1. Introduction
Breast cancer (BC) is the leading cause of
cancer-associated amongst women worldwide. Considering the most
updated data on BC from 2020, 2.261.419 new cases of BC were
registered worldwide (accounting for 24.5% of all tumours in
women). Of these, 531,086 cases (25.8%) in Europe were detected,
with 55,133 new diagnoses (28.1%) in Italy (1).
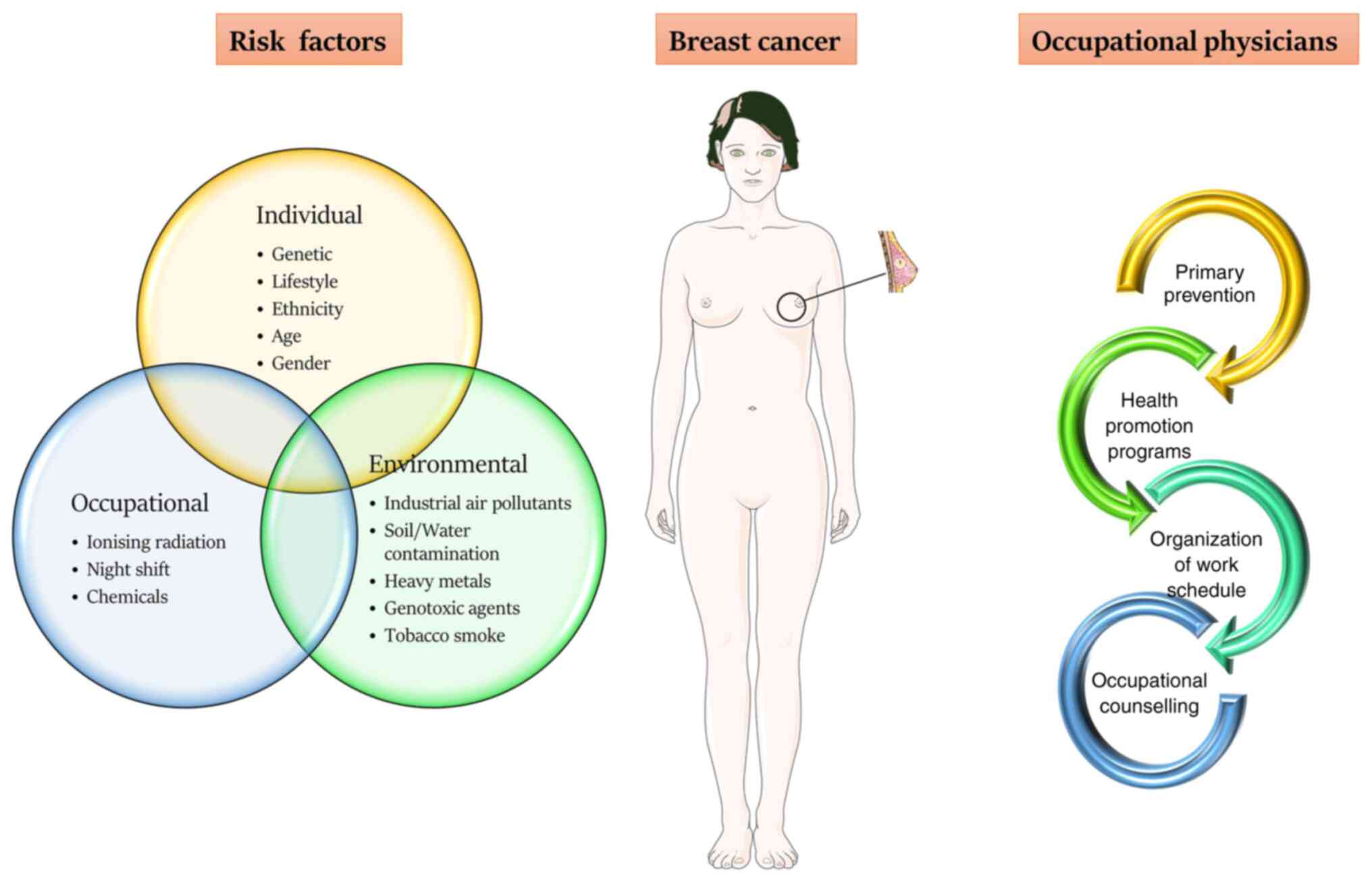
BC is a multifactorial disease; however, the
different risk factors established for pathological development do
not fully explain the aetiology of the disease. The results of
several studies showed that individual, environmental and
occupational factors serve a critical role in the onset of this
pathology (2-6).
In women, the most common individual risk factors causing
hereditary BC are genetic mutations involving the BRCA1 or BRCA2
genes. However, there are also less common inherited mutations in
other genes, including ATM, TP53, CHEK2, PTEN, CDH1, STK11 and
PALB2(7). Lifestyle is an important
risk factor, with lack of physical activity, excessive consumption
of alcohol, a high body mass index (BMI), a high-fat diet and
smoking all increasing the risk (2,8-11).
Ethnicity, age and socio-economic status can influence the risk of
developing BC. White women exhibit the highest incidence of BC,
followed by Black, Asian and Hispanic women. This could be
explained by racial and socioeconomic barriers to early detection
and screening, and unequal access to treatment, as well as
differences in lifestyles (12-16).
Other individual factors are attributable to procreative aspects,
such as the onset of menarche and menopause, as well as the age of
first pregnancy with breastfeeding (17-19).
Last but not least, a familiar BC and/or general predisposition to
cancer may represent important risk factors (17,18),
such as chronic fatigue, sleep disturbance and depression (20-22).
Several studies have investigated the gene-environment interactions
showing that BC risk is related to the common susceptibility
variants, which can be altered by environmental factors, such as
industrial air pollutants, soil or water contamination due to heavy
metals and genotoxic agents, active or passive tobacco smoke
(3,10,23-34).
Finally, several occupational factors can expose workers to a
higher risk of BC due to toxic compounds or exposure to ionising
radiation, rotating night shifts or higher-status occupations
(35-42).
This last association between BC and occupational
factors is a topical issue, since it is estimated that exposure to
occupational hazards causes up to 8% of cancers (37,43,44).
According to data from the seventh European Working
Conditions Survey in 2020, 13 and 15% of female workers, in Italy
(ITA) and European Union (EU) respectively, declared exposure to
chemical products or substances for at least 25% of the time in the
workplace. Moreover, 10% (ITA) and 14% (EU) of women stated they
worked ≥1 time a month at night, whereas 18% (ITA) and 21% (EU)
declared to perform work shifts (45).
Occupational exposure to rotating or night shifts
could lead to the onset of tumours such as BC (46,47).
In fact, in 2019, the International Agency for Research on Cancer
(IARC) published a monograph that considered the relationship
between night shift work and carcinogenesis. Based on both
epidemiological and experimental scientific contributions on
animals, the IARC Working Group defined night shift work, which
causes an alteration of circadian rhythms, as a probable human
carcinogen, with classification 2A (limited scientific evidence in
humans, but sufficient evidence in animals) (48).
Although the results of the latest studies are
controversial, the main pathogenetic hypothesis is based on the
impact that night shift work and night light exposure could have on
the rhythmicity of the circadian biological functions (49,50).
These alterations result in disturbance of circadian rhythms,
reduction of melatonin production and sleep perturbation, affecting
several metabolic and physiological processes, including hormone
synthesis and cell cycle progression (51-54).
Amongst the several mechanisms proposed to determine
the effects of artificial light at night on BC, the majority seem
to highlight the importance of inhibition of melatonin secretion
during the night due to sleep deprivation, resulting in
chronodisruption (55). In women
performing shift or night work, melatonin secretion is inhibited by
light, resulting in an increase in estrogen, since it has a
significant anti-estrogenic activity (54,56).
This multifaceted hypothesis may explain why night
workers may have a greater risk of developing BC compared to the
general population (42,52,55,57)
and why work organisations should implement a strategic procedure
to prevent BC. Under these premises and also in light of the new
evidence, this review aims to provide an overview of the most
relevant risk factors, providing recommendations to fill gaps in
risk assessment and suggest novel organisational strategies that
occupational physicians should adopt in the workplace, both for the
prevention and return-to-work process of female workers affected by
BC.
2. Literature search
The review was performed by searching PubMed, Scopus
and Web of Science databases. The inclusion criteria were:
Full-text studies, published in English, published between
2015-2021, and studies associating BC risk with occupational
factors, particularly shift and night work. Certain studies,
although methodologically sound, were excluded as they were
considered irrelevant to the purpose of the study. Most articles
were found using the terms: ‘breast cancer’ AND ‘night shift’ OR
‘breast cancer’ AND ‘night shift work’ OR ‘breast cancer’ AND
‘occupational exposure’ OR ‘breast cancer’ AND ‘occupational risk’.
No restrictions were applied to the country of origin or ethnicity
of the patients recruited. Furthermore, based on titles and
abstracts, the relevance of the topic and admissibility of all the
retrieved publications were further assessed. Finally, further
relevant studies were identified through manual screening of the
selected articles' reference lists and recently published
reviews.
3. Mechanistic pathways
Shift work and melatonin
secretion
Although the literature is controversial, as
summarized in Table I, numerous
studies have suggested that shift work and the night shift may
represent one of the main occupational risks associated with BC
(35,36,52,58).
 | Table ISummary of the main studies
reviewed. |
Table I
Summary of the main studies
reviewed.
| First author,
year | Country | Time span | Study type | Result | (Refs.) |
|---|
| Jung et al,
2016 | Not available | 6-18 Years | Pooled analysis of
20 prospective cohort studies | Alcohol intake was
positively associated with risk of overall BC, ER+, ER-, PR+ and
PR- BC. | (11) |
| Ma et al,
2020 | Not available | Not available | Bioinformatic
analysis | Two lncRNAs
(AL139280.1 and AP000851.1) and three mRNAs (MT1M, HBB and TFPI2)
were identified as differential risk biomarkers in patients with BC
in both the young and old age groups. | (12) |
| Barcenas et
al, 2010 | Not available | 1990-2005 | Systematic
review | Black women had a
significantly poorer overall survival risk compared with white
women. | (14) |
| Wieder et
al, 2016 | United States of
America | 1973-2011 | Retrospective
study | African American
race was demonstrated to be an independent predictive variable for
decreased survival compared to the Caucasian race in women with a
diagnosis of localized BC. | (15) |
| Taheri et
al, 2019 | Iran | Not available | Retrospective
study | No significant
relationship between the metastasis and recurrence of BC with age
of patients and education levels. | (16) |
| Beral et al,
2002 | 30 countries | Not available | Pooled analysis of
47 epidemiological studies | The relative risk
of BC was reduced by 4.3% for each year of breastfeeding, in
addition to a reduction of 7.0% for each birth. | (17) |
| Johansson et
al, 2013 | Italy | 1998-2002 | Double-blind,
placebo-controlled trial | Low-dose tamoxifen
exhibited a positive outcome on the hormonal profile and
fenretinide was associated with a weak anti-aromatase action,
supporting the administration of low-dose tamoxifen or fenretinide
as single agents in the prevention of BC in at-risk women. | (18) |
| Kröz et al,
2017 | Germany | 2011-2013 | Cohort study | The study showed
the effectiveness of a multimodal approach in the treatment of
chronic cancer-related fatigue compared to the standard aerobic
therapy. | (20) |
| Tsaras et
al, 2018 | Greece | 2017 | Cross-sectional
study | Patients with BC
were high risk for developing psychiatric disorders, such as
depression and anxiety. | (21) |
| Fox et al,
2020 | United States of
America | 2000-2010 | Prospective
study | Sleep disturbances
and cancer-related fatigue are the most common symptoms associated
with BC and its treatment, in particular chemotherapy. | (22) |
| Cohn et al,
2019 | United States of
America | 1959-1967 | Prospective
study | DDT was associated
with BC development. The risk was dependant on the timing of first
exposure and diagnosis age. DDT appears to act as endocrine
disruptor with responsive breast targets from in utero to
menopause. | (27) |
| Filippini et
al, 2020 | Not available | Not available | Meta-analysis | Limited evidence of
an association between cadmium and BC. | (29) |
| Wegrzyn et
al, 2017 | United States of
America | Since 1976 | Cohort study | Long-term rotating
night-shift work was associated with a higher risk of BC,
particularly amongst women who performed shifts during young
adulthood. | (35) |
| Fagundo-Rivera
et al, 2020 | Not available | 2010-2020 | Systematic
review | Significant
associations between BC and prolonged rotating night shifts. | (36) |
| Videnros et
al, 2019 | Sweden | 1991-1996 | Prospective cohort
study | Occupational
exposure to chemicals was associated with an elevated risk of BC. A
minor increase in risk was seen after exposure to organic solvents.
Statistically significant elevation of risk after >10 years of
exposure to diesel exhaust was found. | (37) |
| Lee et al,
2019 | Canada | 2005-2010 | Multicentre
Study | Prolonged
occupational exposure to polycyclic aromatic hydrocarbons may
increase BC risk, especially amongst women with a family history of
BC. | (39) |
| Schubauer-Berigan
et al, 2015 | United States | 2002-2005 | Cohort study | Increased BC
incidence in a cohort of flight attendants compared to the general
population. This incidence was not related to cosmic radiation or
to circadian rhythm disruption. | (40) |
| Szkiela et
al, 2020 | Poland | 2015-2019 | Case-control
study | The study supported
the hypothesis that night shift work is a significant risk factor
for BC. | (46) |
| Gómez-Salgado et
al, 2021 | Spain | Up to 2020 | Cross-sectional
study | The number of years
worked, nights worked throughout life, and years working >3
nights per month were identified as significant risk variables for
BC. The risk increased with other factors, such as sleep
disturbance, physical stress or family responsibilities. | (47) |
| Bustamante-Montes
et al, 2019 | Mexico | Not available | Case-control
study | The risk for BC
increased amongst the women who performed night-work, even after
adjusting for potential confounders. | (49) |
| Xiang et al,
2019 | United States of
America | Not available | In vitro
study | Circadian
disruption of melatonin by dim light at night increased resistance
to Paclitaxel via epigenetic mechanisms. | (51) |
| Erdem et al,
2017 | Norway | 1990-2007 | Case-control
study | Telomere shortening
was associated with the duration and intensity of night work, and
may be a contributing factor for BC risk amongst female shift
workers. | (52) |
| Erdem et al,
2017 | Norway | 1990-2007 | Case-control
study | Epigenetic
regulation of CLOCK, BMAL1, CRY1 and PER1 may have contributed to
BC in shift workers. | (53) |
| El-Benhawy et
al, 2021 | Egypt | Not available | Case-control
study | Nightshift workers
had significantly lower levels of melatonin and total antioxidant
capacity, and higher levels of serum inflammatory markers and
cortisol than the control group. Workers occupationally exposed to
ionizing radiation had significantly higher levels of serum
melatonin, malondialdehyde and inflammatory markers, lower levels
of serum cortisol and lower total antioxidant capacity than day
shift workers. | (57) |
| Rosa et al,
2019 | Not available | 2005-2016 | Systematic
review | Shift work is a
risk factor for stress, sleep disorders, metabolic disorders,
diabetes, cardiovascular disorders and BC. | (58) |
| Cordina-Duverger
et al, 2018 | Not available | Up to 2016 | Meta-analysis | Night shift work
increases the risk of BC in pre-menopausal women, particularly
those with high intensity and long duration of exposure. | (59) |
| Sweeney et
al, 2020 | Not available | 2003-2009 | Prospective cohort
study | There was little
evidence that rotating shift work or work at night was associated
with a higher risk of BC. | (60) |
| Pahwa et al,
2019 | Canada | 1961-2000 | Prospective cohort
study | An estimated
2.0-5.2% of newly diagnosed BC cases in 2011 in Canada were
attributable to shift work. This corresponds to 470-1,200 incident
cases of BC. | (61) |
| Pham et al,
2019 | Korea | 2012-2018 | Case-control
study | No association
between night shift work and the risk of BC was identified. | (62) |
| Jones et al,
2019 | United Kingdom | 2003-2014 | Cohort study | The lack of overall
association with dose, duration, and intensity of night shift, does
not support an increased risk of BC from night shift work in
women. | (63) |
| Ritonja et
al, 2020 | Canada | 2005-2010 | Population-based
case-control study | No association
between residential outdoor light at night and BC. Light at night
has a small or no effect on BC risk. | (64) |
| Yang et al,
2019 | China | 2013-2016 | Case-control
study | Light exposure at
night, habitual timing of sleep, night/shift work and frequency of
night-time wakes were associated with an increased risk of BC.
Sleep duration, sleep quality, sleep medication use, insomnia
frequency and daytime nap were not associated with the risk of
BC. | (65) |
| Garcia-Saenz et
al, 2018 | Spain | 2008-2013 | Case-control
study | Prostate cancer and
BC were associated with high estimated exposure to outdoor light at
night in the blue-enriched light spectrum. | (66) |
| Lacerda et
al, 2019 | Not available | Not available | In vitro
study | Melatonin was able
to increase the expression levels of miR-148a-3p and decreased the
gene and protein expression levels of IGF-1R and VEGF, both in
vitro and in vivo. Melatonin also showed an inhibitory
effect on the survival, migration and invasion of BC cells. | (67) |
| Rajabi et
al, 2020 | Not available | Not available | In vitro
study | Melatonin was able
to attenuate DLL4 expression in estrogen-responsive BC cells and
was able to induce apoptosis. | (68) |
| El-Sokkary et
al, 2019 | Not available | Not available | In vitro
study | Melatonin and Taxol
decreased cell migration and invasion at low doses. Melatonin may
assist in preventing BC metastasis through inhibiting a
DJ-1/KLF17/ID-1 signalling axis. The combination of melatonin and
Taxol was a potent combination for management of BC
metastasis. | (69) |
| Liu et al,
2020 | Not available | Not available | In vitro
study | Melatonin regulated
BC progression via a lnc010561/miR-30/FKBP3 axis, which exhibited
anticancer properties. | (70) |
| Salamanca-Fernández
et al, 2018 | Not available | Up to 2017 | Systematic
review | In this systematic
review, 62.5% studies found an association between night shift work
and increased risk of BC and prostate cancer. The evidence of a
possible association between night-shift work and BC risk remains
contested though. | (72) |
| Dun et al,
2020 | Not available | Up to 2019 | Systematic review
and meta-analysis | Cancer risk was not
significantly elevated with the increased light exposure of
night-shift work. | (73) |
| Yuan et al,
2018 | Europe, North
America, Asia, and Australia | Up to 2016 | Meta-analysis | Confirmed the
positive association between night shift work and the risks of
several common types of cancer in women, including BC. Cancer risk
was increased with as the number of years of night shift work
accumulated. | (74) |
| de Castro et
al, 2018 | Brazil | Not available | Case-control
study | Melatonin serum
levels were lower in patients with BC and in nurses working at
night. Higher levels of melatonin and of a metabolite of oxidative
metabolism (acetyl-N-formyl-5-methoxykynurenamine) were related
with the clinical pathological characteristics of the patients with
BC, such as metastasis and lymph node positive status, suggesting a
relationship with the inflammatory response. | (77) |
| Vaughn et
al, 2018 | United States | Up to 2013 | Population-based
case-control study | The association of
sleep quality differed by menopausal status, where mild sleep
disturbance was associated with higher BC mortality in
premenopausal women. | (82) |
| McNeil et
al, 2020 | Canada | 2004-2008 | Cohort study | No associations
were found between shift work or sleep duration on the risk of
breast and colorectal cancer. | (83) |
| Shi et al,
2013 | Denmark | 1993-1997 | Cohort study | Long-term night
shift work exposure may lead to the downregulation of miR-219,
which may in turn lead to the downregulation of antitumor activity,
thus increasing BC risk. | (88) |
| Pham et al,
2019 | Korea | 2012-2017 | Hospital-based
case-control study | Night shift work
increased risk of BC in women who harboured the heterozygote
genotype of CRY2 rs2292912 or carried at least one minor allele of
RORA rs1482057. | (90) |
| Carugno et
al, 2019 | Italy | Not available | Cross-sectional
study | Night shift workers
were associated with estrogen receptor 1, TP53 and BRCA1
hypomethylation. Telomere length was decreased in workers who had
worked night shifts for >12 years. | (91) |
| Leensen et
al, 2017 | Netherlands | 2011-2015 | Longitudinal
prospective intervention study | Return to work of
patients with cancer was higher after completion of the
multidisciplinary rehabilitation program. | (99) |
Cordina-Duverger et al (59) analysed five case-controlled studies
(Australia, Canada, France, Germany and Spain) by pooling the data
into a single standardised dataset, demonstrating that night work
amplified the risk of BC in women in premenopause, especially in
those with a high frequency of night work, measured as the number
of nights per week, and a long period of exposure. Amongst
premenopausal women, the risk of BC was higher in recent night
workers [odds ratio (OR)=1.41; 95% confidence interval (CI),
1.06-1.88] than in those who had stopped night work more than 2
years earlier (59). Similarly,
women who worked at night showed an increased risk of developing BC
when compared to women who never worked nights in Mexico (OR=8.58;
95% CI, 2.19-33.8) (49), as well
as in Poland (OR=2.61; 95% CI, 1.94-3.53) (46). However, there was only a slight
increase in higher BC risk amongst night shift workers in the
Sister Study, with a cohort of 50,884 women who had a sister with
breast tumour, but were BC-free themselves (60). Moreover, in a study conducted in
Canada, Levin's equation was applied to assess attributable
population fractions (PAFs) amongst women who worked night or
rotating shifts in 1961-2000. The PAFs varied from 2.0-5.2, and 38%
of overall incident BC cases in 2011 were detected in women who
worked in health-related occupational environments (61). Nevertheless, this increased risk was
observed in two prospective cohorts (5,971 in the Nurses' Health
Study and 3,570 in the Nurses' Health Study 2, performed in USA),
which showed an association between a high risk of BC and long-term
night shift work (35). Conversely,
in a study conducted on Korean women (62), and another study based on the
Generations Study cohort (63)
demonstrated the lack of association between night shift work and
an increased risk of developing BC.
The causal links between night work and BC have yet
to be established, although a plausible biological mechanism could
be related to night light-related disruption of the circadian
rhythm. The inhibition of night melatonin secretion along with
sleep deprivation and chronodisruption is suggested to be a crucial
mechanism by which artificial light at night could contribute to BC
development (55).
However, even with regard to this proposed
mechanism, the studies have shown contested results; a
case-controlled study performed in Vancouver found no association
between nocturnal artificial light and BC (64); whereas a case-controlled study
performed in China (65) and a
population-based case-controlled study in Spain (66) both showed that there was an
increased risk of BC associated with exposure to night light, in
particular, with external light in the blue spectrum.
To better understand the association between night
work, the disruption of the circadian rhythm and the risk of BC,
several studies investigated the role of the genes involved in the
circadian pathway and melatonin regulation (51,67-71).
However, both the relationship between circadian rhythm and genetic
expression/modification and specific markers continues to be
debated (50,72-75).
Moreover, melatonin is also a powerful antioxidant,
due to its capacity to scavenge free radicals and stimulate
antioxidative enzymes (76). Lower
levels of melatonin were observed in patients with BC, suggesting
that working at night modifies the circulating levels of biomarkers
associated with inflammation and redox reactions (57,77).
Neuroendocrine disruption
In women employed in shift or night work, melatonin
secretion, which has anti-estrogenic activity, is inhibited by
light, resulting in a rise in estrogen levels (56). It is well known that early menarche,
late menopause and no pregnancies, all factors linked to sex
hormones exposure, are associated with an increased risk of BC
(78). Sleep deprivation, resulting
in shift work disorder, characterised by excessive sleepiness and
sleep disturbances (79), has a
strong influence on the neuro-immune-endocrine axis, which can
affect cell proliferation and immune responses, including cytokine
production (80) and some plasma
metabolites, that may play an essential role in the circadian
system (81). In particular, Daly
et al (8) described a 16%
increase in the incidence of BC in women aged 25-49 since 1990s,
which may be at least partly attributed to more improved screening
programs and detection methods, as well as changing lifestyles,
highlighting the role of the endocrine factors in the onset of BC.
In fact, younger women (<40 years) seem to be affected by more
aggressive phenotypes of BC, resulting in a higher mortality rate.
Moreover, young women were more likely to exhibit advanced disease
stage tumours (larger size, lymph node involvement, poorly
differentiated). Still, women aged <40 years seem to have a
higher frequency of familiar history of BC, with a higher frequency
of pathogenic mutations in the BC genes (BRCA1, BRCA2, TP53 and
PALB2) compared with women with BC that develops after the age of
40, thus mutation penetrance appears higher in younger patients
(8).
Regarding the relationship between poor sleep
quality and BC, it is impossible to give a straightforward
interpretation. In general, sleep disturbances are likely to be
associated with aggressive subtypes of BC, as demonstrated by the
Western New York Exposures and Breast Cancer study (82), conducted on a sample of 1,122
subjects with incident BC. This investigation also showed that
sleep quality, well-known to be influenced by occupational features
(42), is also linked to the state
of menopause. However, no association was found between BC risk and
sleep duration, sleep quality, use of sleep medication, frequency
of insomnia and daytime napping in a case-controlled study
conducted in China (65). This
research showed that night shift work and frequency of nocturnal
awakenings were associated with an increased risk of BC
independently from menopausal status and tumour estrogen receptor
status. Conversely, research on 21,804 participants from Alberta's
Tomorrow project evaluated the primary effects of shift work and
sleep duration on cancer incidence, and found no association
between shift work or sleep duration and BC risk (83).
Disruption of circadian genes
Changes in the expression of circadian genes can be
attributed to genetic or epigenetic mechanisms. Certain genes
[Brain and Muscle ARNT-Like 1 (Bmal1), Period Circadian Regulator
(Per)1 and Per2] have been hypothesized to act as tumour
suppressors (84,85). Primary epigenetic controls are
represented by DNA methylation and the modifications of histones
(86). Altered expression of genes
associated with the circadian rhythm is associated with atypical
cell proliferation, impairment of DNA repair systems and apoptosis,
the response to DNA damage, and an increase in drug resistance in
human cancer cells (85). A
previous study observed that mice with a shorter circadian cycle
showed hypermethylation in the promoters of cryptochrome circadian
regulator 1 (Cry1) and Per2 genes and hypomethylation in the
Circadian Locomotor Output Cycles Kaput (CLOCK) promoter (87).
Several studies have demonstrated that lifestyle and
circadian disruption are associated with changes in microRNA
expression, such as miR-127, miR-146b and miR-219. These altered
miRNA profiles may then lead to epigenetic modifications,
supporting the hypothesis that long-term night shift work may
increase BC risk (88,89).
A study conducted on Korean women investigated 22
polymorphisms in 11 genes in 941 cases of BC and 959 controls. In
the analysis of single nucleotide polymorphisms, a correlation
between night shift work and BC was found; specifically, night
shift exposure increased the risk of BC in women carrying the
heterozygous genotype of CRY2 rs2292912 or carrying at least one
minor allele of RORA rs1482057(90). Epigenetic changes in 5-methyl
cytosine in five circadian genes, BMAL1, CLOCK, CRY1, PER1 and
PER2, were also analysed in a population of night shift female
nurses (278 BC cases, 280 controls). The authors suggested that
epigenetic alteration of CLOCK, BMAL1, CRY1 and PER1 may contribute
to BC in night shift workers. An Italian study found that night
shifts were associated with ESR1, TP53 and BRCA1 hypomethylation in
female nurses. |Further analysis showed that these night
shift-associated markers could be of potential interest in the
study of cellular ageing, genomic variability and cancer
development (91). In another
case-controlled study amongst Norwegian nurses, intensity and
length of night work were associated with telomere shortening,
which may contribute to an increased risk of BC amongst women
working in shifts (52).
Bracci et al (92) evaluated alterations in BRCA
expression in shift workers, finding lower levels of BRCA1 and
BRCA2 in shift workers than in day workers, suggesting a potential
role associated with a higher risk of BC (92).
In a murine model of spontaneous mammary
carcinogenesis, authors noticed that mixed-background mice (B6*FVB
PyMT:Luc2) developed BC within 10-12 weeks of age, and demonstrated
that the circadian rhythm disruption considerably enhanced
cancer-cell dissemination and pulmonary metastasis, and affected
the immunosuppressive response showing the role of circadian
dysregulation on breast tumour progression (93).
4. Risk assessment and prevention
strategies
In work schedules characterized by night shifts, the
occupational physicians can play a key role both in risk assessment
and in prevention strategies. In particular, when workers
susceptible to BC are employed, healthcare professionals can
provide strategic contributions for primary prevention, health
promotion and return to work. Primary prevention has different
targets, such as adequately assessing the suitability for shift
work, organising shifts according to ergonomic criteria, and
adopting adequate compensatory measures to avoid significant
disturbances in circadian rhythms, accumulation of sleep loss and
conflicts in social life. Although certain pathologies can
constitute a handicap for rotating or night work, shift work cannot
and must not be a discriminatory criterion for selecting workers.
Particularly, for female workers, more stressful living conditions
can arise in relation to time pressures caused by the conflict
between irregular working hours and habitual domestic commitments,
especially for those women who are married and with children.
Conversely, it may be the specific family conditions and/or
economic needs that force several women to accept shift or night
work. However, it is clear that there is no optimal or best shift
pattern, so each shift scheme must be planned and adopted, taking
into account the specific working conditions, the peculiar
requirements of the tasks, and the particular individual and social
characteristics of the workers. The occupational physician should
evaluate individual health conditions, which could be related to or
aggravated by shift work, to exempt workers from shift work or at
least from the night shifts. It is also necessary that helpful
information and suggestions should be given to workers on how to
best cope with shift and night work, particularly concerning sleep,
diet, stress control and good physical health.
Regarding the organization of work schedules, it is
advisable to reduce night work by adopting rapid rotation schemes
to limit the number of consecutive night shifts as much as
possible, reducing the interference on circadian rhythms and sleep.
In fact, it is suggested to prefer the rotation of shifts in ‘phase
delay’ (Morning/Afternoon/Night), which supports the natural
biological circadian rhythms, interspersed by at least 11 h
intervals between one shift and the next, in order to allow
improved recovery from any sleep loss and fatigue.
The occupational physician can also implement health
promotion programs targeting the primary individual risk factors,
promoting healthy lifestyle behaviours, for example supporting the
introduction of a Mediterranean diet, that has been shown to be
associated with a lower risk of BC incidence in women (94,95).
The typical Mediterranean diet nutrients have shown a positive
influence on the expression of inflammatory biomarkers, oxidative
stress, DNA damage and genetic modifications, all aspects that can
affect BC outcomes (96,97). Moreover, lifestyle modifications can
positively impact BC survival, whose lower health-related quality
of life negatively affects treatment compliance and disease
outcomes (98).
Occupational physicians should encourage BC
screening programs in female workers at risk of BC. It is well
established that inclusion into mammography screening programs can
result in earlier detection, showing a decrease in the rate of
mastectomies, favouring breast conservation surgery. Therefore, BC
screening programs are associated with re-employment; in fact, an
early diagnosis is frequently followed by a more efficient recovery
and improved ability to work. After BC curative treatment, the
assessment of a patient's ability to return to work should consider
the following: Clinical outcomes, lifestyle and occupational
variables. After the sick leave period, employer support to
simplify the employee rehabilitation has been associated with a
positive return to work experience. A rehabilitation program that
combines occupational counselling, physical workout programs and
physiotherapy during chemotherapy resulted in an increased return
rate to work, improved quality of life and better ability to work
(99). In this setting, the role of
occupational physicians is crucial in informing workers on the
benefits of rehabilitation plans, healthy lifestyles and
assistances guaranteed by the law. A virtuous occupational
physician should collaborate with the employer to adjust individual
risk assessment and optimize tailored calibration of work tasks
(Fig. 1).
5. Conclusions
The current review focused on the contribution of
the occupational physician to the prevention and management of BC
in the workplace, with a brief overview of the main individual,
environmental and occupational risk factors associated with BC,
which may have synergistic effects towards the susceptibility,
development and progression of BC.
The overall examination of the reviewed
contributions does not allow to exclude that exposure to shift and
night work is related to an increased risk of developing BC;
moreover, it leads to the hypothesis that this relationship is
supported by both a theoretical background and possible
pathogenetic mechanisms.
Though further studies are needed to establish a
causal link, this review suggests introduction of a novel approach
to management, including screening tools in the workplace,
especially targeting those workers with greater exposure to shift
or night work.
In this context, the role of the occupational
physician is crucial in primary prevention, health promotion and in
the return to work process. In particular, this return to work
phase should take into consideration a multidisciplinary approach,
involving employers adopting working features tailored on the
specific worker's conditions.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
Not applicable.
Authors' contributions
CC, CF and FG conceived the subject of the review.
GB, SI and MT contributed to writing the manuscript. All authors
have read and approved the final manuscript. Data authentication is
not applicable.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
World Health Organization International
Agency for Research on Cancer (IARC): GLOBOCAN 2020, 2020.
|
|
2
|
Kispert S and McHowat J: Recent insights
into cigarette smoking as a lifestyle risk factor for breast
cancer. Breast Cancer (Dove Med Press. 9:127–132. 2017.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Costa C, Teodoro M, Rugolo CA, Alibrando
C, Giambò F, Briguglio G and Fenga C: MicroRNAs alteration as early
biomarkers for cancer and neurodegenerative diseases: New
challenges in pesticides exposure. Toxicol Rep. 7:759–767.
2020.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Kumar S, Sharma A and Kshetrimayum C:
Environmental and occupational exposure and female reproductive
dysfunction. Indian J Med Res. 150:532–545. 2019.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Fenga C: Occupational exposure and risk of
breast cancer (Review). Biomed Rep. 4:282–292. 2016.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Golubnitschaja O, Debald M, Yeghiazaryan
K, Kuhn W, Pešta M, Costigliola V and Grech G: Breast cancer
epidemic in the early twenty-first century: Evaluation of risk
factors, cumulative questionnaires and recommendations for
preventive measures. Tumor Biol. 37:12941–12957. 2016.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Feng Y, Spezia M, Huang S, Yuan C, Zeng Z,
Zhang L, Ji X, Liu W, Huang B, Luo W, et al: Breast cancer
development and progression: Risk factors, cancer stem cells,
signaling pathways, genomics, and molecular pathogenesis. Genes
Dis. 5:77–106. 2018.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Daly AA, Rolph R, Cutress RI and Copson
ER: A review of modifiable risk factors in young women for the
prevention of breast cancer. Breast Cancer (Dove Med Press).
13:241–257. 2021.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2017. CA Cancer J Clin. 67:7–30. 2017.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Rudolph A, Chang-Claude J and Schmidt MK:
Gene-environment interaction and risk of breast cancer. Br J
Cancer. 114:125–133. 2016.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Jung S, Wang M, Anderson K, Baglietto L,
Bergkvist L, Bernstein L, van den Brandt PA, Brinton L, Buring JE,
Eliassen AH, et al: Alcohol consumption and breast cancer risk by
estrogen receptor status: In a pooled analysis of 20 studies. Int J
Epidemiol. 45:916–928. 2016.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Ma X, Liu C, Xu X, Liu L, Gao C, Zhuang J,
Li H, Feng F, Zhou C, Liu Z, et al: Biomarker expression analysis
in different age groups revealed age was a risk factor for breast
cancer. J Cell Physiol. 235:4268–4278. 2020.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Yedjou CG, Sims JN, Miele L, Noubissi F,
Lowe L, Fonseca DD, Alo RA, Payton M and Tchounwou PB: Health and
racial disparity in breast cancer. Adv Exp Med Biol. 1152:31–49.
2019.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Barcenas CH, Wells J, Chong D, French J,
Looney SW and Samuel TA: Race as an independent risk factor for
breast cancer survival: Breast cancer outcomes from the medical
college of Georgia tumor registry. Clin Breast Cancer. 10:59–63.
2010.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Wieder R, Shafiq B and Adam N: African
American race is an independent risk factor in survival from
initially diagnosed localized breast cancer. J Cancer. 7:1587–1598.
2016.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Taheri M, Tavakol M, Akbari ME,
Anoshirvani AA, Aghabozorgi R, Almasi-Hashiani A and Abbasi M:
Socioeconomic inequalities in metastasis, recurrence, stage and
grade of breast cancer: A hospital-based retrospective cohort
study. J Prev Med Hyg. 60:E262–E269. 2019.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Beral V, Bull D, Doll R, Peto R and Reeves
G: Breast cancer and breastfeeding: Collaborative reanalysis of
individual data from 47 epidemiological studies in 30 countries,
including 50 302 women with breast cancer and 96 973 women without
the disease. Lancet. 360:187–195. 2002.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Johansson H, Bonanni B, Gandini S,
Guerrieri-Gonzaga A, Cazzaniga M, Serrano D, Macis D, Puccio A,
Sandri MT, Gulisano M, et al: Circulating hormones and breast
cancer risk in premenopausal women: A randomized trial of low-dose
tamoxifen and fenretinide. Breast Cancer Res Treat. 142:569–578.
2013.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Dall GV and Britt KL: Estrogen effects on
the mammary gland in early and late life and breast cancer risk.
Front Oncol. 7(110)2017.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Kröz M, Reif M, Glinz A, Berger B,
Nikolaou A, Zerm R, Brinkhaus B, Girke M, Büssing A and
Gutenbrunner C: CRF-2 study group. Impact of a combined
multimodal-aerobic and multimodal intervention compared to standard
aerobic treatment in breast cancer survivors with chronic
cancer-related fatigue-results of a three-armed pragmatic trial in
a comprehensive cohort design. BMC Cancer. 17(166)2017.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Tsaras K, Papathanasiou IV, Mitsi D,
Veneti A, Kelesi M, Zyga S and Fradelos EC: Assessment of
depression and anxiety in breast cancer patients: Prevalence and
associated factors. Asian Pacific J Cancer Prev. 19:1661–1669.
2018.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Fox RS, Ancoli-Israel S, Roesch SC, Merz
EL, Mills SD, Wells KJ, Sadler GR and Malcarne VL: Sleep
disturbance and cancer-related fatigue symptom cluster in breast
cancer patients undergoing chemotherapy. Support Care Cancer.
28:845–855. 2020.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Costa C, Briguglio G, Giambò F, Catanoso
R, Teodoro M, Caccamo D and Fenga C: Association between oxidative
stress biomarkers and PON and GST polymorphisms as a predictor for
susceptibility to the effects of pesticides. Int J Mol Med.
45:1951–1959. 2020.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Costa C, Briguglio G, Catanoso R, Giambò
F, Polito I, Teodoro M and Fenga C: New perspectives on cytokine
pathways modulation by pesticide exposure. Curr Opin Toxicol.
19:99–104. 2020.
|
|
25
|
Giambò F, Teodoro M, Costa C and Fenga C:
Toxicology and microbiota: How do pesticides influence gut
microbiota? A review. Int J Environ Res Public Health.
18(5510)2021.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Giambò F, Italia S, Teodoro M, Briguglio
G, Furnari N, Catanoso R, Costa C and Fenga C: Influence of toxic
metal exposure on the gut microbiota (Review). World Acad Sci J.
3(19)2021.
|
|
27
|
Cohn BA, Cirillo PM and Terry MB: DDT and
breast cancer: Prospective study of induction time and
susceptibility windows. J Natl Cancer Inst. 111:803–810.
2019.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Darbre PD: Aluminium and the human breast.
Morphologie. 100:65–74. 2016.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Filippini T, Torres D, Lopes C, Carvalho
C, Moreira P, Naska A, Kasdagli MI, Malavolti M, Orsini N and
Vinceti M: Cadmium exposure and risk of breast cancer: A
dose-response meta-analysis of cohort studies. Environ Int.
142(105879)2020.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Hiatt RA and Brody JG: Environmental
determinants of breast cancer. Annu Rev Public Health. 39:113–133.
2018.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Koual M, Tomkiewicz C, Cano-Sancho G,
Antignac JP, Bats AS and Coumoul X: Environmental chemicals, breast
cancer progression and drug resistance. Environ Health.
19(117)2020.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Pullella K and Kotsopoulos J: Arsenic
exposure and breast cancer risk: A re-evaluation of the literature.
Nutrients. 12(3305)2020.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Yang KJ, Lee J and Park HL:
Organophosphate pesticide exposure and breast cancer risk: A rapid
review of human, animal, and cell-based studies. Int J Environ Res
Public Health. 17(5030)2020.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Costa C, Miozzi E, Teodoro M and Fenga C:
Influence of genetic polymorphism on pesticide-induced oxidative
stress. Curr Opin Toxicol. 13:1–7. 2019.
|
|
35
|
Wegrzyn LR, Tamimi RM, Rosner BA, Brown
SB, Stevens RG, Eliassen AH, Laden F, Willett WC, Hankinson SE and
Schernhammer ES: Rotating night-shift work and the risk of breast
Cancer in the nurses' health studies. Am J Epidemiol. 186:532–540.
2017.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Fagundo-Rivera J, Gómez-Salgado J,
García-Iglesias JJ, Gómez-Salgado C, Camacho-Martín S and
Ruiz-Frutos C: Relationship between night shifts and risk of breast
cancer among nurses: A systematic review. Medicina (Kaunas).
56(680)2020.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Videnros C, Selander J, Wiebert P, Albin
M, Plato N, Borgquist S, Manjer J and Gustavsson P: Postmenopausal
breast cancer and occupational exposure to chemicals. Scand J Work
Environ Heal. 45:642–650. 2019.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Gehlert S and Clanton M: Shift work and
breast cancer. Int J Environ Res Public Health.
17(9544)2020.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Lee DG, Burstyn I, Lai AS, Grundy A,
Friesen MC, Aronson KJ and Spinelli JJ: Women's occupational
exposure to polycyclic aromatic hydrocarbons and risk of breast
cancer. Occup Environ Med. 76:22–29. 2019.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Schubauer-Berigan MK, Anderson JL, Hein
MJ, Little MP, Sigurdson AJ and Pinkerton LE: Breast cancer
incidence in a cohort of U.S. flight attendants. Am J Ind Med.
58:252–266. 2015.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Costa C, Mondello S, Micali E, Indelicato
G, Licciardello AA, Vitale E, Briguglio G, Teodoro M and Fenga C:
Night shift work in resident physicians: Does it affect mood states
and cognitive levels? J Affect Disord. 272:289–294. 2020.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Briguglio G, Teodoro M, Italia S, Verduci
F, Pollicino M, Coco M, De Vita A, Micali E, Alibrandi A, Lembo G,
et al: Salivary biomarkers and work-related stress in night shift
workers. Int J Environ Res Public Health. 18(3184)2021.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Jalilian H, Ziaei M, Weiderpass E, Rueegg
CS, Khosravi Y and Kjaerheim K: Cancer incidence and mortality
among firefighters. Int J Cancer. 145:2639–2646. 2019.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Labrèche F, Kim J, Song C, Pahwa M, Ge CB,
Arrandale VH, McLeod CB, Peters CE, Lavoué J, Davies HW, et al: The
current burden of cancer attributable to occupational exposures in
Canada. Prev Med. 122:128–139. 2019.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Eurofond. European Working Conditions
Survey, 3 Nov, 2020.
|
|
46
|
Szkiela M, Kusideł E, Makowiec-Dabrowska T
and Kaleta D: Night shift work-A risk factor for breast cancer. Int
J Environ Res Public Health. 17(659)2020.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Gómez-Salgado J, Fagundo-Rivera J,
Ortega-Moreno M, Allande-Cussó R, Ayuso-Murillo D and Ruiz-Frutos
C: Night work and breast cancer risk in nurses: Multifactorial risk
analysis. Cancers (Basel). 13(1470)2021.PubMed/NCBI View Article : Google Scholar
|
|
48
|
IARC: IARC monographs on the evaluation of
carcinogenic risks to humans. In: Vol. 93. IARC Monographs on the
Evaluation of Carcinogenic Risks to Humans, pp9-38, 2010.
|
|
49
|
Bustamante-Montes LP, Flores-Meza B,
Hernández-Valero MA, Cárdenas-López A, Dolores-Velázquez R,
Borja-Bustamante P and Borja-Aburto VH: Night shift work and risk
of breast cancer in women. Arch Med Res. 50:393–399.
2019.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Pahwa M, Labrèche F and Demers PA: Night
shift work and breast cancer risk: What do the meta-analyses tell
us? Scand J Work Environ Heal. 44:432–435. 2018.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Xiang S, Dauchy RT, Hoffman AE, Pointer D,
Frasch T, Blask DE and Hill SM: Epigenetic inhibition of the tumor
suppressor ARHI by light at night-induced circadian melatonin
disruption mediates STAT3-driven paclitaxel resistance in breast
cancer. J Pineal Res. 67(e12586)2019.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Erdem JS, Notø HØ, Skare Ø, Lie JS,
Petersen-Øverleir M, Reszka E, Pepłońska B and Zienolddiny S:
Mechanisms of breast cancer risk in shift workers: Association of
telomere shortening with the duration and intensity of night work.
Cancer Med. 6:1988–1997. 2017.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Erdem JS, Skare Ø, Petersen-Øverleir M,
Notø HØ, Lie JS, Reszka E, Pepłońska B and Zienolddiny S:
Mechanisms of breast cancer in shift workers: DNA methylation in
five core circadian genes in nurses working night shifts. J Cancer.
8:2876–2884. 2017.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Cos S, Álvarez-García V, González A,
Alonso CG and Martínez CC: Melatonin modulation of crosstalk among
malignant epithelial, endothelial and adipose cells in breast
cancer. Oncol Lett. 8:487–492. 2014.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Touitou Y, Reinberg A and Touitou D:
Association between light at night, melatonin secretion, sleep
deprivation, and the internal clock: Health impacts and mechanisms
of circadian disruption. Life Sci. 173:94–106. 2017.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Stevens RG and Schernhammer E:
Epidemiology of uriniary melatonin in women and its relation to
other hormones and night work. Cancer Epidemiol Biomarkers Prev.
14(551)2005.PubMed/NCBI View Article : Google Scholar
|
|
57
|
El-Benhawy SA, El-Tahan RA and Nakhla SF:
Exposure to radiation during work shifts and working at night act
as occupational stressors alter redox and inflammatory markers.
Arch Med Res. 52:76–83. 2021.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Rosa D, Terzoni S, Dellafiore F and
Destrebecq A: Systematic review of shift work and nurses' health.
Occup Med (Lond). 69:237–243. 2019.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Cordina-Duverger E, Menegaux F, Popa A,
Rabstein S, Harth V, Pesch B, Brüning T, Fritschi L, Glass DC,
Heyworth JS, et al: Night shift work and breast cancer: A pooled
analysis of population-based case-control studies with complete
work history. Eur J Epidemiol. 33:369–379. 2018.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Sweeney MR, Sandler DP, Niehoff NM and
White AJ: Shift work and working at night in relation to breast
cancer incidence. Cancer Epidemiol Biomarkers Prev. 29:687–689.
2020.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Pahwa M, Labrèche F, Kim J, Harris MA,
Song C, Peters CE, Arrandale VH, Davies H, McLeod CB and Demers PA:
The impact of night shift work on breast cancer: Results from the
burden of occupational cancer in Canada study. Am J Ind Med.
62:635–642. 2019.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Pham TT, Hwang M, Lee ES, Kong SY, Jung
SY, Lee S, Kim J, Ha M, Kim SY and Park B: Night-shift work and
risk of breast cancer in Korean women. Clin Epidemiol. 11:743–751.
2019.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Jones ME, Schoemaker MJ, McFadden EC,
Wright LB, Johns LE and Swerdlow AJ: Night shift work and risk of
breast cancer in women: The Generations Study cohort. Br J Cancer.
121:172–179. 2019.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Ritonja J, McIsaac MA, Sanders E, Kyba
CCM, Grundy A, Cordina-Duverger E, Spinelli JJ and Aronson KJ:
Outdoor light at night at residences and breast cancer risk in
Canada. Eur J Epidemiol. 35:579–589. 2020.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Yang W, Shi Y, Ke X, Sun H, Guo J and Wang
X: Long-term sleep habits and the risk of breast cancer among
Chinese women: A case-control study. Eur J Cancer Prev. 28:323–329.
2019.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Garcia-Saenz A, Sánchez de Miguel A,
Espinosa A, Valentin A, Aragonés N, Llorca J, Amiano P, Martín
Sánchez V, Guevara M, Capelo R, et al: Evaluating the association
between artificial light-at-night exposure and breast and prostate
cancer risk in Spain (MCC-Spain study). Environ Health Perspect.
126(047011)2018.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Lacerda JZ, Ferreira LC, Lopes BC,
Aristizábal-Pachón AF, Bajgelman MC, Borin TF and de Campos Zuccari
DAP: Therapeutic potential of melatonin in the regulation of
MiR-148a-3p and angiogenic factors in breast Cancer. Microrna.
8:237–247. 2019.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Rajabi A, Saber A, Pourmahdi M, Emami A,
Ravanbakhsh R, Khodavirdipour A, Khodaei M, Akbarzadeh M, Abdolahi
S, Hosseinpourfeizi MA and Safaralizadeh R: Anti-cancer effect of
melatonin via downregulation of Delta-like ligand 4 in
Estrogen-responsive breast cancer cells. Recent Pat Anticancer Drug
Discov. 15:329–340. 2020.PubMed/NCBI View Article : Google Scholar
|
|
69
|
El-Sokkary GH, Ismail IA and Saber SH:
Melatonin inhibits breast cancer cell invasion through modulating
DJ-1/KLF17/ID-1 signaling pathway. J Cell Biochem. 120:3945–3957.
2019.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Liu P and Xie X, Yang A, Kong Y,
Allen-Gipson D, Tian Z, Zhou L, Tang H and Xie X: Melatonin
regulates breast cancer progression by the lnc010561/miR-30/FKBP3
Axis. Mol Ther Nucleic Acids. 19:765–774. 2020.PubMed/NCBI View Article : Google Scholar
|
|
71
|
Chuffa LGA, Carvalho RF, Justulin LA, Cury
SS, Seiva FRF, Jardim-Perassi BV, Zuccari DAPC and Reiter RJ: A
meta-analysis of microRNA networks regulated by melatonin in
cancer: Portrait of potential candidates for breast cancer
treatment. J Pineal Res. 69(e12693)2020.PubMed/NCBI View Article : Google Scholar
|
|
72
|
Salamanca-Fernández E, Rodríguez-Barranco
M, Guevara M, Ardanaz E, Olry de Labry Lima A and Sánchez MJ:
Night-shift work and breast and prostate cancer risk: Updating the
evidence from epidemiological studies. An Sist Sanit Navar.
41:211–226. 2018.PubMed/NCBI View Article : Google Scholar
|
|
73
|
Dun A, Zhao X, Jin X, Wei T, Gao X, Wang Y
and Hou H: Association between night-shift work and cancer risk:
Updated systematic review and meta-analysis. Front Oncol.
10(1006)2020.PubMed/NCBI View Article : Google Scholar
|
|
74
|
Yuan X, Zhu C, Wang M, Mo F, Du W and Ma
X: Night shift work increases the risks of multiple primary cancers
in women: A systematic review and meta-analysis of 61 articles.
Cancer Epidemiol Biomarkers Prev. 27:25–40. 2018.PubMed/NCBI View Article : Google Scholar
|
|
75
|
Lee HE, Lee J, Jang TW, Kim IA, Park J and
Song J: The relationship between night work and breast cancer. Ann
Occup Environ Med. 30(11)2018.PubMed/NCBI View Article : Google Scholar
|
|
76
|
Zhou L, Chen X, Liu T, Gong Y, Chen S, Pan
G, Cui W, Luo ZP, Pei M, Yang H and He F: Melatonin reverses
H2O2-induced premature senescence in mesenchymal stem cells via the
SIRT1-dependent pathway. J Pineal Res. 59:190–205. 2015.PubMed/NCBI View Article : Google Scholar
|
|
77
|
de Castro TB, Bordin-Junior NA, de Almeida
EA and de Campos Zuccari DAP: Evaluation of melatonin and AFMK
levels in women with breast cancer. Endocrine. 62:242–249.
2018.PubMed/NCBI View Article : Google Scholar
|
|
78
|
Samavat H and Kurzer MS: Estrogen
metabolism and breast cancer. Cancer Lett. 356:231–243.
2015.PubMed/NCBI View Article : Google Scholar
|
|
79
|
Costa G, Haus E and Stevens R: Shift work
and cancer-Considerations on rationale, mechanisms, and
epidemiology. Scand J Work Environ Heal. 36:163–179.
2010.PubMed/NCBI View Article : Google Scholar
|
|
80
|
Everson CA, Henchen CJ, Szabo A and Hogg
N: Cell injury and repair resulting from sleep loss and sleep
recovery in laboratory rats. Sleep. 37:1929–1940. 2014.PubMed/NCBI View Article : Google Scholar
|
|
81
|
Davies SK, Ang JE, Revell VL, Holmes B,
Mann A, Robertson FP, Cui N, Middleton B, Ackermann K, Kayser M, et
al: Effect of sleep deprivation on the human metabolome. Proc Natl
Acad Sci USA. 111:10761–10766. 2014.PubMed/NCBI View Article : Google Scholar
|
|
82
|
Vaughn CB, Freudenheim JL, Nie J,
Sucheston-Campbell L, Wactawski-Wende J, Marian C, Shields PG,
Kallakury BV, Trevisan M and Ochs-Balcom HM: Sleep and breast
cancer in the western New York exposures and breast cancer (WEB)
study. J Clin Sleep Med. 14:81–86. 2018.PubMed/NCBI View Article : Google Scholar
|
|
83
|
McNeil J, Heer E, Willemsen RF,
Friedenreich CM and Brenner DR: The effects of shift work and sleep
duration on cancer incidence in Alberta's Tomorrow Project cohort.
Cancer Epidemiol. 67(101729)2020.PubMed/NCBI View Article : Google Scholar
|
|
84
|
Shostak A: Circadian clock, cell division,
and cancer: From molecules to organism. Int J Mol Sci.
18(873)2017.PubMed/NCBI View Article : Google Scholar
|
|
85
|
Hernández-Rosas F, López-Rosas CA and
Saavedra-Vélez MV: Disruption of the molecular circadian clock and
cancer: An Epigenetic Link. Biochem Genet. 58:189–209.
2020.PubMed/NCBI View Article : Google Scholar
|
|
86
|
Sahar S and Sassone-Corsi P: Circadian
clock and breast cancer: A molecular link. Cell Cycle. 6:1329–1331.
2007.PubMed/NCBI View Article : Google Scholar
|
|
87
|
Xia L, Ma S, Zhang Y, Wang T, Zhou M, Wang
Z and Zhang J: Daily variation in global and local DNA methylation
in mouse livers. PLoS One. 10(e0118101)2015.PubMed/NCBI View Article : Google Scholar
|
|
88
|
Shi F, Chen X, Fu A, Hansen J, Stevens R,
Tjonneland A, Vogel UB, Zheng T and Zhu Y: Aberrant DNA methylation
of miR-219 promoter in long-term night shiftworkers. Environ Mol
Mutagen. 54:406–413. 2013.PubMed/NCBI View Article : Google Scholar
|
|
89
|
Kochan DZ, Ilnytskyy Y, Golubov A, Deibel
SH, McDonald RJ and Kovalchuk O: Circadian disruption-induced
microRNAome deregulation in rat mammary gland tissues. Oncoscience.
2(428)2015.PubMed/NCBI View Article : Google Scholar
|
|
90
|
Pham TT, Lee ES, Kong SY, Kim J, Kim SY,
Joo J, Yoon KA and Park B: Night-shift work, circadian and
melatonin pathway related genes and their interaction on breast
cancer risk: Evidence from a case-control study in Korean women.
Sci Rep. 9(10982)2019.PubMed/NCBI View Article : Google Scholar
|
|
91
|
Carugno M, Maggioni C, Crespi E, Bonzini
M, Cuocina S, Dioni L, Tarantini L, Consonni D, Ferrari L and
Pesatori AC: Night shift work, DNA methylation and telomere length:
An investigation on hospital female nurses. Int J Environ Res
Public Health. 16(2292)2019.PubMed/NCBI View Article : Google Scholar
|
|
92
|
Bracci M, Ciarapica V, Zabaleta ME,
Tartaglione MF, Pirozzi S, Giuliani L, Piva F, Valentino M, Ledda
C, Rapisarda V, et al: BRCA1 and BRCA2 gene expression: Diurnal
variability and influence of shift work. Cancers (Basel).
11(1146)2019.PubMed/NCBI View Article : Google Scholar
|
|
93
|
Hadadi E, Taylor W, Li XM, Aslan Y,
Villote M, Rivière J, Duvallet G, Auriau C, Dulong S,
Raymond-Letron I, et al: Chronic circadian disruption modulates
breast cancer stemness and immune microenvironment to drive
metastasis in mice. Nat Commun. 11(3193)2020.PubMed/NCBI View Article : Google Scholar
|
|
94
|
Montagnese C, Porciello G, Vitale S,
Palumbo E, Crispo A, Grimaldi M, Calabrese I, Pica R, Prete M,
Falzone L, et al: Quality of life in women diagnosed with breast
cancer after a 12-month treatment of lifestyle modifications.
Nutrients. 13(136)2020.PubMed/NCBI View Article : Google Scholar
|
|
95
|
Porciello G, Montagnese C, Crispo A,
Grimaldi M, Libra M, Vitale S, Palumbo E, Pica R, Calabrese I,
Cubisino S, et al: Mediterranean diet and quality of life in women
treated for breast cancer: A baseline analysis of DEDiCa
multicentre trial. PLoS One. 15(e0239803)2020.PubMed/NCBI View Article : Google Scholar
|
|
96
|
Briguglio G, Costa C, Pollicino M, Giambò
F, Catania S and Fenga C: Polyphenols in cancer prevention: New
insights (Review). Int J Funct Nutr. 1(9)2020.
|
|
97
|
Falzone L, Grimaldi M, Celentano E,
Augustin LS and Libra M: Identification of modulated micrornas
associated with breast cancer, diet, and physical activity. Cancers
(Basel). 12(2555)2020.PubMed/NCBI View Article : Google Scholar
|
|
98
|
Montagnese C, Porciello G, Vitale S,
Palumbo E, Crispo A, Grimaldi M, Calabrese I, Pica R, Prete M,
Falzone L, et al: Quality of life in women diagnosed with breast
cancer after a 12-month treatment of lifestyle modifications.
Nutrients. 13(136)2020.PubMed/NCBI View Article : Google Scholar
|
|
99
|
Leensen MC, Groeneveld IF, van der Heide
I, Rejda T, van Veldhoven PLJ, Berkel SV, Snoek A, Harten WV,
Frings-Dresen MH and de Boer AG: Return to work of cancer patients
after a multidisciplinary intervention including occupational
counselling and physical exercise in cancer patients: A prospective
study in the Netherlands. BMJ Open. 7(e014746)2017.PubMed/NCBI View Article : Google Scholar
|