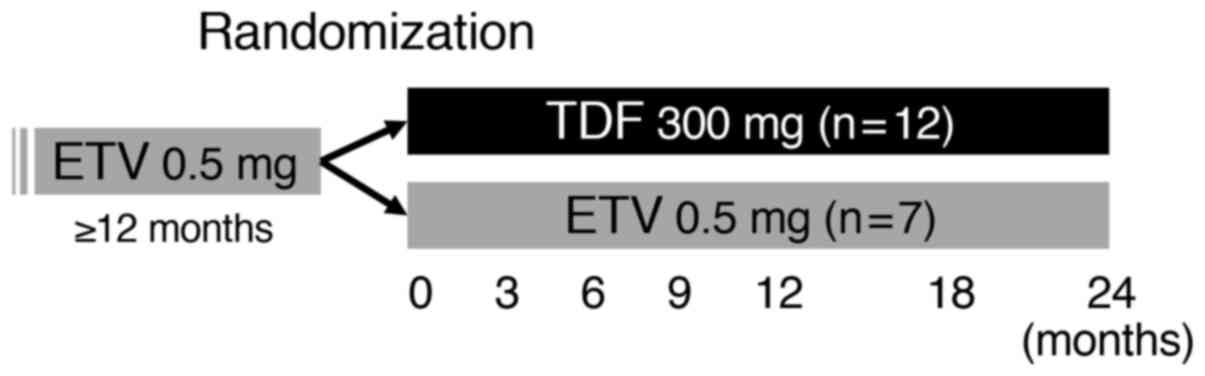
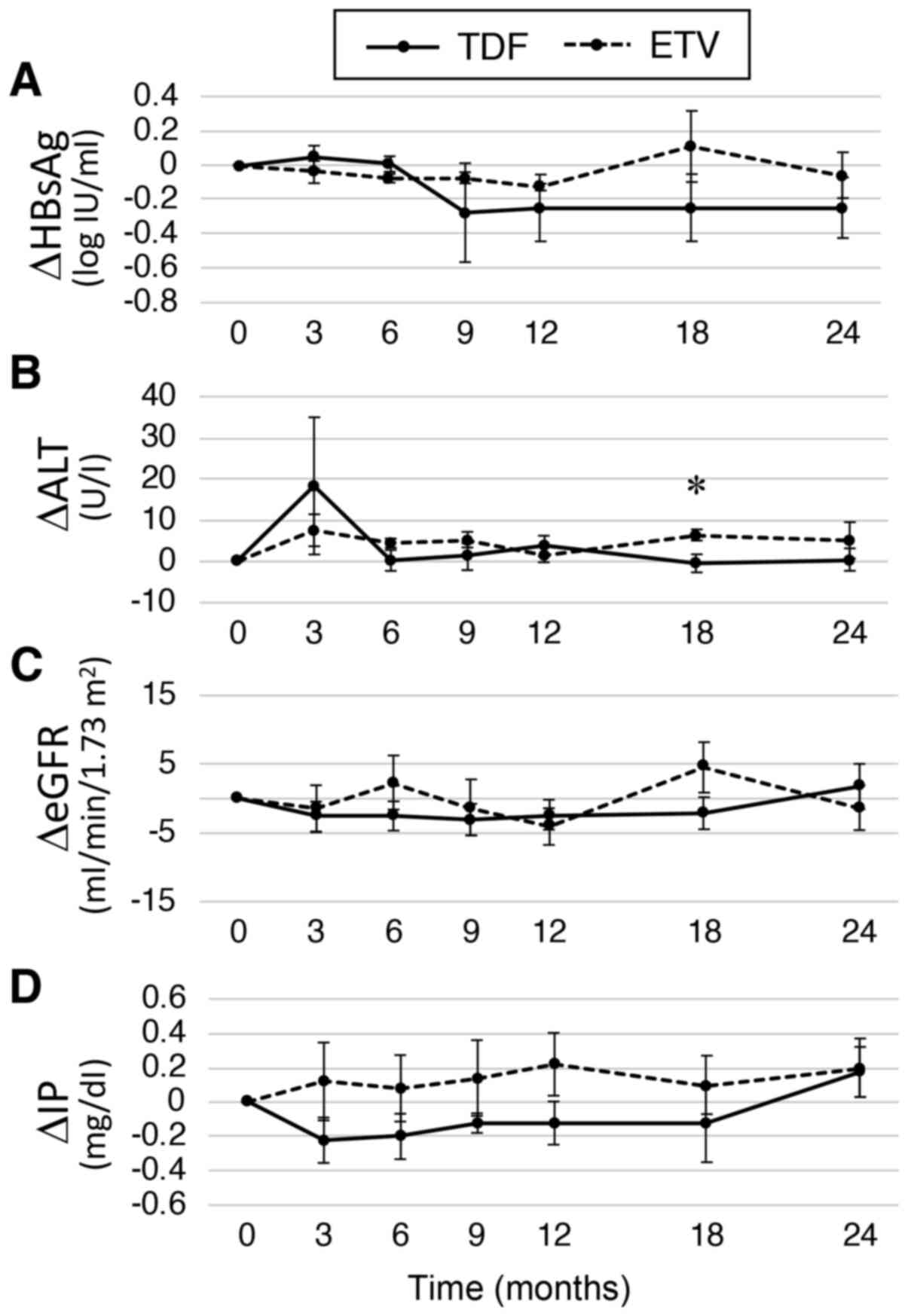
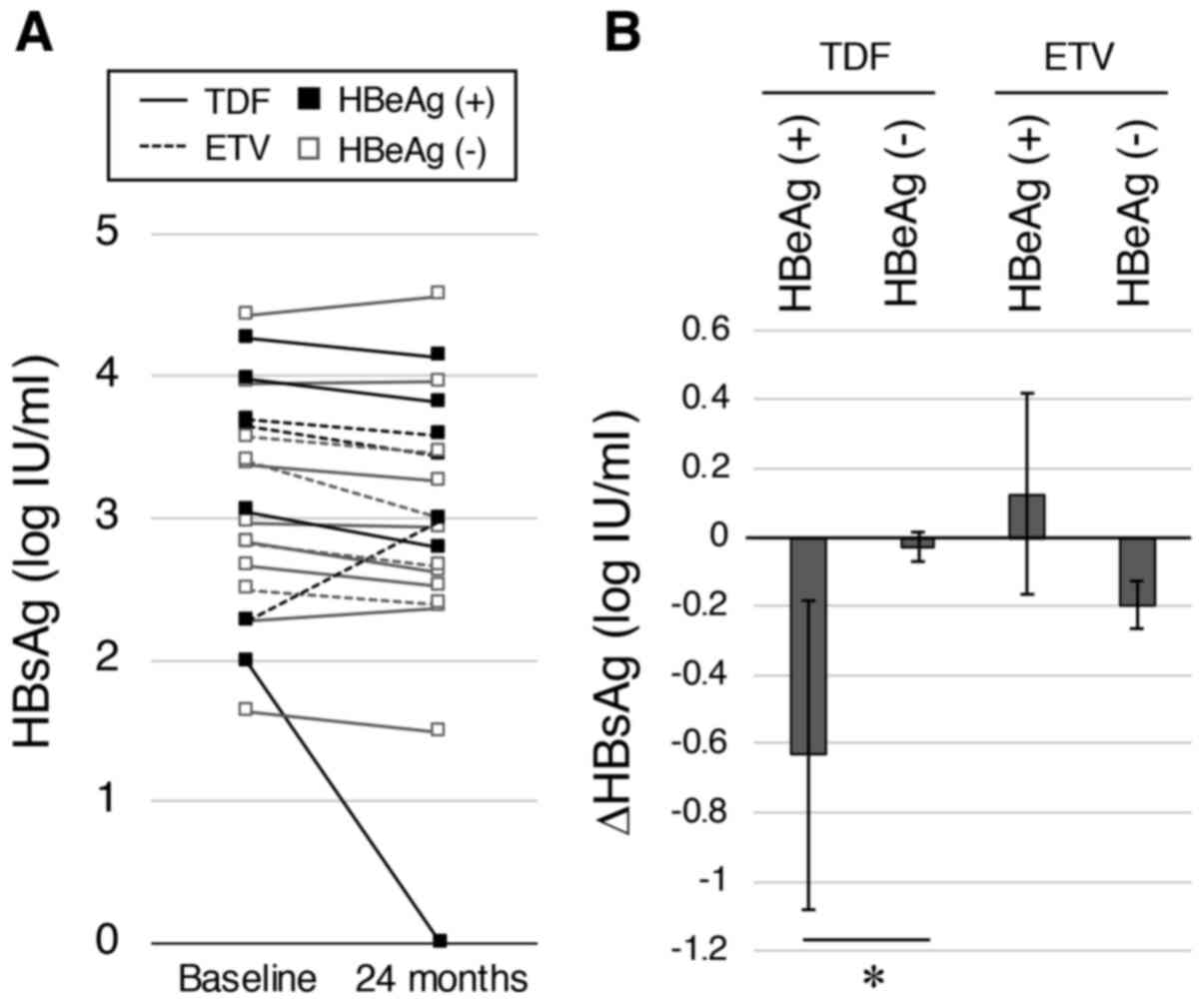
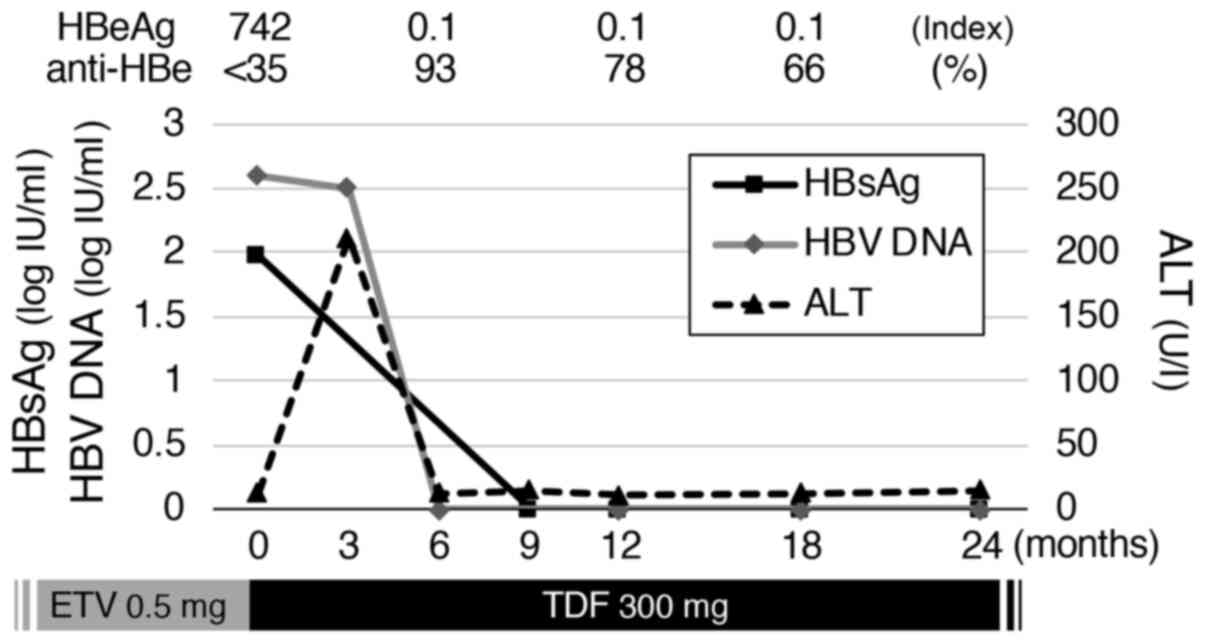
|
1
|
Polaris Observatory Collaborators. Global
prevalence, treatment, and prevention of hepatitis B virus
infection in 2016: A modelling study. Lancet Gastroenterol Hepatol.
3:383–403. 2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Inoue J, Ninomiya M, Shimosegawa T and
McNiven MA: Cellular membrane trafficking machineries used by the
hepatitis viruses. Hepatology. 68:751–762. 2018.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Cornberg M, Wong VW, Locarnini S, Brunetto
M, Janssen HL and Chan HL: The role of quantitative hepatitis B
surface antigen revisited. J Hepatol. 66:398–411. 2017.PubMed/NCBI View Article : Google Scholar
|
|
4
|
European Association for the Study of the
Liver. Electronic address: simpleeasloffice@easloffice.eu;
European Association for the Study of the Liver. EASL 2017 Clinical
Practice Guidelines on the management of hepatitis B virus
infection. J Hepatol. 67:370–398. 2017.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Terrault NA, Lok ASF, McMahon BJ, Chang
KM, Hwang JP, Jonas MM, Brown RS Jr, Bzowej NH and Wong JB: Update
on prevention, diagnosis, and treatment of chronic hepatitis B:
AASLD 2018 hepatitis B guidance. Hepatology. 67:1560–1599.
2018.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Marcellin P, Ahn SH, Ma X, Caruntu FA, Tak
WY, Elkashab M, Chuang WL, Lim SG, Tabak F, Mehta R, et al:
Combination of tenofovir disoproxil fumarate and peginterferon α-2a
increases loss of hepatitis B surface antigen in patients with
chronic hepatitis B. Gastroenterology. 150:134–144.e10.
2016.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Tamaki N, Kurosaki M, Kusakabe A, Orito E,
Joko K, Kojima Y, Kimura H, Uchida Y, Hasebe C, Asahina Y, et al:
Hepatitis B surface antigen reduction by switching from long-term
nucleoside/nucleotide analogue administration to pegylated
interferon. J Viral Hepat. 24:672–678. 2017.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Tatsukawa Y, Tsuge M, Kawakami Y, Hiyama
Y, Murakami E, Kurihara M, Nomura M, Tsushima K, Uchida T, Nakahara
T, et al: Reduction of hepatitis B surface antigen in sequential
versus add-on pegylated interferon to nucleoside/nucleotide
analogue therapy in HBe-antigen-negative chronic hepatitis B
patients: A pilot study. Antivir Ther. 23:639–646. 2018.PubMed/NCBI View
Article : Google Scholar
|
|
9
|
Asselah T, Loureiro D, Boyer N and
Mansouri A: Targets and future direct-acting antiviral approaches
to achieve hepatitis B virus cure. Lancet Gastroenterol Hepatol.
4:883–892. 2019.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Tseng TC, Liu CJ, Yang HC, Su TH, Wang CC,
Chen CL, Kuo SF, Liu CH, Chen PJ, Chen DS and Kao JH: High levels
of hepatitis B surface antigen increase risk of hepatocellular
carcinoma in patients with low HBV load. Gastroenterology.
142:1140–1149.e3; quiz e13-4. 2012.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Tanaka E and Matsumoto A: Guidelines for
avoiding risks resulting from discontinuation of
nucleoside/nucleotide analogs in patients with chronic hepatitis B.
Hepatol Res. 44:1–8. 2014.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Papatheodoridis G, Vlachogiannakos I,
Cholongitas E, Wursthorn K, Thomadakis C, Touloumi G and Petersen
J: Discontinuation of oral antivirals in chronic hepatitis B: A
systematic review. Hepatology. 63:1481–1492. 2016.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Koike K, Suyama K, Ito H, Itoh H and
Sugiura W: Randomized prospective study showing the non-inferiority
of tenofovir to entecavir in treatment-naive chronic hepatitis B
patients. Hepatol Res. 48:59–68. 2018.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Lim YS, Gwak GY, Choi J, Lee YS, Byun KS,
Kim YJ, Yoo BC, Kwon SY and Lee HC: Monotherapy with tenofovir
disoproxil fumarate for adefovir-resistant vs. entecavir-resistant
chronic hepatitis B: A 5-year clinical trial. J Hepatol. 71:35–44.
2019.PubMed/NCBI View Article : Google Scholar
|
|
15
|
World Medical Association, Inc.:
Declaration of Helsinki. urihttps://www.wma.net/what-we-do/medical-ethics/declaration-of-helsinki/simplehttps://www.wma.net/what-we-do/medical-ethics/declaration-of-helsinki/.
Accessed October 16, 2020.
|
|
16
|
Wong GL, Seto WK, Wong VW, Yuen MF and
Chan HL: Review article: Long-term safety of oral anti-viral
treatment for chronic hepatitis B. Aliment Pharmacol Ther.
47:730–737. 2018.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Inoue J, Akahane T, Nakayama H, Kimura O,
Kobayashi T, Kisara N, Sato T, Morosawa T, Izuma M, Kakazu E, et
al: Comparison of hepatitis B virus genotypes B and C among
chronically hepatitis B virus-infected patients who received
nucleos(t)ide analogs: A multicenter retrospective study. Hepatol
Res. 49:1263–1274. 2019.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Bedossa P and Poynard T: An algorithm for
the grading of activity in chronic hepatitis C. The METAVIR
Cooperative Study Group. Hepatology. 24:289–293. 1996.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Iida-Ueno A, Enomoto M, Kozuka R, Tamori A
and Kawada N: Switching to tenofovir disoproxil fumarate vs
continuing treatment in patients with chronic hepatitis B who
maintain long-term virological response to entecavir therapy: A
randomized trial. J Med Virol. 91:1295–1300. 2019.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Yim HJ, Kim IH, Suh SJ, Jung YK, Kim JH,
Seo YS, Yeon JE, Kim CW, Kwon SY, Park SH, et al: Switching to
tenofovir vs. continuing entecavir for hepatitis B virus with
partial virologic response to entecavir: A randomized controlled
trial. J Viral Hepat. 25:1321–1330. 2018.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Chen LM, Fan XG, Ma J, He B and Jiang YF:
Molecular mechanisms of HBeAg in persistent HBV infection. Hepatol
Int. 11:79–86. 2017.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Inoue J, Krueger EW, Chen J, Cao H,
Ninomiya M and McNiven MA: HBV secretion is regulated through the
activation of endocytic and autophagic compartments mediated by
Rab7 stimulation. J Cell Sci. 128:1696–1706. 2015.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Inoue J, Kondo Y, Umetsu T, Yamamoto T,
Miura M, Mano Y, Kobayashi T, Obara N, Niitsuma H, Kogure T, et al:
Shifting hepatitis B virus genotypes of acute hepatitis B patients
in northeast Japan. J Med Virol. 88:69–78. 2016.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Suzuki K, Suda G, Yamamoto Y, Furuya K,
Baba M, Kimura M, Maehara O, Shimazaki T, Yamamoto K, Shigesawa T,
et al: Entecavir treatment of hepatitis B virus-infected patients
with severe renal impairment and those on hemodialysis. Hepatol
Res. 49:1294–1304. 2019.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Ezinga M, Wetzels JF, Bosch ME, van der
Ven AJ and Burger DM: Long-term treatment with tenofovir:
Prevalence of kidney tubular dysfunction and its association with
tenofovir plasma concentration. Antivir Ther. 19:765–771.
2014.PubMed/NCBI View
Article : Google Scholar
|
|
26
|
Agarwal K, Brunetto M, Seto WK, Lim YS,
Fung S, Marcellin P, Ahn SH, Izumi N, Chuang WL, Bae H, et al: 96
weeks treatment of tenofovir alafenamide vs. tenofovir disoproxil
fumarate for hepatitis B virus infection. J Hepatol. 68:672–681.
2018.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Kaneko S, Kurosaki M, Tamaki N, Itakura J,
Hayashi T, Kirino S, Osawa L, Watakabe K, Okada M, Wang W, et al:
Tenofovir alafenamide for hepatitis B virus infection including
switching therapy from tenofovir disoproxil fumarate. J
Gastroenterol Hepatol. 34:2004–2010. 2019.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Surial B, Ledergerber B, Calmy A,
Cavassini M, Gunthard HF, Kovari H, Stöckle M, Bernasconi E, Schmid
P, Fux CA, et al: Changes in renal function after switching from
TDF to TAF in HIV-infected individuals: A prospective cohort study.
J Infect Dis. 222:637–645. 2020.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Kumada T, Toyoda H, Tada T, Yasuda S,
Miyake N and Tanaka J: Comparison of the impact of tenofovir
alafenamide and entecavir on declines of hepatitis B surface
antigen levels. Eur J Gastroenterol Hepatol: Apr 10, 2020.
|
|
30
|
Hagiwara S, Nishida N, Ida H, Ueshima K,
Minami Y, Takita M, Komeda Y and Kudo M: Switching from entecavir
to tenofovir alafenamide versus maintaining entecavir for chronic
hepatitis B. J Med Virol. 91:1804–1810. 2019.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Uchida Y, Nakao M, Tsuji S, Uemura H,
Kouyama JI, Naiki K, Motoya D, Sugawara K, Nakayama N, Imai Y, et
al: Significance of switching of the nucleos(t)ide analog used to
treat Japanese patients with chronic hepatitis B virus infection
from entecavir to tenofovir alafenamide fumarate. J Med Virol.
92:329–338. 2020.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Murata K, Asano M, Matsumoto A, Sugiyama
M, Nishida N, Tanaka E, Inoue T, Sakamoto M, Enomoto N, Shirasaki
T, et al: Induction of IFN-λ3 as an additional effect of
nucleotide, not nucleoside, analogues: A new potential target for
HBV infection. Gut. 67:362–371. 2018.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Murata K, Tsukuda S, Suizu F, Kimura A,
Sugiyama M, Watashi K, Noguchi M and Mizokami M: Immunomodulatory
Mechanism of Acyclic Nucleoside phosphates in treatment of
hepatitis B virus infection. Hepatology. 71:1533–1545.
2020.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Baudi I, Inoue T and Tanaka Y: Novel
biomarkers of hepatitis B and hepatocellular carcinoma: Clinical
significance of HBcrAg and M2BPGi. Int J Mol Sci.
21(949)2020.PubMed/NCBI View Article : Google Scholar
|