1. Introduction
Advanced glycation end-products (AGEs) represent a
broad heterogeneous group of compounds formed by nonenzymatic
reactions between reducing sugars or oxidized lipids and the free
amino groups of proteins, amino phospholipids or nucleic acids.
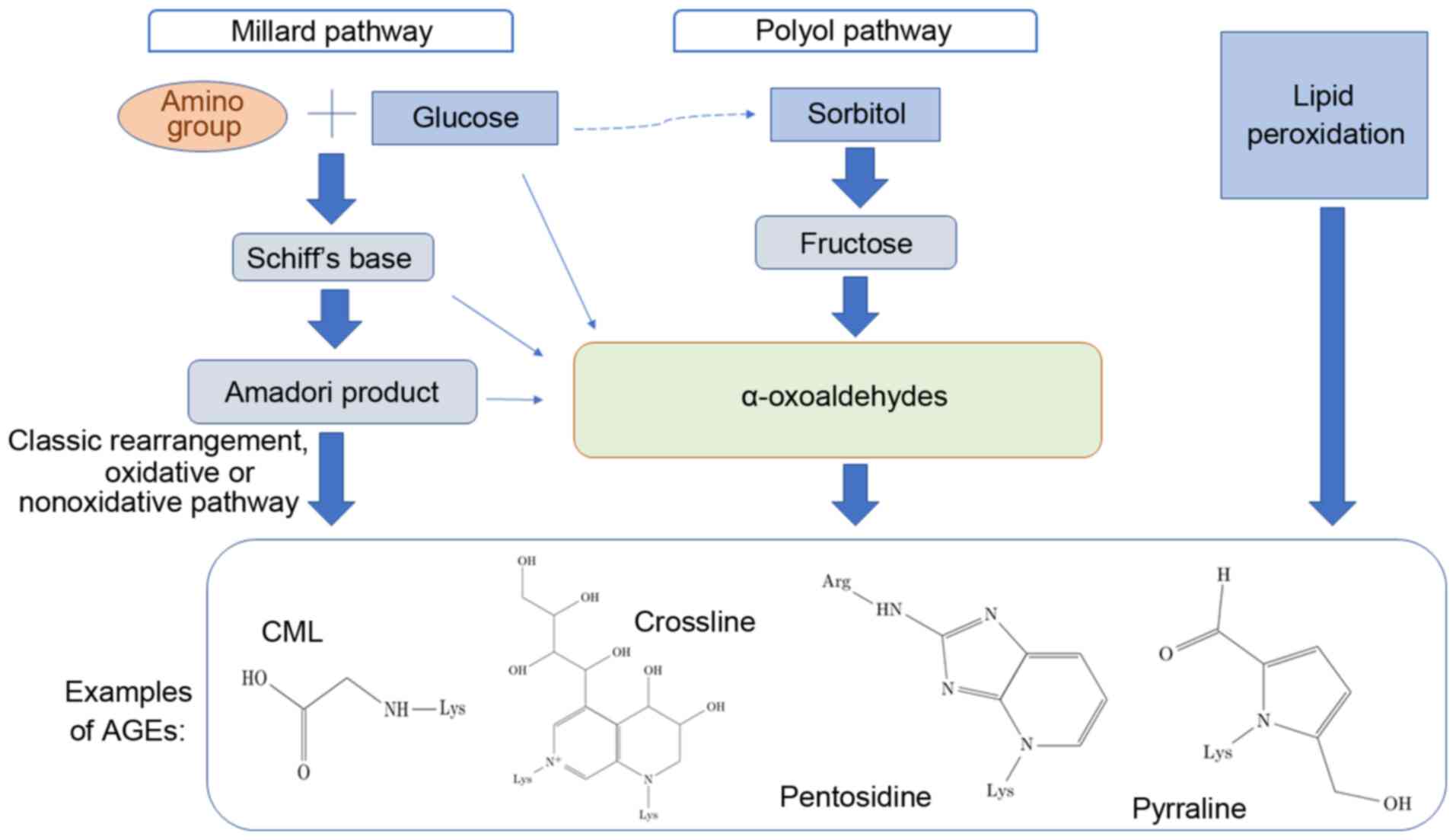
There are three different methods of AGE formation, which are
schematically depicted in Fig. 1
(1-4).
The initial process, known as the Maillard reaction,
leads to the formation of glycated molecules termed Amadori
products or early glycation products. Further rearrangement,
oxidation, reduction, dehydration, condensation, fragmentation and
cyclization of an Amadori product results in the formation of
relevant irreversible AGEs. Incubation of proteins with lipid
peroxidation products is an alternative method of generating AGEs.
The polyol pathway leads to the conversion of glucose into
fructose, and also promotes glycation; fructose may further be
converted to 3-deoxyglucose and fructose-3-phosphate, both of which
are very potent nonenzymatic glycation agents (5-7).
Detailed information on AGE formation is reviewed elsewhere
(8-11).
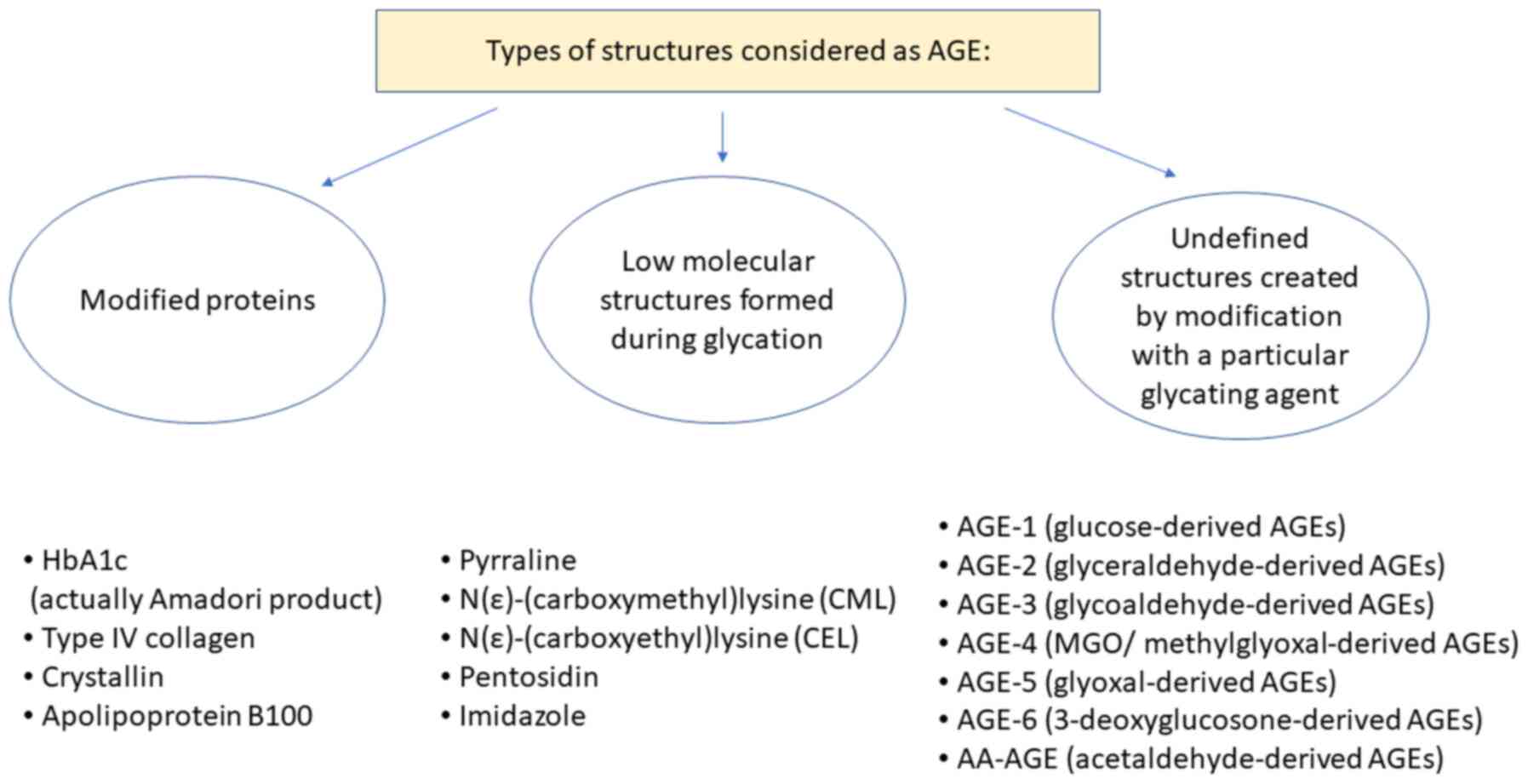
In studies on AGE, two classifications for the
nomenclature regarding AGEs are used, which are either focused on
the structure of the AGE or instead focused on the proteins being
modified. In the first classification system, the most extensively
studied AGEs are N-carboxymethyllysine (CML), pentosidine,
crossline, pyrraline and hydroimidazolone (2). The second group includes AGE-1
(glucose-derived AGEs), AGE-2 (glyceraldehyde-derived AGEs), AGE-3
(glycolaldehyde-derived AGEs), AGE-4 (MGO/methylglyoxal-derived
AGEs), AGE-5 (glyoxal-derived AGEs), AGE-6
(3-deoxyglucosone-derived AGEs) and acetaldehyde-derived AGEs
(AA-AGEs) (12,13). Specific modifications of proteins are
also considered AGEs; for example, haemoglobin (also referred to as
HbA1c, is in fact an Amadori product, not an AGE), albumin, eye
crystallin, collagen type IV and others (Fig. 2) (1-3,14).
It has been reported that AGEs adversely affect
several processes by two primary mechanisms: Directly through
trapping and cross-linking of proteins, and indirectly by binding
to specific receptors for AGE on the surface of various cells. Upon
binding to receptors, AGEs activate several signalling pathways,
including NF-κB, MAPKs, and Jun N-terminal kinase, which regulate
the transcription of proteins, such as cytokines, chemokines,
growth factors, adhesion molecules and extracellular matrix
proteins. In doing so, AGEs can result alter chemotaxis,
angiogenesis, oxidative stress, cell proliferation and programmed
cell death (5,7).
AGE molecules (free, peptide-bound and
protein-bound) are found in the blood plasma in high
concentrations, particularly in diabetic patients. This is
explained by their high concentration of the substrates; glucose
and its derivatives in blood (5).
AGEs are also found in aging patients and those with degenerative
diseases, where AGEs accumulate in cells and tissues, such as
arteries, neurons and hepatocytes (6).
Glycation may also occur whilst cooking foods, for
example during frying, baking, heating food in a microwave oven,
and especially during caramelization. The resulting products have a
strong taste and aroma. Another source of exogenous AGEs is
cigarette smoke (15). Some of the
exogenous AGEs are carcinogenic, for example acrylamide or
heterocyclic amines, whereas others interfere with cell signal
transduction or expression of numerous genes (16-18).
Both endogenous and exogenous AGEs bind to specific receptors, and
cause oxidative stress and promote inflammatory processes (16,19).
It is postulated that AGEs participate in the
pathological mechanism of cardiovascular disease, nephropathy,
rheumatoid arthritis, dysfunctions in bone remodelling and
neurological diseases (for example, Alzheimer's and Parkinson's
disease), cancer growth, metastasis and other degenerative diseases
(9,20-22).
The list of pathological conditions associated with AGE with a
short description of the relationship between glycation and the
disease is presented in Table I.
 | Table IEffects of AGEs in various
diseases. |
Table I
Effects of AGEs in various
diseases.
|
Disease/condition | Types of AGEs
implicated in disease | Role of AGEs in
disease | (Refs.) |
|---|
| Diabetic
retinopathy | AGE-2, AGE-3 | Accelerates
retinopathy by upregulating VEGF mRNA expression levels, and
stimulating DNA synthesis and tube formation in microvascular
endothelial cell through interacting with RAGEs | (12) |
| Diabetic
nephropathy | TAGE | Induces apoptotic
cell death in human mesangial cells, and causes hyperfiltration and
microalbuminuria by stimulating secretion of VEGF and monocyte
chemoattractant protein-1 | (12) |
| Diabetic
neuropathy | MGO-derived
AGEs | Associated with the
development of large- and small-fibre dysfunction | (51) |
| Other neuropathies
(including Parkinson's disease) | AGE-albumin | Neurodegeneration
associated with upregulation of RAGEs and the MAPK pathway; AGEs
also induce protein aggregation and cross-linking between
molecules, the formation of Lewy bodies and neuronal apoptosis | (52,53) |
| Malignant
melanoma | AGE-2, AGE-3 | Stimulate the
growth and migration of human melanoma cells | (12) |
| Other types of
cancer | CML, CEL, and
argpyrimidine and other ligands for RAGE | AGEs, via binding
to RAGEs, result in sustained inflammation, which leads to
metabolic reprogramming, and genomic instability, and may result in
oncogenic transformation, telomere elongation and increased
angiogenesis | (17,18) |
| Alzheimer's disease
and dementia | AGE-1, MGO-derived
AGEs | May affect
hippocampal neurons, and increase the percentage of apoptotic
neurones in the hippocampus | (22,51,54) |
|
Atherosclerosis | TAGEs and other
AGE | AGEs trigger
inflammation and cell proliferation, contributing to the
development of vascular dysfunction; glycation of apolipoprotein
B100 increases the atherogenicity of low-density lipoproteins; AGEs
influence platelet activation, thrombosis and
hypercoagulability | (45,55) |
| Other
cardiovascular diseases | AGE-3a | Causes fibrosis,
hypertrophy, oxidative stress and an exacerbated inflammatory
response | (56) |
| Hypertension | MGO-derived
AGEs | Vascular
endothelial damage by increasing oxidative stress, reduction of
NO-dependent vasorelaxation; increasing arterial stiffness by
cross-linking of extracellular matrix proteins | (45,51,57) |
| Osteoporosis,
osteoarthritis, and sarcopenia | Undefined AGE | AGEs accumulate in
bones, joints and skeletal muscles, impairing the biomechanical
properties of the tissues; AGE-induced chronic inflammation may
stimulate osteoblasts, osteoclasts and myocytes, resulting in
degradation of bones, cartilage and muscles | (10,58) |
AGEs may also be considered glycotoxins, due to
their toxic effects on certain cells and tissues (5). The term ‘TAGE’ is the abbreviation for
toxic advanced glycation end-products, which applies to some
(AGE-2, AGE-3 and AA-AGE), but not all AGEs. These molecules induce
reactions primarily by interaction with receptors for advanced
glycation end-products (RAGEs), and exert their toxic effects in
the blood vessels, liver and retinas, and can also promote the
development of several types of cancer as well as infertility
(12,13,21-23).
There is considerable research showing that non-TAGE molecules,
such as CML, pentosidine, pyrraline and crossline may also be
cytotoxic (24-26).
2. Cytotoxicity of AGEs
The viability of various types of cells in the
presence of AGEs has been extensively studied. In all reported
studies, changes in the survival rate of cells in the presence of
most types of AGEs was demonstrated. Below, a brief overview of the
studies examining the effects of AGEs on viability of cells is
discussed.
The toxicity of CML (the best-defined AGE) was
investigated in mice. The estimated LD50. of CML was
>5 mg/kg. Administration of 2 or 5 g/kg CML did not induce
mortality within 14 days. However, some biochemical and
histopathological changes were observed: Markers of aberrant
hepatic and renal function were elevated, antioxidant enzyme (SOD
and GSH-Px) activities were reduced, the levels of a marker of
lipid peroxidation (MDA) were increased and histological changes
were observed in the lungs, liver, kidney and spleen (24).
The effect of glycated albumin has been investigated
in numerous different cell lines. For example, the impact of
BSA-AGE (BSA incubated for 12 weeks with glucose) on cell cultures
of amniotic and embryonic origin (WISH and MRC-5 cell lines) and on
placental villi explants were determined. The observed effects
included: Condensation of chromatin, formation of apoptotic bodies
and elevated expression of cytokeratin 18 and Caspase 3. It was
concluded that BSA-AGE had a direct toxic effect on the cell
viability in a time and dose-dependent manner, and that some
pregnancy complications may arise due to the formation of AGEs
(5).
The effect of glycated albumin was also analysed on
other mammalian cell lines: Peripheral blood mononuclear cells, 293
cells, normal human fibroblasts and Chinese hamster ovary cells.
Glycated albumin was significantly more toxic than native human
serum albumin (LC50 values between 35.00-48.34 vs.
5.47-9.10 µg/ml, respectively) (2).
The addition of rosmarinic acid suppressed the cytotoxic effects
and inhibited the activity of elastase and collagenase (2).
The cytotoxicity of BSA-AGE was investigated in BHK
21 hamster fibroblast cells and SH-SY5Y human neuroblastoma cells.
It was found that BSA-AGE significantly induced cell death in a
dose-dependent manner, which was confirmed by three different
methods (Thiazole Blue assay, lactate dehydrogenase assay, Neutral
Red assay). Notably, the cytotoxic effects of AGEs were attenuated
by antioxidants, such as thioctic acid and N-acetylcysteine, which
lead to the conclusion that the toxic effects of AGEs was
associated with the oxidative stress (3). Chowdhury et al (27) drew similar conclusions in a study
performed using D-galactose-derived AGEs. The experiments were
performed using a mouse model; galactose was injected and one group
of mice were administered antioxidants, which abolished the toxic
effects (27). Similar results were
observed when assessing the effects of AGEs generated from ribose.
In a study conducted on Chinese hamster ovary cells, the effect was
suppressed by glucose-regulated protein 78 kDa (GRP78). Glycation
with a ribose is called ribosylation (6). GRP78 acts as a chaperone, and when it
is ribosylated, which induces ER stress, it leads to cell death
(6).
3. Methylglyoxal (MGO)-AGE
MGO is a by-product of glycolysis. This highly
reactive dicarbonyl compound is also a major precursor of AGE-4, a
methylglyoxal-derived AGE. MGO is not classified as a TAGE;
however, its cytotoxicity has been repeatedly observed.
Sampath et al (28) showed the cytotoxic effects of MGO on
Human Retinal Pigment Epithelial cells. It was found that
MGO-induced cytotoxicity resulted in increased levels of AGEs, such
as CML, as well as expression of various RAGEs and glutathione. In
the presence of AGE-MGO, the translocation of Nrf2 from the cytosol
to the nucleus is inhibited, which results in decreased expression
of detoxifying enzymes such as heme oxygenase-1(28). It is also worth quoting the results
of research on the impact of MGOs on bone marrow-derived
endothelial progenitor cells (EPC). MGO increased the levels of
AGEs, and decreased cell viability and protein expression of
vascular endothelial growth factor receptor (VEGFR)-2. The latter
is associated with functional impairments of tube formation.
Inhibition of RAGEs by FPS-ZM1 significantly reverses the decrease
in VEGFR-2 protein expression and angiogenic dysfunction in
MGO-treated EPC (29).
4. TAGEs in diabetes
TAGEs are a subset of AGEs, which include AGE-2,
AGE-3 and i AA-AGE. Diabetic hyperglycaemia may be caused by
elevated production of TAGEs. Histological analysis of healthy
control rats and STZ-induced diabetic rats showed AGE-2 expression
in the brain, pancreas and stomach, but not in the adipose tissue,
intestine, eye, heart, kidneys, spleen or lungs. However, there
seemed to be no difference in the distribution of AGEs between the
control group and experimental group (1).
As a common complication of diabetes is retinopathy,
it is not surprising to find reports showing the involvement of
AGEs in the death of retinal tissue. Takeuchi and Yamagishi
(12) reported that in the presence
of TAGEs, apoptotic cell death of retinal pericytes was observed,
and this was mediated by TAGE interaction with RAGEs. It was found
that AGE-2 and AGE-3 accelerated retinopathy by upregulating
vascular endothelial growth factor (VEGF) mRNA levels, as well as
stimulating DNA synthesis and tube formation in microvascular
endothelial cells via the interactions with RAGEs (12).
Another common complication of diabetes is
nephropathy. It has been demonstrated that TAGEs influence
nephropathy via two mechanisms: i) They induce apoptotic cell death
in human mesangial cells; and ii) cause hyperfiltration and
microalbuminuria by stimulating secretion of VEGF and monocyte
chemoattractant protein-1(12).
5. TAGEs in the nervous system
Of all the TAGEs, AGE-2 and AA-AGE appear to be
particularly neurotoxic. It was demonstrated that AGE-2 exhibits
potent neurotoxic effects in cortical neuronal cells, and the
effect was stronger than that of Glu-AGEs and CML. Additionally,
another line of evidence which has shown that GA-AGE is involved in
neurodegeneration, is that the neurotoxic effects of serum AGE
fractions from diabetic nephropathy in haemodialysis patients were
completely attenuated by the addition of an anti-GA-AGE antibody,
but not by antibodies against other types AGEs (30).
On the basis of histological analysis and
determination of serum AGE-2 levels, the relationship between the
presence of this antigen and Alzheimer's disease has been
suggested. AGE-2 is primarily detected in the cytosol of neurons of
the hippocampus and para-hippocampal gyrus, but not in the senile
plaques in the brains of AD patients, where CML was present
(31).
Acetaldehyde, a product of ethanol metabolism, is a
two-carbon carbonyl compound that can react with nucleophiles to
form covalent addition products. It has been shown that AA-AGE may
participate in the degeneration of neurons as the AA-AGE epitope
has been detected in the brains of alcoholic individuals (12).
In the context of neurodegenerative diseases, the
proper functioning of both neurons and the glial cells is of
paramount importance. The effects of TAGEs (AGE-2 and AGE-3) on
cultured Schwann cells has been assessed. Cell viability,
proliferation and the production of proinflammatory cytokines were
all substantially affected by treatment with TAGEs (12).
6. TAGEs in cancer
AGEs have been known to promote mutagenesis and
stimulate proliferation and migration of cancer cells through
activation of RAGEs. Stimulation of the receptors upon binding of
its ligands, for example with interalia AGE, leads to the
activation of several molecular signalling pathways, including the
PI3K/AKT, JAK/STAT, NF-κB, Ras/MAPK, Rac1/cdc42, p44/p42, p38 and
SAP/JNK/MAPK pathways, which contribute to the expression of
transcription factors, such as NF-κB, STAT3, HIF-1α, AP-1 and CREB.
AGE-RAGE interactions also result in activation of NADPH oxidases,
leading to increased intracellular oxidative stress. These
inflammatory mediators induce epigenetic changes in pre-malignant
lesions and silence tumour suppressor genes (17).
RAGE expression is upregulated in a vast majority of
cancers, but its expression is very low under physiological
conditions. Specific examples of types of cancer in which RAGE
expression is upregulated include colorectal cancer (32), pancreatic cancer (33), prostate cancer (34), lung cancer (35) and breast cancer, and in breast
cancer, it has been reported that the RAGE rs1800624 polymorphism
may increase the risk of breast cancer (36).
It has been shown that TAGEs may contribute to the
pathogenesis of neoplasia and cancer development. In human
pancreatic cancer cells treated with 1-4 mmol/l GA for 24 h, the
cell viability and intracellular levels of GA-AGEs, as well as heat
shock proteins 90α, 90β, 70, 27 were analyzed. Cell viability
decreased to almost 0% when cells were treated with 4 mmol/l GA,
and the levels of heat shock proteins increased significantly. It
was concluded that intracellular GA-AGEs induce pancreatic cancer
cell death. Additionally, their secretion may promote the
proliferation of other cancer cells (37).
7. Dietary AGEs (dAGEs)
AGEs can also be a toxic component of food. It is
estimated that an average human diet consists of ~75 mg AGEs per
day (38). It is not clear how much
dAGE is absorbed in the digestive system; one study has stated that
10-30% is absorbed (10), whereas
another report has stated 30-80% is abosrbed (38). Absorbed glycation end-products are
biotransformed and excreted, or otherwise accumulate in various
tissues. The levels of accumulated AGEs in tissues can be estimated
using the non-invasive skin autofluorescence measurement method
(39).
The body's ability to detoxify AGE products is
difficult to assess. Glycated proteins, particularly large ones,
are resistant to proteolytic enzymes, and this makes it several
times more difficult to eliminate them from the body. However,
certain AGEs are bound to AGER1 receptors located on macrophages,
T-lymphocytes, endothelial cells, mesangial cells, fibroblasts,
smooth muscle cells and neuronal cells, which are then excreted by
the kidney (10). It is estimated
that ~30% of dAGEs are removed in the urine, provided the patient
has a healthy kidney, otherwise this percentage will be lower
(40).
8. TAGEs for diagnosis and treatment of
diseases
It has been suggested that the serum levels of toxic
AGEs are correlated with the progression of degenerative diseases,
such as atherosclerosis or diabetes. It was also found that higher
levels of TAGEs are associated with overeating, a lack of exercise
or excessive ingestion of sugars and dAGEs. It is therefore
proposed that the serum levels of TAGEs may be a promising novel
biomarker for the onset and progression of lifestyle-related
diseases (41).
Sato et al (13) proposed TAGEs may serve as biomarkers
for Alzheimer's disease, and suggested that assessing TAGE levels
as a diagnostic tool may improve diagnosis and thus treatment of
patients with this disorder.
There are also specific proposals to apply knowledge
regarding AGEs and TAGEs for therapeutic purposes. It was suggested
that inhibition of the interactions between a TAGE and its RAGE,
using an anti-RAGE antibody, was a suitable candidate for treatment
of patients with malignant melanoma (12). FPS-ZM1 or other specific antagonists
for RAGEs may also be useful in inhibiting RAGEs, and may thus also
serve as a potential therapeutic option for management of diabetic
vascular complications (29).
Other approaches to reduce the detrimental effects
of AGEs may be to inhibit their formation, or the use of compounds
that can break the cross-linked bonds, such as aminoguanidine and
pyridoxamine, phenylthiazole, 4,5-dimethyl-3-phenyl-acyl-thiazole
chloride and ALT-711(42). These are
synthetic substances, and, unfortunately, no endogenous analogues
have been identified as of yet. The downside of these compounds is
that they may exert a certain degree of cytotoxicity, and this has
complicated the development of a clinically suitable anti-AGE
treatment. It has not been clearly established whether these
compounds affects both TAGE and non-TAGE metabolism; however, it is
worth exploring the therapeutic value of such strategies, and
possibly applying them in clinical practice.
9. Methodology for determining AGEs in
biological materials
Determination of AGEs in tissues is problematic. It
is important to pay particular attention to the diversity of
compounds that should be analysed (4,11).
Additionally, a multitude of conditions that can influence the
glycation of AGEs (such as pH, presence and the amount of free
radicals and metal ions, amongst others), and the type and
concentration of substrates need to be taken into consideration
(4). Moreover, AGEs are typically
present in small amounts in vivo (14,43).
Their isolation from tissues can cause undesirable chemical
modifications and the formation of artefacts (44). Nevertheless, research on the content
of AGEs in serum, in tissues and in food is very desirable,
particularly for diagnostic and therapeutic purposes (14,21,38,41,45,46).
A number of methods for determining AGEs in samples
are available. Amongst the most frequently used are chromatographic
methods coupled with mass spectroscopy (11,14,44,46).
Equally popular are immunoenzymatic methods using antibodies
(1,4,44,47);
however, the specificity of the antibodies (11,14) or
the low quantities of antigens in the sample can cause difficulties
(1). The natural properties of AGEs
associated with fluorescence can also be exploited in fluorometric
methods (43,45,47);
unfortunately, the fluorescence of other components in the sample
often obfuscates the results (11).
Thus, at present, there is no one specific and sensitive method to
measure AGE content in samples (11).
10. Summary and conclusions
Despite the fact that methods for determination of
the entire range of possible AGEs in biological samples is limited
and challenging, it is still recommended to attempt measurement to
reduce errors. Numerous studies have shown the association between
AGEs and their negative effects, such as oxidative stress,
inflammatory processes, and the formation of cross-links, which can
alter the biochemical properties of proteins (12,48).
AGEs can be classified as toxic or non-toxic AGEs,
however it seems that the effect of each type of AGE may vary based
on the specific conditions. A significant challenge in the study of
AGEs is that the structures of AGE-1 through AGE-4 and AA-AGE are
not defined. Additionally, the epitopes recognized by the
antibodies in the study of TAGEs have not been determined. Another
problem faced by researchers examining AGEs is the impact of
albumin-AGE. The formation of AGE is complex, and is partially
dependent on non-enzymatic glycation, and it is difficult to
control the course of this process to obtain a homogeneous reaction
product. The levels of AGEs or TAGEs in serum or tissues is also
very small, hence the difficulty in accurately determining their
concentration in biological samples. Thus, there is an urgent need
to improve our understanding of the effects of glycated agents, as
it may improve our understanding of diabetic complications, and
other processes associated with oxidative stress caused by
glycation.
It is also important to educate individuals on the
risk of AGE toxicity, instilling the importance of a healthy diet,
and encouraging them to choose food products that are not processed
in high temperature conditions. It is also worth raising patients'
awareness on preventing the effects of glycation, used to protect
food supplies. Natural anti-glycation agents include anthocyanins
and ellagic acid, which are derived from vegetables and fruits, and
are also active ingredients contained in green tea; garlic;
resveratrol; red wine; curcumin; cinnamic acid derivatives, such as
ferulic acid; quercetin, which is found in several plants, caffeic
acid from Ilex paraguariensis and numerous other food stuffs
(49,50). Lowering the intake of dAGEs can
reduce the risk of diabetes, cardiovascular complications and other
glycation-related diseases.
Due to the ability of AGEs to increase the risk of
diseases and promote neoplastic changes, AGEs are becoming an
increasingly popular subject of study in the field of toxicology.
However, it is extremely difficult to define the strict
toxicological parameters of the various types of AGEs in the
literature. At present the following parameters are used:
No-Observed-Effect-Level, No-Observed-Adverse-Effect-Level,
Permissible Exposure Limit and Time-Weighted Average. An increasing
interest in the field of AGEs may emphasize the importance of the
role of AGEs in the pathological mechanisms of various diseases,
and improve public awarness of the risks associated with ingestion
of food-derived AGEs.
Acknowledgements
I would like to thank Miss Karolina Nowakowska
(Department of Internal Medicine, Pneumology and Allergology,
Wroclaw Medical University, Poland) for preparing the figures and
linguistic correction, and Dr Mariusz Bromke (Department of Medical
Biochemistry, Wroclaw Medical University, Poland) for proofreading
this review.
Funding
Funding: No funding was received.
Availability of data and materials
Not applicable.
Authors' contributions
AK wrote, edited and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Morioka Y, Teshigawara K, Tomono Y, Wang
D, Izushi Y, Wake H, Liu K, Takahashi HK, Mori S and Nishibori M:
The specific localization of advanced glycation end-products (AGEs)
in rat pancreatic islets. J Pharmacol Sci. 134:218–224.
2017.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Miroliaei M, Aminjafari A, Ślusarczyk S,
Nawrot-Hadzik I, Rahimmalek M and Matkowski A: Inhibition of
glycation-induced cytotoxicity, protein glycation, and activity of
proteolytic enzymes by extract from Perovskia atriplicifolia
roots. Pharmacogn Mag. 13 (Suppl 3):S676–S683. 2017.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Loske C, Neumann A, Cunningham AM, Nichol
K, Schinzel R, Riederer P and Münch G: Cytotoxicity of advanced
glycation endproducts is mediated by oxidative stress. J Neural
Transm (Vienna). 105:1005–1015. 1998.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Gkogkolou P and Böhm M: Advanced glycation
end products: Keyplayers in skin aging? Dermatoendocrinol.
4:259–270. 2012.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Boyanova M and Huppertz B: Cytotoxic
effect of advanced glycation end products. Biotechnol Biotechnol
Equip. 23:1072–1078. 2009.
|
|
6
|
Wu B, Yu L, Hu P, Lu Y, Li J, Wei Y and He
R: GRP78 protects CHO cells from ribosylation. Biochim Biophys Acta
Mol Cell Res. 1865:629–637. 2018.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Stinghen AE, Massy ZA, Vlassara H, Striker
GE and Boullier A: Uremic toxicity of advanced glycation end
products in CKD. J Am Soc Nephrol. 27:354–370. 2016.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Sergi D, Boulestin H, Campbell FM and
Williams LM: The role of dietary advanced glycation end products in
metabolic dysfunction. Mol Nutr Food Res.
65(e1900934)2021.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Ali Khan MW, Banga K and Ali W:
Gluco-oxidation of proteins in etiology of diabetic retinopathy.
In: Diabetic retinopathy. InTech, 2012.
|
|
10
|
Chen JH, Lin X, Bu C and Zhang X: Role of
advanced glycation end products in mobility and considerations in
possible dietary and nutritional intervention strategies. Nutr
Metab (Lond). 15(72)2018.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Perrone A, Giovino A, Benny J and
Martinelli F: Advanced glycation end products (AGEs): Biochemistry,
signaling, analytical methods, and epigenetic effects. Oxid Med
Cell Longev. 2020(3818196)2020.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Takeuchi M and Yamagishi S: TAGE (toxic
AGEs) hypothesis in various chronic diseases. Med Hypotheses.
63:449–452. 2004.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Sato T, Shimogaito N, Wu X, Kikuchi S,
Yamagishi S and Takeuchi M: Toxic advanced glycation end products
(TAGE) theory in Alzheimer's disease. Am J Alzheimers Dis Other
Demen. 21:197–208. 2006.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Ashraf JM, Ahmad S, Choi I, Ahmad N,
Farhan M, Tatyana G and Shahab U: Recent advances in detection of
AGEs: Immunochemical, bioanalytical and biochemical approaches.
IUBMB Life. 67:897–913. 2015.PubMed/NCBI View
Article : Google Scholar
|
|
15
|
Federico G, Gori M, Randazzo E and
Vierucci F: Skin advanced glycation end-products evaluation in
infants according to the type of feeding and mother's smoking
habits. SAGE Open Med. 4(2050312116682126)2016.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Sellier C, Boulanger E, Maladry F, Tessier
FJ, Lorenzi R, Nevière R, Desreumaux P, Beuscart JB, Puisieux F and
Grossin N: Acrylamide induces accelerated endothelial aging in a
human cell model. Food Chem Toxicol. 83:140–145. 2015.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Palanissami G and Paul SFD: RAGE and its
ligands: Molecular interplay between glycation, inflammation, and
hallmarks of cancer-a review. Horm Cancer. 9:295–325.
2018.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Schröter D and Höhn A: Role of advanced
glycation end products in carcinogenesis and their therapeutic
implications. Curr Pharm Des. 24:5245–5251. 2019.PubMed/NCBI View Article : Google Scholar
|
|
19
|
ALjahdali N and Carbonero F: Impact of
maillard reaction products on nutrition and health: Current
knowledge and need to understand their fate in the human digestive
system. Crit Rev Food Sci Nutr. 59:474–487. 2019.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Cho HJ, Xie C and Cai H: AGE-induced
neuronal cell death is enhanced in G2019S LRRK2 mutation with
increased RAGE expression. Transl Neurodegener. 7(1)2018.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Takeuchi M, Sakasai-Sakai A, Takata T,
Ueda T, Takino J, Tsutsumi M, Hyogo H and Yamagishi S: Serum levels
of toxic AGEs (TAGE) may be a promising novel biomarker in
development and progression of NASH. Med Hypotheses. 84:490–493.
2015.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Takeuchi M and Yamagishi S: Involvement of
Toxic AGEs (TAGE) in the pathogenesis of diabetic vascular
complications and Alzheimer's disease. J Alzheimers Dis.
16:845–858. 2009.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Sakasai-Sakai A, Takata T, Takino J and
Takeuchi M: Impact of intracellular glyceraldehyde-derived advanced
glycation end-products on human hepatocyte cell death. Sci Rep.
7(14282)2017.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Liu X, Zheng L, Zhang R, Liu G, Xiao S,
Qiao X, Wu Y and Gong Z: Toxicological evaluation of advanced
glycation end product Nε-(carboxymethyl)lysine: Acute and subacute
oral toxicity studies. Regul Toxicol Pharmacol. 77:65–74.
2016.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Chou SM, Wang HS, Taniguchi A and Bucala
R: Advanced glycation endproducts in neurofilament conglomeration
of motoneurons in familial and sporadic amyotrophic lateral
sclerosis. Mol Med. 4:324–332. 1998.PubMed/NCBI
|
|
26
|
Bassi AM, Ledda S, Valentini S, De Pascale
MC, Rossi S, Odetti P and Cottalasso D: Damaging effects of
advanced glycation end-products in the murine macrophage cell line
J774A.1. Toxicol In Vitro. 16:339–347. 2002.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Chowdhury AA, Gawali NB, Bulani VD,
Kothavade PS, Mestry SN, Deshpande PS and Juvekar AR: In vitro
antiglycating effect and in vivo neuroprotective activity of
Trigonelline in d-galactose induced cognitive impairment. Pharmacol
Rep. 70:372–377. 2018.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Sampath C, Zhu Y, Sang S and Ahmedna M:
Bioactive compounds isolated from apple, tea, and ginger protect
against dicarbonyl induced stress in cultured human retinal
epithelial cells. Phytomedicine. 23:200–213. 2016.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Kim JH, Kim KA, Shin YJ, Kim H, Majid A
and Bae ON: Methylglyoxal induced advanced glycation end products
(AGE)/receptor for AGE (RAGE)-mediated angiogenic impairment in
bone marrow-derived endothelial progenitor cells. J Toxicol Environ
Health A. 81:266–277. 2018.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Takeuchi M, Takino JI, Sakasai-Sakai A,
Takata T and Tsutsumi M: Toxic AGE (TAGE) theory for the
pathophysiology of the Onset/Progression of NAFLD and ALD.
Nutrients. 9(634)2017.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Takeuchi M, Sato T, Takino J, Kobayashi Y,
Furuno S, Kikuchi S and Yamagishi S: Diagnostic utility of serum or
cerebrospinal fluid levels of toxic advanced glycation end-products
(TAGE) in early detection of Alzheimer's disease. Med Hypotheses.
69:1358–1366. 2007.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Azizian-Farsani F, Abedpoor N, Hasan
Sheikhha M, Gure AO, Nasr-Esfahani MH and Ghaedi K: Receptor for
advanced glycation end products acts as a fuel to colorectal cancer
development. Front Oncol. 10(552283)2020.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Swami P, Thiyagarajan S, Vidger A,
Indurthi VSK, Vetter SW and Leclerc E: Rage up-regulation
differently affects cell proliferation and migration in pancreatic
cancer cells. Int J Mol Sci. 21(7723)2020.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Akkus G, Izol V, Ok F, Evran M, Inceman M,
Erdogan S, Kaplan HM, Sert M and Tetiker T: Possible role of the
receptor of advanced glycation end products (RAGE) in the clinical
course of prostate neoplasia in patients with and without type 2
diabetes mellitus. Int J Clin Pract: Sep 21, 2020 (Epub ahead of
print). doi: 10.1111/ijcp.13723.
|
|
35
|
Chen MC, Chen KC, Chang GC, Lin H, Wu CC,
Kao WH, Teng CJ, Hsu SL and Yang TY: RAGE acts as an oncogenic role
and promotes the metastasis of human lung cancer. Cell Death Dis.
11(265)2020.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Zhang W, Deng X, Tang R and Wang H:
Receptor for advanced glycation end-product rs1800624 polymorphism
contributes to increase breast cancer risk: Evidence from a
meta-analysis. Medicine (Baltimore). 99(e22775)2020.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Takata T, Ueda T, Sakasai-Sakai A and
Takeuchi M: Generation of glyceraldehyde-derived advanced glycation
end-products in pancreatic cancer cells and the potential of tumor
promotion. World J Gastroenterol. 23:4910–4919. 2017.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Waqas K, Chen J, van der Eerden BCJ, Ikram
MA, Uitterlinden AG, Voortman T and Zillikens M: Dietary advanced
glycation end-products (dAGEs) intake and bone health: A
cross-sectional analysis in the rotterdam study. Nutrients.
12(2377)2020.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Yamagishi S and Matsui T: Role of ligands
of receptor for advanced glycation end products (RAGE) in
peripheral artery disease. Rejuvenation Res. 21:456–463.
2018.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Tessier FJ, Boulanger E and Howsam M:
Metabolic transit of dietary advanced glycation end-products-the
case of NƐ-carboxymethyllysine. Glycoconj J: Sep 29,
2020 (Epub ahead of print). doi: 10.1007/s10719-020-09950-y.
|
|
41
|
Takeuchi M: Serum levels of toxic AGEs
(TAGE) may be a promising novel biomarker for the onset/progression
of lifestyle-related diseases. Diagnostics (Basel).
6(23)2016.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Kuzan A, Chwiłkowska A, Kobielarz M,
Pezowicz C and Gamian A: Glycation of extracellular matrix proteins
and its role in atherosclerosis. Postepy Hig Med Dosw (Online).
66:804–809. 2012.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Kuzan A, Chwiłkowska A, Maksymowicz K,
Bronowicka-Szydełko A, Stach K, Pezowicz C and Gamian A: Advanced
glycation end products as a source of artifacts in immunoenzymatic
methods. Glycoconj J. 35:95–103. 2018.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Humeny A, Kislinger T, Becker CM and
Pischetsrieder M: Qualitative determination of specific protein
glycation products by matrix-assisted laser desorption/ionization
mass spectrometry Peptide mapping. J Agric Food Chem. 50:2153–2160.
2002.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Yamagishi S and Matsui T: Role of
Hyperglycemia-induced advanced glycation end product (AGE)
accumulation in atherosclerosis. Ann Vasc Dis. 11:253–258.
2018.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Kim Y, Keogh JB, Deo P and Clifton PM:
Differential effects of dietary patterns on advanced glycation end
products: A randomized crossover study. Nutrients.
12(1767)2020.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Münch G, Keis R, Wessels A, Riederer P,
Bahner U, Heidland A, Niwa T, Lemke HD and Schinzel R:
Determination of advanced glycation end products in serum by
fluorescence spectroscopy and competitive ELISA. Eur J Clin Chem
Clin Biochem. 35:669–677. 1997.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Kuzan A, Michel O and Gamian A: Glycation
of matrix proteins in the artery inhibits migration of smooth
muscle cells from the media to the intima. Folia Biol (Praha).
63:105–114. 2017.PubMed/NCBI
|
|
49
|
Younus H and Anwar S: Prevention of
Non-enzymatic glycosylation (Glycation): Implication in the
treatment of diabetic complication. Int J Health Sci (Qassim).
10:261–277. 2016.PubMed/NCBI
|
|
50
|
Hashemzaei M, Tabrizian K, Alizadeh Z,
Pasandideh S, Rezaee R, Mamoulakis C, Tsatsakis A, Skaperda Z,
Kouretas D and Shahraki J: Resveratrol, curcumin and gallic acid
attenuate glyoxal-induced damage to rat renal cells. Toxicol Rep.
7:1571–1577. 2020.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Schalkwijk CG and Stehouwer CDA:
Methylglyoxal, a highly reactive dicarbonyl compound, in diabetes,
its vascular complications and other age-related diseases. Physiol
Rev. 100:407–461. 2020.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Byun K, Bayarsaikhan D, Bayarsaikhan E,
Son M, Oh S, Lee J, Son HI, Won MH, Kim SU, Song BJ and Lee B:
Microglial AGE-albumin is critical in promoting alcohol-induced
neurodegeneration in rats and humans. PLoS One.
9(e104699)2014.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Bayarsaikhan E, Bayarsaikhan D, Lee J, Son
M, Oh S, Moon J, Park HJ, Roshini A, Kim SU, Song BJ, et al:
Microglial AGE-albumin is critical for neuronal death in
Parkinson's disease: A possible implication for theranostics. Int J
Nanomedicine. 10:281–292. 2016.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Fleitas C, Piñol-Ripoll G, Marfull P,
Rocandio D, Ferrer I, Rampon C, Egea J and Espinet C: proBDNF is
modified by advanced glycation end products in Alzheimer's disease
and causes neuronal apoptosis by inducing p75 neurotrophin receptor
processing. Mol Brain. 11(68)2018.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Moldogazieva NT, Mokhosoev IM, Mel'nikova
TI, Porozov YB and Terentiev AA: Oxidative stress and advanced
lipoxidation and glycation end products (ALEs and AGEs) in aging
and age-related diseases. Oxid Med Cell Longev.
2019(3085756)2019.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Wang X, Chen XX, Yu HT, Tan Y, Lin Q,
Keller BB, Zheng Y and Cai L: Engineered cardiac tissues: A novel
in vitro model to investigate the pathophysiology of mouse diabetic
cardiomyopathy. Acta Pharmacol Sin: Oct 9, 2020 (Epub ahead of
print). doi: 10.1038/s41401-020-00538-8.
|
|
57
|
Wang WC, Lee JA and Chou CK: Open access
short review evolving evidence of methylglyoxal and dicarbonyl
stress related diseases from diabetic to non-diabetic models. Pharm
Anal Acta. 7(4)2016.
|
|
58
|
Takata T, Sakasai-Sakai A and Takeuchi M:
Impact of intracellular toxic advanced glycation end-products
(TAGE) on murine myoblast cell death. Diabetol Metab Syndr.
12(54)2020.PubMed/NCBI View Article : Google Scholar
|