Introduction
Type 2 diabetes mellitus (T2DM) has become a major
public health issue and is associated with a high incidence of
cognitive impairment and dementia disorders, particularly
Alzheimer's disease (AD) (1-3).
Previous studies reported that the relative risk of AD in patients
with T2DM is ~2x higher than that in non-diabetic patients
(4,5). The burden of patients of AD with
diabetes mellitus in the future may even worsen as the prevalence
of T2DM continues to increase (3).
Diabetes mellitus induces some toxic effects, such as
hyperglycemia, vascular dementia, brain insulin resistance and
neurodegeneration, which increase the risk of cognitive impairment
and dementia (1,6). Previous studies reported that cognitive
impairment and dementia are related to the inflammation (7).
Obesity increases the risk of diabetes mellitus
(8-10).
Obese individuals exhibit pathological proliferation (hyperplasia)
and enlargement (hypertropia) of adipose tissue as a response to
excessive nutrition, which may reduce tissue oxygenation and cause
cell hypoxia (11,12). Inflammation in adipose tissue may
accelerate insulin resistance and systemic inflammation response by
activating several pro-inflammatory cytokines, such as
interleukin-6 (IL-6) and tumor nuclear factor-α (TNF-α), and
increasing the generation of reactive oxygen species (ROS)
(11,13). The high IL-6 and TNF-α levels in
diabetes mellitus have been shown by several studies in animals and
humans (7,14-16).
The generation of the antioxidative stress enzyme superoxide
dismutase (SOD) has been correlated with neurodegenerative diseases
(17,18).
The progression of AD has been correlated with the
activity of nuclear factor-κB (NF-κB) (19-22).
The NF-κB signal serves a crucial role in maintaining brain
homeostasis (19). It essentially
participates in synaptic plasticity and in balancing brain
functions that are associated with learning and memory (19). Destruction of this signaling pathway
triggers several neuronal changes, such as brain inflammation,
glial activation, oxidative stress generation and apoptotic cell
death stimulation, which lead to neuronal degeneration, the initial
stage of AD (19-22).
Potent treatments are thus required to prevent
diabetes-induced suppression of neuronal function. Mangosteen
(Garcinia mangostana Linn) of the Guttiferae family is
deemed as the ‘Queen of Fruits’ because of its distinctive and
delectable tropical taste (23,24). It
is a popular fruit in tropical countries, mostly in Southeast Asia
and has been used as a medicine for hundreds of years around the
world (23,25). WHO has proven its safety as a
traditional fruit without reported mutagenicity or teratogenicity
for over 100 years, and is recommended for consumption by humans,
as there are no reports of acute or chronic hepatic damage or
immunological activities following consumption of fruits and
commercial beverages containing pericarp (26). A previous study reported that daily
intervention with 220-280 mg pericarp extract for 12 weeks,
followed by a double dose for the next 12 weeks was adequately safe
for oral administration in the humans (27). Mangosteen leaves, bark, whole fruit
and pericarp contain secondary metabolites with potential
biological effects (23,25,28,29).
Xanthones are a class of polyphenolic compounds, of which α- and
γ-mangosteen are the most abundant (23,27-29).
This fruit reportedly exhibits various therapeutic effects, such as
anti-inflammatory, anti-dyslipidemia, antioxidant, hypoglycemic and
anti-obesity effects (24,30-36).
The glycemic index of mangosteen fruit was considered low; however,
there are ~10x more phenolic compounds and 20x more antioxidant
activity in the pericarp compared with the edible part of the fruit
(37,38). Thus, in the present study, the
pericarp extract was used. Whether or not mangosteen pericarp
extract exerts a protective effect of brain function in patients
with diabetes has not been assessed previously, to the best of our
knowledge.
The aim of the present study was to analyze the
mechanism of cerebral inflammation by identifying the activation of
glial NF-κB and the expression of serum IL-6, TNF-α and SOD under
diabetic conditions. It also aimed to investigate the protective
effects of Garcinia mangostana pericarp (GMP) extract on
brain function in T2DM rats. The mechanism underlying the effects
of GMP on T2DM-suppressed brain function has not been sufficiently
investigated. Therefore, the effect of GMP on the brain function of
obese rats with and without T2DM was assessed.
Materials and methods
Animals and treatment protocol
A total of 36 male Wistar rats, aged 2-3 months,
with an average body weight of 150-200 g were used in the present
study. The Wistar rats were used in the animal building with an
environmental setting of 23±2˚C, 50±5% humidity, a 12-12 h
light-dark cycle, and ad libitum access to a standard chow
and tap water. Rats were randomly divided into six groups with the
following interventions: Normal control (C1), administered with a
standard normal diet; mangosteen control (C2), administered a
high-fat diet (HFD) and GMP extract at 200 g/kg BW/day; diabetic
control (C3), diabetic Wistar rats treated with HFD; mangosteen
intervention 1 (M1), diabetic Wistar rats treated with HFD and 100
g/kg body weight (BW)/day GMP extract; mangosteen intervention 2
(M2), diabetic Wistar rats treated with HFD 200 g/kg BW/day GMP
extract; and mangosteen intervention 3 (M3), diabetic rats treated
with HFD and 400 g/kg BW/day GMP extract. GMP extract was prepared
as a solution (as described below) and administered by oral gavage
technique to Wistar rats. A previous study used mangosteen pericarp
ethanolic extract at doses of 200, 400 and 800 mg/kg, and found
that the oral intervention of 800 mg/kg decreased vasa vasorum
angiogenesis through H2O2, HIF-1α, NF-κB and
iNOS inhibition in hypercholesterolemic Wistar rats (39). Another study reported that pericarp
extract at 100 mg/kg via oral gavage could protect mice from the
memory degrading effects of scopolamine and improved memory
retention (40). Therefore, in the
present study, doses of 100, 200 and 400 mg/kg body weight of
Wistar rats were used. No behavioral changes were observed, and
there were no deaths of rats during the experiment, suggesting that
the doses used in the present study did not cause any notable toxic
effects.
The Wistar rats were allowed to acclimatize for 1
week before being treated with HFD consisting of 90% comfeed
standard II, 10% pork fat and 1.25% pure cholesterol at a dose of
20 g BW/day for 6 weeks to induce obesity. The body weight was
measured every week to monitor the weight gain of the animals.
Streptozotocin (STZ) (Nacalai Tesque, Inc.; 45 mg/kg BW dissolved
with sodium citrate buffer 1.5 ml/100 g BW) and nicotinamide (NA)
(Nacalai Tesque, Inc.; 110 mg/kg BW dissolved with NaCl 1.5 ml/kg
BW) were intraperitoneally administered at day 42 to induce T2DM in
the rats.
In a previous study, Wistar rats showed significant
impairment of memory, as well as ability and restoration of
learning at 8 weeks after being diagnosed as diabetic (17). Therefore, the intervention with GMP
extract in groups 3 to 6 was started at weeks 8 after a diabetic
status was confirmed.
The termination of rats was performed under
ether-induced anesthesia by cervical dislocation. For anesthesia,
~30% of anesthetic ether was added in a glass jar containing cotton
pads with an average volume of 6.5 ml/h. Each rat was placed in the
glass jar for ~4.5 min, after which cervical dislocation was
performed.
The research protocols were performed in accordance
with the National Institute of Health Guidelines for Animal Care
(41), and has been reviewed and
approved by the Medical and Health Research Ethics Committee,
Faculty of Medicine Diponegoro University, Semarang Indonesia
(approval no. 115/EC/H/KEPK/FK-UNDIP/VIII/2019).
Preparation of GMP extract
GMP was collected from Surabaya, and voucher
specimens were deposited at the Department of Pharmacognosy and
Phytochemistry, Faculty of Pharmacy, Universitas Airlangga,
Indonesia. The extract preparation was performed as described
previously (39,42). Fresh pericarps of Garcinia
mangostana (10 kg) were cut into small pieces and dried under
indirect sunlight. The drying process was performed to minimize the
amount of water. Then, the dried pericarps were powdered (1 kg) and
extracted with 70% ethanol (4 liters, 3 times) through maceration
for 24 h. The 70% ethanol solutions were evaporated using a rotary
evaporator and dried in a drying machine (oven) to obtain the 70%
ethanol extract (302 x g).
Blood samples and tissue
collection
Blood was collected from the periorbital sinus after
10 h of fasting; ~2 ml blood was collected from the periorbital
sinus and placed in a heparin tube (One Med Health Care) and
centrifuged (447 x g, 4˚C) to obtain the serum. The rats were
sacrificed as described above, and brain tissues were collected and
placed in 10% formalin buffer solution overnight at room
temperature, and subsequently embedded in paraffin a block, and
sectioned into 3-4 µm thick slices.
Measurement of fasting blood glucose
concentrations
Fasting blood glucose levels (mg/dl) were measured
after 3 days of STZ/NA intervention to determine the diabetic
status in the rats and on the last day after intervention, using
the glucose oxidase phenol 4-amino phenazone method (DiaSys
Diagnostic Systems GmbH, cat. no. 10 026). Each of blood serum
sample (10 µl), standard solution (10 µl) and blank (10 µl) was
added to the dilution solution (1 ml), vortexed and then incubated
for 20 min at 20-25˚C. The absorbance was measured using a
spectrophotometer (Thermo Fisher Scientific, Inc.) at a wavelength
of 500 nm.
Measurement of IL-6, TNF-α and SOD
levels
The expression levels of IL-6 (cat. no. M6000B),
TNF-α (cat. no. MTA00B) and SOD (cat. no. DYC3419-2) were examined
in rat blood serum using sandwich ELISA according to the
manufacturer's protocol (R&D Systems, Inc.). Absorbance was
determined using a spectrophotometer at a wavelength of 450 nm. The
expression levels of IL-6, TNF-α and SOD were compared between the
normal control and obese-T2DM groups, as well as between the GMP
intervention groups in obese and obese-T2DM.
NF-κB immunostaining
Immunostaining was performed on the formalin-fixed
paraffin-embedded samples with using a UltraTek HRP
(Anti-Polyvalent) Ready-To-Use kit (cat. no. AMF080-IFU, ScyTek
Laboratories, Inc.) at room temperature. The primary antibody used
was a rabbit polyclonal anti-NF-κB (cat. no. ab7970; Abcam; 1:300).
Slides were deparaffinized in xylene followed by rehydration using
a graded series of alcohol solutions (95, 80 and 70%), treated with
heat-induced epitope retrieval using Tris EDTA solution with pH 9.0
at 95˚C for 10 min, followed by Super Block (cat. no. AMF080-IFU,
ScyTek Laboratories, Inc.) for 10 min, and an overnight antibody
incubation at 4˚C. UltraTek Anti-Polyvalent and subsequently
UltraTek HRP were both applied for 10 min each at room temperature.
DAB staining was administered for 3 min. A blinded observer
assessed the immunostaining for glial and neuronal cells at both
hippocampi. Immuno-positive glial and neuron cells were defined
with positive cytoplasm staining. The evaluation was conducted
using a bright field microscope at x400 magnification (BX41;
Olympus Corporation) with manual counter. All immune-positive glial
cells in the parenchyma area and neuron cells in the cornu ammonis
(CA)1, CA4, and dentate gyrus areas were counted.
Statistical analysis
Data are presented as the mean ± standard deviation.
The distribution data of study were determined using a Shapiro-Wilk
test and then analyzed statistically using a Wilcoxon Signed-Rank
test to compare body weights and fasting blood glucose of the rats
before and after intervention. A Kruskal Wallis test followed by a
Dunn's post hoc test was used to determine differences in glial
NF-κB levels, and IL-6, TNF-α and SOD expression between the
control and GMP intervention groups.
Results
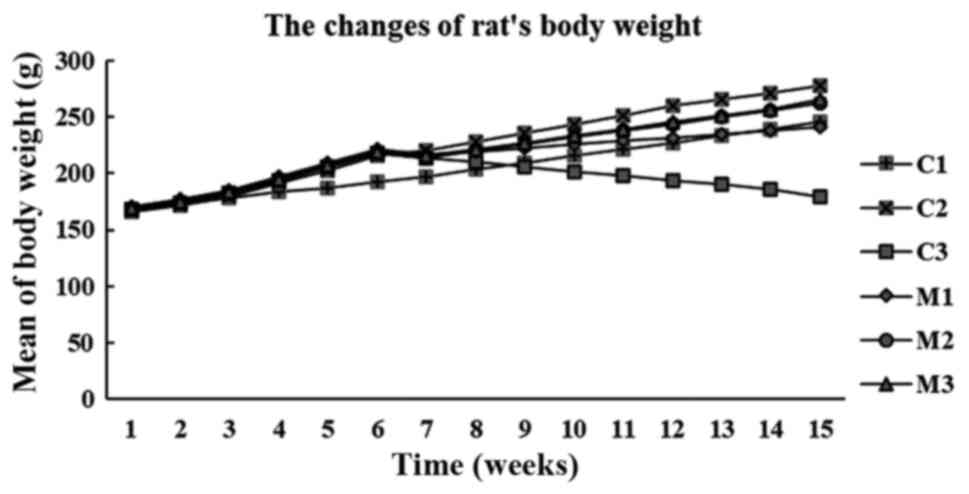
Changes in rat BW
The BW of the rats significantly increased in the
group fed a HFD compared with the normal control rats that received
a standard diet (168.31±3.48 vs. 214.5±10.37 g; P<0.001;
Wilcoxon Signed-Rank test). Compared with the starting BW, after 7
weeks of GMP intervention, BW significantly increased in the normal
and mangosteen control rats (198±2.43 vs. 246±2.80 g; 220±2.83 vs.
278±2.28 g, respectively; both P=0.024; Wilcoxon Signed Ranks test)
but decreased continuously in the T2DM rats (214±3.29 vs. 180±3.89
g; P=0.026; Wilcoxon Signed Ranks test) (Fig. 1).
Effects of GMP extract treatment on
fasting blood glucose levels in obese-T2DM rats
Fig. 2 shows the mean
fasting blood glucose levels before and after GMP intervention in
Wistar rats. The fasting blood glucose levels in all GMP groups
(C2, M1, M2 and M3) were significantly different between the pre-
and post-GMP intervention (all P<0.001; Wilcoxon Signed-Rank
test). The administration of GMP at all doses effectively reduced
fasting blood glucose levels in all diabetic groups, particularly
at a dose of 400 mg/kg (from 277.86±1.48 to 102.45±4.78 mg/dl).
This effect was also observed in an obese non-diabetic group that
was orally treated with 200 mg/kg of GMP extract (from 168.33±4.13
to 84.68±5.04 mg/dl).
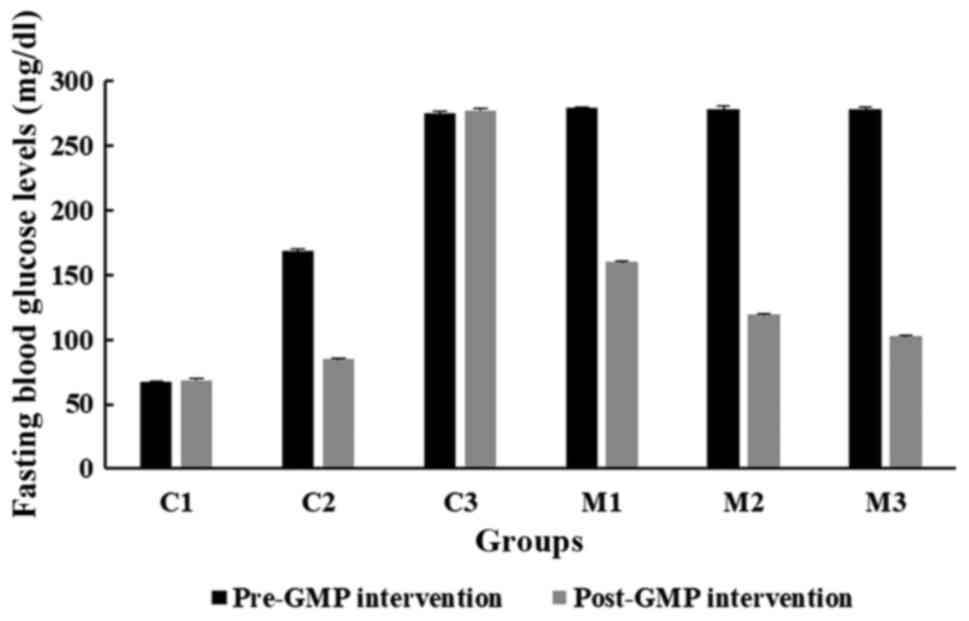 | Figure 2Comparison of fasting blood glucose
levels before and after GMP intervention in the C1, C2, C3, M1, M2
and M3 groups. HFD, high-fat diet; GMP, Garcinia mangostana
pericarp; BW, body weight; C1, normal control rats administered
with a standard normal diet; C2, mangosteen control administered a
HFD and GMP extract at 200 g/kg BW/day; C3, diabetic Wistar rats
treated with HFD; M1, diabetic Wistar rats treated with HFD and 100
g/kg BW/day GMP extract; M2, diabetic Wistar rats treated with HFD
200 g/kg BW/day GMP extract; M3, diabetic rats treated with HFD and
400 g/kg BW/day GMP extract. |
Effect of GMP extract treatment on
glial NF-κB expression in obese-T2DM rats
Fig. 3 shows the
expression of glial NF-κB in the hippocampal area at a
magnification of x400 in 10 fields of view. The number of dark
brown color, absence of visible a nucleus, and irregularly shaped
cells, which were considered as highly positively immunostaining
glial cells, were significantly different in their number amongst
the intervention groups (P=0.007; Kruskal Wallis test). The
hippocampal area of the obese-diabetic rats showed significantly
higher positive expression than that of the normal control group
(132.9±56.41 vs. 73.6±24.44 positive glial cells; P=0.009; Dunn's
test). The treatment of GMP extract, particularly at 200 g/kg
BW/day, effectively reduced the number of activated glial cells
(72.1±17.18 positive glial cells; P=0.016; Dunn's test; compared
with the obese-diabetic group). The results of immune-positive
neuronal cells was not significantly different between the groups
(data not shown).
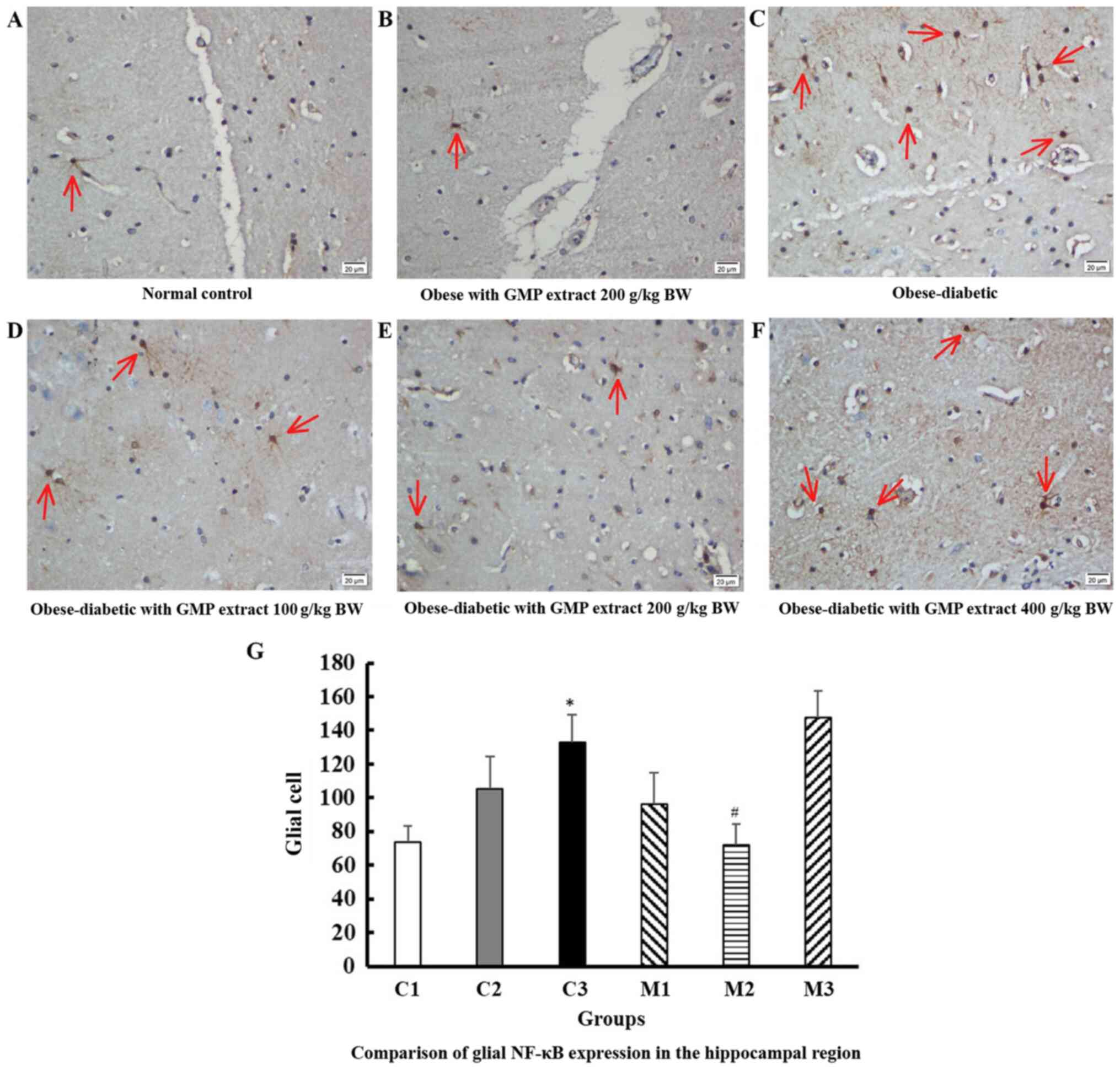 | Figure 3NF-κB expression in glial cells in
the hippocampal region. NF-κB expression in glial cells (red arrow)
in the (A) control group, (B) obese rats treated with GMP,
(C) obese-diabetic rats, and obese-diabetic groups treated with (D)
100, (E) 200 and (F) 400 g/kg BW/day GMP extract. NF-κB stained
glial cells appeared dark brown in color, without a visible nucleus
and were irregularly-shaped cells. The number of stained cells was
counted in 10 fields of view at a magnification of x400. (G)
Comparison of glial NF-κB expression in the hippocampal region
shows a significant difference before (C3) and after (M2)
intervention with 200 g/kg BW/day GMP in obese-diabetic
rats. Error bars indicate the standard error of the mean.
*P<0.001 vs. C1; #P<0.05 vs. C3. NF-κB,
HFD, high-fat diet; GMP, Garcinia mangostana pericarp; BW,
body weight; C1, normal control rats administered with a standard
normal diet; C2, mangosteen control administered a HFD and GMP
extract at 200 g/kg BW/day; C3, diabetic Wistar rats treated with
HFD; M1, diabetic Wistar rats treated with HFD and 100 g/kg BW/day
GMP extract; M2, diabetic Wistar rats treated with HFD 200 g/kg
BW/day GMP extract; M3, diabetic rats treated with HFD and 400 g/kg
BW/day GMP extract. |
Effect of GMP extract treatment on the
IL-6 and TNF-α expression of obese-T2DM rats
The T2DM rats showed significantly higher levels of
IL-6 and TNF-α compared with the normal control rats (IL-6,
122.94±4.86 vs. 76.13±2.47 pg/ml, TNF-α, 14.15±0.23 vs. 6.14±0.17
pg/ml, respectively; P≤0.001; Dunn's test). GMP extract,
particularly 100, 200 and 400 g/kg BW/day, significantly suppressed
T2DM-induced IL-6 levels compared with the untreated rats
(106.18±3.98, 98.04±5.21 and 88.25±2.36 vs. 122.94±4.86 pg/ml,
respectively; P<0.001; Kruskal Wallis test) and TNF-α expression
(9.15±0.12, 8.07±0.38 and 7.18±0.35 vs. 14.15±0.23, respectively;
P<0.001; Kruskal Wallis test). GMP extract also effectively
reduced the upregulation in IL-6 and TNF-α expression in the
obese-non-diabetic rats (92.23±3.212 vs. 122.94±4.856; 7.26±0.233
vs. 14.15±0.227; respectively; both P≤0.01; Dunn's test) (Fig. 4).
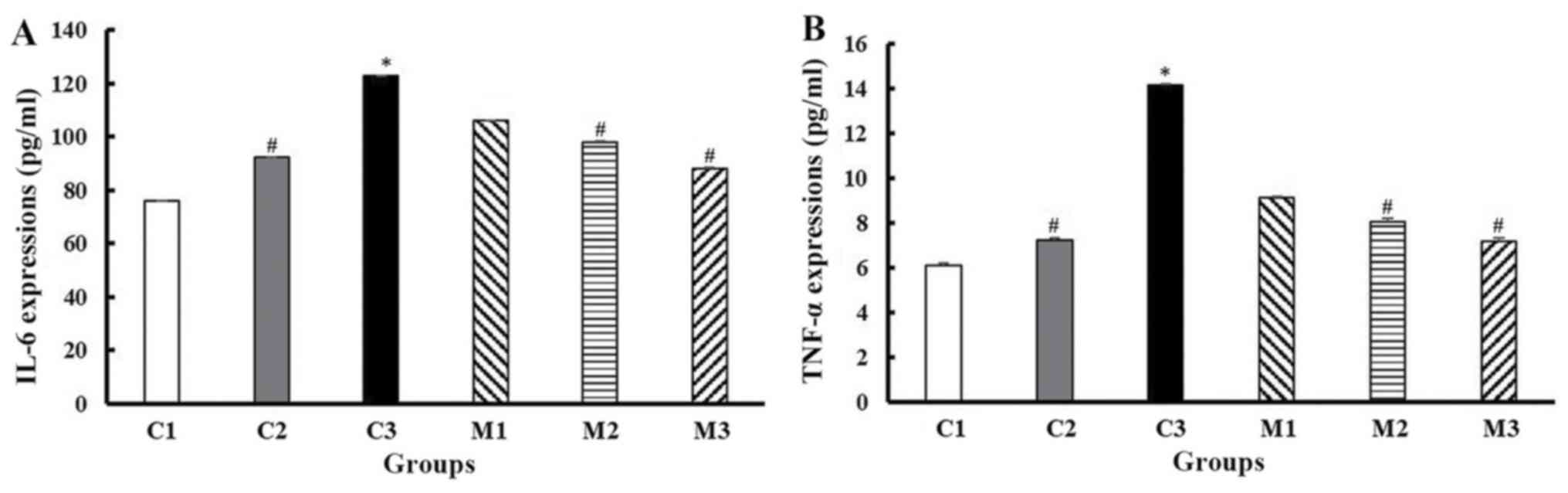 | Figure 4Upregulation of IL-6 (A) and TNF-α
expression (B) in the obese-diabetic control group (C3) and its
suppression in GMP intervention at 100 (M1), 200 (M2), and 400 (M3)
g/kg BW/day. C1 and C2 are the control groups of the normal and
obese rats treated with 200 g/kg BW/day GMP extract, respectively.
Error bars indicate the standard error of the mean.
*P<0.001 vs. C1; #P<0.05 vs. C3. TNF-α,
tumor necrosis factor-α; IL-6, interleukin-6; HFD, high-fat diet;
GMP, Garcinia mangostana pericarp; BW, body weight; C1,
normal control rats administered with a standard normal diet; C2,
mangosteen control administered a HFD and GMP extract at 200 g/kg
BW/day; C3, diabetic Wistar rats treated with HFD; M1, diabetic
Wistar rats treated with HFD and 100 g/kg BW/day GMP extract; M2,
diabetic Wistar rats treated with HFD 200 g/kg BW/day GMP extract;
M3, diabetic rats treated with HFD and 400 g/kg BW/day GMP
extract. |
Effect of GMP extract treatment on SOD
generation in obese-T2DM rats
The T2DM rats exhibited significantly lower levels
of SOD compared with the normal control rats (28.43±3.18% vs.
89.46±2.16%; P≤0.001; Dunn's test). GMP extract, particularly at
100, 200 and 400 g/kg BW/day, significantly attenuated the
T2DM-induced reduction in SOD expression compared with the
untreated T2DM rats (45.34±4.50%, 68.14±3.18%, 75.74±2.75 vs.
28.43±3.18%, respectively; P<0.001; Kruskal Wallis test) in the
blood serum. GMP extract also effectively increased SOD generation
in the obese-non-diabetic rats (81.37±4.712 vs. 28.43±3.18%,
P≤0.001; Dunn's test) (Fig. 5).
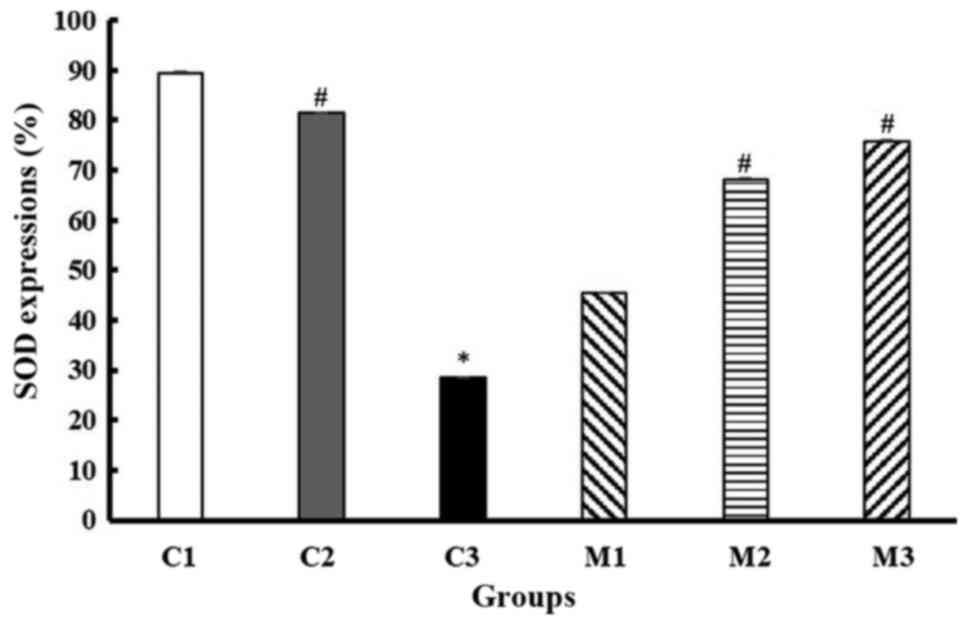 | Figure 5Detection of SOD generation in the
obese-diabetic control group (C3) and its suppression in the 100
(M1), 200 (M2), and 400 (M3) g/kg BW/day GMP intervention groups.
C1 and C2 are the control groups of the normal and obese rats
treated with 200 g/kg BW/day GMP extract, respectively. Error bars
indicate the standard error of the mean. *P<0.001 vs.
C1; #P<0.05 vs. C3. SOD, superoxide dismutase; HFD,
high-fat diet; GMP, Garcinia mangostana pericarp; BW, body
weight; C1, normal control rats administered with a standard normal
diet; C2, mangosteen control administered a HFD and GMP extract at
200 g/kg BW/day; C3, diabetic Wistar rats treated with HFD; M1,
diabetic Wistar rats treated with HFD and 100 g/kg BW/day GMP
extract; M2, diabetic Wistar rats treated with HFD 200 g/kg BW/day
GMP extract; M3, diabetic rats treated with HFD and 400 g/kg BW/day
GMP extract. |
Discussion
The aim of the present study was evaluate the
protective effects of GMP extract on brain tissues against
inflammation, particularly the hippocampus, which is involved in
the early stages of neurocognitive impairment, such as Dementia and
AD, and the prevalence of these diseases is higher in diabetic
patients (4,5). The results of the present study showed
that the expression of NF-κB was upregulated in the glial cells of
the obese-diabetic rats, and that the IL-6 and TNF-α expression, as
well as SOD generation in the blood serum were increased. These
results indicated that obesity with diabetes can stimulate systemic
and cerebral inflammation. NF-κB is an inflammatory factor that can
be released by activated glial cells and is associated with white
matter astrogliosis and cerebral inflammation (43). It also serves an important role in
synaptic signaling in the nervous system that maintains learning
and memory functions (44,45). The NF-κB pathway is activated by
numerous stimuli, including ligands of the TNF receptor families,
pattern-recognition receptors, cytokine receptors and receptors of
B-cells and T-cells (46). It is
involved in the expression of IL-6 and TNF-α, which are secreted by
glial cells in high amounts and related to the production of
several inflammatory cytokines (44,47).
Inhibition of NF-κB expression in reactive astrocytes exerts an
effect on vascular cognitive impairment, such as repairing gliosis
and axonal loss, maintaining the integrity of white matter
structure and improving memory function (43). Compared with glial cells,
immune-positive neuronal cells were not significantly observed in
this study, suggesting that glial cells serve a crucial role in the
early stage of T2DM-induced brain inflammation. Thus, upregulation
of NF-κB expression in hippocampal glial cells may serve as an
early indicator of the pathogenic process of cognitive impairment
in the brain, prior to symptoms of cognitive function impairment in
the rats.
The activation of IL-6 and TNF-α expression has been
reported in patients with T2DM with mild cognitive impairment, and
increases in their levels has been shown to be related to aging in
humans (7,48). The high levels of TNF-α in the
cerebrospinal fluid and high levels of IL-6 in the blood serum of
patients with AD indicate that these two pro-inflammatory cytokines
participate in the early stages of AD through inflammatory
mechanisms (49,50). Other studies reported high expression
levels of IL-6 and TNF-α in the brain micro vessels in diabetic
mice with AD, suggesting an increase in the pathogenesis of
cognitive dysfunction under diabetic conditions by promoting
inflammatory mechanisms (14). The
expression of IL-6 in the plasma has an inverse association with
the volume of hippocampal gray matter, which is a critical
structure involved in memory and cognitive functions (51). The results of the present study are
in agreement with previous studies, indicating that IL-6 and TNF-α
inflammatory cytokines are important biological markers of
cognitive impairment in T2DM, particularly in the initial stage
(14,49,50).
Therefore, they may serve as potential biomarkers for the early
detection of neurocognitive impairment in diabetic patients.
Previous studies have shown an association between
diabetes and increased generation of antioxidants (52-55).
The generation of ROS and suppression of SOD levels are
significantly increased under hyperglycemic conditions in diabetes,
and their enhancement has been observed in rats with
diabetes-induced severe learning and memory deficits associated
with endothelial dysfunction; this result indicates their crucial
roles in cognitive impairment, particularly in vascular dementia
(17,18). Diabetes causes the dysfunction of
endothelial tissue and increases the levels of oxidative stress,
leading to diabetic neurodegeneration and encephalopathic disorders
(53,56,57).
Animal studies reported the decrease of eNOS expression in the
vascular tissue and SOD levels in the thoracic aorta and serum in
the diabetic group (17,52-55).
In agreement with those previous studies, the significant
impairment of SOD generation in diabetic rats was also observed in
the present study.
In the last decade, Garcinia mangostana Linn
of the Guttiferae family has been widely used as a potential
preventative agent for numerous degenerative diseases, including
diabetes, due to its anti-inflammatory, antioxidant and
antihyperglycemic activities (32-35).
Its pericarp contents are rich in flavonoids called xanthones,
which have beneficial effects on metabolic syndrome by inhibiting
α-glucosidase and post-prandial hyperglycemia, thereby reducing
glucose absorption (27). The
xanthone bioactive compounds (particularly in α-mangosteen) have a
similar structure and chromatographic behaviors to that of
flavonoids (58), and ~25% of the
α-mangosteen was observed in mangosteen pericarp (59). When 20 mg/kg α-mangosteen was orally
administered, the bioavailability was estimated to be just 0.4%
(60), and it could reach maximum
plasma levels within 63 min (61).
This low bioavailability is caused by the metabolism of xanthones
that takes a place in the liver and intestine, and the other
compounds in mangosteen extract may obstruct the multiple CYP450
isoforms, such as CYP1A and CYP2C, and inhibit the conjugation of
glucuronide and/or sulfate of α-mangosteen, which has an impact on
reducing the metabolic process in the liver and intestine (62-65).
The anti-inflammatory and antioxidant properties of xanthones may
lower the expression of inflammatory genes, such as TNF-α, IL-6 and
INF-γ inducible protein-10, as well as NF-κB in macrophages and
adipocytes (30,57). Its suppressive effects on NF-κB
activation are exerted by the inhibition of IκBα degradation and
p65 nuclear translocation (66,67). In
the present study, it was shown that the GMP extract could
attenuate the activation of NF-κB expression in cerebral tissue,
particularly in glial cells. The absorbed constituents of GMP
extract, which included xanthones, could penetrate the blood-brain
barrier and exert its effects in hippocampal glial cells, an
important component of the brain that is associated with the
pathogenesis of neurocognitive impairment, such as in dementia and
AD. The effects of the extract also regulated the activation of
TNF-α and IL-6, and increased SOD levels in the serum, indicating
that the activation of NF-κB in the hippocampal tissue is
concurrent with the expression of these proinflammatory cytokines
and antioxidative stress in the blood. Therefore, GMP extracts may
be used as a potential biomarker for the early detection of
neurocognitive impairment in diabetic patients.
In conclusion, the increase in glial NF-κB levels
followed by the increase in IL-6 and TNF-α expression, and the
reduction in SOD activity in the serum of obese-T2DM rats indicates
that obesity with T2DM increases the risk of brain inflammation,
which is related to neurodegenerative disorders, such as AD.
Treatment with GMP extract effectively reduced the levels of these
inflammatory factors, suggesting that xanthones may potentially
prevent brain inflammation in obese-T2DM rats. Further study is
required to establish the advanced neuroprotective effects of
xanthones in humans.
Acknowledgements
We would like to thank Mr Yulianto (Animal
Laboratory of Center for Food and Nutrition Studies, Universitas
Gadjah Mada) for his valuable assistance during the animal
experiment.
Funding
The present study was supported by the Ministry of Research and
Technology/National Research and Innovation Regency Republic of
Indonesia (grant no. 201-04/UN7.6.1/PP/2020).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
MM wrote the manuscript. MM and YN designed the
experiments, performed the animal experiments, analyzed the data,
and edited the manuscript. VK analyzed the data. YP was involved in
the data interpretation and writing the manuscript. NDR analyzed
the data, and edited the manuscript. RW prepared and analyzed the
GMP extract. SS was involved in the conception of study, the
interpretation of the study results and writing the manuscript. All
authors confirmed the authenticity of all the raw data. All authors
have read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Medical and
Health Research Ethics Committee, Faculty of Medicine Diponegoro
University Semarang Indonesia (approval no.
115/EC/H/KEPK/FK-UNDIP/VIII/2019).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Gudala K, Bansal D, Schifano F and
Bhansali A: Diabetes mellitus and risk of dementia: A meta-analysis
of prospective observational studies. J Diabetes Investig.
4:640–650. 2013.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Cheng G, Huang C, Deng H and Wang H:
Diabetes as a risk factor for dementia and mild cognitive
impairment: A meta-analysis of longitudinal studies. Intern Med J.
42:484–491. 2012.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Strachan MW, Price JF and Frier BM:
Diabetes, cognitive impairment, and dementia. BMJ.
336(6)2008.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Profenno LA, Porsteinsson AP and Faraone
SV: Meta-analysis of Alzheimer's disease risk with obesity,
diabetes, and related disorders. Biol Psychiatry. 67:505–512.
2010.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Dybjer E, Nilsson PM, Engström G, Helmer C
and Nägga K: Pre-diabetes and diabetes are independently associated
with adverse cognitive test results: A cross-sectional,
population-based study. BMC Endocr Disord. 18(91)2018.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Cholerton B, Baker LD, Montine TJ and
Craft S: Type 2 diabetes, cognition, and dementia in older adults:
Toward a precision health approach. Diabetes Spectr. 29:210–219.
2016.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Zheng M, Chang B, Tian L, Shan C, Chen H,
Gao Y, Huang G and Zhang M: Relationship between inflammatory
markers and mild cognitive impairment in Chinese patients with type
2 diabetes: A case-control study. BMC Endocr Disord.
19(73)2019.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Meldrum DR, Gambone JC, Morris MA,
Esposito K, Giugliano D and Ignarro LJ: Lifestyle and metabolic
approaches to maximizing erectile and vascular health. Int J Impot
Res. 24:61–68. 2012.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Buvat J, Maggi M, Gooren L, Guay AT,
Kaufman J, Morgentaler A, Schulman C, Tan HM, Torres LO, Yassin A,
et al: Endocrine aspects of male sexual dysfunctions. J Sex Med.
7:1627–1656. 2010.PubMed/NCBI View Article : Google Scholar
|
|
10
|
García-Cruz E, Leibar-Tamayo A, Romero J,
Piqueras M, Luque P, Cardeñosa O and Alcaraz A: Metabolic syndrome
in men with low testosterone levels: Relationship with
cardiovascular risk factors and comorbidities and with erectile
dysfunction. J Sex Med. 10:2529–2538. 2013.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Xu H: Obesity and metabolic inflammation.
Drug Discov Today Dis Mech. 10(10)2013.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Monteiro R and Azevedo I: Chronic
inflammation in obesity and the metabolic syndrome. Mediators
Inflamm. 2010(289645)2010.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Otis CR, Wamhoff BR and Sturek M:
Hyperglycemia-induced insulin resistance in diabetic dyslipidemic
Yucatan swine. Comp Med. 53:53–64. 2003.PubMed/NCBI
|
|
14
|
Takeda S, Sato N, Uchio-Yamada K, Sawada
K, Kunieda T, Takeuchi D, Kurinami H, Shinohara M, Rakugi H and
Morishita R: Diabetes-accelerated memory dysfunction via
cerebrovascular inflammation and Abeta deposition in an Alzheimer
mouse model with diabetes. Proc Natl Acad Sci USA. 107:7036–7041.
2010.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Zhao SJ, Guo CN, Wang MQ, Chen WJ and Zhao
YB: Serum levels of inflammation factors and cognitive performance
in amnestic mild cognitive impairment: A Chinese clinical study.
Cytokine. 57:221–225. 2012.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Lai KSP, Liu CS, Rau A, Lanctôt KL, Köhler
CA, Pakosh M, Carvalho AF and Herrmann N: Peripheral inflammatory
markers in Alzheimer's disease: A systematic review and
meta-analysis of 175 studies. J Neurol Neurosurg Psychiatry.
88:876–882. 2017.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Gocmez SS, Şahin TD, Yazir Y, Duruksu G,
Eraldemir FC, Polat S and Utkan T: Resveratrol prevents cognitive
deficits by attenuating oxidative damage and inflammation in rat
model of streptozotocin diabetes induced vascular dementia. Physiol
Behav. 201:198–207. 2019.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Versari D, Daghini E, Virdis A, Ghiadoni L
and Taddei S: Endothelial dysfunction as a target for prevention of
cardiovascular disease. Diabetes Care. 32 (Suppl 2):S314–S321.
2009.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Jha NK, Jha SK, Kar R, Nand P, Swati K and
Goswami VK: Nuclear factor-kappa β as a therapeutic target for
Alzheimer's disease. J Neurochem. 150:113–137. 2019.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Kawahara TLA, Michishita E, Adler AS,
Damian M, Berber E, Lin M, McCord RA, Ongaigui KCL, Boxer LD, Chang
HY, et al: SIRT6 links histone H3 lysine 9 deacetylation to
NF-kappaB-dependent gene expression and organismal life span. Cell.
136:62–74. 2009.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Natoli G: When sirtuins and NF-kappaB
collide. Cell. 136:19–21. 2009.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Feng Y, Li X, Zhou W, Lou D, Huang D, Li
Y, Kang Y, Xiang Y, Li T, Zhou W, et al: Regulation of SET gene
expression by NFκB. Mol Neurobiol. 54:4477–4485. 2017.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Tousian Shandiz H, Razavi BM and
Hosseinzadeh H: Review of Garcinia mangostana and its
Xanthones in Metabolic Syndrome and Related Complications.
Phytother Res. 31:1173–1182. 2017.PubMed/NCBI View
Article : Google Scholar
|
|
24
|
Ibrahim MY, Hashim NM, Mariod AA, Mohan S,
Abdulla MA, Abdelwahab SI and Arbab IA: α-Mangostin from
Garcinia mangostana Linn: An updated review of its
pharmacological properties. Arab J Chem. 9:317–329. 2016.
|
|
25
|
Obolskiy D, Pischel I, Siriwatanametanon N
and Heinrich M: Garcinia mangostana L.: A phytochemical and
pharmacological review. Phytother Res. 23:1047–1065.
2009.PubMed/NCBI View
Article : Google Scholar
|
|
26
|
World Health Organization: Programme on
Traditional Medicine. General guidelines for methodologies on
research and evaluation of traditional medicine. World Health
Organization, 2000.
|
|
27
|
Suthammarak W, Numpraphrut P, Charoensakdi
R, Neungton N, Tunrungruangtavee V, Jaisupa N, Charoensak S,
Moongkarndi P and Muangpaisan W: Antioxidant-enhancing property of
the polar fraction of mangosteen pericarp extract and evaluation of
its safety in humans. Oxid Med Cell Longev.
2016(1293036)2016.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Jung HA, Su BN, Keller WJ, Mehta RG and
Kinghorn AD: Antioxidant xanthones from the pericarp of Garcinia
mangostana (Mangosteen). J Agric Food Chem. 54:2077–2082.
2006.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Wang MH, Zhang KJ, Gu QL, Bi XL and Wang
JX: Pharmacology of mangostins and their derivatives: A
comprehensive review. Chin J Nat Med. 15:81–93. 2017.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Bumrungpert A, Kalpravidh RW, Chuang CC,
Overman A, Martinez K, Kennedy A and McIntosh M: Xanthones from
mangosteen inhibit inflammation in human macrophages and in human
adipocytes exposed to macrophage-conditioned media. J Nutr.
140:842–847. 2010.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Cho BO, Ryu HW, So Y, Lee CW, Jin CH, Yook
HS, Jeong YW, Park JC and Jeong IY: Anti-inflammatory effect of
mangostenone F in lipopolysaccharide-stimulated RAW264.7
macrophages by suppressing NF-κB and MAPK activation. Biomol Ther
(Seoul). 22:288–294. 2014.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Syam S, Bustamam A, Abdullah R, Sukari MA,
Hashim NM, Mohan S, Looi CY, Wong WF, Yahayu MA and Abdelwahab SI:
β Mangostin suppress LPS-induced inflammatory response in RAW 264.7
macrophages in vitro and carrageenan-induced peritonitis in vivo. J
Ethnopharmacol. 153:435–445. 2014.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Liu Q, Li D, Wang A, Dong Z, Yin S, Zhang
Q, Ye Y, Li L and Lin L: Nitric oxide inhibitory xanthones from the
pericarps of Garcinia mangostana. Phytochemistry.
131:115–123. 2016.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Chin YW, Jung HA, Chai H, Keller WJ and
Kinghorn AD: Xanthones with quinone reductase-inducing activity
from the fruits of Garcinia mangostana (Mangosteen).
Phytochemistry. 69:754–758. 2008.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Taher M, Tg Zakaria TMFS, Susanti D and
Zakaria ZA: Hypoglycaemic activity of ethanolic extract of
Garcinia mangostana Linn. in normoglycaemic and
streptozotocin-induced diabetic rats. BMC Complement Altern Med.
16(135)2016.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Liu QY, Wang YT and Lin LG: New insights
into the anti-obesity activity of xanthones from Garcinia
mangostana. Food Funct. 6:383–393. 2015.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Sukatta U, Takenaka M, Ono H, Okadome H,
Sotome I, Nanayama K, Thanapase W and Isobe S: Distribution of
major xanthones in the pericarp, aril, and yellow gum of mangosteen
(Garcinia mangostana Linn.) fruit and their contribution to
antioxidative activity. Biosci Biotechnol Biochem. 77:984–987.
2013.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Ashton MM, Dean OM, Walker AJ, Bortolasci
CC, Ng CH, Hopwood M, Harvey BH, Möller M, McGrath JJ, Marx W, et
al: The therapeutic potential of mangosteen pericarp as an
adjunctive therapy for bipolar disorder and schizophrenia. Front
Psychiatry. 10(115)2019.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Wihastuti TA, Sargowo D, Tjokroprawiro A,
Permatasari N, Widodo MA and Soeharto S: Vasa vasorum
anti-angiogenesis through H2O2, HIF-1α,
NF-κB, and iNOS inhibition by mangosteen pericarp ethanolic extract
(Garcinia mangostana Linn) in hypercholesterol-diet-given
Rattus norvegicus Wistar strain. Vasc Health Risk Manag.
10:523–531. 2014.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Sattayasai J, Chaonapan P, Arkaravichie T,
Soi-Ampornkul R, Junnu S, Charoensilp P, Samer J, Jantaravinid J,
Masaratana P, Suktitipat B, et al: Protective effects of mangosteen
extract on H2O2-induced cytotoxicity in
SK-N-SH cells and scopolamine-induced memory impairment in mice.
PLoS One. 8(e85053)2013.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Council NR: Guide for the Care and Use of
Laboratory Animals. 8th edition. The National Academies Press,
Washington, DC, 2011.
|
|
42
|
Janardhanan S, Mahendra J, Mahendra L and
Devarajan N: Cytotoxic effects of mangosteen pericarp extracts on
oral cancer and cervical cancer cells. Asian Pac J Cancer Prev.
21:2577–2583. 2020.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Saggu R, Schumacher T, Gerich F, Rakers C,
Tai K, Delekate A and Petzold GC: Astroglial NF-κB contributes to
white matter damage and cognitive impairment in a mouse model of
vascular dementia. Acta Neuropathol Commun. 4(76)2016.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Mattson MP and Camandola S: NF-kappaB in
neuronal plasticity and neurodegenerative disorders. J Clin Invest.
107:247–254. 2001.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Albensi BC and Mattson MP: Evidence for
the involvement of TNF and NF-kappaB in hippocampal synaptic
plasticity. Synapse. 35:151–159. 2000.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Liu T, Zhang L, Joo D and Sun SC: NF-κB
signaling in inflammation. Signal Transduct Target Ther.
2(17023)2017.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Schwaninger M, Sallmann S, Petersen N,
Schneider A, Prinz S, Libermann TA and Spranger M: Bradykinin
induces interleukin-6 expression in astrocytes through activation
of nuclear factor-kappaB. J Neurochem. 73:1461–1466.
1999.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Brüünsgaard H and Pedersen BK: Age-related
inflammatory cytokines and disease. Immunol Allergy Clin North Am.
23:15–39. 2003.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Tarkowski E, Liljeroth AM, Minthon L,
Tarkowski A, Wallin A and Blennow K: Cerebral pattern of pro- and
anti-inflammatory cytokines in dementias. Brain Res Bull.
61:255–260. 2003.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Schuitemaker A, Dik MG, Veerhuis R,
Scheltens P, Schoonenboom NS, Hack CE, Blankenstein MA and Jonker
C: Inflammatory markers in AD and MCI patients with different
biomarker profiles. Neurobiol Aging. 30:1885–1889. 2009.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Marsland AL, Gianaros PJ, Abramowitch SM,
Manuck SB and Hariri AR: Interleukin-6 covaries inversely with
hippocampal grey matter volume in middle-aged adults. Biol
Psychiatry. 64:484–490. 2008.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Arrick DM, Sun H, Patel KP and Mayhan WG:
Chronic resveratrol treatment restores vascular responsiveness of
cerebral arterioles in type 1 diabetic rats. Am J Physiol Heart
Circ Physiol. 301:H696–H703. 2011.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Hadi HAR and Suwaidi JA: Endothelial
dysfunction in diabetes mellitus. Vasc Health Risk Manag.
3:853–876. 2007.PubMed/NCBI
|
|
54
|
Sharma B and Singh N: Pitavastatin and
4'-hydroxy-3'-methoxyacetophenone (HMAP) reduce cognitive
dysfunction in vascular dementia during experimental diabetes. Curr
Neurovasc Res. 7:180–191. 2010.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Sharma B and Singh N: Attenuation of
vascular dementia by sodium butyrate in streptozotocin diabetic
rats. Psychopharmacology (Berl). 215:677–687. 2011.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Biessels GJ, van der Heide LP, Kamal A,
Bleys RL and Gispen WH: Ageing and diabetes: Implications for brain
function. Eur J Pharmacol. 441:1–14. 2002.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Bumrungpert A, Kalpravidh RW,
Chitchumroonchokchai C, Chuang CC, West T, Kennedy A and McIntosh
M: Xanthones from mangosteen prevent lipopolysaccharide-mediated
inflammation and insulin resistance in primary cultures of human
adipocytes. J Nutr. 139:1185–1191. 2009.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Negi JS, Bisht VK, Singh P, Rawat MS and
Joshi GP: Naturally occurring xanthones: Chemistry and biology. J
Appl Chem. 2013(621459)2013.
|
|
59
|
Quan GH, Oh SR, Kim JH, Lee HK, Kinghorn
AD and Chin YW: Xanthone constituents of the fruits of Garcinia
mangostana with anticomplement activity. Phytother Res.
24:1575–1577. 2010.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Li L, Brunner I, Han AR, Hamburger M,
Kinghorn AD, Frye R and Butterweck V: Pharmacokinetics of
α-mangostin in rats after intravenous and oral application. Mol
Nutr Food Res. 55 (Suppl 1):S67–S74. 2011.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Syamsudin F and Rahayu L: HPLC Analysis
mangostin after orally administration in rats. Asian J Chem.
22:6729–6733. 2010.
|
|
62
|
Foti RS, Pearson JT, Rock DA, Wahlstrom JL
and Wienkers LC: In vitro inhibition of multiple cytochrome P450
isoforms by xanthone derivatives from mangosteen extract. Drug
Metab Dispos. 37:1848–1855. 2009.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Li L, Han AR, Kinghorn AD, Frye RF,
Derendorf H and Butterweck V: Pharmacokinetic properties of pure
xanthones in comparison to a mangosteen fruit extract in rats.
Planta Med. 79:646–653. 2013.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Chitchumroonchokchai C, Riedl KM,
Suksumrarn S, Clinton SK, Kinghorn AD and Failla ML: Xanthones in
mangosteen juice are absorbed and partially conjugated by healthy
adults. J Nutr. 142:675–680. 2012.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Chitchumroonchokchai C, Thomas-Ahner JM,
Li J, Riedl KM, Nontakham J, Suksumrarn S, Clinton SK, Kinghorn AD
and Failla ML: Anti-tumorigenicity of dietary α-mangostin in an
HT-29 colon cell xenograft model and the tissue distribution of
xanthones and their phase II metabolites. Mol Nutr Food Res.
57:203–211. 2013.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Chen L, Zhang J, Zhang Y, Wang Y and Wang
B: Improvement of inflammatory responses associated with NF-κB
pathway in kidneys from diabetic rats. Inflamm Res. 57:199–204.
2008.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Tewtrakul S, Wattanapiromsakul C and
Mahabusarakam W: Effects of compounds from Garcinia
mangostana on inflammatory mediators in RAW264.7 macrophage
cells. J Ethnopharmacol. 121:379–382. 2009.PubMed/NCBI View Article : Google Scholar
|