the TDF group, whose serum IP levels
were 2.6 mg/dl at randomization showed hypophosphatemia (2.0 mg/dl)
at 12 months and TDF was switched to TAF. No data regarding this
patient were included in the analysis after this time point. In
this patient, switching back to ETV was not recommended as the
slight signal of serum HBV DNA was detected during ETV
administration. The IP levels slightly recovered to 2.2 mg/dl 6
months after switching to TAF.
HBsAg dependent changes on HBeAg
positivity
As HBeAg affects the efficacies of antiviral
treatments (17), the HBsAg decrease
after 24 months between the HBeAg-positive and HBeAg-negative
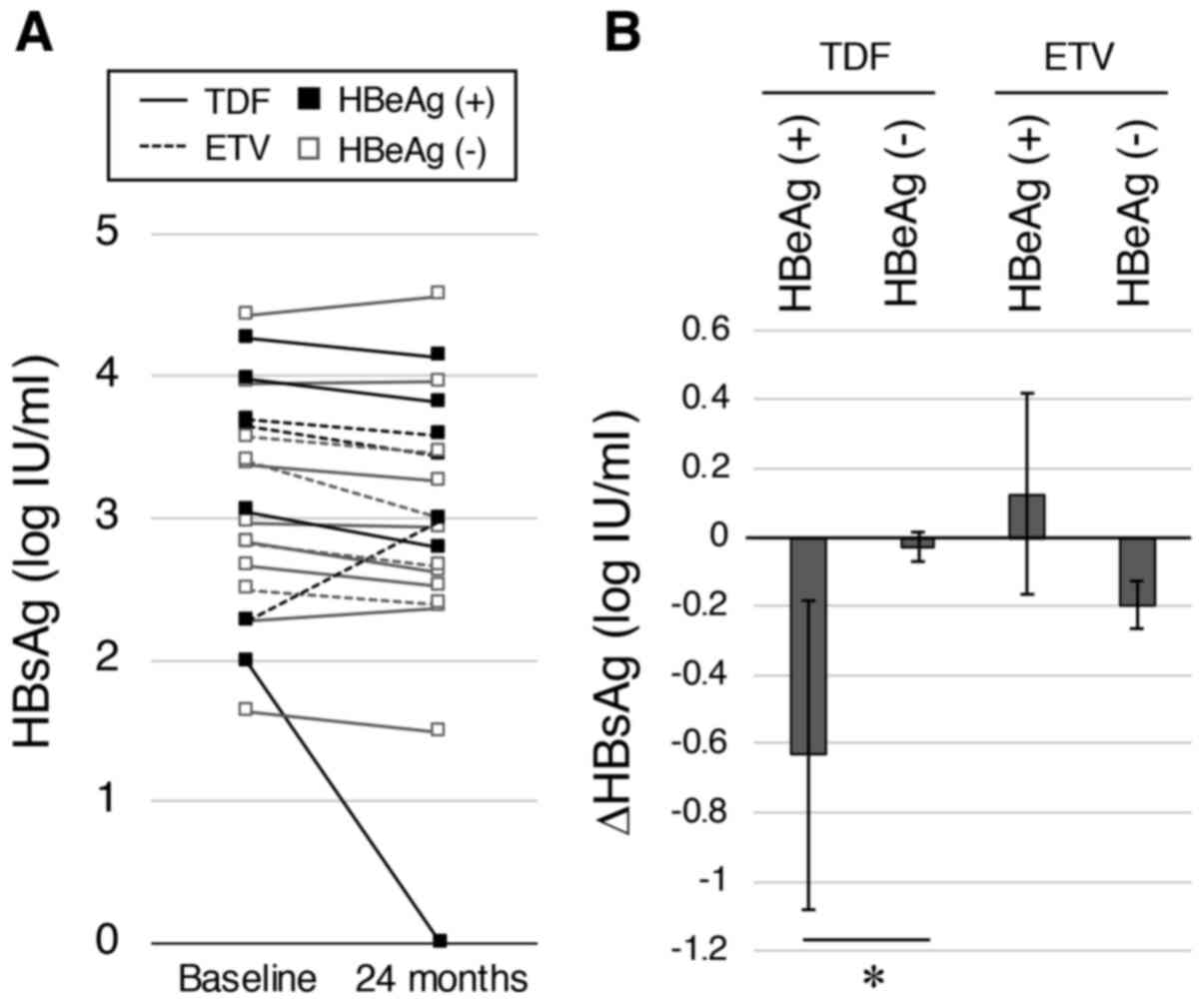
patients were compared. The HBsAg levels at baseline and at 24
months in each patient are shown in Fig.
3A. Notably, an HBeAg-positive patient in the TDF-switching
group lost their HBsAg signal. In the TDF-switching group, a
significantly greater decrease in HBsAg was observed in
HBeAg-positive patients than in the HBeAg-negative patients (-0.63
vs. -0.03 log IU/ml; P=0.030; Fig.
3B). In contrast, no differences between HBeAg-positive and
HBeAg-negative patients was observed in the ETV-continuing
group.
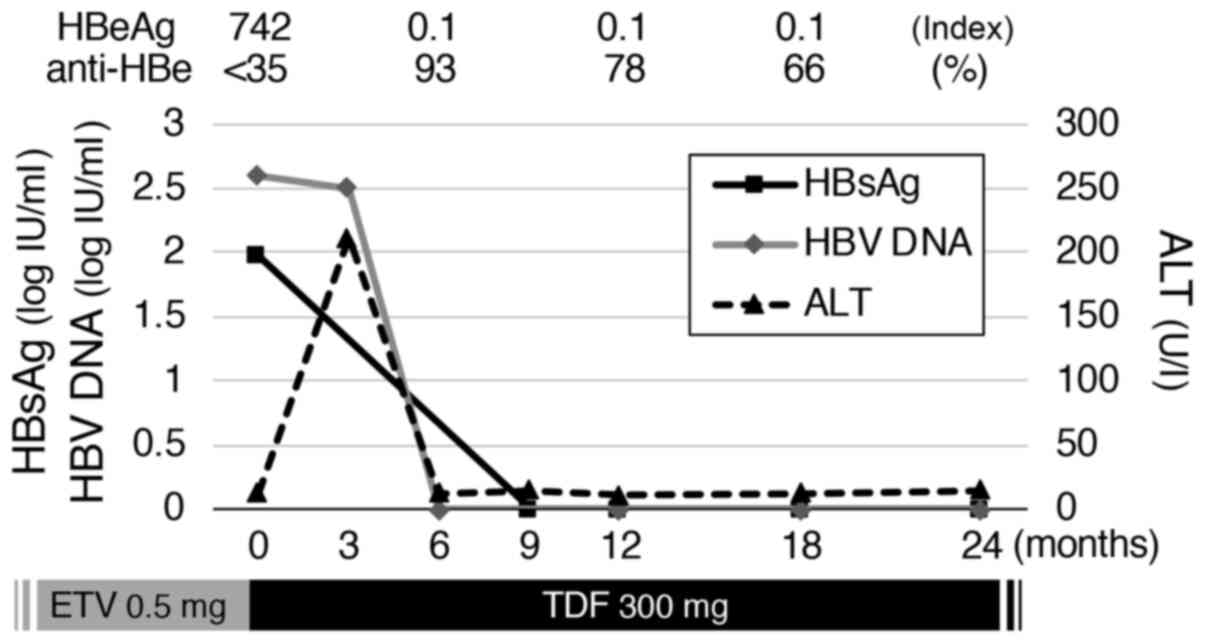
Case presentations
A patient who showed ALT flare-ups after switching
from ETV to TDF exhibited loss of HBsAg subsequently. The patient
was a 77-year-old male and a liver biopsy 8 years before the
enrollment showed METAVIR scores (18) of F2 and A2. He had a history of
diabetes mellitus and underwent an operation for esophageal cancer.
He started administration of ETV 8 years prior to inclusion, and it
was stopped once 4.5 years later. After that, a hepatitis relapse
with HBV DNA of 7.6 log IU/ml and HBsAg of 2.98 log IU/ml was
observed and ETV was restarted 2.5 years ago, and has been
continued for 30 months before switching to TDF. The clinical
course after the treatment switch to TDF is shown in Fig. 4. The HBV genotype was C and HBcrAg
levels evaluated 9 months after the treatment switch was 4.6 log
U/ml. No other patients showed such ALT elevations.
Discussion
In the present pilot study, the effects of switching
to TDF in ETV-treated patients without drug resistance was
assessed. The primary aim was to evaluate differences in the
decrease in HBsAg signal after the treatment switch, but they were
not significant in the overall analysis. A similar result for
changes in HBsAg was reported recently in a 48-week randomized
trial targeting patients who had been treated with ETV for >5
years (19). However, when analyzed
in the TDF-switching group in the present study, a greater decrease
in HBsAg signal in HBeAg-positive patients compared with
HBeAg-negative patients was observed. Of note, even when a patient
whose HBsAg disappeared rapidly (Fig.
4) was removed from the analysis, there was still a similar
tendency (P=0.068). As most patients (85%) were HBeAg-negative in
the previous study (19), it is
possible that switching to TDF in ETV-treated patients may have had
an additional effect on the HBsAg decrease only in HBeAg-positive
patients. Consistent with this, in treatment-naïve patients, TDF
was reported to result in a greater decrease in HBsAg signal in
HBeAg-positive patients compared with HBeAg-negative patients
(-0.37 vs. 0.07 log IU/ml) (13).
Additionally, a randomized controlled study in South Korea in
patients whose responses to ETV were partial showed that switching
from ETV to TDF was superior than continuing ETV for the
suppression of HBV DNA (20). In
this previous study, all patients in the TDF-switching group were
HBeAg-positive (n=22). Based on the aforementioned previous study
and the present study, switching ETV to TDF may be considered in
ETV-treated patients whose HBeAg is still positive. The mechanisms
underlying the more prominent decrease in HBsAg in the
HBeAg-positive patients are still unclear, but they may be related
to the fact that HBeAg positivity during ETV administration
indicates limited suppression of HBV mRNA transcription from
cccDNA, even if HBV DNA is undetectable in the serum. In such
patients, TDF may exert additional effects. Another possible
mechanism is that HBeAg may affect the anti-viral effects of TDF.
HBeAg is known to modulate immune responses (21). Furthermore, it was previously shown
that HBeAg may modulate intracellular vesicle trafficking (22). Such potential effects of HBeAg may
alter the effect of TDF, but further investigation is required.
The different HBV genotypes are known to have
varying effects on the clinical course in chronic infections. In
our previous study, it was shown that the HBsAg decrease was
greater in patients with HBV genotype B than in those with HBV
genotype C (17). A higher frequency
of HBV genotype B in individuals from northeast Japan, where our
institutions are located, than overall in Japan, has been
established previously (23). In the
present study including HBV genotype B at 26%, differences between
genotypes could not be found (data not shown), possibly due to the
small number of patients.
It has been reported that ETV does not affect renal
function, even in patients with severe renal dysfunction (24). In contrast, TDF is known to have a
potential side effect on renal toxicity (25). Proximal tubular dysfunction causes a
decrease in phosphorus reabsorption leading to decreased bone
mineral density. The present study showed no statistical
differences in eGFR and IP levels, but there was a tendency to
reduction of IP levels in the TDF group and a patient in the TDF
group presented with hypophosphatemia. Therefore, the treatment
switch from ETV to TDF should be considered carefully in older
patients, who are more likely to also suffer from chronic kidney
diseases or osteoporosis. As TAF was reported to have fewer side
effects on the kidneys and bones (26), it may be an option to switch NAs to
TAF in such patients. Switching from TDF to TAF was reported to
contribute to recovery of renal dysfunction in patients with HBV
(27) and in those infected with
human immunodeficiency virus (28).
A previous study assessing switching from ETV to TAF showed a
greater decrease in HBsAg levels (29), and another study showed that such a
decrease was observed in patients with a low baseline HBsAg
(30). Additionally, it has been
shown that HBsAg is decreased more prominently in patients with
non-liver cirrhosis, HBV genotype B, HBeAg negative patients or
patients with low hepatitis B core-related antigen (HBcrAg)
(31). Although no similar tendency
was observed in the present study, further studies with larger
cohorts are required to clarify the differences between switching
from ETV to TDF with that to TAF.
Recent studies have shown that only acyclic
nucleoside phosphonates (ANPs) such as adefovir dipivoxil and TDF,
increased IFN-λ3 levels in the gastrointestinal tract (32), inhibited lipopolysaccharide-mediated
interleukin (IL)-10 production and induced IL-12p70 in peripheral
blood mononuclear cells towards HBV elimination (33). Such additional immunomodulatory
effects with ANPs, which were not observed with ETV, may have a
favorable effect on the HBsAg decrease, particularly in
HBeAg-positive patients. In our HBeAg-positive case with HBsAg
disappearance after switching from ETV to TDF, such an
immunomodulatory effect may have played a role. Clarification of
the HBeAg effects on TDF is needed.
There are some limitations to the present study.
First, this study was a small pilot study, and the number of
HBeAg-positive patients were limited; therefore, the results should
be verified in larger studies. The allocation of groups was
intended to be equal in the study design, but the difference in
patient numbers was made unintentionally due the small study size.
Second, although HBcrAg has been reported to be associated with
cccDNA in the liver (34), the
changes in HBcrAg could not be evaluated due to a lack of relevant
data. Third, TDF may affect bone mineral density (26), but this could not be evaluated. These
parameters should be analyzed sequentially in future studies.
In conclusion, the present pilot randomized control
study showed that switching to TDF did not have additional effects
on the decrease in HBsAg in previously ETV-treated patients.
However, when analyzing only the TDF-switching group, the
HBeAg-positive patients showed a greater decrease in HBsAg than the
HBeAg-negative patients. Further studies are required to confirm
the effects and to determine the underlying mechanisms.
Acknowledgements
Not applicable.
Funding
This study was supported in part by a Grant-in-Aid
from the Japan Society for the Promotion of Science (grant no.
19K08385).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
JI designed the study, analyzed the data and wrote
the manuscript. TA and TK designed the study, collected the data
and critically reviewed the manuscript. NO, TU, EK, MN, TI, AS, MT
and KS collected the data. AM designed the study and critically
reviewed the manuscript. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The study protocol used in the present study
conformed to the guidelines described in the Declaration of
Helsinki, and has been approved by the Medical Ethics Committee of
Tohoku University (approval no. 2016-2-11-1). Written informed
consent was obtained from each patient.
Patient consent for publication
Not applicable.
Competing interests
JI received research funding from Gilead Sciences
and AbbVie. The other authors declare that they have no conflict of
interests.
References
|
1
|
Polaris Observatory Collaborators. Global
prevalence, treatment, and prevention of hepatitis B virus
infection in 2016: A modelling study. Lancet Gastroenterol Hepatol.
3:383–403. 2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Inoue J, Ninomiya M, Shimosegawa T and
McNiven MA: Cellular membrane trafficking machineries used by the
hepatitis viruses. Hepatology. 68:751–762. 2018.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Cornberg M, Wong VW, Locarnini S, Brunetto
M, Janssen HL and Chan HL: The role of quantitative hepatitis B
surface antigen revisited. J Hepatol. 66:398–411. 2017.PubMed/NCBI View Article : Google Scholar
|
|
4
|
European Association for the Study of the
Liver. Electronic address: simpleeasloffice@easloffice.eu;
European Association for the Study of the Liver. EASL 2017 Clinical
Practice Guidelines on the management of hepatitis B virus
infection. J Hepatol. 67:370–398. 2017.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Terrault NA, Lok ASF, McMahon BJ, Chang
KM, Hwang JP, Jonas MM, Brown RS Jr, Bzowej NH and Wong JB: Update
on prevention, diagnosis, and treatment of chronic hepatitis B:
AASLD 2018 hepatitis B guidance. Hepatology. 67:1560–1599.
2018.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Marcellin P, Ahn SH, Ma X, Caruntu FA, Tak
WY, Elkashab M, Chuang WL, Lim SG, Tabak F, Mehta R, et al:
Combination of tenofovir disoproxil fumarate and peginterferon α-2a
increases loss of hepatitis B surface antigen in patients with
chronic hepatitis B. Gastroenterology. 150:134–144.e10.
2016.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Tamaki N, Kurosaki M, Kusakabe A, Orito E,
Joko K, Kojima Y, Kimura H, Uchida Y, Hasebe C, Asahina Y, et al:
Hepatitis B surface antigen reduction by switching from long-term
nucleoside/nucleotide analogue administration to pegylated
interferon. J Viral Hepat. 24:672–678. 2017.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Tatsukawa Y, Tsuge M, Kawakami Y, Hiyama
Y, Murakami E, Kurihara M, Nomura M, Tsushima K, Uchida T, Nakahara
T, et al: Reduction of hepatitis B surface antigen in sequential
versus add-on pegylated interferon to nucleoside/nucleotide
analogue therapy in HBe-antigen-negative chronic hepatitis B
patients: A pilot study. Antivir Ther. 23:639–646. 2018.PubMed/NCBI View
Article : Google Scholar
|
|
9
|
Asselah T, Loureiro D, Boyer N and
Mansouri A: Targets and future direct-acting antiviral approaches
to achieve hepatitis B virus cure. Lancet Gastroenterol Hepatol.
4:883–892. 2019.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Tseng TC, Liu CJ, Yang HC, Su TH, Wang CC,
Chen CL, Kuo SF, Liu CH, Chen PJ, Chen DS and Kao JH: High levels
of hepatitis B surface antigen increase risk of hepatocellular
carcinoma in patients with low HBV load. Gastroenterology.
142:1140–1149.e3; quiz e13-4. 2012.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Tanaka E and Matsumoto A: Guidelines for
avoiding risks resulting from discontinuation of
nucleoside/nucleotide analogs in patients with chronic hepatitis B.
Hepatol Res. 44:1–8. 2014.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Papatheodoridis G, Vlachogiannakos I,
Cholongitas E, Wursthorn K, Thomadakis C, Touloumi G and Petersen
J: Discontinuation of oral antivirals in chronic hepatitis B: A
systematic review. Hepatology. 63:1481–1492. 2016.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Koike K, Suyama K, Ito H, Itoh H and
Sugiura W: Randomized prospective study showing the non-inferiority
of tenofovir to entecavir in treatment-naive chronic hepatitis B
patients. Hepatol Res. 48:59–68. 2018.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Lim YS, Gwak GY, Choi J, Lee YS, Byun KS,
Kim YJ, Yoo BC, Kwon SY and Lee HC: Monotherapy with tenofovir
disoproxil fumarate for adefovir-resistant vs. entecavir-resistant
chronic hepatitis B: A 5-year clinical trial. J Hepatol. 71:35–44.
2019.PubMed/NCBI View Article : Google Scholar
|
|
15
|
World Medical Association, Inc.:
Declaration of Helsinki. urihttps://www.wma.net/what-we-do/medical-ethics/declaration-of-helsinki/simplehttps://www.wma.net/what-we-do/medical-ethics/declaration-of-helsinki/.
Accessed October 16, 2020.
|
|
16
|
Wong GL, Seto WK, Wong VW, Yuen MF and
Chan HL: Review article: Long-term safety of oral anti-viral
treatment for chronic hepatitis B. Aliment Pharmacol Ther.
47:730–737. 2018.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Inoue J, Akahane T, Nakayama H, Kimura O,
Kobayashi T, Kisara N, Sato T, Morosawa T, Izuma M, Kakazu E, et
al: Comparison of hepatitis B virus genotypes B and C among
chronically hepatitis B virus-infected patients who received
nucleos(t)ide analogs: A multicenter retrospective study. Hepatol
Res. 49:1263–1274. 2019.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Bedossa P and Poynard T: An algorithm for
the grading of activity in chronic hepatitis C. The METAVIR
Cooperative Study Group. Hepatology. 24:289–293. 1996.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Iida-Ueno A, Enomoto M, Kozuka R, Tamori A
and Kawada N: Switching to tenofovir disoproxil fumarate vs
continuing treatment in patients with chronic hepatitis B who
maintain long-term virological response to entecavir therapy: A
randomized trial. J Med Virol. 91:1295–1300. 2019.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Yim HJ, Kim IH, Suh SJ, Jung YK, Kim JH,
Seo YS, Yeon JE, Kim CW, Kwon SY, Park SH, et al: Switching to
tenofovir vs. continuing entecavir for hepatitis B virus with
partial virologic response to entecavir: A randomized controlled
trial. J Viral Hepat. 25:1321–1330. 2018.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Chen LM, Fan XG, Ma J, He B and Jiang YF:
Molecular mechanisms of HBeAg in persistent HBV infection. Hepatol
Int. 11:79–86. 2017.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Inoue J, Krueger EW, Chen J, Cao H,
Ninomiya M and McNiven MA: HBV secretion is regulated through the
activation of endocytic and autophagic compartments mediated by
Rab7 stimulation. J Cell Sci. 128:1696–1706. 2015.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Inoue J, Kondo Y, Umetsu T, Yamamoto T,
Miura M, Mano Y, Kobayashi T, Obara N, Niitsuma H, Kogure T, et al:
Shifting hepatitis B virus genotypes of acute hepatitis B patients
in northeast Japan. J Med Virol. 88:69–78. 2016.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Suzuki K, Suda G, Yamamoto Y, Furuya K,
Baba M, Kimura M, Maehara O, Shimazaki T, Yamamoto K, Shigesawa T,
et al: Entecavir treatment of hepatitis B virus-infected patients
with severe renal impairment and those on hemodialysis. Hepatol
Res. 49:1294–1304. 2019.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Ezinga M, Wetzels JF, Bosch ME, van der
Ven AJ and Burger DM: Long-term treatment with tenofovir:
Prevalence of kidney tubular dysfunction and its association with
tenofovir plasma concentration. Antivir Ther. 19:765–771.
2014.PubMed/NCBI View
Article : Google Scholar
|
|
26
|
Agarwal K, Brunetto M, Seto WK, Lim YS,
Fung S, Marcellin P, Ahn SH, Izumi N, Chuang WL, Bae H, et al: 96
weeks treatment of tenofovir alafenamide vs. tenofovir disoproxil
fumarate for hepatitis B virus infection. J Hepatol. 68:672–681.
2018.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Kaneko S, Kurosaki M, Tamaki N, Itakura J,
Hayashi T, Kirino S, Osawa L, Watakabe K, Okada M, Wang W, et al:
Tenofovir alafenamide for hepatitis B virus infection including
switching therapy from tenofovir disoproxil fumarate. J
Gastroenterol Hepatol. 34:2004–2010. 2019.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Surial B, Ledergerber B, Calmy A,
Cavassini M, Gunthard HF, Kovari H, Stöckle M, Bernasconi E, Schmid
P, Fux CA, et al: Changes in renal function after switching from
TDF to TAF in HIV-infected individuals: A prospective cohort study.
J Infect Dis. 222:637–645. 2020.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Kumada T, Toyoda H, Tada T, Yasuda S,
Miyake N and Tanaka J: Comparison of the impact of tenofovir
alafenamide and entecavir on declines of hepatitis B surface
antigen levels. Eur J Gastroenterol Hepatol: Apr 10, 2020.
|
|
30
|
Hagiwara S, Nishida N, Ida H, Ueshima K,
Minami Y, Takita M, Komeda Y and Kudo M: Switching from entecavir
to tenofovir alafenamide versus maintaining entecavir for chronic
hepatitis B. J Med Virol. 91:1804–1810. 2019.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Uchida Y, Nakao M, Tsuji S, Uemura H,
Kouyama JI, Naiki K, Motoya D, Sugawara K, Nakayama N, Imai Y, et
al: Significance of switching of the nucleos(t)ide analog used to
treat Japanese patients with chronic hepatitis B virus infection
from entecavir to tenofovir alafenamide fumarate. J Med Virol.
92:329–338. 2020.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Murata K, Asano M, Matsumoto A, Sugiyama
M, Nishida N, Tanaka E, Inoue T, Sakamoto M, Enomoto N, Shirasaki
T, et al: Induction of IFN-λ3 as an additional effect of
nucleotide, not nucleoside, analogues: A new potential target for
HBV infection. Gut. 67:362–371. 2018.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Murata K, Tsukuda S, Suizu F, Kimura A,
Sugiyama M, Watashi K, Noguchi M and Mizokami M: Immunomodulatory
Mechanism of Acyclic Nucleoside phosphates in treatment of
hepatitis B virus infection. Hepatology. 71:1533–1545.
2020.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Baudi I, Inoue T and Tanaka Y: Novel
biomarkers of hepatitis B and hepatocellular carcinoma: Clinical
significance of HBcrAg and M2BPGi. Int J Mol Sci.
21(949)2020.PubMed/NCBI View Article : Google Scholar
|