Introduction
Small interfering RNAs (siRNAs) inhibit the
expression of mRNAs bearing complementary sequences (1). The first siRNA therapeutic, patisiran,
was approved by the FDA in 2018, and the second siRNA therapeutic,
givosiran, was approved the following year (2). Several studies have focused on RNA
therapy, especially siRNA-based therapeutics. Screening with siRNA
libraries is an efficient method for identifying genes as
therapeutic targets (3). For
screening with a large set of siRNAs, reliable and reproducible
transfections in multi-well plates is required (4). However, siRNAs are unable to diffuse
passively into cells on their own; therefore, they are often
introduced into cells using cationic liposomes (5). Several studies have demonstrated that
siRNA and cationic liposome complexes (siRNA lipoplexes) are
unstable when stored in solution at room temperature (6). A relatively simple method to store
siRNA lipoplexes at room temperature for longer periods of time is
to transform the siRNA lipoplex suspension into a freeze-dried
siRNA lipoplex. Solid-phase reverse (Rev)-transfection using
freeze-dried siRNA lipoplexes is a valid means of easy and reliable
siRNA transfection with cationic liposomes (7,8). In
Rev-transfection with freeze-dried siRNA lipoplexes, siRNA lipoplex
solutions are freeze-dried in multi-well plates, and at the time of
transfection, cell suspensions are added to the wells.
Freeze-drying is widely used to stabilize liposomes
for long-term storage at room temperature (9,10).
However, siRNA lipoplexes increase in size following
dehydration-rehydration cycles, resulting in a decrease in gene
knockdown activity if they are freeze-dried without appropriate
stabilizers (7). Although
cryoprotectants, including saccharides (such as sucrose and
trehalose) (6,11-13)
and amino acids (14), are employed
to stabilize liposomes and lipid nanoparticles during the
freeze-drying and rehydration processes, the mechanism of
protection of these molecules is not yet fully understood. After
decades of studying saccharides, two hypotheses, water replacement
and vitrification, have been well accepted. In the water
replacement hypothesis, instead of water molecules, saccharides
associate with the polar head groups of the hydrated phospholipids
of the liposomal membrane, stabilizing the liposome (15). Alternatively, the vitrification
hypothesis indicates that saccharides around the bilayer form a
vitreous layer, which depresses the transition temperature of the
lipids (16). Alternatively, amino
acids are not only able to offer hydrogen bonds, but also provide
electrostatic interactions for effective lyophilization (14). To improve the stability of proteins,
amino acids are often added as stabilizing excipients to prevent
protein inactivation during freeze-drying and storage (17,18).
Furthermore, the addition of lysine to liposomes during
freeze-drying appears to have protective effects similar to those
of trehalose (14). However, to the
best of our knowledge, there are no studies assessing the
application of amino acids as cryoprotectants in the freeze-drying
of siRNA lipoplexes.
In our previous study, it was shown that
Rev-transfection with siRNA lipoplexes freeze-dried in the presence
of a disaccharide or trisaccharide solution resulted in a high
level of gene-knockdown activity without exerting a notable
cytotoxic effect (7,8). In the present study, to investigate the
appropriate use of amino acids as cryoprotectants to retain this
high efficiency of Rev-transfection using siRNA lipoplexes,
freeze-dried siRNA lipoplexes were prepared with 15 types of amino
acids, and their efficiency in gene knockdown in cells was
assessed. The presence of amino acids during the freeze-drying of
siRNA lipoplexes resulted in gene knockdown activity; however,
certain amino acids strongly upregulated or downregulated gene
expression in cells transfected with negative control siRNA. In
addition, the types of amino acids used during freeze-drying
affected the size of the siRNA lipoplexes following rehydration.
Amongst the amino acids tested in the present study, siRNA
lipoplexes freeze-dried with asparagine showed specific gene
suppression, forming large cakes after freeze-drying, whilst
retaining a relatively small size following rehydration. The
results of the present study provide valuable information regarding
the optimal amino acids for use as cryoprotectants for
Rev-transfection using freeze-dried siRNA lipoplexes.
Materials and methods
Materials
1,2-Dioleoyl-3-trimethylammonium-propane methyl
sulfate salt (DOTAP) was obtained from Avanti Polar Lipids Inc.
1,2-Dioleoyl-sn-glycero-3-phosphoethanolamine (DOPE, COATSOME; cat.
no. ME-8181) was obtained from NOF Co., Ltd. L-Alanine, L-arginine
hydrochloride, L-asparagine monohydrate, L-cysteine, L-glutamine,
glycine, L-histidine, L-isoleucine, L-leucine, L-lysine,
L-methionine, L-proline, L-serine, L-threonine and L-valine were
obtained from Wako Pure Chemical Industries, Ltd. All the other
chemicals used were of the highest available grade, and were
obtained from commercial sources.
Small interfering (si)RNAs
siRNAs targeting nucleotides of firefly luciferase
(Luc siRNA) and non-targeting siRNA control (Cont siRNA) as a
negative control for Luc siRNA were synthesized by Sigma Genosys.
The siRNA sequences of the Luc siRNA were as follows: Sense strand,
5'-CCGUGGUGUUCGUGUCUAAGA-3' and antisense strand,
5'-UUAGACACGAACACCACGGUA-3' (19),
and the siRNA sequences of the Cont siRNA were as follows: Sense
strand, 5'-GUACCGCACGUCAUUCGUAUC-3' and antisense strand,
5'-UACGAAUGACGUGCGGUACGU-3' (20).
Appearance of cakes after
freeze-drying the amino acid solution
To compare the cake volumes after freeze-drying, 5
ml 100 mM alanine, arginine, asparagine, cysteine, glutamine,
glycine, histidine, isoleucine, leucine, lysine, methionine,
proline, serine, threonine and valine solution were transferred
into a 5 ml vial, and then frozen at -80˚C, followed by drying
under a high vacuum pressure (10-20 Pa) using a FDU-540
freeze-dryer (Tokyo Rikakikai Co.) equipped with a DRC-2L dry
chamber (Tokyo Rikakikai Co.).
Preparation of cationic liposomes and
siRNA lipoplexes
Cationic liposomes were prepared from DOTAP and DOPE
at a molar ratio of 1:1(21). For
the preparation of cationic liposomes using the thin-film hydration
method, DOTAP and DOPE were dissolved in chloroform, and the
chloroform was evaporated under vacuum on a rotary evaporator at
60˚C to obtain a thin film. The thin film was hydrated with water
at 60˚C through vortexing. The hydrated liposomes were placed in an
eggplant flask and sonicated using a bath-type sonicator
(Bransonic® 2510 J-MTH, 42 kHz, 100 W; Branson UL
Trasonics Co.) for 5-10 min at room temperature.
To prepare cationic liposome/siRNA complexes (siRNA
lipoplexes), each liposome preparation was added to siRNA at a
charge ratio (+:-) of 4:1 via vortex mixing for 10 sec, leaving the
solution at room temperature for 15 min. The charge ratio (+:-) of
liposomes:siRNA is expressed as the molar ratio of cationic lipid
to siRNA phosphate.
Size of reconstituted siRNA
lipoplexes
To measure the size of siRNA lipoplexes, siRNA
lipoplexes were formed through the addition of cationic liposomes
to 5 µg Cont siRNA with vortex-mixing for 10 sec and leaving the
solution at room temperature for 15 min. In the preparation of
freeze-dried siRNA lipoplexes, each lipoplex containing 5 µg Cont
siRNA was diluted in 933 µl 100 mM amino acid solution sterilized
using a 0.45-µm filter [125 µl amino acid solution per 50 pmol
(0.67 µg) siRNA], and then transferred to a 6-well plate, followed
by freezing at -80˚C. The frozen plates were dried under a high
vacuum using a freeze-dryer. Freeze-dried siRNA lipoplexes were
reconstituted to an appropriate volume (~1 ml) with water, and the
particle size (cumulant average particle size) and polydispersity
index (PDI) of the siRNA lipoplexes were measured using the
cumulant method with a ELS-Z2 light-scattering photometer (Otsuka
Electronics Co., Ltd.) at 25˚C.
Cell culture
Human breast cancer MCF-7 cells stably expressing
firefly luciferase (MCF-7-Luc), constructed via transfection with a
pcDNA3 plasmid containing the firefly luciferase gene from the
plasmid psiCHECK2 (Promega Corporation), were donated by Dr Kenji
Yamato of the University of Tsukuba. MCF-7-Luc cells were grown in
RPMI-1640 medium (Wako Pure Chemical Industries, Ltd.) supplemented
with 10% heat-inactivated FBS (Invitrogen; Thermo Fisher
Scientific, Inc.) and 1.2 mg/ml G418 (Santa Cruz Biotechnology,
Inc.) at 37˚C in a 5% CO2 humidified atmosphere.
Effect of amino acid types used in the
freeze-drying of siRNA lipoplexes on gene knockdown by
Rev-transfection
For Rev-transfection, siRNA lipoplexes were formed
through the addition of cationic liposomes to 50 pmol Cont siRNA or
Luc siRNA by vortexing for 10 sec, and then leaving the solutions
to stand at room temperature for 15 min. Each lipoplex was diluted
in a 125 µl solution containing different concentrations of amino
acids (10, 25, 50, 100 or 150 mM), transferred to a 12-well plate
(50 pmol siRNA/well), followed by freezing at -80˚C. The frozen
plates were dried under high vacuum using a freeze-dryer and stored
at room temperature in a desiccator until required. For
Rev-transfection using freeze-dried siRNA lipoplexes, MCF-7-Luc
cells (1x105 cells) were suspended in 1 ml culture
medium supplemented with 10% FBS, and this suspension was added to
each well (final siRNA concentration, 50 nM). The medium, after the
rehydration of siRNA lipoplexes freeze-dried with 10, 25, 50, 100
or 150 mM amino acid solutions, contained 1.25, 3.125, 6.25, 12.5
or 18.75 mM extra amino acids, respectively. A total of 48 h after
transfection, the cells were lysed through the addition of 125 µl
cell lysis buffer (Pierce Luciferase Cell Lysis Buffer; Thermo
Fisher Scientific Inc.) and subjected to one cycle of freezing
(-80˚C) and thawing (37˚C), followed by centrifugation at 15,000 x
g for 10 sec at 4˚C. A total of 10 µl aliquots of the cell lysate
supernatants were mixed with 50 µl PicaGene MelioraStar-LT
Luminescence Reagent (Toyo Ink Mfg. Co., Ltd.), and the
luminescence was measured as counts per second (cps) with a
chemoluminometer (ARVO X2; Perkin Elmer, Inc.). The protein
concentration of the supernatants was determined using BCA reagent
(Pierce BCA Protein assay kit; Thermo Fisher Scientific Inc.), with
bovine serum albumin (BSA; Sigma-Aldrich; Merck KGaA) as a
standard, and the luciferase activity (cps/µg protein) was
calculated. Luciferase activity (%) was calculated relative to the
luciferase activity (cps/µg protein) of untransfected cells.
To investigate the effect of amino acids on
luciferase expression in MCF-7-Luc cells, 125 µl 100 mM amino acid
solution was transferred to a 12-well plate, followed by
freeze-drying. MCF-7-Luc cells (1x105) were suspended in
1 ml medium supplemented with 10% FBS and the resulting cell
suspension was added to the wells. Luciferase activity was measured
as described above.
Cytotoxicity caused by
Rev-transfection with freeze-dried siRNA lipoplexes
Each lipoplex containing 5 pmol Cont siRNA was
diluted with a 12.5 µl solution containing 100 mM amino acid, and
then transferred to each well of a 96-well plate (5 pmol
siRNA/well). After freezing at -80˚C, the plates were dried under a
high vacuum using a freeze-dryer. MCF-7-Luc cells
(1x104) were suspended in 100 µl culture medium
supplemented with 10% FBS, and the suspension was added to each
well (final siRNA concentration of 50 nM). After 24 h of
incubation, cell viability was determined using a Cell Counting
Kit-8 assay (Dojindo Laboratories). The relative cell viability (%)
was calculated as the percentage of the cells added to the wells
without freeze-dried lipoplexes.
Statistical analysis
Data are presented as the mean ± standard deviation
of at least three repeats. The statistical significance of the
differences between mean values was determined using Student's
t-test or a one-way ANOVA followed by a post-hoc Tukey's test in
GraphPad Prism version 4.0 (GraphPad Software Inc.). P<0.05 was
considered to indicate a statistically significant difference.
Results
Appearance of cakes after
freeze-drying amino acid solution
In the present study, 15 amino acids (alanine,
arginine, asparagine, cysteine, glutamine, glycine, histidine,
isoleucine, leucine, lysine, methionine, proline, serine, threonine
and valine) were used as cryoprotectants during the freeze-drying
of siRNA lipoplexes, and their effects on gene knockdown efficiency
after Rev-transfection using freeze-dried siRNA lipoplexes were
investigated. As aspartic acid, glutamic acid, phenylalanine,
tyrosine and tryptophan did not completely dissolve in water at a
concentration of 100-150 mM, these were excluded as potential
cryoprotectants. The appearance of cakes (dry powder) after
freeze-drying may be an indicator of product quality. Thus, first,
a 100 mM amino acid solution was prepared and freeze-dried in a
vial. Histidine and threonine formed shrunken cakes (Fig. 1B) after freeze-drying; arginine,
glycine and proline formed collapsed cakes (Fig. 1C); and lysine formed puffing cakes
(Fig. 1D). In contrast, the other
amino acids formed large cakes (Fig.
1A), similar to previously reported disaccharides (7,8). This
result suggests that different amino acid types affect the
appearance of cakes after freeze-drying.
 | Figure 1Appearance of cakes after
freeze-drying of amino acid solutions. A total of 5 ml 100 mM
alanine, arginine, asparagine, cysteine, glutamine, glycine,
histidine, isoleucine, leucine, lysine, methionine, proline,
serine, threonine and valine solutions were transferred into 5 ml
vials, followed by freezing at -80˚C. The frozen vials were dried
under a high vacuum using a freeze-dryer. (A) Appearance of cakes
formed using serine, methionine, asparagine, leucine, alanine,
cysteine, glutamine, isoleucine and valine; (B) histidine and
threonine; (C) proline, glycine, and arginine; and (D) lysine
solutions. |
Effect of amino acid types in
freeze-dried siRNA lipoplexes on gene knockdown in Rev-transfected
cells
Reverse transfection is a method in which siRNA
lipoplexes are attached to the bottom of cell culture plates
through freeze-drying, then, at the time of transfection, a cell
suspension is added to the culture plate (7,8). To
assess the gene-knockdown effects of siRNA lipoplexes freeze-dried
in the presence of amino acids, freeze-dried siRNA lipoplexes in
12-well plates were reconstituted with MCF-7-Luc cells suspended in
culture medium. Here, DOTAP was used as a cationic lipid and DOPE
as a helper lipid, and cationic liposomes were prepared at a molar
ratio of 1:1(8). In our previous
study, it was reported that cationic liposomes composed of DOTAP
and DOPE could efficiently deliver siRNA into the cells via
conventional transfection (forward transfection) and strongly
suppress the expression of target genes (>80% knockdown)
(7). siRNA lipoplexes were formed at
a charge ratio (+:-) of 4:1, diluted with solutions containing
different concentrations of amino acids, and then added into the
wells of a 12-well plate, followed by freeze-drying (Fig. S1). When siRNA lipoplexes were
freeze-dried without amino acids, the lipoplexes with Luc siRNA did
not suppress luciferase activity after Rev-transfection (Fig. 2A-C). However, increasing
concentrations of amino acids used in the freeze-drying of siRNA
lipoplexes were associated with increased gene-knockdown activity
after Rev-transfection. Freeze-drying of lipoplexes containing Luc
siRNA in the presence of 50-100 mM amino acids strongly suppressed
luciferase activity regardless of the amino acid type.
Interestingly, luciferase activity after Rev-transfection with
lipoplexes of Cont siRNA differed depending on the amino acid used.
The results could be divided into three groups: The presence of
amino acids in freeze-drying that exhibited increased luciferase
activity (Fig. 2A), decreased
luciferase activity (Fig. 2B) or no
notable change in luciferase activity (Fig. 2C). Increasing concentrations of
alanine, glutamine, glycine, serine, threonine or valine in the
freeze-drying of siRNA lipoplexes increased luciferase activity
(~200% compared with the untreated cells) after Rev-transfection
with lipoplexes of Cont siRNA (Fig.
2A). In contrast, the presence of arginine, cysteine or lysine
during freeze-drying decreased luciferase activity at increasing
concentrations, whereas at concentrations >100 mM, luciferase
activity decreased to ~20% compared with the untreated cells
(Fig. 2B). However, the presence of
asparagine, histidine, isoleucine, leucine, methionine or proline
during freeze-drying did not notably affect luciferase activity
(Fig. 2C). To confirm the effects of
amino acids on luciferase activity, MCF-7-Luc cells were added to
each well of a multi-well plate, which was freeze-dried with 100 mM
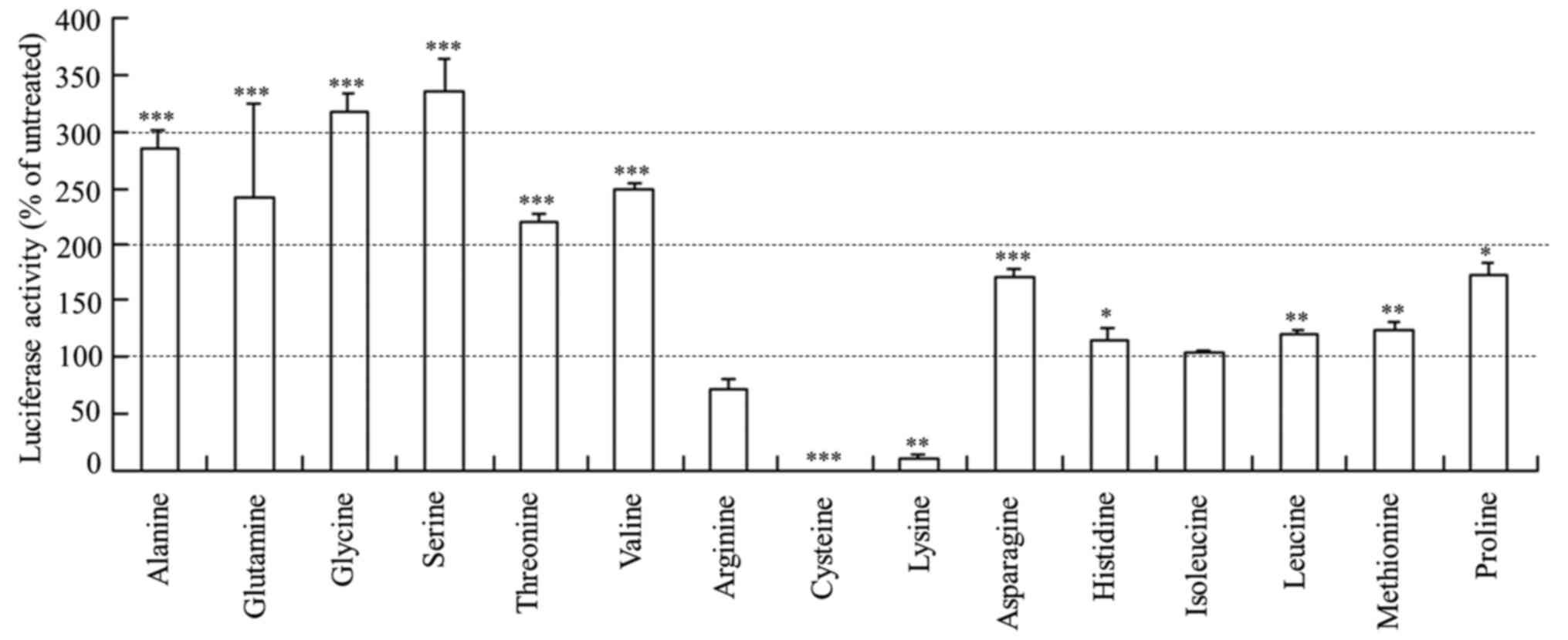
amino acids without siRNA lipoplexes (Fig. 3). As a result, the extra amino acids
affected luciferase activity similarly to those after
Rev-transfection with the lipoplexes of Cont siRNA freeze-dried
with amino acids (Fig. 2),
indicating that the presence of extra amino acids in the culture
medium may affect luciferase expression or luciferase activity.
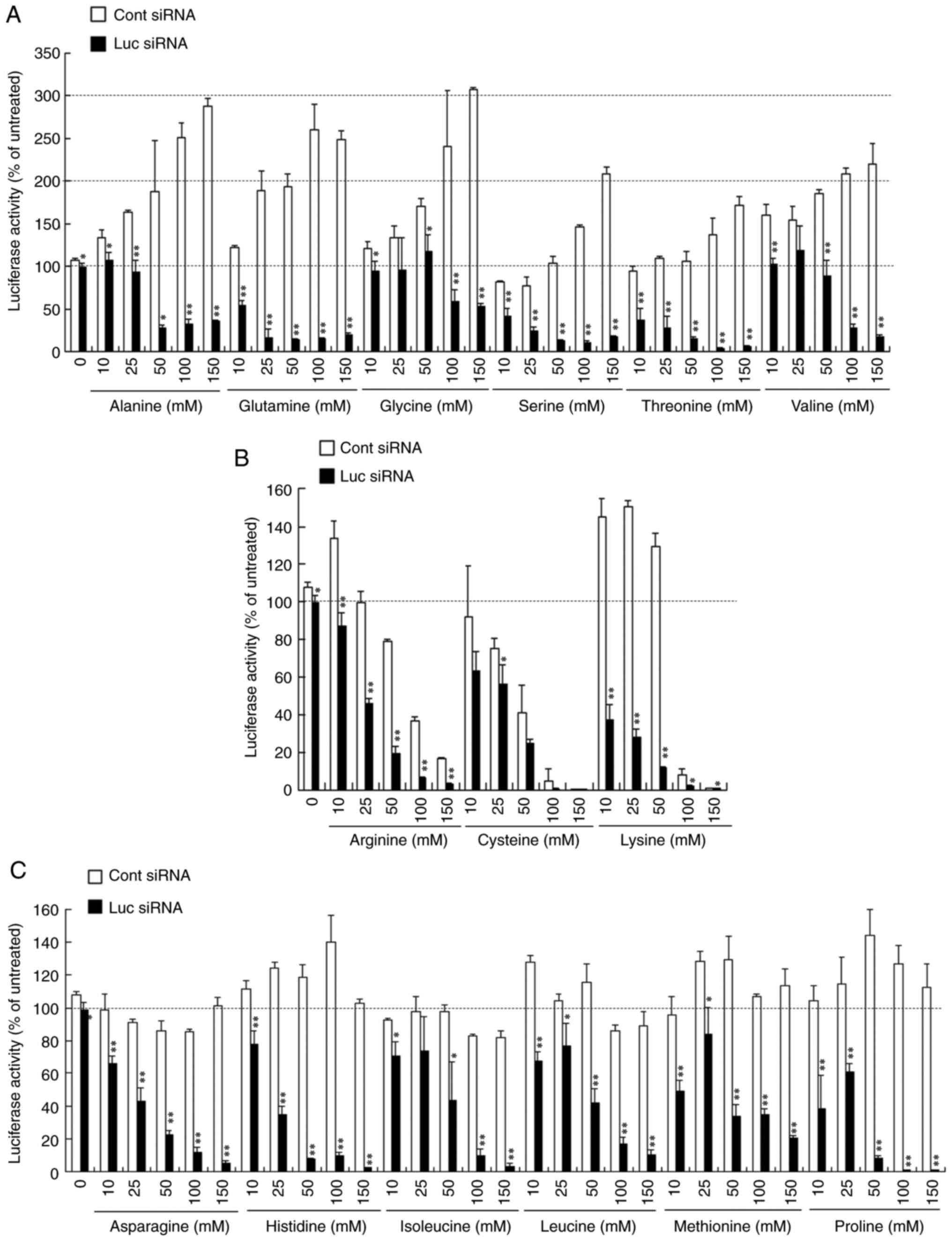 | Figure 2Effect of amino acids in the
freeze-drying on the suppression of luciferase expression in
MCF-7-Luc cells after reverse transfection with siRNA lipoplexes.
Lipoplexes with 50 pmol Cont siRNA or Luc siRNA were diluted in a
125 µl solution containing 10, 25, 50, 100 or 150 mM (A) alanine,
glutamine, glycine, serine, threonine or valine; (B) arginine,
cysteine or lysine; or (C) asparagine, histidine, isoleucine,
leucine, methionine or proline, and transferred to 12-well plates,
followed by freeze-drying. MCF-7-Luc cells suspended in culture
medium (1 ml) were added to each well, and luciferase assays were
performed after incubation at 37˚C for 48 h. Data are presented as
the mean ± standard deviation of three repeats.
*P<0.05, **P<0.01 vs. Cont siRNA. Luc,
luciferase; Cont, control; siRNA, small interfering RNA; lipoplex,
liposome complex. |
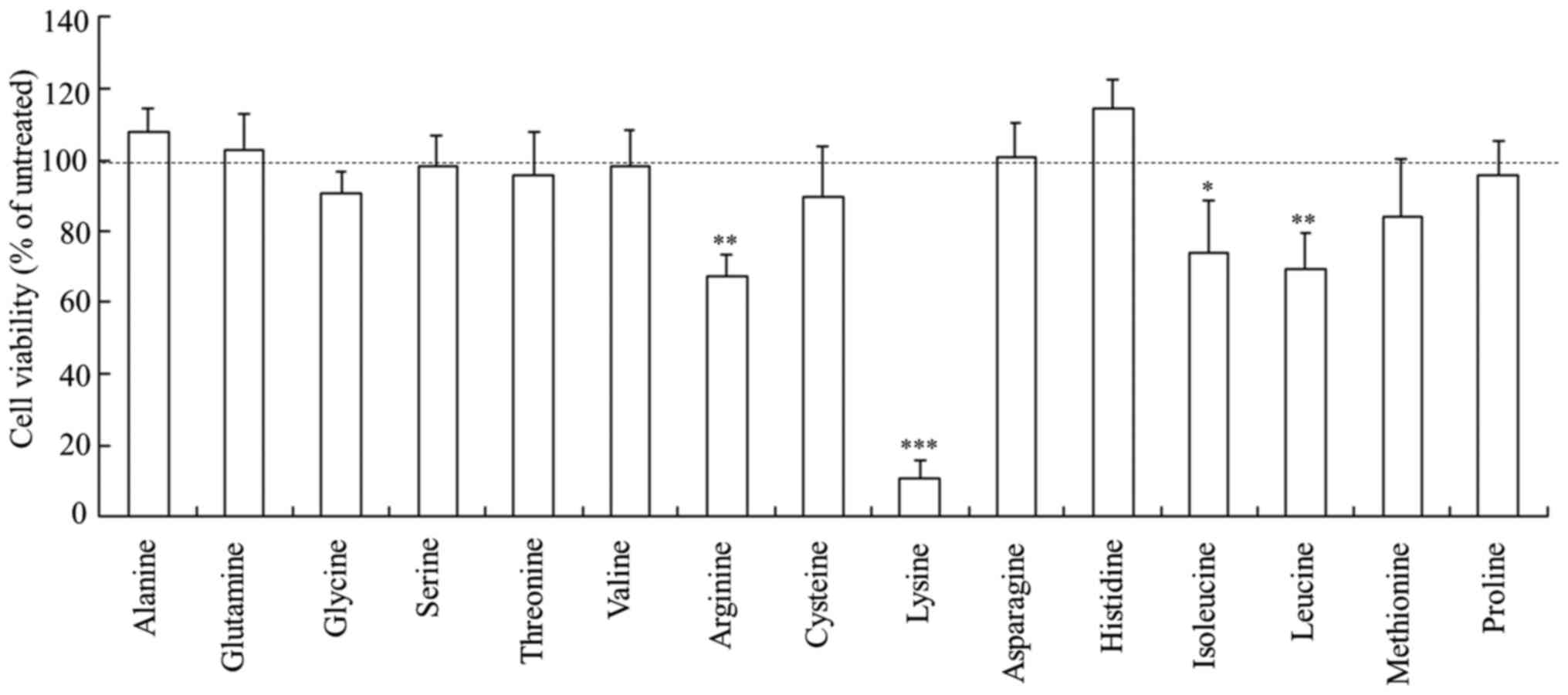
Cytotoxicity of Rev-transfection with
freeze-dried siRNA lipoplexes
Cell viability was measured 24 h after
Rev-transfection with freeze-dried siRNA lipoplexes into MCF-7-Luc
cells. The siRNA lipoplexes freeze-dried with arginine, isoleucine
and leucine showed slightly increased cytotoxicity (67-74% cell
viability), whereas those freeze-dried with lysine exhibited strong
cytotoxicity (11% cell viability) (Fig.
4). In contrast, the siRNA lipoplexes freeze-dried with the
other amino acids did not induce a significant cytotoxic
effect.
Characterization of freeze-dried siRNA
lipoplexes after reconstitution
To examine whether the amino acids used during
freeze-drying affected the size of siRNA lipoplexes after
rehydration, freeze-dried siRNA lipoplexes in the presence of 100
mM amino acid solutions were prepared, and the sizes of the
resulting siRNA lipoplexes after rehydration were measured. The
cationic liposomes were ~100 nm in size, and the siRNA lipoplexes
were ~180 nm (data not shown) before freeze-drying. After
freeze-drying and rehydration, the sizes of the siRNA lipoplexes
ranged from 190-2,900 nm, although the lipoplexes freeze-dried with
arginine, histidine, leucine and lysine were aggregated (Table I). The siRNA lipoplexes freeze-dried
with cysteine, glycine, isoleucine, methionine or valine had larger
sizes (800-2,900 nm). In contrast, siRNA lipoplexes freeze-dried
with alanine, asparagine, glutamine, proline, serine or threonine
were relatively smaller (~340, ~370, ~360, ~540, ~420 and ~190 nm,
respectively) with a monodisperse distribution (0.17-0.24 in PDI).
Amongst these, polar but uncharged hydrophilic amino acids
(asparagine, glutamine, serine and threonine) appeared effective
for production of stable siRNA lipoplexes in size after
freeze-drying (200-400 nm), indicating that amino acids that
possessed an -OH or -CONH2 moiety in the side chain may
interact with the polar head groups on the surface of the siRNA
lipoplexes, and prevent the aggregation of siRNA lipoplexes caused
by freeze-drying. From these results, the types of amino acids used
during freeze-drying affected the size of the siRNA lipoplexes
after rehydration.
 | Table IParticle size of small interfering RNA
lipoplexes after rehydration of freeze-dried lipoplexes. |
Table I
Particle size of small interfering RNA
lipoplexes after rehydration of freeze-dried lipoplexes.
| Amino acid used for
freeze-dryinga | Sizeb,c, nm | Polydispersity
indexc |
|---|
| Alanine | 344.1±90.0 | 0.18±0.02 |
| Arginine | Aggregation | N.D. |
| Asparagine | 366.3±39.2 | 0.20±0.02 |
| Cysteine | 840.6±15.6 | 0.32±0.01 |
| Glutamine | 361.5±35.4 | 0.17±0.01 |
| Glycine | 2,443.6±178.6 | 0.73±0.06 |
| Histidine | Aggregation | N.D. |
| Isoleucine | 1,153.0±8.7 | 0.36±0.01 |
| Leucine | Aggregation | N.D. |
| Lysine | Aggregation | N.D. |
| Methionine | 2,916.5±100.2 | 0.65±0.01 |
| Proline | 540.6±42.3 | 0.24±0.02 |
| Serine | 420.8±12.6 | 0.20±0.01 |
| Threonine | 192.6±4.5 | 0.24±0.02 |
| Valine | 2,045.5±565.1 | 0.60±0.16 |
Discussion
In Rev-transfection, freeze-drying of lipoplexes
containing Luc siRNA in the presence of 50-100 mM amino acids
strongly suppressed luciferase activity regardless of the amino
acid type. However, the presence of alanine, glutamine, glycine,
serine, threonine or valine in freeze-drying increased luciferase
activity in a concentration-dependent manner upon Rev-transfection
with lipoplexes of Cont siRNA. It has been reported that genes were
specifically upregulated in response to supra-physiological
concentrations of amino acids (22).
Increasing the concentration of amino acids from physiological to
supraphysiological levels stimulates protein synthesis (23). Therefore, it was speculated that the
increase in amino acid levels may activate the transcription and/or
translation of certain genes in cells, resulting in an increase in
luciferase activity. In contrast, a decrease in luciferase activity
in the cells was observed after Rev-transfection with lipoplexes of
Cont siRNA freeze-dried with arginine, cysteine or lysine. However,
it was not clear why they exhibited decreased luciferase activity.
Arginine and lysine are basic amino acids, and the presence of
positively charged amino acids may lead to the downregulation of
luciferase activity via cytotoxicity. With cysteine, it was
speculated that the thiol groups of cysteine residues may affect
luciferase activity, as cysteine is a potent nucleophile and is
often linked to another cysteine to form a covalent disulfide bond.
Furthermore, the presence of asparagine, histidine, isoleucine,
leucine, methionine or proline in freeze-drying did not
significantly affect the luciferase activity after Rev-transfection
with lipoplexes of Cont siRNA, indicating that these amino acids
may be suitable for freeze-drying siRNA lipoplexes. Amongst the
amino acids tested in the present study, asparagine was suitable
for Rev-transfection since siRNA lipoplexes freeze-dried with
asparagine at concentrations >100 mM exhibited specific gene
suppression, produced large cakes after freeze-drying and remained
relatively small in size after rehydration (Table I). However, it is not clear why
asparagine is an effective cryoprotectant for the freeze-drying of
siRNA lipoplexes. In our previous study, it was shown that
Rev-transfection with siRNA lipoplexes freeze-dried in a 25 mM
disaccharide or trisaccharide solution could induce efficient gene
knockdown, and that the presence of 100 mM saccharide during
freeze-drying did not substantially increase the size of the siRNA
lipoplexes (~200 nm) (7,8). Based on these findings, saccharides
might be more suitable as cryoprotectants for siRNA lipoplexes than
amino acids. Forney-Stevens et al (24) demonstrated that 15 amino acids can
significantly improve the storage stability of sucrose-based
protein formulations after freeze-drying. Therefore, a combination
of saccharides and amino acids may be an effective cryoprotectant
for the freeze-drying of siRNA lipoplexes. However, further studies
are required to investigate the optimal combination of saccharides
and amino acids as a cryoprotectant for Rev-transfection.
In conclusion, in the present study, the effect of
amino acids in the freeze-drying of siRNA lipoplexes on gene
knockdown by Rev-transfection was assessed, and the results showed
that the types of amino acids affected gene knockdown activity and
lipoplex size after rehydration. Amongst the amino acids tested,
the presence of asparagine in the freeze-drying of siRNA lipoplexes
showed strong gene knockdown activity without a notable cytotoxic
effect and had satisfactory particle sizes after rehydration. These
findings provide valuable information regarding amino acids as
cryoprotectants for Rev-transfection using freeze-dried siRNA
lipoplexes for the efficient delivery of siRNA into cells.
Supplementary Material
Appearance of cakes in 12-well plates
after freeze-drying siRNA lipoplexes in solutions containing
various concentrations of amino acids. siRNA lipoplexes with 50
pmol siRNA were diluted in 125 μl 10, 25, 50, 100 or 150 mM amino
acid solutions and then transferred to 12-well plates, followed by
freeze-drying. siRNA, small interfering RNA; lipoplex, liposome
complex.
Acknowledgements
We would like to thank Mr Ayato Kubota, Ms Natsuki
Inagaki and Mr Shun Fujishita (Department of Molecular
Pharmaceutics, Hoshi University) for assistance with the
experimental work (in vitro gene-knockdown effect).
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
YH conceived and designed the study. Experiments
were performed by MT. YH and MT wrote the manuscript. Both authors
have read and approved the final manuscript. MT and YH confirm the
authenticity of all the raw data
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Wilson RC and Doudna JA: Molecular
mechanisms of RNA interference. Annu Rev Biophys. 42:217–239.
2013.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Zhang MM, Bahal R, Rasmussen TP, Manautou
JE and Zhong XB: The growth of siRNA-based therapeutics: Updated
clinical studies. Biochem Pharmacol. 189(114432)2021.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Thapa B, Remant KC and Uludağ H: siRNA
library screening to identify complementary therapeutic pairs in
triple-negative breast cancer cells. Methods Mol Biol. 1974:1–19.
2019.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Erfle H, Neumann B, Liebel U, Rogers P,
Held M, Walter T, Ellenberg J and Pepperkok R: Reverse transfection
on cell arrays for high content screening microscopy. Nat Protoc.
2:392–399. 2007.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Zhang S, Zhi D and Huang L: Lipid-based
vectors for siRNA delivery. J Drug Target. 20:724–735.
2012.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Yadava P, Gibbs M, Castro C and Hughes JA:
Effect of lyophilization and freeze-thawing on the stability of
siRNA-liposome complexes. AAPS PharmSciTech. 9:335–341.
2008.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Hattori Y, Hu S and Onishi H: Effects of
cationic lipids in cationic liposomes and disaccharides in the
freeze-drying of siRNA lipoplexes on gene silencing in cells by
reverse transfection. J Liposome Res. 30:235–245. 2020.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Tang M, Hu S and Hattori Y: Effect of pre
freezing and saccharide types in freeze drying of siRNA lipoplexes
on gene silencing effects in the cells by reverse transfection. Mol
Med Rep. 22:3233–3244. 2020.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Franzé S, Selmin F, Samaritani E,
Minghetti P and Cilurzo F: Lyophilization of liposomal
formulations: Still necessary, still challenging. Pharmaceutics.
10(10)2018.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Abdelwahed W, Degobert G, Stainmesse S and
Fessi H: Freeze-drying of nanoparticles: Formulation, process and
storage considerations. Adv Drug Deliv Rev. 58:1688–1713.
2006.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Ball RL, Bajaj P and Whitehead KA:
Achieving long-term stability of lipid nanoparticles: Examining the
effect of pH, temperature, and lyophilization. Int J Nanomedicine.
12:305–315. 2016.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Kundu AK, Chandra PK, Hazari S, Ledet G,
Pramar YV, Dash S and Mandal TK: Stability of lyophilized siRNA
nanosome formulations. Int J Pharm. 423:525–534. 2012.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Andersen MØ, Howard KA, Paludan SR,
Besenbacher F and Kjems J: Delivery of siRNA from lyophilized
polymeric surfaces. Biomaterials. 29:506–512. 2008.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Mohammed AR, Coombes AG and Perrie Y:
Amino acids as cryoprotectants for liposomal delivery systems. Eur
J Pharm Sci. 30:406–413. 2007.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Crowe JH and Crowe LM: Factors affecting
the stability of dry liposomes. Biochim Biophys Acta. 939:327–334.
1988.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Koster KL, Webb MS, Bryant G and Lynch DV:
Interactions between soluble sugars and POPC
(1-palmitoyl-2-oleoylphosphatidylcholine) during dehydration:
Vitrification of sugars alters the phase behavior of the
phospholipid. Biochim Biophys Acta. 1193:143–150. 1994.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Stärtzel P: Arginine as an excipient for
protein freeze-drying: A mini review. J Pharm Sci. 107:960–967.
2018.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Paik SH, Kim YJ, Han SK, Kim JM, Huh JW
and Park YI: Mixture of three amino acids as stabilizers replacing
albumin in lyophilization of new third generation recombinant
factor VIII GreenGene F. Biotechnol Prog. 28:1517–1525.
2012.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Hattori Y, Nakamura T, Ohno H, Fujii N and
Maitani Y: siRNA delivery into tumor cells by lipid-based
nanoparticles composed of hydroxyethylated cholesteryl triamine.
Int J Pharm. 443:221–229. 2013.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Hattori Y, Hara E, Shingu Y, Minamiguchi
D, Nakamura A, Arai S, Ohno H, Kawano K, Fujii N and Yonemochi E:
siRNA delivery into tumor cells by cationic cholesterol
derivative-based nanoparticles and liposomes. Biol Pharm Bull.
38:30–38. 2015.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Hattori Y, Nakamura M, Takeuchi N, Tamaki
K, Ozaki K and Onishi H: Effect of cationic lipid type in pegylated
liposomes on siRNA delivery following the intravenous injection of
siRNA lipoplexes. World Acad Sci. 1:74–85. 2019.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Varga J, Li L, Mauviel A, Jeffrey J and
Jimenez SA: L-Tryptophan in supraphysiologic concentrations
stimulates collagenase gene expression in human skin fibroblasts.
Lab Invest. 70:183–191. 1994.PubMed/NCBI
|
|
23
|
Vary TC, Jefferson LS and Kimball SR:
Amino acid-induced stimulation of translation initiation in rat
skeletal muscle. Am J Physiol. 277:E1077–E1086. 1999.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Forney-Stevens KM, Bogner RH and Pikal MJ:
Addition of amino acids to further stabilize lyophilized
sucrose-based protein formulations: I. Screening of 15 amino acids
in two model proteins. J Pharm Sci. 105:697–704. 2016.PubMed/NCBI View Article : Google Scholar
|