Introduction
The transient receptor potential canonical 6 (TRPC6)
channel plays an important role in the physiology and
pathophysiology of blood vessels. The TRPC6 channel is a
membrane-bound, nonselective, calcium-permeable cation channel. As
such, it is significantly involved in the regulation of the calcium
balance in cells expressing this channel (1). The typical pathway of TRPC6 channel
activation is via a G-protein coupled mechanism. Through a ligand
on the G-protein, phospholipase C (PLC) is activated. PLC
phosphorylates the membrane bound
phosphatidylinositol-4,5-bisphosphate and cleaves this into
inositol-1,4,5-trisphosphate and diacylglycerol (DAG). DAG can
directly activate the TRPC6 channel (2,3). In
addition, external DAG analogues can also activate the TRPC6
channel, such as 1-oleoyl-2-acetyl-sn-glycerol (4). The orally bioavailable and selective
antagonist, BI-749327 has been shown to be a possible option to
modulate the TRPC6 channel (5).
Therefore, TRPC6 channels are an interesting target for future
pharmacological therapies. Current knowledge regarding the function
of the TRPC6 channel in vessels is primarily based on animal models
(6) or cell cultures (7). Studies in a mouse model show that the
TRPC6 channel has similar functions to the α-adrenoreceptor and is
important for regulation of systemic blood pressure (8). Calcium influx directly mediates
cellular actions, such as the activation of myosin light chain
kinase, which leads to vasoconstriction (9). In relation to animal models, the
presence of TRPC6 in the vascular endothelium would be in agreement
with the current view; studies have shown that calcium influx via
TRP channels is crucial in endothelial cell physiology (10,11).
By activating the synthesis of nitric oxide (NO) the TRPC6 channel
contributes to vasoactivity. NO leads to vasodilation, thus
reducing blood pressure (12,13).
The physiological Baylis effect, also termed myogenic
autoregulation describes the vasoconstriction triggered by
increased blood pressure, or vasodilation caused by an acute drop
in blood pressure (14). This
mechanism is essential for constant blood flow to the brain and
kidney. In mouse models, the TRPC6 channel can be assigned a
leading role in the Baylis effect (15,16).
TRPC6 is also involved in the hypoxic vasoconstriction (Euler
Liljestrand mechanism), which regulates blood flow to capillaries
around alveoli based on alveolar ventilation (17,18).
In an ischemic brain model, improved circulation in the penumbra
with less degraded TRPC6 expression/function was observed,
indicating a vasoactive role for TRPC6 and highlighting its
potential clinical relevance (19).
These examples underpin the vasoactive relevance of TRPC6 channel
in vivo. Due to the relevance of the TRPC6 channel in
physiological processes, it is hypothesized that it is also
involved in certain pathophysiological events.
A well known role of TRPC6 channels in human
diseases is focal segmental glomerulosclerosis (20). An alteration of the TRPC6 gene leads
to defective formation of podocyte processes and consequently to
nephrotic syndrome (21,22).
A disease that affects the vascular system and in
which the TRPC6 channel is established to play a role in the
development of in humans is idiopathic pulmonary arterial
hypertension (IPAH). Pathophysiologically, proliferation of the
vessel walls and the microenvironment with a consecutive increase
in pressure in the pulmonary vascular system is relevant (23). In IPAH patients, increased
expression of the TRPC6 channel in pulmonary vessels may be
detected, which could be responsible for the development of the
disease (24,25). In addition, inflammation plays an
important role in the development of IPAH (26,27).
In certain IPAH patients, gene variations of the TRPC6 channel have
been detected, which possess special binding sites for NF-κB (a
proinflammatory transcription factor), in the promoter region and
thus increase the expression of TRPC6 channels through inflammatory
processes (28,29). Re-stenosis processes are still
feared complications after interventional procedures, such as stent
implantation (30). Neointima
formation and further fibroblast differentiation play a crucial
role in the process of restenosis. Studies have shown that the
TRPC6 channel is involved in both neointima formation (31) and fibroblast activation (32,33).
Furthermore, this finding raises the hypothesis that the TRPC6
channel is related to intimal proliferation in atherosclerosis.
This would suggest etiological involvement of TRPC6 in
atherosclerosis and associated diseases, such as coronary artery
disease or stroke (34).
In the process of aging in humans, dysregulation of
calcium hemostasis plays an important role in vascular dysfunction.
Co-participation of the TRPC6 channel in these processes is also
indicated through altered regulation (35). Studies have determined expression of
TRPC6 channels at the gene level in humans (35-37).
However, thus far, there are no systematic descriptions of the
localization of TRPC6 in human samples of vessels, to the best of
our knowledge. TRPC6 channels are interesting targets that can be
modulated pharmacologically (37),
thus determining their physiological localization may have clinical
relevance.
The aim of this pilot study was the investigation of
TRPC6 channels in human vessels to assess the translational value
of animal and cell culture data. Overall, 40 samples of vessels
from different locations from nine body donations were
included.
Materials and methods
Immunohistochemistry and controls
Various vessel samples (Table I) were obtained from a total of nine
body donors from the Anatomical Institute of Saarland University
and immersion-fixed in formaldehyde (Table II). The study was approved by the
Permanent Ethics Committee of the Saarland Medical Association,
Homburg/Saar, Germany (approval no. 163/20). The time post-mortem
was <72 h until the corpses were fixed using Weigners protocol
(38). The specimens were then
embedded in paraffin and 7 µm thin sections were prepared using a
microtome. The specimens were first stained with hematoxylin and
eosin (H&E) and assessed to determine the morphology of the
vessels and whether they were intact, as a basic requirement for
immunohistochemistry (IHC) analysis. The first step of IHC was
antigen retrieval using 1%-citrate-buffered-solution for 60 min at
95˚C in heating incubator, after which the samples were allowed to
passively cool down for 30 min in the citrate. After washing the
samples twice for 2 min in PBS each time, they were incubated with
a knockout-validated antibody against TRPC6 channel antigen
structures (Alomone Labs; cat. no. ACC-017). The specificity and
quality of the antibody was assessed using peptide-blocked control
samples (Alomone Labs; cat. no. BLP-CC017). For antibody
specificity, 40 µg control peptide was dissolved with 20 µl PBS and
then incubated with 40 µl 1:100 diluted TRPC6 primary antibody
overnight at 7˚C in tubes. Negative controls incubated with rabbit
serum (from an untreated rabbit from the Institute for
Biochemistry, Homburg, Germany) instead of TRPC6 antibodies were
included in each staining run. The protein concentration in both
solutions was identical (0.01 mg/ml). Horseradish
peroxidase-conjugated goat anti-rabbit antibodies (Invitrogen;
Thermo Fisher Scientific, Inc.; cat. no. A10547; 1:500) were used
as secondary antibodies. These were incubated for 10-12 h at room
temperature in a humidity chamber. The dilutions were made with
normal goat serum (Invitrogen; Thermo Fisher Scientific, Inc.; cat.
no. 01-6201). The added chromogen DAB became visible as brown
coloration, which was converted by an HRP catalyzed reaction. The
incubation time with the DAB was determined using light microscopy,
and was usually 10 min.
 | Table ILocation of the obtained vessels. |
Table I
Location of the obtained vessels.
| Vessel | Standardized
sampling location | Number of
samples |
|---|
| Arteria renalis
dextra | 2 cm from the
outflow from the aorta | 7 |
| Arteria renalis
sinistra | 2 cm from the
outflow from the aorta | 6 |
| Arteria thoracica
interna dextra | Third intercostal
space | 4 |
| Arteria thoracica
interna sinistra | Third intercostal
space | 2 |
| Arteria radialis
dextra | 2 cm proximal in
hight of Processus styloideus radii | 2 |
| Aorta
(abdominal/thoracal) | 2 cm proximal of
the aortic bifurcation, or ascending aorta | 6 |
| Vena cava
inferior | Paired to the
sampling site of the abdominal aorta | 2 |
| Vena jugularis
interna sinistra | Vagina carotica in
hight of larynx | 2 |
| Truncus
pulmonalis | 2 cm distal of
Valva trunci pulmonalis | 2 |
| Ramus
interventricularis anterior | Middle third,
beginning after the exit of the ramus circumflexus | 2 |
| Arteria iliaca
communis sinistra | 2 cm distal of
bifurcatio aortae | 1 |
| Sinus caroticus
dextra | Complete sinus
caroticus | 2 |
| Vena cava
superior | Between the
junction of the brachiocephalic trunci and the confluence with the
atrium dextrum | 1 |
| Arteria carotis
communis dextra | Ventral of musculus
sternocleidomastoideus, in the vagina carotica | 1 |
 | Table IIData on the body donors. |
Table II
Data on the body donors.
| Proband | Ag, years | Sex | Cause of death
according to death certificate | Co-morbidities | Special features of
the removal |
|---|
| 1 | 90 | Female | Dementia as a
consequence of arterial hypertension and deep vein thrombosis | Arterial
hypertension; deep vein thrombose | |
| 2 | 89 | Male | Decompensated heart
failure due to heart failure and CHD | Diabetes
mellitus | Severe
atherosclerotic changes in the aorta, not directly at the donor
site |
| 3 | 84 | Male | Cardiovascular
arrest due to heart failure | Condition after
STEMI; atherosclerosis; CHD | Partial
atherosclerotic changes of the sampling sites; absence of the
radial artery dextra |
| 4 | 78 | Female | Respiratory
insufficiency due to bronchial carcinoma | Nicotine abuse | Stent of the
abdominal aorta |
| 5 | 70 | Male | Sudden cardiac
death | Condition after
myocardial infarction; arte rial hypertension; Angio sclerosis;
Raynaud's disease | Stent of the
abdominal aorta with bifurcation aortae |
| 6 | 69 | Male | Tumor hemorrhage
due to tumor infiltration of the intrahepatic portal vein system
caused by cancer of unknown primary syndrome. | Peritoneal
carcinomatosis; incomplete deep paraplegia metastasis related; bone
metastases | |
| 7 | 76 | Female | Respiratory
insufficiency due to aspiration | Locked-in syndrome;
meningioma | |
| 8 | 64 | Female | Suspicion of
cardiac arrhythmia due to hyperthyroidism | Graves' disease;
Crohn's disease; liver cirrhosis | |
| 9 | 57 | Female | Central respiratory
paralysis due to advanced Parkinson's disease | Parkinson's
disease | |
Western blotting
As additional evidence of TRPC6 protein expression,
western blotting was performed on skeletonized vessel samples from
unfixed body donors with a post-mortem interval <48 h. For this,
human tissues were collected and frozen until required. Samples
were homogenized in RIPA buffer supplemented with proteinase
inhibitor Complete® (Roche Diagnostics) using a
precellys homogenizer. A total of 80 µg protein extract was loaded
on a 10% SDS gel, resolved using SDS-PAGE, transferred to a PVDF
membrane (Advansta), and incubated with primary antibodies in 5%
non-fat milk in TBS-Tween buffer (0.05% Tween; Merck KGaA) at 4˚C
overnight. The following primary antibodies were used for probing:
anti-TRPC-6 (1:200; Alomone Labs; cat. no. ACC-017) and
anti-β-actin (1:1,000; Sigma-Aldrich; Merck KGaA; cat. no. A5441).
Antibody specificity of the anti-TRPC6 antibody was determined by
peptide-inhibition using the corresponding blocking peptide (cat.
no. BLP-CC017; Alomone Labs) according to the manufacturer's
protocol. After washing the membrane in TBS-Tween for 15 min,
secondary antibody incubation was performed for 1 h at room
temperature using HRP-conjugated goat anti-rabbit (1:7,000; Thermo
Fisher Scientific, Inc.; cat. no. A16096) or goat anti-mouse
(1:7,000; Santa Cruz Biotechnology, Inc.; cat. no. sc-525409)
secondary antibodies. The membrane was washed for 15 min in
TBS-Tween. Development of electrochemiluminescent signals was
performed by incubation with WesternBright Quantum ECL substrate
(Advansta Inc.) for 5 min, and visualized using the ChemiDoc System
with the Image Lab Software (both from Bio-Rad Laboratories,
Inc.).
Statistical analysis
Overall, 40 samples were processed and evaluated
using a light microscope (magnification, x10 or x40). The
evaluation scheme considered the distinction of the three vessel
wall layers: Tunica intima, Tunica media and Adventitia. IHC
detected specific staining that was significantly different from
the negative control or any background staining. For histological
evaluation, the IHC stained specimens were evaluated using a
photomicroscope ((magnification, x10, x20 or x40) in a blinded
manner by two investigators. The signals were categorized into
strongly positive (++), positive (+), uncertain (?) and negative
(0). In this study modal values and relative frequencies were
determined. Image analyses were not performed due to the complexity
of the histological compartments. Stratification was performed into
the intima, media and adventitia and its structures, such as the
endothelium, subendothelium, elastica interna; stratum musculare
and elastica externa, as well as the surrounding connective tissues
with nerves, vasa vasorum and other cell types. Absolute and
relative frequencies were collected and mathematical modes were
determined for comparing the evaluated characteristics (Microsoft
Excel 365; Microsoft Corporation). In this descriptive
investigation no experimental groups were compared.
Results
The systematic evaluation of positive and specific
protein detection of the TRPC6 channel by means of IHC shows
differences in its expression in the layers of the vessel wall, as
well as in different vessel sections. Overall, 14 different
localizations of vessels were assessed based on 40 samples
(Table I). In this study the
primary focus was the renal artery and aortic samples. The other
vessel sections that were evaluated should be seen as random
samples. All evaluations of the samples can be seen in Table III. In renal arteries, positive
signals were seen in the tunica intima in 77% of cases (10 out of
13), in 0% of cases (0 out of 13) in the tunica media and in 77% of
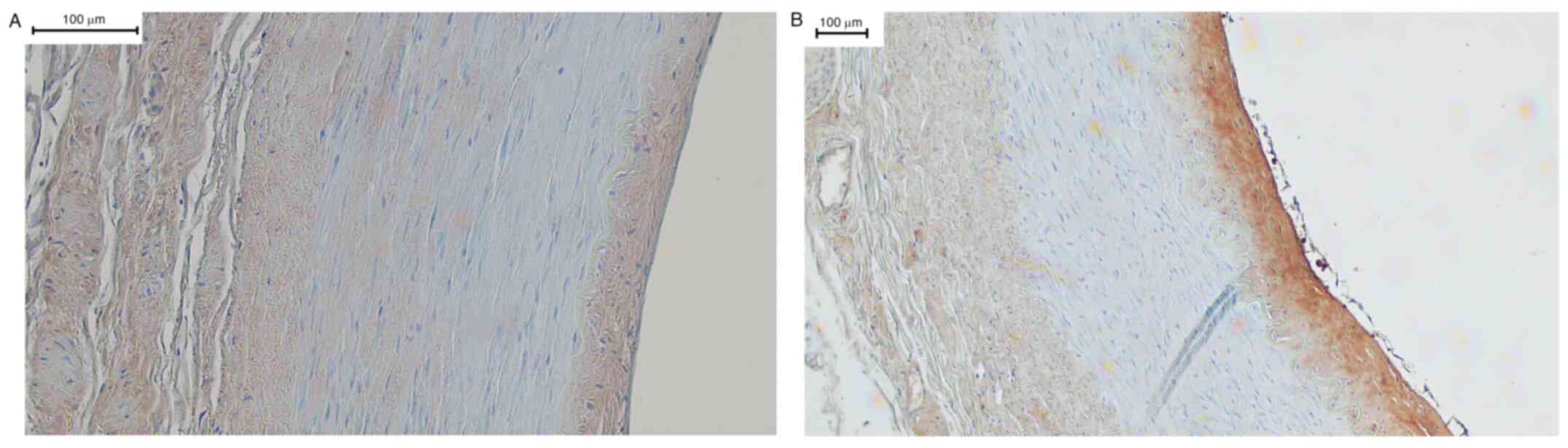
cases (10 out of 13) in the adventitia. Fig. 1A shows a sample of the left renal
artery, incubated with the TRPC6 channel antibody. Here, the tunica
intima with subendothelium is clearly stained brown. This brown
staining is a specific signal for the TRPC6 channel. In addition,
the adventitia, also homogeneously positive, possessed a vasa
vasorum with erythrocytes displaying clear positive signals. In
contrast, no signals were detected in the tunica media, suggesting
an absence of the TRPC6 channel in this layer. Fig. 1B shows the included negative control
incubated with rabbit serum. The quality of specific staining by
the primary antibody used could be confirmed in IHC, as shown in
Fig. 1C. Here, the primary TRPC6
antibody was pre-incubated overnight with the control peptide.
Similar results were obtained in other renal arteries incubated
with the primary antibody. As an example, renal arteries from other
body donors are shown in Fig. 2A
and B.
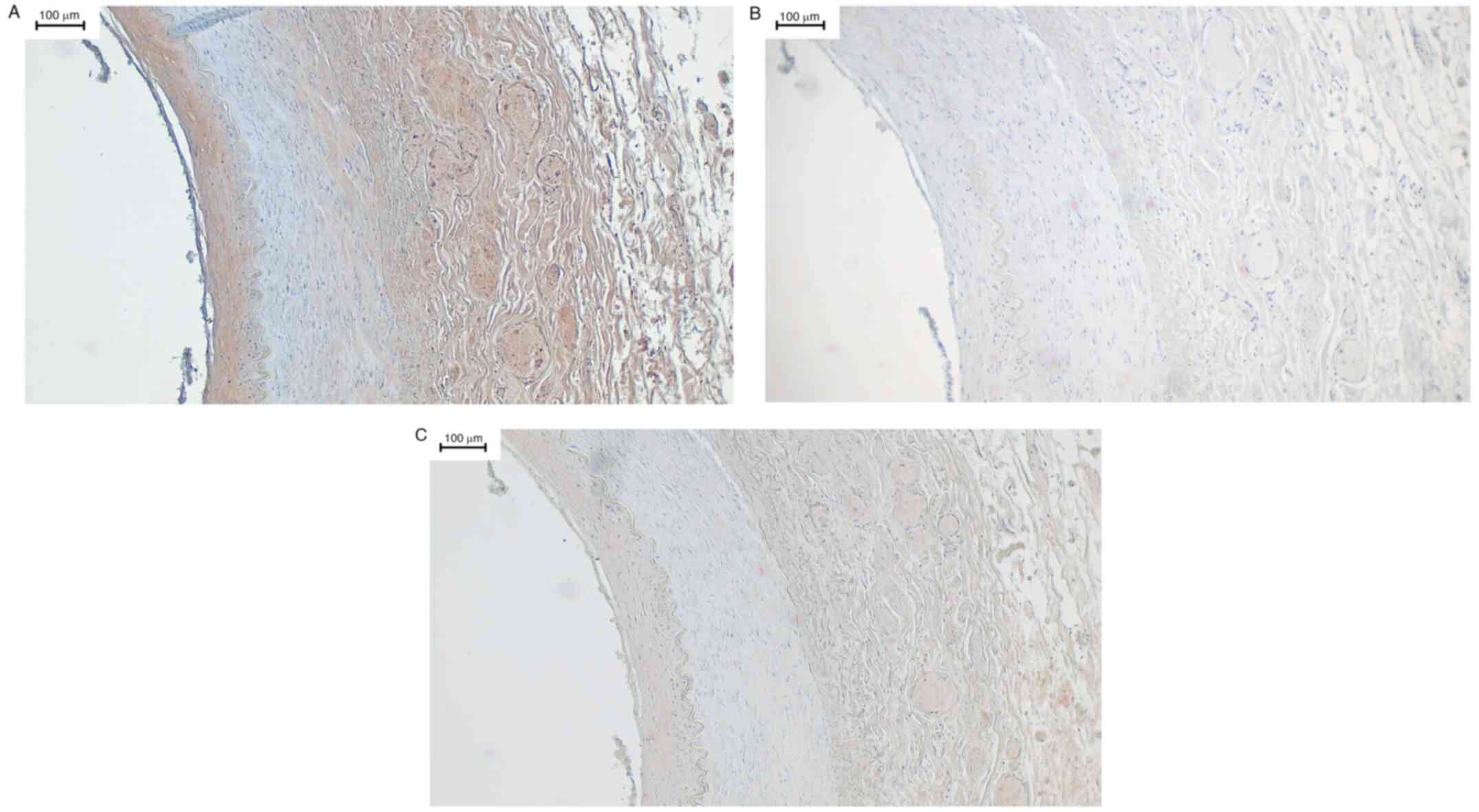 | Figure 1(A-C) Left renal artery. Left renal
artery, immunohistochemistry using a primary antibody against TRPC6
followed by DAB staining. The stratum endothelial, in the picture
on the left, is artefactually lifted in places, but still well
recognizable by the flat cell nuclei. The underlying stratum
subendotheliale can be clearly distinguished from the tunica media
by the membrana elastica interna. Due to the loss of some fibrin
fibers, the elastic fibers of this membrane have contracted as
expected and the membrana elastica interna also appears
artefactual. The tunica media is relatively unchanged and shows no
staining. After the membrana elastica externa, which is clearly
visible, the adventitia is located at the right of the membrana
elastica externa. Its is stroma slightly loosened and impresses
with several vasa vasorum. In these, erythrocytes can be
identified. Apart from these two entities and the surrounding
connective tissue, there are no assessable structures here. (B) The
corresponding negative control did not display any non-specific
staining. Here, all wall layers were negative. The same artefacts
that are present in the positive control can be detected. The
erythrocytes in the vaso vasorum can also be approximated, but
there is no brown staining. (C) The corresponding sections of the
vessel in Panels A and B in the same shape. The staining was
performed using primary antibodies, which were previously incubated
with a control peptide. The staining pattern is the same as in the
positive control, although the intensity of the staining is clearly
reduced. This corresponded with the expected staining and showed
the quality of the antibody in the test series. Magnification, x10.
TRPC6, transient receptor potential canonical 6. |
 | Table IIIOverview of the scoring results of
vessel staining. |
Table III
Overview of the scoring results of
vessel staining.
| Vessel | Tunica intima:
stratum endotheliale | Tunica intima:
stratum subendotheliale | Tunica media | Adventitia |
|---|
| Arteria renalis
dextra | ++ | 3 | ++ | 4 | ++ | 0 | ++ | 4 |
| | + | 2 | + | 1 | + | 0 | + | 0 |
| | ? | 0 | ? | 2 | ? | 1 | ? | 2 |
| | 0 | 2 | 0 | 0 | 0 | 6 | 0 | 1 |
| Arteria renalis
sinistra | ++ | 3 | ++ | 3 | ++ | 0 | ++ | 3 |
| | + | 2 | + | 1 | + | 0 | + | 3 |
| | ? | 0 | ? | 2 | ? | 2 | ? | 0 |
| | 0 | 1 | 0 | 0 | 0 | 4 | 0 | 0 |
| Arteria thoracica
interna dextra | ++ | 0 | ++ | 0 | ++ | 0 | ++ | 0 |
| | + | 0 | + | 0 | + | 0 | + | 1 |
| | ? | 1 | ? | 0 | ? | 0 | ? | 0 |
| | 0 | 3 | 0 | 4 | 0 | 4 | 0 | 3 |
| Arteria thoracica
interna sinistra | ++ | 0 | ++ | 0 | ++ | 0 | ++ | 0 |
| | + | 0 | + | 0 | + | 0 | + | 1 |
| | ? | 0 | ? | 0 | ? | 0 | ? | 0 |
| | 0 | 2 | 0 | 2 | 0 | 2 | 0 | 1 |
| Arteria radialis
dextra | ++ | 0 | ++ | 0 | ++ | 0 | ++ | 0 |
| | + | 0 | + | 0 | + | 0 | + | 0 |
| | ? | 0 | ? | 0 | ? | 0 | ? | 0 |
| | 0 | 2 | 0 | 2 | 0 | 2 | 0 | 2 |
| Aorta
abdominal/thoracal | ++ | 4 | ++ | 4 | ++ | 0 | ++ | 1 |
| | + | 0 | + | 1 | + | 0 | + | 4 |
| | ? | 1 | ? | 0 | ? | 0 | ? | 1 |
| | 0 | 1 | 0 | 1 | 0 | 6 | 0 | 0 |
| Vena cava
inferior | ++ | 0 | ++ | 0 | ++ | 0 | ++ | 0 |
| | + | 0 | + | 0 | + | 0 | + | 1 |
| | ? | 0 | ? | 0 | ? | 0 | ? | 0 |
| | 0 | 2 | 0 | 2 | 0 | 2 | 0 | 1 |
| Vena jugularis
interna sinistra | ++ | 0 | ++ | 0 | ++ | 0 | ++ | 0 |
| | + | 0 | + | 0 | + | 0 | + | 0 |
| | ? | 0 | ? | 0 | ? | 0 | ? | 0 |
| | 0 | 2 | 0 | 2 | 0 | 2 | 0 | 2 |
| Truncus
pulmonalis | ++ | 1 | ++ | 1 | ++ | 0 | ++ | 0 |
| | + | 0 | + | 0 | + | 1 | + | 1 |
| | ? | 1 | ? | 1 | ? | 0 | ? | 1 |
| | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 |
| 10 (Ramus
interventricularis anterior) | ++ | 0 | ++ | 0 | ++ | 0 | ++ | 1 |
| | + | 0 | + | 0 | + | 0 | + | 0 |
| | ? | 0 | ? | 0 | ? | 0 | ? | 1 |
| | 0 | 2 | 0 | 2 | 0 | 2 | 0 | 0 |
| Arteria iliaca
communis sinistra | ++ | 1 | ++ | 1 | ++ | 0 | ++ | 0 |
| | + | 0 | + | 0 | + | 0 | + | 1 |
| | ? | 0 | ? | 0 | ? | 0 | ? | 0 |
| | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 |
| Sinus
carotivus | ++ | 0 | ++ | 1 | ++ | 0 | ++ | 1 |
| | + | 1 | + | 1 | + | 0 | + | 0 |
| | ? | 0 | ? | 0 | ? | 0 | ? | 0 |
| | 0 | 1 | 0 | 0 | 0 | 2 | 0 | 1 |
| Vena cava
superior | ++ | 0 | ++ | 0 | ++ | 0 | ++ | 0 |
| | + | 0 | + | 0 | + | 0 | + | 0 |
| | ? | 0 | ? | 0 | ? | 0 | ? | 1 |
| | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 0 |
| Arteria carotis
communis dextra | ++ | 1 | ++ | 1 | ++ | 0 | ++ | 0 |
| | + | 0 | + | 0 | + | 0 | + | 1 |
| | ? | 0 | ? | 0 | ? | 0 | ? | 0 |
| | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 |
In all sections of the aortic samples, positive
signals were seen in the tunica intima in 83% of cases (5 out of
6), in 0% of cases (0 out of 6) in the tunica media and in 83% of
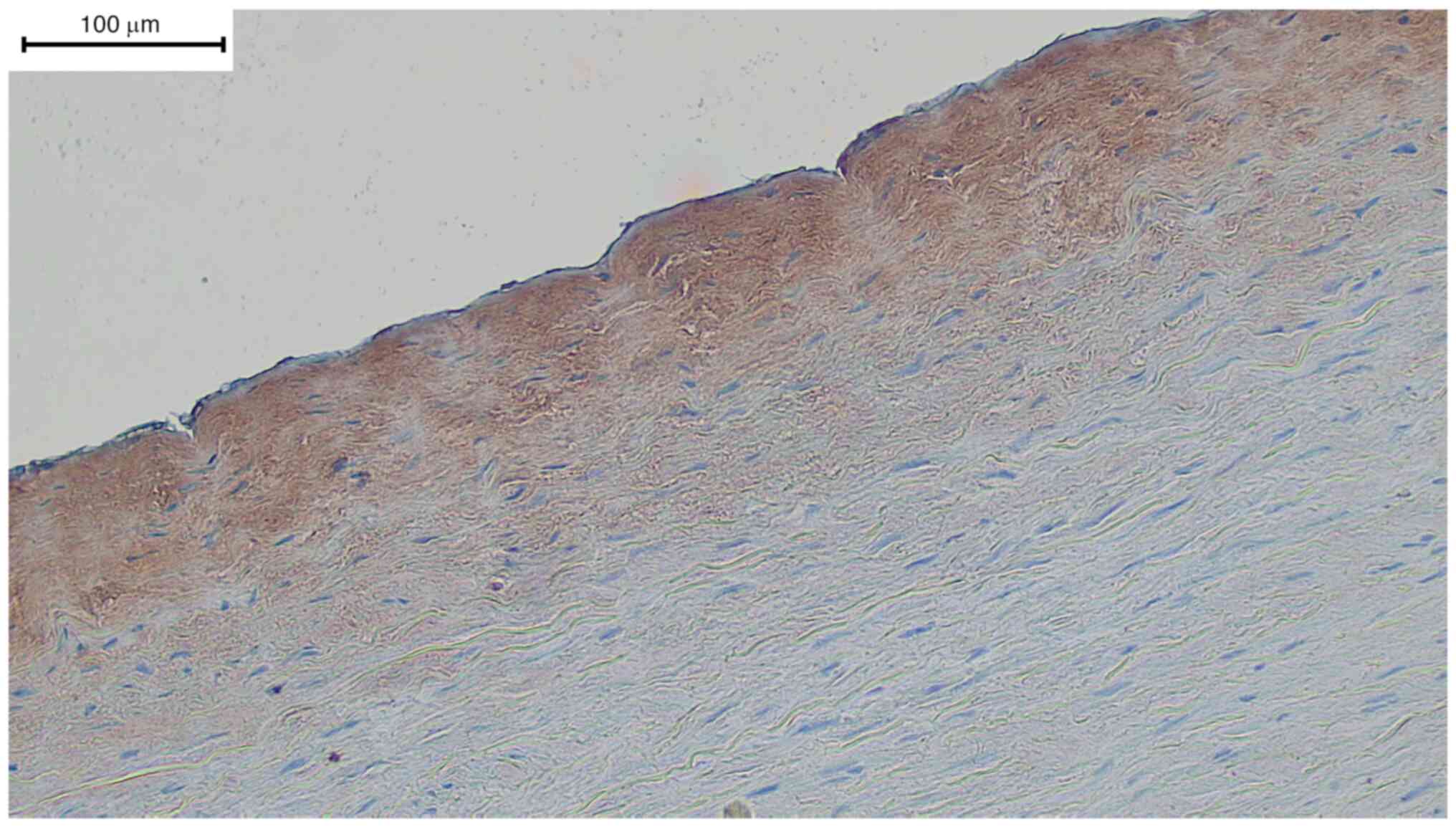
cases (5 out of 6) in the adventitia. Fig. 3 specifically shows the transition
between the tunica intima and media. The subendothelial showed very
strong positive signals in contrast to the endothelial cells. The
entirety of the tunica media showed negative staining (data not
shown).
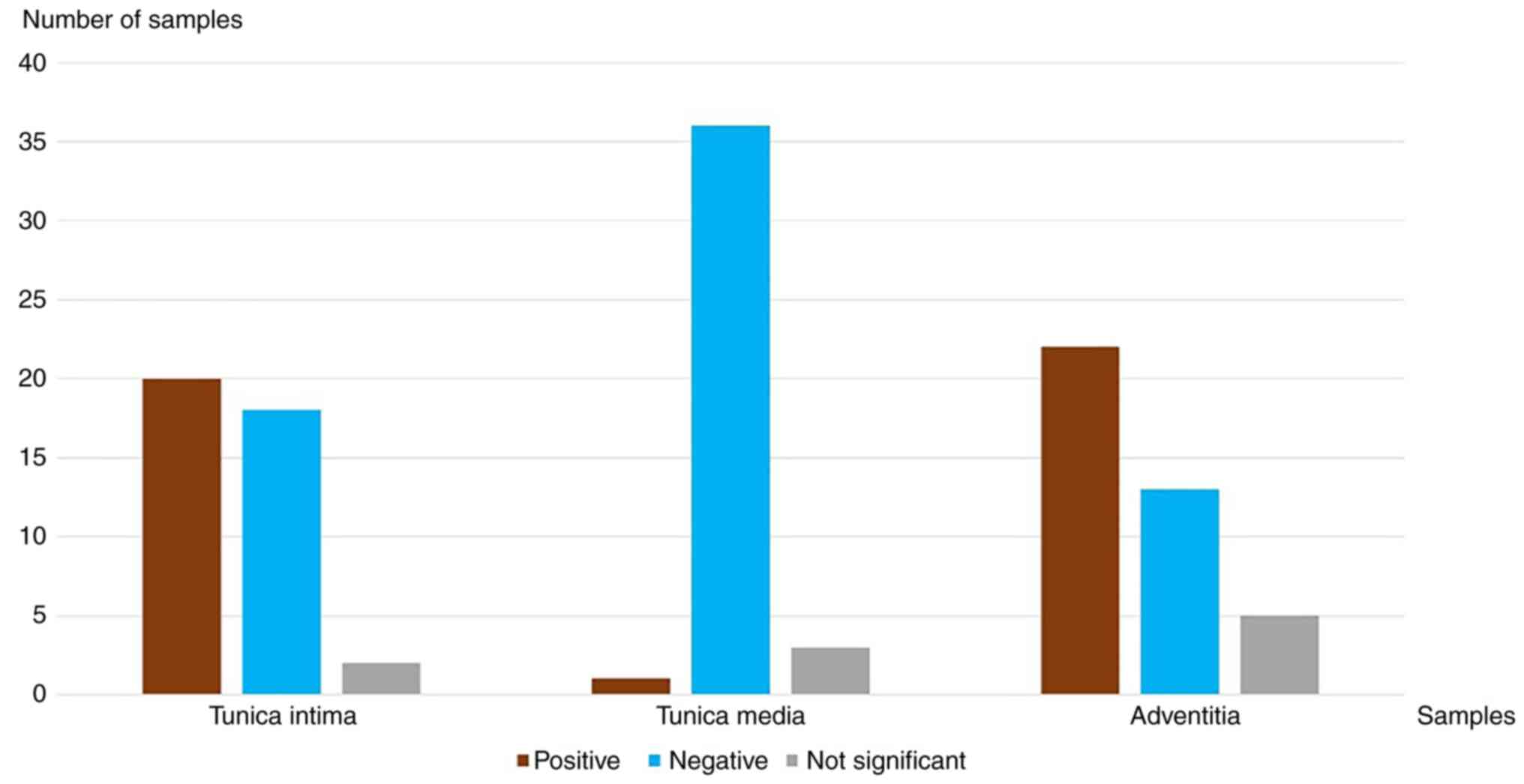
Of the 40 samples, specific staining was observed in
the tunica intima in 50% of cases (20 out of 40). The largest share
of these 20 samples were renal arteries and aortic samples. The
adventitia showed similar results; positive signals in 55% of cases
(22 out of 40). In contrast to the tunica intima, no specific
vascular segment was detected in which the TRPC6 channel expression
was increased. In all samples in which the vaso vasorum was
observable in the adventitia with an intact integrity, the vaso
vasorum itself, as well as the erythrocytes contained therein,
stained positively. There was less staining in the tunica media, in
which vascular smooth muscle cells (VSMCs) were detected, based on
H&E staining. The results demonstrated specific signals in 2.5%
of cases (1 out of 40). All the results are summarized in Fig. 4.
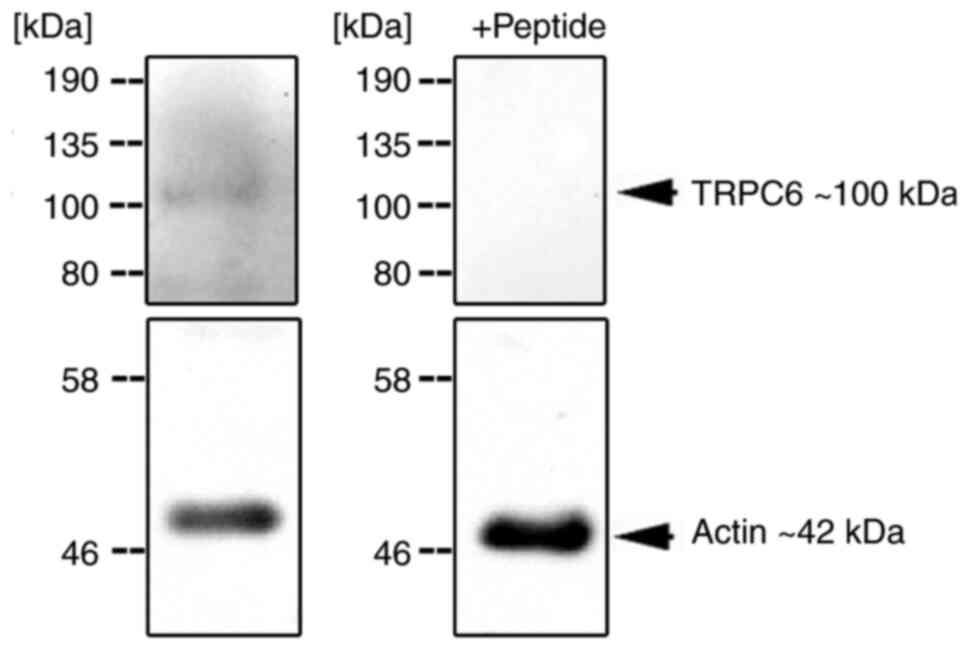
Western blotting detected the presence of TRPC6
protein in skeletonized preparations of the aorta. As shown in
Fig. 5, a protein band appeared at
the expected ~100 kDa marker, which was absent after peptide
inhibition. This is comparable to the results of another study that
used the same antibody in western blotting (19,39).
Discussion
The results of the present study show differences in
the distribution of TRPC6 expression in the vessels. Strong signals
could be detected in the tunica intima of the renal arteries and
aorta samples. Signals were also observed in samples of the
pulmonary trunk, the carotic sinus dextra and the right common
carotid artery. The tunica media showed a negative signal in nearly
all samples. The adventitia showed mixed signaling. In certain
tissues, TRPC6 signals in the adventitia was observed, whereas in
others, the signal was absent. Adventitia showed clustered positive
signals in larger arteries compared with the smaller arteries and
veins. In this study modal values and relative frequencies were
determined. Image analyses were not performed due to the complexity
of the histological compartments.
In the tunica intima the endothelium itself did not
always exhibit a positive signal. This is indicative of the varied
expression of TRPC6 based on the specific vessel segments. However,
there may also be a bias in the results; the endothelium became
slightly detached during the processes of fixation. This may have
led to cell damage and other artefacts that prevented continuous
positive staining. Detached cells from the stratum subendotheliale
or remnants of blood cells may have resulted in false positive
staining. TRPC6 channels were found in erythrocytes in the present,
in agreement with a previous study (40).
Concepts based on animal models of TRPC6 channels in
the vascular endothelium correspond with the results of the present
study. Studies have shown significant calcium influx via TRP
channels, and this is involved in the physiology of endothelial
cells (10,11). The presence of TRPC6 channels on
endothelial cells also fits in with the mechanism of vasodilation
involving NO release, and TRPC6-/- mice were shown to
have a higher systemic blood pressure than wild type mice (41). This could be explained by decreased
NO release from the endothelium due to missing TRPC6 channels. It
is possible that future therapeutics may also lead to improved
blood flow to the penumbra in acute strokes and thus possibly to an
improved outcome (19). The stratum
subendotheliale exhibited localized positive signals in renal
arteries and aortas. This was evidenced by similar patterns in 69%
of the renal arteries. The stratum subendotheliale is delimited by
the membrana elastica interna. The staining of the subendothelium
followed this border and was clearly demarcated from the tunica
media. In aortic samples, subendothelial signals were positive in
83% of cases. In contrast, the demarcation to the tunica media was
harder to delineate than in the renal arteries. A cellular
component of this layer is connective tissue-producing fibroblasts
(42). It has already been
demonstrated that TRPC6 channels are expressed on the surface of
fibroblasts (32). These results
confirm previous hypotheses that certain human fibroblasts possess
TRPC6 channels on their surface in vivo (32). In the pathogenesis of
atherosclerosis, changes often take place in the Tunica intima.
Fibrotic remodeling may result from overactivation of fibroblasts
(43,44). Fibroblast differentiation through
the TRPC6 channel via angiotensin-II and other cytokines has
already been demonstrated using mouse models. Upregulated
expression of the TRPC6 channel on fibroblasts was observed in
wild-type mice vs. TRPC6-/- mice following treatment
with angiotensin-II (33). Thus,
overactivation of the TRPC6 channel could lead to stenosis in
vascular segments. This would explain the increased expression of
TRPC6 channels in IPAH patients (24,25).
Thus, TRPC6 may also contribute to other diseases in which
atherosclerosis is etiologically involved (35). In particular, changes in the renal
arteries would allow for the development of renal artery stenosis
with consecutive renal (45). Renal
artery stenosis is a disease that triggers the
renin-angiotensin-aldosterone system through decreased renal artery
blood flow, resulting in a systemic increase in blood pressure
(46).
The results in the tunica media showed no positive
signal in the majority of vessel sections evaluated. In 2.5% of
cases TRPC6 could not be detected. Only one sample, the truncus
pulmonalis, exhibited TRPC6 staining. The tunica media consists
primarily of smooth muscle cells (47). These results contradict current
research assumptions that the TRPC6 channel is present on human
VSMCs. Supporting a potential false negative result of these
results, and thus a presence of TRPC6 channel on the cell surface
of VSMCs highlight a candidate carrying a relevant calcium influx
in VSMCs. This influx causes contraction of the muscle cells and
leads to vasoconstriction with a consecutive increase in blood
pressure (48). Likewise, the
sympathetic influence on vascular position is hypothesized to be
modulated by TRPC6 channels. Relevant calcium influxes were shown
in experiments comparing the effects of the α1-adrenoreceptor in
mediating vasoconstriction via transmitters with TRPC6 ligands
(49). It appears that
α1-adrenoreceptor mediated calcium influx causes upregulation of
TRPC6 channel density at the cell membrane (50). The physiological Bayliss effect also
argues for a mechanosensitive component that could mediate
constriction of vascular muscle cells through the involvement of
TRPC6 channels (51,52). In support of a valid negative result
observed in the present study however, and thus an absence of TRPC6
channels at the cell surface of vascular muscle cells, a previous
study showed higher blood pressure in TRPC6-/-mice
compared with wild-type mice (42).
This may be explained by an inferior vasodilatory effect due to
reduced NO release from the endothelium and a relatively minor
effect of TRPC6 on smooth muscles. It should be noted that none of
these studies replicate situations observed in humans. The majority
of these assumptions are based on research results from mouse
models (6,9) or cell cultures with human embryonic
cells, so-called HEK cells (53).
For this section, it can be summarized that there are studies that
have systematically detected TRPC6 expression in samples taken
directly from humans previously. This is complicated by the
challenges posed when working with body donors and the limited
number of samples available at any given time. The present study is
the first work in this field, and the results indicate absent TRPC6
channel expression in human vascular muscle cells, in contrast with
the current research opinion based on animal models. Further
studies are required to verify or refute this result.
Of all the layers evaluated, the adventitia was the
one with the most artefacts. The loose connective tissue, which
forms the majority of the adventitia, enables its anchoring and
displaceability in the tissue (54). In the present study, it may have
been destroyed by various mechanical stimuli during dissection. In
further processes, this layer may have been further progressively
destroyed, or become detached, by chemical stimuli. Large artefacts
were already partially visible in the H&E sections. Scattering
of other tissue types or cells into the adventitia cannot be
excluded. Thus, in addition to resident fibroblasts, macrophages,
erythrocytes or other cells may have also migrated in. The results
show in some sections clear positive staining of well preserved
entities, such as the vaso vasorum with erythrocytes. In some
cases, the entire adventitia also showed a homogeneous positive
signal. The morphological appearance did not allow an assessment of
the type of cells. Most of the sections showed only unspecific
staining, and the tissues in the adventitia could not be identified
correctly in many cases. Most of the results were too unspecific
and single specific positive signals should only be considered as a
rough indication for protein detection of the TRPC6 channel. In the
future, other methods should be considered to identify cells
showing positive protein detection of TRPC6 channels. For example,
immunofluorescence could be used to visualize TRPC6 using cell
specific markers (e.g. for fibroblasts).
In conclusion, the systematic evaluation of TRPC6
channel expression using IHC showed differences in its expression
in the layers of the vessel wall as well as in the different vessel
sections. The subendothelial stratum in the tunica intima shows
localized and clear protein detection of the TRPC6 channel in only
a few of the vascular sections examined. Here reproducible patterns
of positive signals was shown in several samples from the left and
right renal artery as well as the aorta. The endothelial cells
themselves also showed evidence of TRPC6 channel expression in some
preparations. Both are in agreement with current research opinion.
The tunica media was negative in almost all cases, which suggests
that the TRPC6 channel is not expressed on cells in this layer. As
some research findings point to vasodilatation rather than
vasoconstriction of vessels following TRPC6 induced NO release,
this result is only partially consistent with current research
findings. However, this result contrasts the results of animal
models, which have shown the TRPC6 channel is present on VSMC in
humans, too. The adventitia showed no clear staining pattern in a
comparison between different vessel sections. Only individual
structures in the adventitia, such as the vasa vasorum, were
labeled with the antibody against TRPC6. Understanding the
pathogenesis of cardiovascular diseases is of importance due to the
high prevalence and mortality of those affected by diseases of the
cardiovascular system. The possibilities of pharmacological
modulation of the TRPC6 channel may open up novel therapeutic
options for certain patient groups.
Acknowledgements
We would like to thanks Ms Irina Scheck, Mr Ronald
Dollwett, Ms Helga Meyer and Ms Barbara Michahelles-Horzella
(Institute of Anatomy and Cell Biology, Saarland University,
Homburg/Saar, Germany) for their support.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
JA and TT conceived the study. DS, TJ and AB
designed the study. JA, AB and TJ performed the experiments. JA
wrote the draft of the manuscript. All authors have read and
approved the manuscript. All authors confirm the authenticity of
the raw data.
Ethics approval and consent to
participate
The study was approved by the Permanent Ethics
Committee of the Saarland Medical Association, Homburg/Saar,
Germany (approval no. 163/20).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Montell C: The TRP superfamily of cation
channels. Sci STKE. 2005(re3)2005.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Hofmann T, Obukhov AG, Schaefer M,
Harteneck C, Gudermann T and Schultz G: Direct activation of human
TRPC6 and TRPC3 channels by diacylglycerol. Nature. 397:259–263.
1999.PubMed/NCBI View
Article : Google Scholar
|
|
3
|
Rohacs T: Regulation of transient receptor
potential channels by the phospholipase C pathway. Adv Biol Regul.
53:341–355. 2013.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Estacion M, Li S, Sinkins WG, Gosling M,
Bahra P, Poll C, Westwick J and Schilling WP: Activation of Human
TRPC6 channels by receptor stimulation. J Biol Chem.
279:22047–22056. 2004.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Lin BL, Matera D, Doerner JF, Zheng N, Del
Camino D, Mishra S, Bian H, Zeveleva S, Zhen X, Blair NT, et al: In
vivo selective inhibition of TRPC6 by antagonist BI 749327
ameliorates fibrosis and dysfunction in cardiac and renal disease.
Proc Natl Acad Sci USA. 116:10156–10161. 2019.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Saleh SN, Albert AP, Peppiatt CM and Large
WA: Angiotensin II activates two cation conductances with distinct
TRPC1 and TRPC6 channel properties in rabbit mesenteric artery
myocytes. J Physiol. 577:479–495. 2006.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Gibon J, Tu P, Bohic S, Richaud P, Arnaud
J, Zhu M, Boulay G and Bouron A: The over-expression of TRPC6
channels in HEK-293 cells favours the intracellular accumulation of
zinc. Biochim Biophys Acta. 1808:2807–2818. 2011.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Inoue R, Okada T, Onoue H, Hara Y, Shimizu
S, Naitoh S, Ito Y and Mori Y: The transient receptor potential
protein homologue TRP6 is the essential component of vascular
alpha(1)-adrenoceptor-activated Ca(2+)-permeable cation channel.
Circ Res. 88:325–332. 2001.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Jung S, Strotmann R, Schultz G and Plant
TD: TRPC6 is a candidate channel involved in receptor-stimulated
cation currents in A7r5 smooth muscle cells. Am J Physiol Cell
Physiol. 282:C347–C359. 2002.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Tiruppathi C, Ahmmed GU, Vogel SM and
Malik AB: Ca2+ signaling, TRP channels, and endothelial
permeability. Microcirculation. 13:693–708. 2006.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Greenberg HZE, Carlton-Carew SRE, Zargaran
AK, Jahan KS, Birnbaumer L and Albert AP: Heteromeric TRPV4/TRPC1
channels mediate calcium-sensing receptor-induced relaxations and
nitric oxide production in mesenteric arteries: Comparative study
using wild-type and TRPC1-/- mice. Channels (Austin).
13:410–423. 2019.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Flammer AJ, Anderson T, Celermajer DS,
Creager MA, Deanfield J, Ganz P, Hamburg NM, Lüscher TF, Shechter
M, Taddei S, et al: The assessment of endothelial function: From
research into clinical practice. Circulation. 126:753–767.
2012.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Takahashi N, Kozai D and Mori Y: TRP
channels: Sensors and transducers of gasotransmitter signals. Front
Physiol. 3(324)2012.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Aaslid R, Lindegaard KF, Sorteberg W and
Nornes H: Cerebral autoregulation dynamics in humans. Stroke.
20:45–52. 1989.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Voets T and Nilius B: TRPCs, GPCRs and the
Bayliss effect. EMBO J. 28:4–5. 2009.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Welsh DG, Morielli AD, Nelson MT and
Brayden JE: Transient receptor potential channels regulate myogenic
tone of resistance arteries. Circ Res. 90:248–250. 2002.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Weissmann N, Dietrich A, Fuchs B, Kalwa H,
Ay M, Dumitrascu R, Olschewski A, Storch U, Mederos y Schnitzler M,
Ghofrani HA, et al: Classical transient receptor potential channel
6 (TRPC6) is essential for hypoxic pulmonary vasoconstriction and
alveolar gas exchange. Proc Natl Acad Sci USA. 103:19093–19098.
2006.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Fuchs B, Rupp M, Ghofrani HA, Schermuly
RT, Seeger W, Grimminger F, Gudermann T, Dietrich A and Weissmann
N: Diacylglycerol regulates acute hypoxic pulmonary
vasoconstriction via TRPC6. Respir Res. 12(20)2011.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Du W, Huang J, Yao H, Zhou K, Duan B and
Wang Y: Inhibition of TRPC6 degradation suppresses ischemic brain
damage in rats. J Clin Invest. 120:3480–3492. 2010.PubMed/NCBI View
Article : Google Scholar
|
|
20
|
Bose B and Cattran D: Toronto
Glomerulonephritis Registry. Glomerular diseases: FSGS. Clin J Am
Soc Nephrol. 9:626–632. 2014.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Mukerji N, Damodaran TV and Winn MP: TRPC6
and FSGS: The latest TRP channelopathy. Biochim Biophys Acta.
1772:859–868. 2007.PubMed/NCBI View Article : Google Scholar
|
|
22
|
De Vriese AS, Sethi S, Nath KA, Glassock
RJ and Fervenza FC: Differentiating primary, genetic, and secondary
FSGS in adults: A Clinicopathologic approach. J Am Soc Nephrol.
29:759–774. 2018.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Schermuly RT, Ghofrani HA, Wilkins MR and
Grimminger F: Mechanisms of disease: Pulmonary arterial
hypertension. Nat Rev Cardiol. 8:443–455. 2011.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Yu Y, Fantozzi I, Remillard CV, Landsberg
JW, Kunichika N, Platoshyn O, Tigno DD, Thistlethwaite PA, Rubin LJ
and Yuan JX: Enhanced expression of transient receptor potential
channels in idiopathic pulmonary arterial hypertension. Proc Natl
Acad Sci USA. 101:13861–13866. 2004.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Yu Y, Sweeney M, Zhang S, Platoshyn O,
Landsberg J, Rothman A and Yuan JX: PDGF stimulates pulmonary
vascular smooth muscle cell proliferation by upregulating TRPC6
expression. Am J Physiol Cell Physiol. 284:C316–C330.
2003.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Hassoun PM, Mouthon L, Barberà JA,
Eddahibi S, Flores SC, Grimminger F, Jones PL, Maitland ML,
Michelakis ED, Morrell NW, et al: Inflammation, growth factors, and
pulmonary vascular remodeling. J Am Coll Cardiol. 54 (Suppl
1):S10–S19. 2009.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Savai R, Pullamsetti SS, Kolbe J, Bieniek
E, Voswinckel R, Fink L, Scheed A, Ritter C, Dahal BK, Vater A, et
al: Immune and inflammatory cell involvement in the pathology of
idiopathic pulmonary arterial hypertension. Am J Respir Crit Care
Med. 186:897–908. 2012.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Yu Y, Keller SH, Remillard CV, Safrina O,
Nicholson A, Zhang SL, Jiang W, Vangala N, Landsberg JW, Wang JY,
et al: A functional single-nucleotide polymorphism in the TRPC6
gene promoter associated with idiopathic pulmonary arterial
hypertension. Circulation. 119:2313–2322. 2009.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Malczyk M, Erb A, Veith C, Ghofrani HA,
Schermuly RT, Gudermann T, Dietrich A, Weissmann N and Sydykov A:
The role of transient receptor potential Channel 6 Channels in the
pulmonary vasculature. Front Immunol. 8(707)2017.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Byrne RA, Joner M and Kastrati A: Stent
thrombosis and restenosis: What have we learned and where are we
going? The Andreas Grüntzig Lecture ESC 2014. Eur Heart J.
36:3320–3331. 2015.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Wierer M, Werner J, Wobst J, Kastrati A,
Cepele G, Aherrahrou R, Sager HB, Erdmann J, Dichgans M, Flockerzi
V, et al: A proteomic atlas of the neointima identifies novel
druggable targets for preventive therapy. Eur Heart J.
42:1773–1785. 2021.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Yue L, Xie J and Nattel S: Molecular
determinants of cardiac fibroblast electrical function and
therapeutic implications for atrial fibrillation. Cardiovasc Res.
89:744–753. 2011.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Davis J, Burr AR, Davis GF, Birnbaumer L
and Molkentin JD: A TRPC6-dependent pathway for myofibroblast
transdifferentiation and wound healing in vivo. Dev Cell.
23:705–715. 2012.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Negri S, Faris P, Berra-Romani R, Guerra G
and Moccia F: Endothelial transient receptor potential channels and
vascular remodeling: Extracellular Ca2+ entry for
angiogenesis, arteriogenesis and vasculogenesis. Front Physiol.
10(1618)2020.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Harraz OF and Jensen LJ: Vascular calcium
signalling and ageing. J Physiol. 599:5361–5377. 2021.PubMed/NCBI View
Article : Google Scholar
|
|
36
|
Yip H, Chan WY, Leung PC, Kwan HY, Liu C,
Huang Y, Michel V, Yew DT and Yao X: Expression of TRPC homologs in
endothelial cells and smooth muscle layers of human arteries.
Histochem Cell Biol. 122:553–561. 2004.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Bai Y, Yu X, Chen H, Horne D, White R, Wu
X, Lee P, Gu Y, Ghimire-Rijal S, Lin DC and Huang X: Structural
basis for pharmacological modulation of the TRPC6 channel. Elife.
9(e53311)2020.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Janczyk P, Weigner J, Luebke-Becker A,
Kaessmeyer S and Plendl J: Nitrite pickling salt as an alternative
to formaldehyde for embalming in veterinary anatomy-A study based
on histo- and microbiological analyses. Ann Anat. 193:71–75.
2011.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Zhang Y, Lu W, Yang K, Xu L, Lai N, Tian
L, Jiang Q, Duan X, Chen M and Wang J: Bone morphogenetic protein 2
decreases TRPC expression, store-operated Ca(2+) entry, and basal
[Ca(2+)]i in rat distal pulmonary arterial smooth muscle cells. Am
J Physiol Cell Physiol. 304:833–843. 2013.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Föller M, Kasinathan RS, Koka S, Lang C,
Shumilina E, Birnbaumer L, Lang F and Huber SM: TRPC6 contributes
to the Ca2(+) leak of human erythrocytes. Cell Physiol Biochem.
21:183–192. 2008.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Dietrich A, Mederos y Schnitzler M,
Gollasch M, Gross V, Storch U, Dubrovska G, Obst M, Yildirim E,
Salanova B, Kalwa H, et al: Increased vascular smooth muscle
contractility in TRPC6-/- mice. Mol Cell Biol.
25:6980–6989. 2005.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Kramer RH, Fuh GM, Bensch KG and Karasek
MA: Synthesis of extracellular matrix glycoproteins by cultured
microvascular endothelial cells isolated from the dermis of
neonatal and adult skin. J Cell Physiol. 123:1–9. 1985.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Xu Q, Oberhuber G, Gruschwitz M and Wick
G: Immunology of atherosclerosis: Cellular composition and major
histocompatibility complex class II antigen expression in aortic
intima, fatty streaks, and atherosclerotic plaques in young and
aged human specimens. Clin Immunol Immunopathol. 56:344–359.
1990.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Kay HR, Korns ME, Flemma RJ, Tector AJ and
Lepley D Jr: Atherosclerosis of the internal mammary artery. Ann
Thoracic Surg. 21:504–507. 1976.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Elliott WJ: Renovascular hypertension: An
update. J Clin Hypertension (Greenwich). 10:522–533.
2005.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Klassen PS and Svetkey LP: Diagnosis and
management of renovascular hypertension. Cardiol Rev. 8:17–29.
2000.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Mozafari H, Zhou C and Gu L: Mechanical
contribution of vascular smooth muscle cells in the tunica media of
artery. Nanotechnol Rev. 8:50–60. 2019.
|
|
48
|
Nelson MT, Patlak JB, Worley JF and
Standen NB: Calcium channels, potassium channels, and voltage
dependence of arterial smooth muscle tone. Am J Physiol.
259:C3–C18. 1990.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Schilling WP: TRP proteins: Novel
therapeutic targets for regional blood pressure control? Circ Res.
88:256–259. 2001.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Kong F, Ma L, Zou L, Meng K, Ji T, Zhang
L, Zhang R and Jiao J: Alpha1-adrenergic receptor activation
stimulates calcium entry and proliferation via TRPC6 channels in
cultured human mesangial cells. Cell Physiol Biochem. 36:1928–1938.
2015.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Spassova MA, Hewavitharana T, Xu W,
Soboloff J and Gill DL: A common mechanism underlies stretch
activation and activation of TRPC6 channels. Proc Natl Acad Sci
USA. 103:16586–16591. 2006.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Inoue R, Jensen LJ, Jian Z, Shi J, Hai L,
Lurie AI, Henriksen FH, Salomonsson M, Morita H, Kawarabayashi Y,
et al: Synergistic activation of vascular TRPC6 channel by receptor
and mechanical stimulation via phospholipase C/Diacylglycerol and
phospholipase A2/omega-hydroxylase/20-HETE pathways. Circ Res.
104:1399–1409. 2009.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Shi J, Mori E, Mori Y, Mori M, Li J, Ito Y
and Inoue R: Multiple regulation by calcium of murine homologues of
transient receptor potential proteins TRPC6 and TRPC7 expressed in
HEK293 cells. J Physiol. 561:415–432. 2004.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Witter K, Tonar Z and Schöpper H: How many
Layers Has the adventitia?-Structure of the arterial tunica Externa
revisited. Anat Histol Embryol. 46:110–120. 2017.PubMed/NCBI View Article : Google Scholar
|