Introduction
Renal cell carcinoma (RCC) accounts for 2.2% of all
cancer diagnoses worldwide. It is the 7th most common type of
cancer in developed countries, with the highest incidence in North
America, followed by Western Europe (1). RCC is responsible for 1.8% of global
cancer deaths and 2.4% of cancer-related deaths in the US (2). The 5-year survival rate for all
individuals with RCC is 76%, making it one of the deadliest
urological cancers (1). RCC is
routinely diagnosed by pathohistological analysis using the
protocol devised by the College of American Pathologists, and
histological type is determined based on the 2016 WHO
classification of tumors of the urinary system (3,4).
Localized RCCs are treated by surgical resection, whereas
metastatic RCCs are treated by targeted therapy according to the
National Comprehensive Cancer Network guidelines (5). The most common histological type is
clear cell RCC (ccRCC), which constitutes 70-90% of all RCCs.
Papillary RCC (pRCC) and chromophobe chRCC (chRCC) are the next
most common types, constituting 10-15% and 3-5% of all RCC cases,
respectively (6). ccRCC has a worse
prognosis and is more likely to be diagnosed in the first instance
at a higher stage than pRCC or chRCC (7). Prior research found that 90% of ccRCCs
have mutations present in the von Hippel-Lindau tumor suppressor
(VHL) gene (8). The VHL protein has
a role in the degradation of hypoxia-inducible factors (HIFs) and
in its absence, HIFs become stabilized (9,10).
Stabilized HIFs act as transcription factors and bind to hypoxia
response elements on target genes, upregulating the expression of
proteins involved in metabolic reprogramming, induction of
angiogenesis, as well as other oncogenic processes (11). HIFs affect cell metabolism primarily
by increasing the expression of glucose transporter 1 (GLUT1)
protein (9). GLUT1 is one of 14
members of a family of integral membrane proteins whose purpose is
the transport of monosaccharides (primarily glucose) and other
small carbon compounds across the cell membrane (12). Cancer cells depend on glucose
metabolism for energy production and the rate-limiting step of
glucose metabolism is uptake across the membrane (13,14).
Thus, it comes as no surprise that expression of glucose
transporters, such as GLUT1, is upregulated in several types of
cancer cells (15). GLUT1
expression is positively correlated with tumor metabolic activity
in several types of cancer, including colorectal cancer, ovarian
cancer and melanoma (16-18).
High expression of GLUT1 has also been used as a prognostic
biomarker, indicating poor survival, in several types of cancer,
such as colorectal, ovarian, bladder and esophageal carcinoma
(19-23).
In addition, GLUT proteins have been suggested as targets for
cancer therapy; it has been shown that inhibition of glucose uptake
can cause metabolic stress and activate tumor suppressor pathways
in cells (24,25). From the GLUT family, GLUT1 is the
most extensively researched member, and anti-GLUT1 therapy exhibits
induction of growth inhibition and/or cell death in several cancer
cell types (26-29).
Glucose uptake inhibitors can also sensitize cancer cells to
cytotoxic therapy, suggesting the possibility of its use as an
adjuvant (13,30).
The aim of the present study was to determine the
correlation between GLUT1 expression and the clinical
characteristics of RCCs, specifically histological type, nuclear
grade and tumor size.
Materials and methods
Tissue samples
The present study was performed at the Institute of
Pathology, Forensic Medicine and Cytology, Split University
Hospital Centre (Split, Croatia). The present study was approved by
the Hospital Ethics Committee of the University Hospital Centre in
Split, Croatia (approval no. 2181-147-01/06/M.S.-20-9), and was
performed in accordance with the ethical standards described in the
1964 Declaration of Helsinki and its later amendments (31). Consent from patients was not
required as the study was retrospective, and patients had provided
consent for use of their data/samples during their visits to the
hospital. Specifically, considering that the hospital is a
university hospital, all patients were informed prior to surgical
and/or diagnostic procedures that the material obtained during
these procedures may be used for education and/or research
purposes, and they signed consent forms for these procedures and
the use of their data/materials.
The institute's database of pathohistological
reports was searched to select appropriate RCC samples for the
study. Samples were taken from the institute's archive; all samples
were initially obtained from patients between January 2015 and
December 2018. All chosen samples were from primary kidney tumors
of adults with only one tumor focus present at the time of
nephrectomy. To avoid confounding factors, samples were chosen in
such a manner that there was no large difference between the sex
and age of patients with RCC. Additionally, the samples that were
chosen had no marked lymphovascular invasion, necrosis, sarcomatoid
and/or rhabdoid features present, to make the samples as uniform as
possible and to assess the immunohistochemical expression more
accurately. Additionally, samples where the tumor was limited to
the kidney were used to obtain precise measurements of tumor size.
Overall, 19 paraffin blocks containing formalin-fixed RCC samples
were collected from the institute's archive, 8 of those being ccRCC
(median age 58.5, age range 49-86, 4 males and 4 females), 7 pRCC
(median age 80, age range 56-83, 5 males and 2 females) and 4 chRCC
(median age 54, age range 44-67, 3 males and 1 female). Out of the
pRCC cases, 4 were type I and 3 were type II.
RCC tissue preparation and
immunohistochemistry
From each paraffin block, a 4 µm-thick RCC section
was cut, mounted and dried at 37˚C. Subsequently, sections were
stained with hematoxylin and eosin using an Automatic Stainer
HE600, according to the manufacturer's protocol (VentanaRoche;
Roche Diagnostics GmbH). All the sections were re-examined by a
pathologist according to the standard protocol described by the
College of American Pathologists (3). The histopathological analysis was
performed manually using a light microscope (Olympus BX41; Olympus
Corporation) at x400 magnification, and Cell D1 Image analysis
software version Cellsense (Olympus Corporation). Immunostaining
was performed on the same serial section of each RCC sample, as
follows: Paraffin sections were mounted on super frost slides
(Thermo Fisher Scientific, Inc.) and processed in an automatic
stainer (Ventana Bench Mark Ultra Autostainer; VentanaRoche; Roche
Diagnostics GmbH). For the detection of GLUT1-positive RCC cells,
primary ‘ready-to-use’ monoclonal mouse antibodies
(RRID:AB_10578246; cat. no. SPM498; 1:100; Novus Biologicals, Ltd.)
were applied (Fig. 1). The
UltraView Universal DAB Detection kit (RRID:AB_2753116; cat. no.
G32061; VentanaRoche; Roche Diagnostics GmbH) was used as the
secondary antibody (supplied ready to use). Brown staining of the
cell membrane and/or cytoplasm was considered positive.
Erythrocytes present in blood vessels inside the RCC tissue were
used as a positive control. The expression of GLUT1 in the samples
was determined by the HSCORE method using the equation: HSCORE=Σ
Pi (i + 1), where i=intensity of staining with
a value of 1 (weak), 2 (moderate), or 3 (strong), and Pi is
the percentage of stained RCC cells of each intensity (32). For every RCC sample, 10
representative fields of view were chosen and an HSCORE was
calculated for each of them. The HSCORE of the entire sample was
the arithmetic mean of HSCOREs of the 10 individual fields of
view.
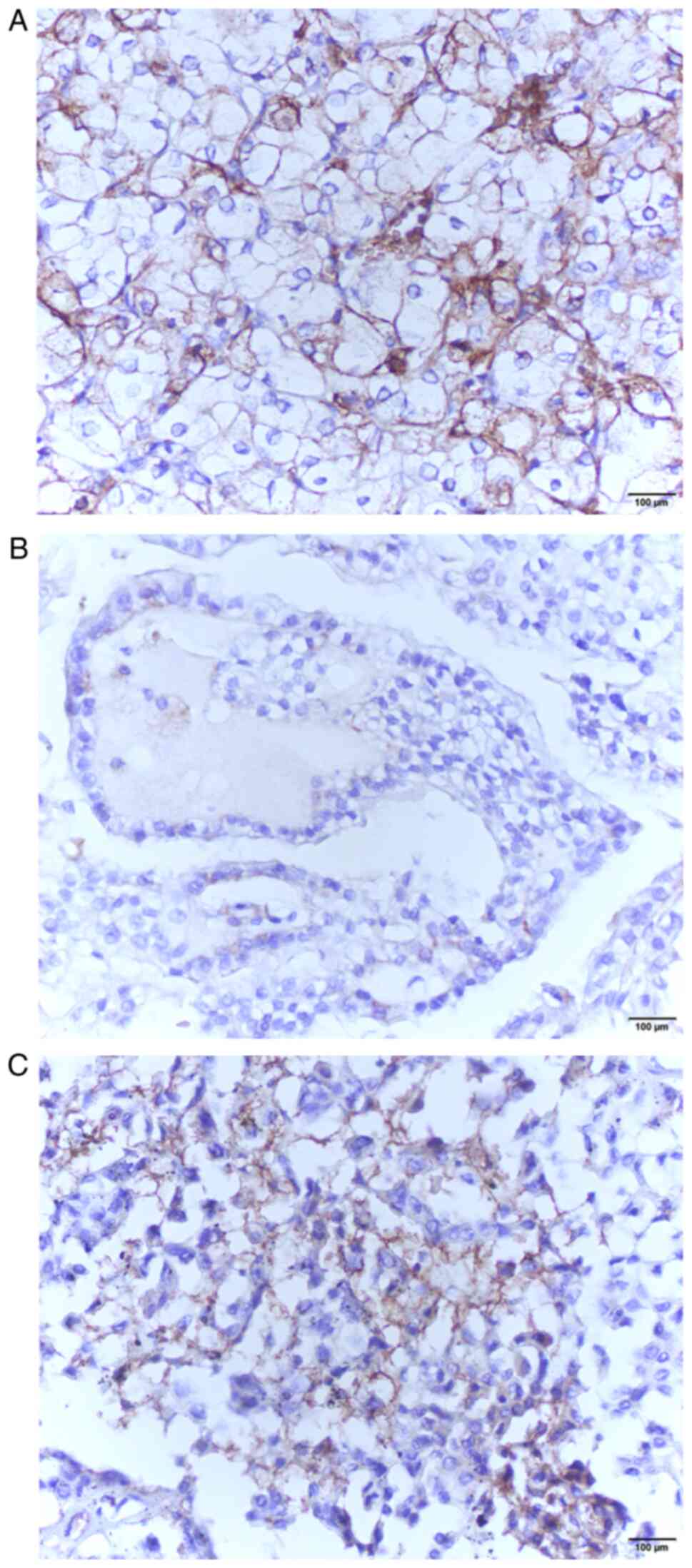 | Figure 1Light microscopy of RCC samples
stained with anti-GLUT1 antibodies. (A) A moderate to strong,
diffuse, membranous staining for GLUT1 can be observed in an
example of clear cell RCC, (B) whilst only weak, focal, cytoplasmic
staining can be observed in a papillary RCC sample, and (C) weak to
moderate, cytoplasmic, with some foci of strong membranous staining
in a chromophobe RCC sample. Strongly stained erythrocytes in
spaces between RCC cells were used as an internal positive
controls. Magnification, x400. Scale bar, 100 µm. RCC, renal cell
carcinoma; GLUT1, glucose transporter 1. |
Statistical analysis
The normality of distributions was tested using a
Shapiro-Wilk test. The mean and standard deviation were used as the
measures of central tendency and variance for normally distributed
data, whereas the median and interquartile range were used for data
that was not normally distributed. A Fisher's exact test was used
to determine the differences between the nominal characteristics of
the groups of RCCs. A Student's t-test (unpaired) and ANOVA with
Scheffé post-hoc test were performed to assess the differences in
the age of the patients according to the type of RCC. A
Mann-Whitney U and Kruskal-Wallis with a Conover post-hoc test was
used to assess the differences in GLUT1 expression for groups
without normal distributions, whereas an Independent sample t-test
was used for groups that exhibited normal distribution. Pearson's
correlation analysis was used to determine the correlation between
tumor size and GLUT1 expression. All data were analyzed using
MedCalc Statistical Software version 19.1.2 (MedCalc Software,
Ostend, Belgium; medcalc.org; 2019, RRID:SCR_015044).
P<0.05 was considered to indicate a statistically significant
difference and all confidence intervals (CI) are stated at the 95%
level.
Results
To compare the immunohistochemical expression of
GLUT1 in different histological types of RCCs, the tissues were
split into 2 groups: ccRCCs (n=8) and non-ccRCCs (n=11). There was
no statistically significant difference in terms of age, sex or
nuclear grade between the two groups. There was a statistically
significant difference in tumor size measured by the greatest
diameter of the tumor (P=0.038) between the two groups; the ccRCC
group was larger in size (Table I).
There was also a statistically significant difference in GLUT1
expression based on the HSCORE (P=0.044) between the two groups,
with the ccRCC group exhibiting higher expression (Table II). Even after assessing the non-cc
group separately by types, there was still a statistically
significant difference in GLUT1 expression between ccRCCs and pRCCs
(P=0.021) or chRCCs (P=0.023), again with the cc group exhibiting
higher expression (Table II).
There was no statistically significant difference in GLUT1
expression between pRCCs and chRCCs, or between type I and II
pRCCs. To compare GLUT1 expression between RCCs of different
grades, the tissues were separated into two groups, low-grade
(containing nuclear grades 1 and 2) and high-grade (containing
grades 3 and 4). When comparing RCCs of different nuclear grades,
there was no statistically significant difference in age, sex,
tumor size or GLUT1 expression between the groups (Table II). Even after separating the
groups into individual grades (1-4),
there was still no statistically significant difference in any of
the characteristics. There was a weak correlation between GLUT1
expression and tumor size (r=0.33), however, it was not
statistically significant (P=0.168).
 | Table ICharacteristics of the patients with
RCC. |
Table I
Characteristics of the patients with
RCC.
|
Characteristics | Clear cell RCC,
n=8 | Non-clear cell RCC,
n=11 | P-value |
|---|
| Age,
yearsb,d | 58.5 (49-86) | 67 (44-83) | 0.495 |
| Sexc, n (%) | | | |
|
Male | 4 (21.1) | 8 (42.1) | 0.324 |
|
Female | 4 (21.1) | 3 (15.8) | |
| Gradec, n (%) | | | |
|
Low | 4 (21.1) | 4 (21.1) | 0.658 |
|
High | 4 (21.1) | 7 (36.8) | |
| Tumor
sizeb,e | 7.31±3.32 | 4.36±2.4 | 0.038a |
 | Table IIComparison of GLUT1 expression using
HSCORE between groups of RCCs based on their histological type or
nuclear grade. |
Table II
Comparison of GLUT1 expression using
HSCORE between groups of RCCs based on their histological type or
nuclear grade.
| Group | HSCORE | P-value |
|---|
| ccRCCb | 2.44
(2.06-2.61) | 0.044a |
|
Non-ccRCCb | 2.01
(2.00-2.03) | |
| ccRCCc | 2.40±0.35 | 0.021a |
| pRCCc | 2.03±0.04 | |
| ccRCCc | 2.40±0.35 | 0.023a |
| chRCCc | 2.04±0.08 | |
| pRCCb | 2.02
(2.01-2.03) | 0.285 |
| chRCCb | 2.00
(2.00-2.04) | |
| Low grade
RCCb | 2.02
(2.00-2.28) | 0.557 |
| High grade
RCCb | 2.12
(2.00-2.37) | |
Discussion
The results of the present study showed higher
expression of GLUT1 in ccRCC tissues compared with pRCC and chRCC
tissues. This aligns with the fact that most ccRCCs have a
homozygous loss of the VHL gene, which leads to the stabilization
of HIFs, increased GLUT1 mRNA transcription and ultimately,
increased GLUT1 protein expression (6,9). Other
studies have already demonstrated higher expression of GLUT1 in
ccRCCs; however, considering that there is no consensus in the
literature on the quantification of the expression levels of GLUT1,
these studies have used a variety of quantification methods, such
as the German immunoreactive score (33-35).
Certain studies have also attempted to correlate increased GLUT1
expression with clinical characteristics of RCCs, such as tumor
size, tumor stage, nuclear grade and overall prognosis. The results
of those studies are not consistent when taking into account
certain clinical parameters, such as tumor stage and prognosis
(34-37).
Whilst most studies have not found a statistically significant
correlation between GLUT1 expression and tumor nuclear grade or
prognosis, Ambrosetti et al (34) determined that there was a
statistically significant positive correlation between increased
GLUT1 expression with both higher nuclear grade tumors and worse
overall prognosis in ccRCCs. Furthermore, Lidgren et al
(37) found a statistically
significant correlation between increased GLUT1 expression and
higher tumor stage at diagnosis, but only for pRCC. In the present
study, a statistically significant difference between GLUT1
expression in RCCs of different nuclear grades was not found, nor
was a correlation between GLUT1 expression and tumor size, in
agreement with most of the referenced studies (35-37).
The greater size of ccRCCs compared to non-ccRCCs corresponds to
the fact that ccRCCs are more likely to be diagnosed in the first
instance at a more advanced stage than the other two types of RCC
assessed (7). Taking into
consideration that other studies on GLUT1 expression in RCCs have
used varying methods to interpret immunohistochemical expression,
whilst obtaining contrasting results, the HSCORE method was used in
the present study. To the best of our knowledge, no other study has
used the HSCORE method for the interpretation of GLUT1 expression
in RCCs, making the present study unique. An advantage that this
method has over other methods of interpretation of
immunohistochemical expression is the manner of data
quantification. Namely, methods used in other studies quantify the
proportion of positive cells in such a manner that a range of
proportions is given a specific value. For example, 0-20% of
positive cells in a sample is categorized with the value of 1,
21-40% categorized with the value of 2, and so on. The HSCORE
method, on the contrary, takes the specific proportion of positive
cells and uses that exact value to calculate the final score
(32,34-36).
Variables that describe immunohistochemical expression are usually
ordinal or discrete numerical variables for the majority of
semi-quantitative methods, but in the case of the HSCORE method,
continuous numerical variables are used. Continuous variables allow
for more precise statistical analysis and interpretation of data.
Additionally, fields of view were chosen in such a manner that a
representative sample of the entire tumor was examined, whilst
other studies specifically chose those fields of view that had the
highest intensity of immunohistochemical expression and analyzed
them (36). Another advantage of
the present study was that there was no statistically significant
between-group differences according to a patients sex or age at the
time of diagnosis, and tumor nuclear grade, reducing potential
confounding factors in the interpretation of the results. However,
using immunohistochemistry as a semi-quantitative method of
evaluation that is liable to the investigator's subjectivity was
the main drawback of the present study. Other substantial caveats
of the present study include the relatively small sample size and
the fact that it was conducted in only one center. Also, no
conclusions or causal relationships can be drawn due to the
cross-sectional design of the study. In the future, larger,
multicenter studies should be performed to increase the validity of
the results. Additionally, other methods besides
immunohistochemistry, such as western blotting or PCR, should be
used to confirm expression.
The increased expression of GLUT1 is associated with
a worse prognosis for several different types of solid tumors,
which makes GLUT1 expression one of the key prognostic factors for
those tumors (38); concerning RCC,
most studies have not demonstrated this connection; however, it is
hypothesized that the relationship is indirectly present (35-37).
It is a fact that most RCCs are of the cc type, and that ccRCCs
have a worse overall prognosis than other common histological types
(6). It is also known that ccRCCs,
unlike other histological types, exhibit increased expression of
GLUT1 due to the genetic changes present in most ccRCCs (6,9,33).
Taking these facts into account, it can be assumed that the
increased expression of GLUT1 may participate in the worse
prognosis of patients with ccRCC compared with other histological
types, which also coincides with studies that have demonstrated
that glucose uptake is the limiting factor for further tumor growth
(39,40). Other than enabling enough glucose
uptake for aerobic glycolysis, which maintains metabolic activity
in tumor cells, the increased GLUT1 expression in RCCs is
associated with reduced CD8+ lymphocyte infiltration of
tumor tissues, which contributes to tumor tissue survival (40). The majority of studies attempting to
identify the connection between GLUT1 expression and RCC prognosis
observe an association either in ccRCCs alone, or in all RCC cases
regardless of the histological type, most of which are ccRCC
(34-37).
Considering that most RCCs, wherein the majority of cases are
ccRCC, exhibit increased GLUT1 expression, the differences in
prognosis between individual patients likely depends on other
factors, and no correlation between GLUT1 expression and prognosis
has been found, to the best of our knowledge. The differences in
GLUT1 expression between ccRCC cases that have the same genetic
mutation, loss of VHL, can be explained by, amongst other things,
different polymorphisms of the GLUT1 gene, which is supported by
the fact that some GLUT1 polymorphisms have a protective role in
carcinogenesis (41). GLUT1 is not
studied just as a prognostic factor, but also as a predictive
factor. Multiple studies have assessed the effects of GLUT1
inhibitors on cancer cells (26-29,42,43).
Some studies have specifically assessed the value of GLUT1 as a
therapeutic target for RCC (44,45).
Even though GLUT1 inhibitors have been proven to be ineffective as
a monotherapy, newer generations of anti-GLUT1 drugs show promising
results when combined with conventional therapy, increasing the
therapeutic benefits and lowering side effects (43). Considering that RCC is resistant to
chemotherapy and radiotherapy, the treatment regimens for this
metastatic disease includes immunotherapy and/or targeted therapy
(5). Additionally, the official
guidelines from the National Comprehensive Cancer Network even
allow RCC treatment with drugs currently in clinical trials in
certain cases (5). In the case of
cancer progression, after all lines of therapy have been exhausted,
a combination of a GLUT1 inhibitor and a conventional drug can be
used as salvation therapy, if an increased expression of GLUT1 was
previously proven in the same cancer, either by
immunohistochemistry or by other methods.
In conclusion, the present study confirmed reports
from previous studies that GLUT1 expression is increased in ccRCC
compared with pRCC and chRCC. It also confirmed that GLUT1
expression does not correlate with tumor nuclear grade or tumor
size. Future studies should include a larger sample size of RCCs
and focus on non-clear cell RCCs with the intention to correlate
GLUT1 expression with prognosis as most ccRCCs have an inherently
increased expression of GLUT1, due to the genetic background of the
tumor. Even though GLUT1 expression cannot be used as a prognostic
marker, it might be used as a predictive marker, if future studies
confirm the efficacy of anti-GLUT1 treatment on RCCs.
Acknowledgements
We would like to thank Professor Merica Glavina
Durdov (Institute for Pathology, University Hospital Split) for her
assistance in obtaining the antibodies needed for this research and
Assistant Professor Shelly Pranić (School of Medicine, University
of Split) for her expertise in language editing.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
MO, AS and SZT contributed to the conception and
design of the present study. MO performed the data collection and
analysis. MO, AS and SZT interpretated the results. MO drafted the
manuscript. MO, AS and SZT revised the manuscript. All authors have
read and approved the final manuscript. MO and SZT confirm the
authenticity of all the raw data.
Ethical approval and consent to
participate
The present study was approved by the Hospital
Ethics Committee of the University Hospital Centre in Split,
Croatia (approval no. 2181-147-01/06/M.S.-20-9), and was performed
in accordance with the ethical standards described in the 1964
Declaration of Helsinki and its later amendments. Consent from
patients was not required as the study was retrospective, and
patients had provided consent for use of their data/samples during
their visits to the hospital. Specifically, considering that the
hospital is a university hospital, all patients were informed prior
to surgical and/or diagnostic procedures that the material obtained
during these procedures may be used for education and/or research
purposes, and they signed consent forms for these procedures and
the use of their data/materials.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Padala SA and Barsouk A, Thandra KC,
Saginala K, Mohammed A, Vakiti A, Rawla P and Barsouk A:
Epidemiology of renal cell carcinoma. World J Oncol. 11:79–87.
2020.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Srigley JR, Amin MB, Delahunt B, Campbell
SC, Chang A, Grignon DJ, Humphrey PA, Leibovich BC, Montironi R,
Renshaw AA, et al: Protocol for the examination of specimens from
patients with invasive carcinoma of renal tubular origin. Arch
Pathol Lab Med. 134:e25–e30. 2010.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Moch H, Cubilla AL, Humphrey PA, Reuter VE
and Ulbright TM: The 2016 WHO classification of tumours of the
urinary system and male genital organs-part A: Renal, penile, and
testicular tumours. Eur Urol. 70:93–105. 2016.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Motzer RJ, Jonasch E, Agarwal N, Bhayani
S, Bro WP, Chang SS, Choueiri TK, Costello BA, Derweesh IH, Fishman
M, et al: Kidney cancer, version 2.2017, NCCN clinical practice
guidelines in oncology. J Natl Compr Canc Netw. 15:804–834.
2017.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Warren AY and Harrison D: WHO/ISUP
classification, grading and pathological staging of renal cell
carcinoma: Standards and controversies. World J Urol. 36:1913–1926.
2018.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Leibovich BC, Lohse CM, Crispen PL,
Boorjian SA, Thompson RH, Blute ML and Cheville JC: Histological
subtype is an independent predictor of outcome for patients with
renal cell carcinoma. J Urol. 183:1309–1315. 2010.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Nickerson ML, Jaeger E, Shi Y, Durocher
JA, Mahurkar S, Zaridze D, Matveev V, Janout V, Kollarova H, Bencko
V, et al: Improved identification of von Hippel-Lindau gene
alterations in clear cell renal tumors. Clin Cancer Res.
14:4726–4734. 2008.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Sadri N and Zhang PJ: Hypoxia-inducible
factors: Mediators of cancer progression; prognostic and
therapeutic targets in soft tissue sarcomas. Cancers (Basel).
5:320–333. 2013.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Keith B, Johnson RS and Simon MC: HIF1α
and HIF2α: Sibling rivalry in hypoxic tumour growth and
progression. Nat Rev Cancer. 12:9–22. 2011.PubMed/NCBI View
Article : Google Scholar
|
|
11
|
Semenza GL: Hypoxia-inducible factors:
Mediators of cancer progression and targets for cancer therapy.
Trends Pharmacol Sci. 33:207–214. 2012.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Mueckler M and Thorens B: The SLC2 (GLUT)
family of membrane transporters. Mol Aspects Med. 34:121–138.
2013.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Barron CC, Bilan PJ, Tsakiridis T and
Tsiani E: Facilitative glucose transporters: Implications for
cancer detection, prognosis and treatment. Metabolism. 65:124–139.
2016.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Hatanaka M: Transport of sugars in tumor
cell membranes. Biochim Biophys Acta. 355:77–104. 1974.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Navale AM and Paranjape AN: Glucose
transporters: Physiological and pathological roles. Biophys Rev.
8:5–9. 2016.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Gu J, Yamamoto H, Fukunaga H, Danno K,
Takemasa I, Ikeda M, Tatsumi M, Sekimoto M, Hatazawa J, Nishimura T
and Monden M: Correlation of GLUT-1 overexpression, tumor size, and
depth of invasion with 18F-2-fluoro-2-deoxy-D-glucose uptake by
positron emission tomography in colorectal cancer. Dig Dis Sci.
51:2198–2205. 2006.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Kurokawa T, Yoshida Y, Kawahara K,
Tsuchida T, Okazawa H, Fujibayashi Y, Yonekura Y and Kotsuji F:
Expression of GLUT-1 glucose transfer, cellular proliferation
activity and grade of tumor correlate with
[F-18]-fluorodeoxyglucose uptake by positron emission tomography in
epithelial tumors of the ovary. Int J Cancer. 109:926–932.
2004.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Park SG, Lee JH, Lee WA and Han KM:
Biologic correlation between glucose transporters, hexokinase-II,
Ki-67 and FDG uptake in malignant melanoma. Nucl Med Biol.
39:1167–1172. 2012.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Sung JY, Kim GY, Lim SJ, Park YK and Kim
YW: Expression of the GLUT1 glucose transporter and p53 in
carcinomas of the pancreatobiliary tract. Pathol Res Pract.
206:24–29. 2010.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Haber RS, Rathan A, Weiser KR, Pritsker A,
Itzkowitz SH, Bodian C, Slater G, Weiss A and Burstein DE: GLUT1
glucose transporter expression in colorectal carcinoma: A marker
for poor prognosis. Cancer. 83:34–40. 1998.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Cantuaria G, Fagotti A, Ferrandina G,
Magalhaes A, Nadji M, Angioli R, Penalver M, Mancuso S and Scambia
G: GLUT-1 expression in ovarian carcinoma: Association with
survival and response to chemotherapy. Cancer. 92:1144–1150.
2001.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Hoskin PJ, Sibtain A, Daley FM and Wilson
GD: GLUT1 and CAIX as intrinsic markers of hypoxia in bladder
cancer: Relationship with vascularity and proliferation as
predictors of outcome of ARCON. Br J Cancer. 89:1290–1297.
2003.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Tohma T, Okazumi S, Makino H, Cho A,
Mochizuki R, Shuto K, Kudo H, Matsubara K, Gunji H, Matsubara H and
Ochiai T: Overexpression of glucose transporter 1 in esophageal
squamous cell carcinomas: A marker for poor prognosis. Dis
Esophagus. 18:185–189. 2005.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Yeluri S, Madhok B, Prasad KR, Quirke P
and Jayne DG: Cancer's craving for sugar: An opportunity for
clinical exploitation. J Cancer Res Clin Oncol. 135:867–877.
2009.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Hardie DG and Ashford ML: AMPK: Regulating
energy balance at the cellular and whole body levels. Physiology
(Bethesda). 29:99–107. 2014.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Melstrom LG, Salabat MR, Ding XZ, Milam
BM, Strouch M, Pelling JC and Bentrem DJ: Apigenin inhibits the
GLUT-1 glucose transporter and the phosphoinositide 3-kinase/Akt
pathway in human pancreatic cancer cells. Pancreas. 37:426–431.
2008.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Wood TE, Dalili S, Simpson CD, Hurren R,
Mao X, Saiz FS, Gronda M, Eberhard Y, Minden MD, Bilan PJ, et al: A
novel inhibitor of glucose uptake sensitizes cells to FAS-induced
cell death. Mol Cancer Ther. 7:3546–3555. 2008.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Zhang D, Wang Y, Dong L, Huang Y, Yuan J,
Ben W, Yang Y, Ning N, Lu M and Guan Y: Therapeutic role of EF24
targeting glucose transporter 1-mediated metabolism and metastasis
in ovarian cancer cells. Cancer Sci. 104:1690–1696. 2013.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Rastogi S, Banerjee S, Chellappan S and
Simon GR: Glut-1 antibodies induce growth arrest and apoptosis in
human cancer cell lines. Cancer Lett. 257:244–251. 2007.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Wang YD, Li SJ and Liao JX: Inhibition of
glucose transporter 1 (GLUT1) chemosensitized head and neck cancer
cells to cisplatin. Technol Cancer Res Treat. 12:525–535.
2013.PubMed/NCBI View Article : Google Scholar
|
|
31
|
World Medical Association: World medical
association declaration of Helsinki. Ethical principles for medical
research involving human subjects. JAMA. 310:2191–2194.
2013.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Tomas SZ, Prusac IK, Roje D and Tadin I:
Trophoblast apoptosis in placentas from pregnancies complicated by
preeclampsia. Gynecol Obstet Invest. 71:250–255. 2011.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Nagase Y, Takata K, Moriyama N, Aso Y,
Murakami T and Hirano H: Immunohistochemical localization of
glucose transporters in human renal cell carcinoma. J Urol.
153:798–801. 1995.PubMed/NCBI
|
|
34
|
Ambrosetti D, Dufies M, Dadone B, Durand
M, Borchiellini D, Amiel J, Pouyssegur J, Rioux-Leclercq N, Pages
G, Burel-Vandenbos F and Mazure NM: The two glycolytic markers
GLUT1 and MCT1 correlate with tumor grade and survival in
clear-cell renal cell carcinoma. PLoS One.
13(e0193477)2018.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Ozcan A, Shen SS, Zhai QJ and Truong LD:
Expression of GLUT1 in primary renal tumors: Morphologic and
biologic implications. Am J Clin Pathol. 128:245–254.
2007.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Kobayashi M, Uematsu T, Tokura Y, Takei K,
Sakamoto K, Narimatsu T, Nukui A and Kamai T: Immunohistochemical
expressionof sodium-dependent glucose transporter-2 (SGLT-2) in
clear cell renal carcinoma: Possible prognostic implications. Int
Braz J Urol. 45:169–178. 2019.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Lidgren A, Bergh A, Grankvist K, Rasmuson
T and Ljungberg B: Glucose transporter-1 expression in renal cell
carcinoma and its correlation with hypoxia inducible factor-1
alpha. BJU Int. 101:480–484. 2008.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Wang J, Ye C, Chen C, Xiong H, Xie B, Zhou
J, Chen Y, Zheng S and Wang L: Glucose transporter GLUT1 expression
and clinical outcome in solid tumors: A systematic review and
meta-analysis. Oncotarget. 8:16875–16886. 2017.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Singer K, Kastenberger M, Gottfried E,
Hammerschmied CG, Büttner M, Aigner M, Seliger B, Walter B,
Schlösser H, Hartmann A, et al: Warburg phenotype in renal cell
carcinoma: High expression of glucose-transporter 1 (GLUT-1)
correlates with low CD8(+) T-cell infiltration in the tumor. Int J
Cancer. 128:2085–2095. 2011.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Adekola K, Rosen ST and Shanmugam M:
Glucose transporters in cancer metabolism. Curr Opin Oncol.
24:650–654. 2012.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Page T, Hodgkinson AD, Ollerenshaw M,
Hammonds JC and Demaine AG: Glucose transporter polymorphisms are
associated with clear-cell renal carcinoma. Cancer Genet Cytogenet.
163:151–155. 2005.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Vera JC, Reyes AM, Velasquez FV, Rivas CI,
Zhang RH, Strobel P, Slebe JC, Núñez-Alarcón J and Golde DW: Direct
inhibition of the hexose transporter GLUT1 by tyrosine kinase
inhibitors. Biochemistry. 40:777–790. 2001.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Zambrano A, Molt M, Uribe E and Salas M:
Glut 1 in cancer cells and the inhibitory action of resveratrol as
a potential therapeutic strategy. Int J Mol.
20(3374)2019.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Chan DA, Sutphin PD, Nguyen P, Turcotte S,
Lai EW, Banh A, Reynolds GE, Chi JT, Wu J, Solow-Cordero DE, et al:
Targeting GLUT1 and the Warburg effect in renal cell carcinoma by
chemical synthetic lethality. Sci Transl Med.
3(94ra70)2011.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Shuch B, Linehan WM and Srinivasan R:
Aerobic glycolysis: A novel target in kidney cancer. Expert Rev
Anticancer Ther. 13:711–719. 2013.PubMed/NCBI View Article : Google Scholar
|















