Introduction
Percutaneous aspiration with sclerotherapy is a
minimally invasive, simple, safe, inexpensive and reasonably
effective treatment for cystic diseases, such as simple hepatic,
renal, splenic, pancreatic and ovarian cysts, as well as for
lymphoceles (1-4).
Bleomycin has been used successfully for management of cysts
(3,4); it is an antibiotic chemotherapeutic
agent derived from Streptomyces verticillus, which was first
used as a sclerosant for malignant pleural effusions in
1976(5). Intralesional bleomycin
injection has been used successfully for the treatment of cystic
diseases, including lymphoceles since 1977(6), cystic craniopharyngiomas since
1985(7), bronchogenic cysts since
1992(8), simple renal cysts since
2012(9) and simple hepatic cysts
since 2015(10). Advantages of
percutaneous bleomycin sclerotherapy for treatment of cystic
diseases are minimal inflammatory reactions, modest cost as only a
single treatment session is required, good tolerance as it causes
little pain and no pulmonary toxicity when performed with the
proper precautions (3,9-11).
The sclerosing mechanisms of intracyst bleomycin
injection are not understood. For example, they may be similar to
those of pulmonary fibrosis induced by intratracheal bleomycin
instillation (12); however, that
inference has not been confirmed by histopathological examination,
and there are no animal models for evaluation of percutaneous
sclerotherapy of hepatic cystic disease. Rodent models of
drug-induced polycystic diseases (including liver, kidney and
ovary) have been widely used, but they have limitations. For
example, their pathogenesis is different from that of simple cysts,
their modeling time is long and they have multiple small cysts
(13-15).
Thus, such animal models are not suitable for studying percutaneous
sclerotherapy of cysts. The animal model of simple ovarian cysts,
established by unilateral total salpingectomy, has been used
successfully to evaluate the effectiveness and safety of aspiration
sclerotherapy, but only 70% of experimental animals formed
macroscopic ovarian cysts of ~10 mm (16). Therefore, in the present study, a new
animal model of a simple hepatic cyst was established, and the
chronological histopathological changes after intracyst bleomycin
injection was assessed.
Materials and methods
Ethical considerations
The present study was approved by the Biomedical
Ethics Committee of Animal Experiments of Guangdong Medical
Laboratory Animal Center (Guangdong, China; approval no.
B201610-5). An Accreditation Certificate from the China National
Accreditation Service for Conformity Assessment has been granted to
this facility. All experimental procedures were performed in
accordance with the Guide for the Care and Use of Laboratory
Animals (National Institutes of Health) (17). Every effort was made to minimize
animal suffering and to use only the number of animals necessary
for the acquisition of reliable data.
Animal model
A total of 32 New Zealand rabbits of either sex (11
males, 21 females), weighing 2.6 kg (range, 2-3 kg) at the
beginning of the study, were obtained from Guangdong Medical
Laboratory Animal Center, China (order no. 44411600002998). Rabbits
were housed in separate cages in the animal experimental center,
with an ambient temperature of 18-24˚C and a 12 h light/dark cycle.
Rabbits were provided ad libitum access to food and water
during the experimental period. Rabbits were anesthetized by
injection of 30 mg/kg sodium pentobarbital in the ear vein
(Shanghai Chemical Reagent Company, China National Pharmaceutical
Group Corporation) and intramuscular injection of 5 mg/kg xylazine
hydrochloride (Shanghai Chemical Reagent Company, China National
Pharmaceutical Group Corporation) after 12 h of fasting. By means
of surgical laparotomy, the cholecystic duct was ligated with a 3/0
surgical suture, whereas cystic vessels were retained to ensure
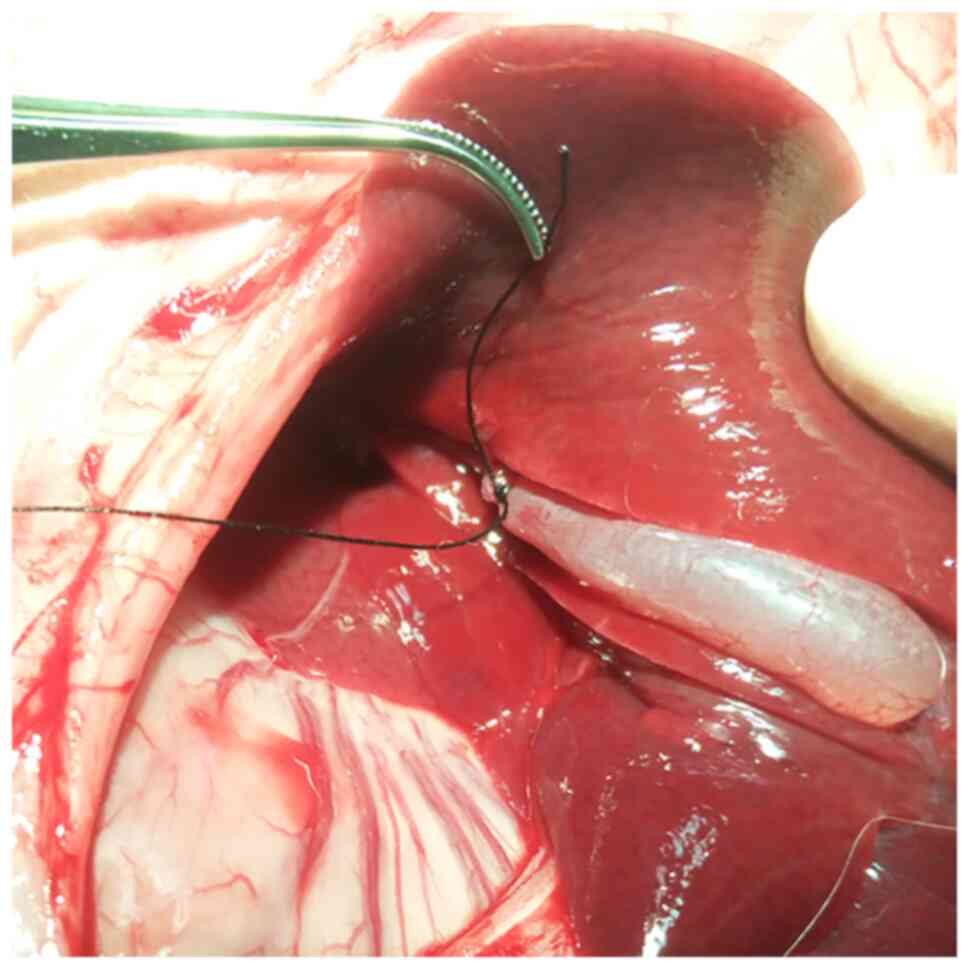
good blood supply and to avoid ischemic necrosis (Fig. 1). Direct gallbladder puncture was
performed as far as possible from gallbladder vessels using a
22-gauge butterfly needle. Bile was aspirated, and the gallbladder
was irrigated 3-4 times with saline until clear. The surgical time
ran between 45 and 60 min. Anesthesia with a standard dose was well
tolerated.
Experimental design
The rabbits were randomly divided into a control
group and seven experimental groups according to different time
points after bleomycin sclerotherapy (1, 7, 14, 28, 42, 56 and 84
days; n=4 per group). In animals in the experimental group, after
gallbladder content was aspirated as thoroughly as possible, 2 mg
dissolved in 1 ml saline bleomycin hydrochloride (Takasaki Plant;
Nippon Kayaku Co., Ltd.) was injected and allowed to remain in the
evacuated gallbladder until the experiment ended. For animals in
the control group, 1 ml normal saline was injected, which remained
in the evacuated gallbladder. Rabbits received penicillin 10
million units/kg per day intramuscularly for three consecutive days
after completion of the procedure, and were sacrificed at the end
of the experimental period. At 1, 7, 14, 28, 56 and 84 days after
the bleomycin injection, animals in experimental groups were
euthanized by intravenous injection of 150 mg/kg potassium chloride
(Shanghai Chemical Reagent Company; China National Pharmaceutical
Group Corporation) under general anesthesia induced by intravenous
injection of 30 mg/kg pentobarbital sodium via the marginal ear
vein; animals in the control group were euthanized at day 14. The
gallbladders and the surrounding liver parenchyma were resected and
evaluated histologically and immunohistochemically.
Histopathological processing
The cholecystic tissue specimens were fixed in 4%
paraformaldehyde for 24 h at room temperature, trimmed
appropriately, dehydrated in graded series of alcohol solutions and
embedded in paraffin according to standard procedures (18). Paraffin sections of 4 µm thickness
were cut and mounted on glass microscope slides.
Hematoxylin and eosin (H&E) staining was
performed using a H&E Staining kit (cat. no. G1005; Wuhan
Servicebio Technology Co., Ltd.), as described previously (14). Masson's trichrome staining was
performed using a Masson's Trichrome Stain kit (cat. no. G1006;
Wuhan Servicebio Technology Co., Ltd.) as described previously
(19).
Infiltration by T-lymphocytes, B-lymphocytes and
macrophages into the tissue was detected immunohistochemically
using a panel of monoclonal antibodies against CD20, CD43 and
CD68(20). Immunohistochemical
staining was performed using the Streptavidin-Biotin-Complex
method, according to the Sixth Edition of Dako's Educational
Guidebook to Immunohistochemical Staining Methods (21). Sections were deparaffinized in xylene
(2x10 min) and rehydrated using an alcohol gradient: 100% ethanol
(2x10 min), 95% ethanol (1x8 min), 80% ethanol (1x5 min) and 70%
ethanol (1x5 min), followed double-distilled H2O (1x10 min).
Nonspecific background staining was blocked using Background Sniper
(cat. no. BS966M; Biocare Medical, LLC), 3% BSA (Sigma-Aldrich;
Merck KGaA) in PBS, and 5% normal goat-serum solution (cat. no.
SP-9000; OriGene Technologies, Inc.) for 30 min at room
temperature. Slides were incubated overnight at 4˚C for 1 h at room
temperature with primary antibodies in 3% BSA-PBS. Primary
antibodies used were anti-CD20 (1:3,000; cat. no. GB11281; Beijing
Solarbio Science & Technology Co., Ltd.), anti-CD43 (1:1,500;
cat. no. GB11066; Beijing Solarbio Science & Technology Co.,
Ltd.) and anti-CD68 (1:1,500; cat. no. GB11067; Beijing Solarbio
Science & Technology Co., Ltd.). After washing five times with
0.05% Brij-35 (Abmole Bioscience, Inc.) in PBS for 1 min, slides
were incubated with horseradish peroxidase-conjugated goat
anti-rabbit/mouse IgG secondary antibody at 1:1,000 dilution (cat.
no. K5007; Dako; Agilent Technologies GmbH) for 1 h at room
temperature. The criterion for positive staining with the CD20,
CD48 and CD68 antibodies was the presence of pale brown particles
in cell nuclei.
Microscope slides were examined by a pathologist
with >15 years of working experience, and who was blinded to the
experimental conditions. At a magnification of x200, two sections
in five random fields of view in each region of interest were
selected for further analysis. Images were taken using a Nikon
Eclipse Ti inverted fluorescence microscope (Nikon Corporation)
equipped with the Nikon NIS-Elements imaging analysis software
version 4.60.00. The average positive stained area percentage
(APSAP) of collagen fiber revealed by Masson's trichrome staining
was quantitatively analyzed using Image-Pro Plus version 7.0 (Media
Cybernetics, Inc.) (22). The
immunohistochemical images with integrated optical density (IOD) of
positive expression, which reflected the dynamic changes and
distribution characteristics of T cells, B cells and macrophages in
gallbladder tissues, were quantitatively analyzed using Image-Pro
Plus (23).
Statistical analysis
Data are presented as the mean ± standard error of
the mean. Statistical comparisons of the intensities of
immunohistochemical staining and Masson's trichrome staining were
performed using a one-way ANOVA followed by a post-hoc Tukey's
test. All statistical analyses were performed using SPSS version 20
(IBM, Corp.) Graphs were generated using GraphPad Prism version 6
(GraphPad Software, Inc.). P<0.05 was considered to indicate a
statistically significant difference.
Results
Histopathological evaluation of the
rabbit model of simple hepatic cysts
All rabbits in the control group survived after the
simple hepatic cyst model was established, with normal appetite,
activity and excretion. A total of 14 days after intracyst saline
injection, autopsies revealed an intact structure and clear
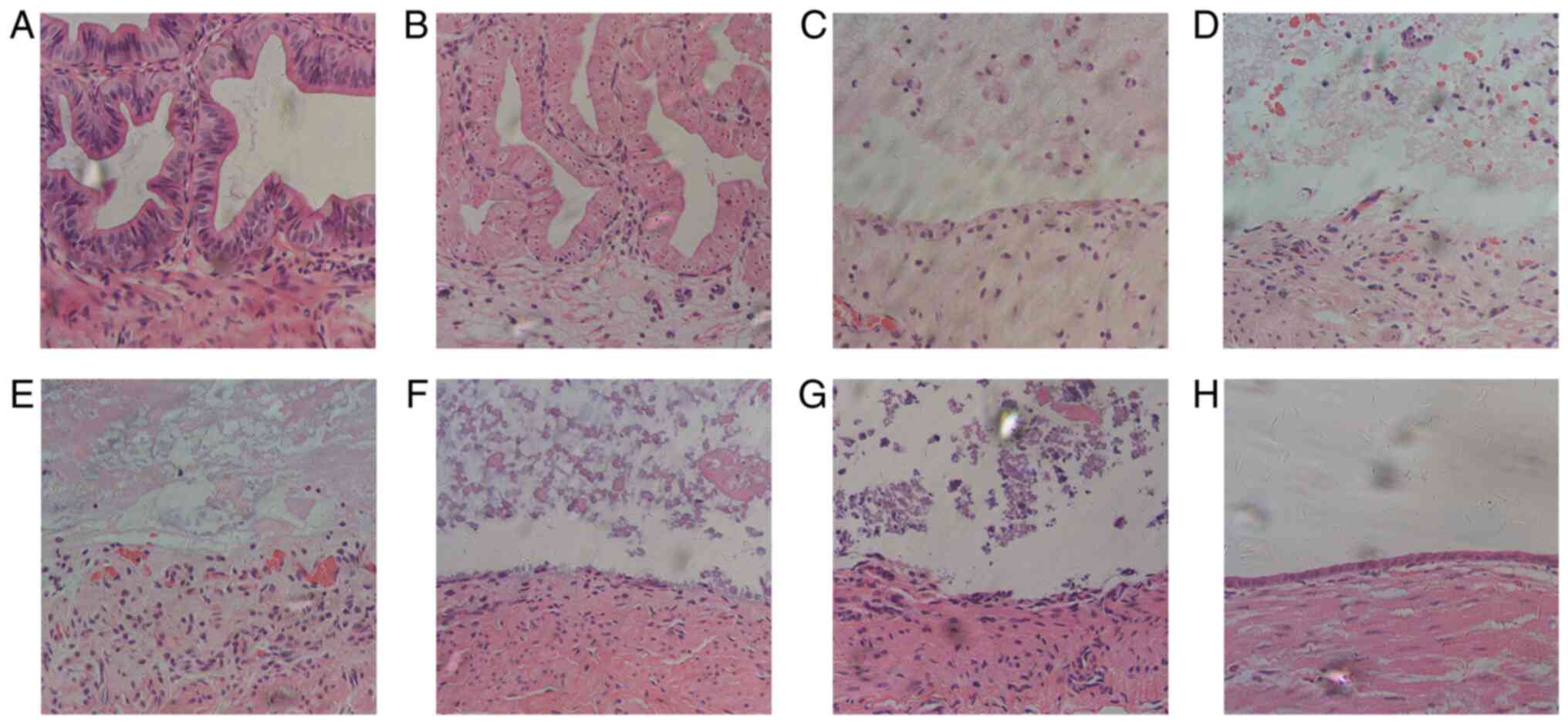
boundary between the gallbladder and hepatic capsule. H&E
staining demonstrated that epithelial cells of the gallbladder were
arranged neatly with prominent nucleoli and abundant cytoplasm, and
the mucosa plica were flourishing without morphological changes
(Fig. 2A). Immunohistochemical
staining of CD20, CD43 and CD68 demonstrated no inflammatory-cell
infiltration in the cyst wall, whether by B-lymphocytes 2 (Fig. 3A), T-lymphocytes (Fig. 4A) or macrophages (Fig. 5A). Masson staining revealed no
collagenous fibers in the mucous layer, and the basal side of
epithelial cells was fixed tightly by a small amount of fibrin;
similarly, collagen fibers in the lamina propria mucosae and
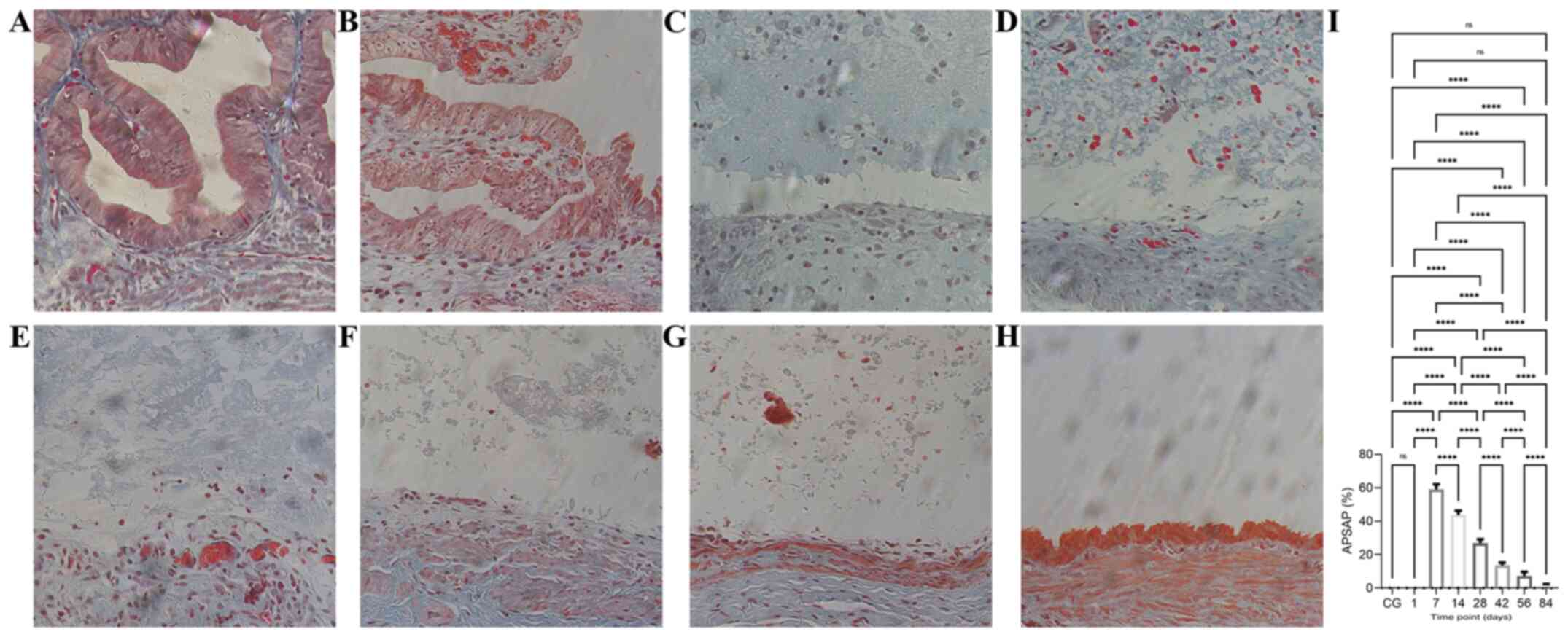
submucous layer were lined up tightly (Fig. 6A).
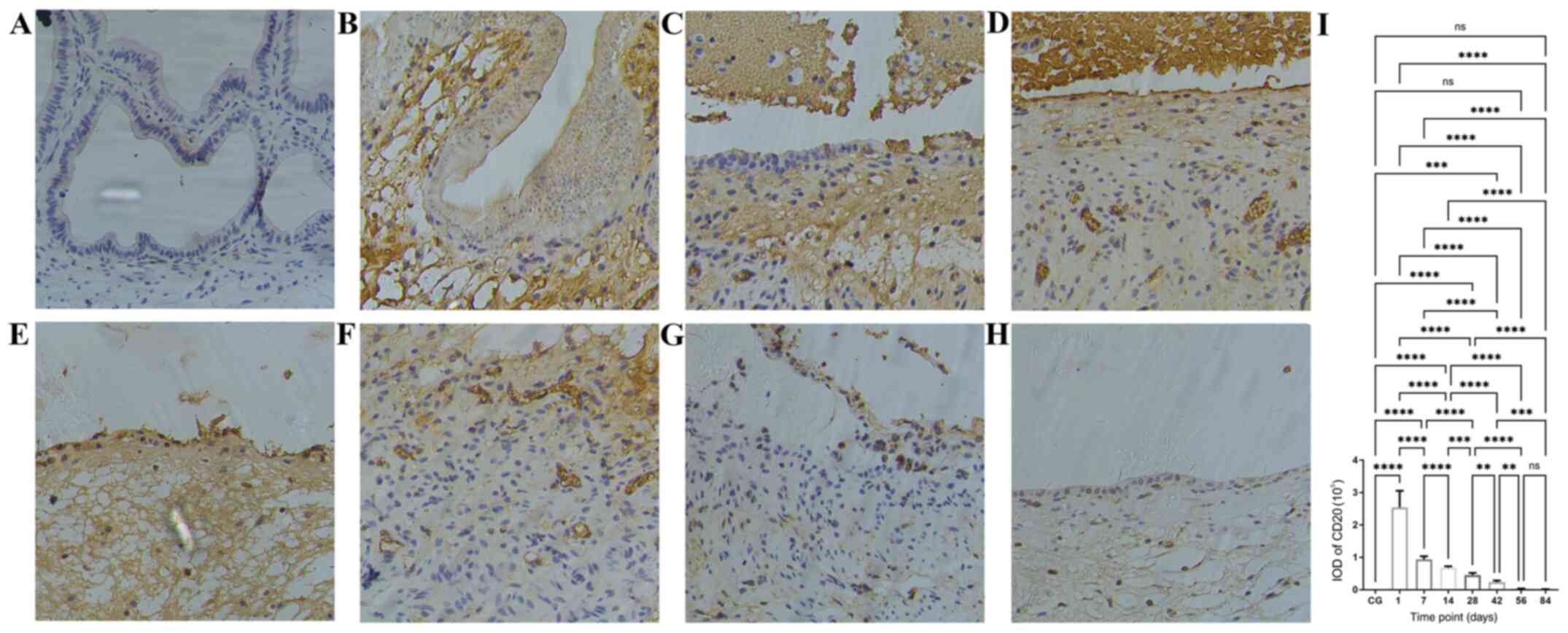 | Figure 3Time course of B-lymphocyte
infiltration following bleomycin sclerotherapy in the rabbit model
of simple hepatic cysts based on anti-CD20 antibody
immunohistochemical staining. (A) In the control group, there were
no B-lymphocytes in the cyst wall. (B) Following intracyst
bleomycin injection, after 1 day, B-lymphocytes had extensively
infiltrated the submucosal layer and lamina propria. (C and D) At 7
and 14 days after intracyst bleomycin injection, B-lymphocytes
extensively infiltrated the submucosal layer, lamina propria, and
the destroyed mucosal areas. (E-H) At 28 and 84 days after
intracyst bleomycin injection, B-lymphocyte infiltration decreased
but persisted throughout the experiment. (I) Quantitative analysis
of IODs of anti-CD20 immunohistochemical staining at the different
time-points. Detailed data and statistics to produce this graph are
listed in Table SI. Magnification,
x200. **P<0.01, ***P<0.001,
****P<0.0001. IOD, integrated optical density; CG,
control group. |
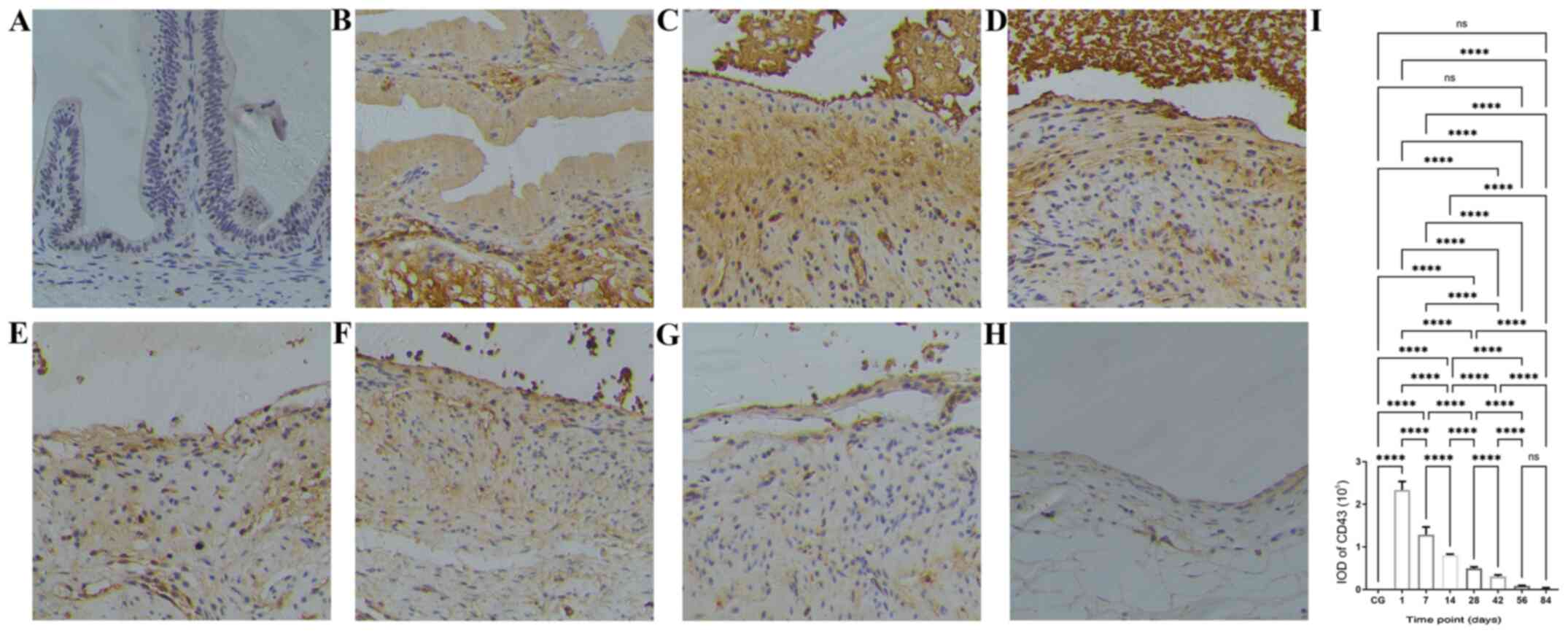
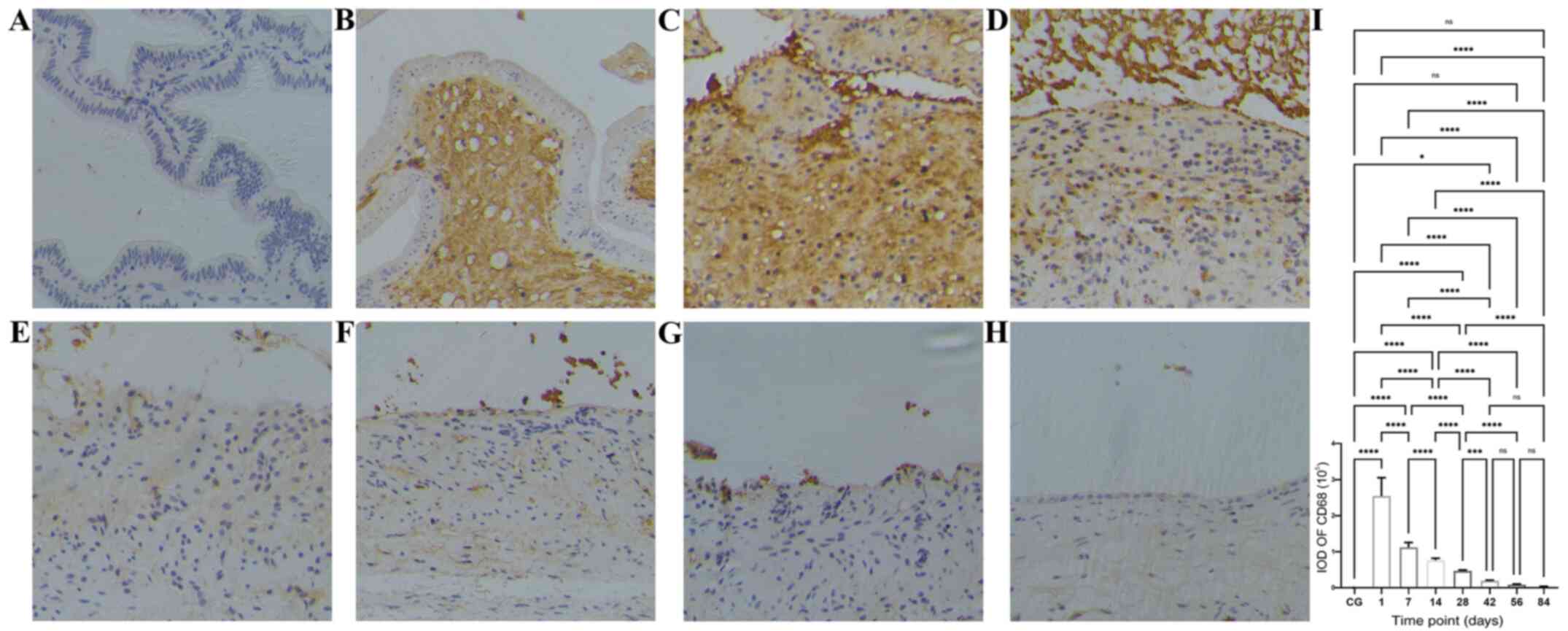 | Figure 5Time course of macrophage
infiltration after bleomycin sclerotherapy in the rabbit model of
simple hepatic cysts based on anti-CD68 antibody
immunohistochemical staining. (A) Control group, no macrophages
were present in the gallbladder wall. (B) At 1 day after intracyst
bleomycin injection, macrophages extensively infiltrated the
submucosal layer and lamina propria. (C and D) At 7 and 14 days
after intracyst bleomycin injection, macrophages extensively
infiltrated the submucosal layer, lamina propria and the destroyed
mucosal areas. (E-H) At 28 and 84 days after intracyst bleomycin
injection, macrophages gradually decreased, but exhibited a
persistent presence throughout the duration of the experiment. (I)
Quantitative analysis of IODs of anti-CD68 immunohistochemical
staining at the different time-points. Detailed data and statistics
to produce this graph are listed in Table SIII. Magnification, x200.
*P<0.05, ***P<0.001,
****P<0.0001. IOD, integrated optical density; CG,
control group. |
Time course of cyst epithelial changes
after bleomycin sclerotherapy in the rabbit model of simple hepatic
cysts
All 28 rabbits in the experimental group survived
the entire duration of the experimental procedure, with normal
behavior, mental state and body weight, and no complications were
observed.
The first day after bleomycin sclerotherapy, the
epithelial cells were swollen and had balloon-like degeneration;
the nuclei were pyknotic and deformed, appearing as small circles.
The lamina propria was edematous, with a few neutrophils and
fibroblasts present (Fig. 2B).
A total of 7 days after bleomycin sclerotherapy, the
mucosal structures were destroyed and had disappeared, and the
lamina propria was detached by the mixed cellular infiltrate of
fibroblasts, collagen fibers and inflammatory cells (Fig. 2C).
From days 14-84 following bleomycin sclerotherapy,
the lamina propria edema decreased gradually and disappeared, and
fibrosis and inflammatory response continually decreased (Fig. 2D-H). At 56 days, the mucous layer
contained irregularly flattened epithelial cells (Fig. 2G), which were replaced almost
completely at 84 days (Fig. 2H).
Time course of inflammatory cell
infiltration after bleomycin sclerotherapy in the rabbit model of
simple hepatic cysts
The time course in the IODs of inflammatory cells in
the cyst wall at various time points after intracyst bleomycin
injection is shown in Fig. 3,
Fig. 4 and Fig. 5 and Table
SI, Table SII and Table SIII. The number of B cells
(CD20-positive, Fig. 3I), T cells
(CD43-positive, Fig. 4I) and
macrophages (CD68-positive, Fig. 5I)
peaked at the first day after intracyst bleomycin injection, and
then gradually declined, with each day's values lower than the
preceding day's values (P<0.01). IODs at 84 days after bleomycin
were still significantly higher than in the control group
(P<0.01).
One day after intracyst bleomycin injection,
inflammatory cells (B-lymphocytes, T-lymphocytes and macrophages)
were extensively present in the submucosal layer and lamina propria
(Fig. 3B, 4B and 5B).
The IODs of stained inflammatory cells were significantly higher
after 1 day than at other time-points (Fig. 3I, 4I
and 5I; P<0.01). A total of 7 and
14 days after bleomycin injection, inflammatory-cell infiltration
was present in the submucosal layer and lamina propria as well as
in the destroyed mucosal areas (Figs.
3C and D, 4C-D and 5C-D).
From 28 to 84 days after intracyst bleomycin
injection, inflammatory cell infiltration in the submucosal layer
decreased gradually, but was present throughout the experiment
(Figs. 3E-H, 4E-H and 5E-H).
Time course of collagen proliferation
after bleomycin sclerotherapy in the rabbit model of simple hepatic
cysts
The time course of the APSAP of collagen fibers
stained with Masson's trichrome in the cyst wall at the various
time points after intracyst bleomycin injection is given in
Fig. 6 and Table SIV. Maximum staining was recorded on
day 7, which gradually decreased and almost disappeared by day 84
(Fig. 6I, P<0.01). At 1 day after
intracyst bleomycin injection, a few new collagen fibers were
present in the submucosal layer (Fig.
6B). At 7 days, abundant collagen fibers were evident amidst
destroyed mucosal areas (Fig. 6C),
and the APSAP was significantly increased compared with that of day
1 (Fig. 6I, P<0.01). From days
14-84 after intracyst bleomycin injection, staining of collagen
fibers decreased gradually and almost disappeared (Fig. 6D-H).
Discussion
The results of the present study indicate that the
time course of histopathological changes in the rabbit gallbladder
as a model for studying bleomycin sclerotherapy of simple hepatic
cysts can be divided into four phases: i) The epithelial
destruction phase, occurring within 14 days; ii) the inflammatory
infiltration phase, peaking the first day after bleomycin
sclerotherapy and persisting throughout the experiment; iii) the
collagenous proliferation phase, demonstrated at day 7 after
bleomycin sclerotherapy, then gradually decreasing and eventually
disappearing; and iv) the epithelial regeneration phase of a single
layer of flat cells from 56 days after bleomycin sclerotherapy.
To the best of our knowledge, the animal model of a
simple hepatic cyst described in the present study is the only
available study on creating such a model in the English literature.
Simple hepatic cysts consist histologically of a single layer of
cuboidal or flattened epithelium, which is derived from
developmental anomalies of biliary epithelium, and arise from
misplaced or detached biliary anlage (24). These features are the theoretical
basis for the animal model of this study. Furthermore, the
anatomical site of the rabbit gallbladder is convenient for making
surgical procedures and is independent of the digestive system,
such that its function and survival are not affected after
operation. The practical basis of the rabbit gallbladder model is
derived from animal experiments of chemical ablation of the
gallbladder, with various sclerosants that emerged from the late
1980's to the early 2000's (25-27).
The results of the present study suggest that the animal model of a
simple hepatic cyst established by ligating the cholecystic duct
and preserving cholecystic vessels is more reliable, operable and
repeatable than other reported models of cystic diseases.
Destruction of the epithelial lining of the inner
surface of the cyst wall to prevent intracystic fluid secretion is
the goal of percutaneous aspiration and sclerotherapy for cystic
diseases (1-4).
Studies using experimental animal models of bleomycin-induced
pulmonary fibrosis have confirmed that the destruction of the
alveolar epithelium is an initiating event in the pathogenesis of
lung fibrosis and in acute lung injury (28). Bleomycin, in the presence of iron and
oxygen, can generate reactive oxygen species and damage DNA by
causing single- and double-strand breaks, a mechanism thought to be
the basis for its antitumor activity and presumed mechanism of
cytotoxicity and tissue injury (29). However, the doses used in the rodent
model of fibrosis do not appear to cause significant DNA damage
(12), thus making it unclear if
this is the basis for of lung fibrosis induced by bleomycin
(12,28). A bleomycin concentration of 2 mg/ml
used in the present study is based on the experimental model of
bleomycin-induced lung and skin fibrosis (29,30). The
total amount of bleomycin saline solution injected into the cyst
cavity (1 ml) is sufficient to fill the rabbit gallbladder, based
on the amount of cyst fluid aspirated initially. The epithelial
destruction resulting from this bleomycin dose appeared slightly
earlier than that of bleomycin-induced lung injury in animals; that
is, epithelial degeneration was seen on the first day, and the
mucosa disappeared within 7 days, whereas alveolar epithelial cell
injury and death occurred 7-14 days after intratracheal bleomycin
instillation (28). Whether this
difference is caused by the organ specificity of bleomycin
hydrolase, which is necessary to inactivate the bleomycin (12,28), is
unknown.
Bleomycin-induced inflammation in animal models of
pulmonary fibrosis is initiated in response to epithelial-cell
damage and release of pro-inflammatory mediators, which results in
enhanced vascular permeability for the recruitment of leukocytes
(28,31). This process also results in the
recruitment and activation of immune cells, including macrophages
and lymphocytes, that produce cytokines and chemokines (32). In the present study, infiltration of
the gallbladder wall with T-lymphocytes, B-lymphocytes and
macrophages suggested that similar inflammatory activities
occurred. In clinical practice, several minor complications after
percutaneous bleomycin sclerotherapy for treatment of cystic
diseases can be encountered, including transient low-grade fever,
local skin ulceration, local swelling and redness at the site of
injection (3,7,9,33), which may be the result of
bleomycin-induced inflammation. This prolonged inflammatory
response may explain the clinical phenomenon in which cysts refill
partially in the initial stages following bleomycin sclerotherapy
and regress gradually thereafter, and even disappear completely
(9,10). Therefore, the optimal follow-up time
for assessing the therapeutic outcome should be after resolution of
inflammation, and this should be no less than 12 weeks (84 days)
after intracyst bleomycin injection.
Tissue fibrosis followed by cyst shrinking is the
treatment outcome of percutaneous aspiration and sclerotherapy for
cystic diseases (1-4).
Bleomycin-induced pulmonary fibrosis is characterized by the
remodeling of fibrotic tissue and collagen deposition, which
primarily results from proliferation of aberrant fibroblasts and
trans-differentiation to myofibroblasts (28,31).
This process results from alveolar epithelial injury and the
recruitment and activation of immune and/or inflammatory cells, as
well as the production and release of cytokines and chemokines.
These inflammatory cells and substances induce the activation of
fibroblasts and myofibroblasts to result in the activation of
transforming growth factor-β1 and the deposition of an excessively
stiff and biochemically abnormal extracellular matrix (32). In intratracheal bleomycin models of
pulmonary fibrosis, fibrosis development occurs from day 14 onward,
and worsens until disease resolution or animal sacrifice (28). The results of the present study
showed that the collagen fibers were abundant at day 7, then
gradually decreased and eventually disappeared from 14 to 84 days.
The different types of inflammatory cells contribute to the
activation of fibroblasts and myofibroblasts and the deposition of
collagen, and activated T-lymphocytes, B-lymphocytes and
macrophages likely collaborate to orchestrate post-injury tissue
remodeling and fibrosis (32). The
present study also revealed that collagen fibrous proliferation is
preceded by inflammatory cell infiltration, with the latter lasting
longer throughout the experiment.
The late epithelial regeneration by a single layer
of flat cells after chemical ablation of the gallbladder is like
that reported in animal experiments with different sclerosants
(25-27).
In animal models of bleomycin-induced pulmonary fibrosis, alveolar
epithelial regeneration is hypothesized to an important repair
process following lung injury, which is regulated by various
cellular and physiological mechanisms (34). In the present study, the regenerating
epithelium consisted of a single layer of flat cells that were
different from the original columnar epithelium of the gallbladder,
and thus did not possess secretory function. Therefore, it is
reasonable that epithelial regeneration after bleomycin
sclerotherapy is the repair response to bleomycin-induced
epithelial injury.
The present study has some limitations. First, it
had few early observation time points, so early or ultra-early
histopathological changes, such as the epithelial injury and
inflammatory processes, may have been undetected. Second, the
molecular and cellular mechanisms of bleomycin sclerotherapy for
cystic diseases were not investigated; thus, the mechanisms of
bleomycin-induced pulmonary and dermal fibrosis may be different
from those of bleomycin sclerotherapy for cystic diseases. Finally,
the gallbladder is a hypervascular structure, particularly when
compared with simple hepatic cysts, with thick muscular layers
beneath the epithelium, which may result in substantial differences
in the complex inflammatory processes.
In conclusion, the present study described a rabbit
model of simple hepatic cyst, established by ligating the
cholecystic duct and preserving cholecystic vessels. The time
course of histopathological changes occurring after bleomycin
sclerotherapy consisted of an epithelial destruction phase,
inflammatory infiltration phase, collagenous proliferation phase
and epithelial regeneration phase. Information derived from this
model may help in understanding the cellular mechanisms involved in
the sclerosis induced by intracyst bleomycin injection of hepatic
cysts and the time course of their resolution. Furthermore, these
findings suggest that the pathogenesis of complications after
percutaneous bleomycin sclerotherapy for treatment of cystic
diseases is related to the bleomycin-induced inflammatory reaction.
The optimal follow-up time of the therapeutic outcome assessment
should be after inflammation has been resolved.
Supplementary Material
Detailed statistical results of
multiple comparisons for IODs of anti-CD20 immunohistochemical
staining at different time-points following bleomycin sclerotherapy
in the rabbit model of simple hepatic cysts.
Detailed statistical results of
multiple comparisons for IODs of anti-CD43 immunohistochemical
staining at different time-points following bleomycin sclerotherapy
in the rabbit model of simple hepatic cysts.
Detailed statistical results of
multiple comparisons for IODs of anti-CD68 immunohistochemical
staining at different time-points following bleomycin sclerotherapy
in the rabbit model of simple hepatic cysts.
Detailed statistical results of
multiple comparisons for APSAPs of collagen fibers using Masson's
trichrome staining following bleomycin sclerotherapy in the rabbit
model of simple hepatic cysts.
Acknowledgements
Not applicable.
Funding
The present study was funded by the Medical Scientific Research
Foundation of Guangdong Province of China (grant no. A2015530).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
LL and YHL conceived and designed the study. LL, YHL
and NZ acquired, analyzed and interpreted the data, and wrote and
revised the manuscript. LL and YHL performed the statistical
analysis. All authors have read and approved the final manuscript.
LL and YHL confirm the authenticity of all the raw data.
Ethics approval and consent to
participate
The present study was approved by the Biomedical
Ethics Committee of Animal Experiments of Guangdong Medical
Laboratory Animal Center (Guangdong, China; approval no.
B201610-5). An Accreditation Certificate from the China National
Accreditation Service for Conformity Assessment has been granted to
this facility. All experimental procedures were performed in
accordance with the Guide for the Care and Use of Laboratory
Animals (National Institutes of Health). Every effort was made to
minimize animal suffering and to use only the number of animals
necessary for the acquisition of reliable data.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Dietrich CF, Chiorean L, Potthoff A, Ignee
A, Cui X and Sparchez Z: Percutaneous sclerotherapy of liver and
renal cysts, comments on the EFSUMB guidelines. Z Gastroenterol.
54:155–166. 2016.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Wijnands TF, Görtjes AP, Gevers TJ,
Jenniskens SF, Kool LJ, Potthoff A, Ronot M and Drenth JP: Efficacy
and safety of aspiration sclerotherapy of simple hepatic cysts: A
systematic review. AJR Am J Roentgenol. 208:201–207.
2017.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Lam SC and Yuen HK: Medical and sclerosing
agents in the treatment of orbital lymphatic malformations: What's
new? Curr Opin Ophthalmol. 30:380–385. 2019.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Eissa A, El Sherbiny A, Martorana E,
Pirola GM, Puliatti S, Scialpi M, Micali S, Rocco B, Liatsikos E,
Breda A, et al: European Section of Uro-Technology (ESUT):
Non-conservative management of simple renal cysts in adults: A
comprehensive review of literature. Minerva Urol Nefrol.
70:179–192. 2018.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Paladine W, Cunningham TJ, Sponzo R,
Donavan M, Olson K and Horton J: Intracavitary bleomycin in the
management of malignant effusions. Cancer. 38:1903–1908.
1976.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Yura J, Hashimoto T, Tsuruga N and Shibata
K: Bleomycin treatment for cystic hygroma in children. Nippon Geka
Hokan. 46:607–614. 1977.PubMed/NCBI
|
|
7
|
Takahashi H, Nakazawa S and Shimura T:
Evaluation of postoperative intratumoral injection of bleomycin for
craniopharyngioma in children. J Neurosurg. 62:120–127.
1985.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Johnston SR, Adam A, Allison DJ, Smith P
and Ind PW: Recurrent respiratory obstruction from a mediastinal
bronchogenic cyst. Thorax. 47:660–662. 1992.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Li L, Chen CC and Zeng XQ: One-year
results of single-session sclerotherapy with bleomycin in simple
renal cysts. J Vasc Interv Radiol. 23:1651–1656. 2012.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Souftas VD, Kosmidou M, Karanikas M,
Souftas D, Menexes G and Prassopoulos P: Symptomatic abdominal
simple cysts: Is percutaneous sclerotherapy with hypertonic saline
and bleomycin a treatment option? Gastroenterol Res Pract.
2015(489363)2015.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Li L, Zeng XQ and Li YH: CT-guided
percutaneous large-needle aspiration and bleomycin sclerotherapy
for bronchogenic cyst: Report of four cases. J Vasc Interv Radiol.
21:1045–1049. 2010.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Della Latta V, Cecchettini A, Del Ry S and
Morales MA: Bleomycin in the setting of lung fibrosis induction:
From biological mechanisms to counteractions. Pharmacol Res.
97:122–130. 2015.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Temmerman F, Chen F, Libbrecht L, Vander
Elst I, Windmolders P, Feng Y, Ni Y, De Smedt H, Nevens F and van
Pelt J: Everolimus halts hepatic cystogenesis in a rodent model of
polycystic-liver-disease. World J Gastroenterol. 23:5499–5507.
2017.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Yu Y, Shumway KL, Matheson JS, Edwards ME,
Kline TL and Lyons LA: Kidney and cystic volume imaging for disease
presentation and progression in the cat autosomal dominant
polycystic kidney disease large animal model. BMC Nephrol.
20(259)2019.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Divyashree S, Janhavi P, Ravindra PV and
Muthukumar SP: Experimental models of polycystic ovary syndrome: An
update. Life Sci. 237(116911)2019.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Atilgan R, Ozkan ZS, Kuloglu T, Kocaman N,
Baspinar M, Can B, Şimşek M and Sapmaz E: Impact of intracystic
ethanol instillation on ovarian cyst diameter and adjacent ovarian
tissue. Eur J Obstet Gynecol Reprod Biol. 174:133–136.
2014.PubMed/NCBI View Article : Google Scholar
|
|
17
|
National Research Council (US): Committee
for the Update of the Guide for the Care and Use of Laboratory
Animals: Guide for the Care and Use of Laboratory Animals. 8th
edition. National Academies Press, Washington, DC, pp1-154,
2011.
|
|
18
|
Feldman AT and Wolfe D: Tissue processing
and hematoxylin and eosin staining. Methods Mol Biol. 1180:31–43.
2014.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Goldner J: A modification of the masson
trichrome technique for routine laboratory purposes. Am J Pathol.
14:237–243. 1938.PubMed/NCBI
|
|
20
|
Rehg JE, Bush D and Ward JM: The utility
of immunohistochemistry for the identification of hematopoietic and
lymphoid cells in normal tissues and interpretation of
proliferative and inflammatory lesions of mice and rats. Toxicol
Pathol. 40:345–374. 2012.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Taylor CR and Rudbeck L (eds): Dako
Education Guide: Immunohistochemical Staining Methods. 6th edition.
pp1-110, 2013.
|
|
22
|
Schmidt MJ, Tschoeke A, Noronha L, Moraes
RS, Mesquita RA, Grégio AM, Alanis LR, Ignácio SA, Santos JN, Lima
AA, et al: Histochemical analysis of collagen fibers in giant cell
fibroma and inflammatory fibrous hyperplasia. Acta Histochem.
118:451–455. 2016.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Varghese F, Bukhari AB, Malhotra R and De
A: IHC Profiler: An open source plugin for the quantitative
evaluation and automated scoring of immunohistochemistry images of
human tissue samples. PLoS One. 9(e96801)2014.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Terada T, Nakanuma Y, Ohta T, Nagakawa T,
Motoo Y, Harada A, Hamato N and Inaba T: Mucin-histochemical and
immunohistochemical profiles of epithelial cells of several types
of hepatic cysts. Virchows Arch A Pathol Anat Histopathol.
419:499–504. 1991.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Uchiyama N, Stridbeck H and Stenram U:
Chemical sclerosis of the gallbladder. An experimental study in
pigs of the effect of absolute ethanol and polidocanol on
gallbladder epithelium. Acta Radiol. 30:427–431. 1989.PubMed/NCBI
|
|
26
|
Aagaard BD, Wetter LA, Montgomery CK and
Gordon RL: Heat ablation of the normal gallbladder in pigs. J Vasc
Interv Radiol. 5:331–339. 1994.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Lee JH, Won JH, Bae JI, Kim JH, Lee HS and
Jung SM: Chemical ablation of the gallbladder with acetic acid. J
Vasc Interv Radiol. 20:1471–1476. 2009.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Williamson JD, Sadofsky LR and Hart SP:
The pathogenesis of bleomycin-induced lung injury in animals and
its applicability to human idiopathic pulmonary fibrosis. Exp Lung
Res. 41:57–73. 2015.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Liu T, De Los Santos FG and Phan SH: The
bleomycin model of pulmonary fibrosis. Methods Mol Biol.
1627:27–42. 2017.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Yamamoto T: Intradermal injections of
bleomycin to model skin fibrosis. Methods Mol Biol. 1627:43–47.
2017.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Hadjicharalambous MR and Lindsay MA:
Idiopathic pulmonary fibrosis: Pathogenesis and the emerging role
of long non-coding RNAs. Int J Mol Sci. 21(524)2020.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Heukels P, Moor CC, von der Thüsen JH,
Wijsenbeek MS and Kool M: Inflammation and immunity in IPF
pathogenesis and treatment. Respir Med. 147:79–91. 2019.PubMed/NCBI View Article : Google Scholar
|
|
33
|
MacIntosh PW, Yoon MK and Fay A:
Complications of intralesional bleomycin in the treatment of
orbital lymphatic malformations. Semin Ophthalmol. 29:450–455.
2014.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Basil MC, Katzen J, Engler AE, Guo M,
Herriges MJ, Kathiriya JJ, Windmueller R, Ysasi AB, Zacharias WJ,
Chapman HA, et al: The cellular and physiological basis for lung
repair and regeneration: Past, present, and future. Cell Stem Cell.
26:482–502. 2020.PubMed/NCBI View Article : Google Scholar
|