Introduction
Globally, breast cancer is the most prevalent
cancer, accounting for 11.7% of all cancer cases and 6.9% of cancer
deaths (1). It has been estimated
that 284,200 new breast cancer cases were diagnosed in 2021 in the
US (2). In Jordan, 2,403 new breast
cancer cases were diagnosed last year accounting for 38.5% of all
cases of cancer in women (3). Tumor
metastasis is a major cause of breast cancer mortality (4). This multi-step process encompasses
local tumor invasion, migration of primary cells, and colonization
at distal sites (5). Metastatic
breast cancer (MBC) represents 6% of newly diagnosed breast cancer
cases. However, 20-30% of early-stage breast cancer cases
eventually develop into MBC (6).
Systemic therapies are the primary treatment options for MBC
including chemotherapy, endocrine therapy, and targeted therapy
(7). Although combination
chemotherapy is commonly used in MBC, it has been associated with
increased toxicity (8,9). Furthermore, the emergence of
chemotherapeutic resistance limits the effectiveness of breast
cancer treatments, thereby increasing disease relapse and death
(10). Therefore, the
identification of novel strategies targeting the primary tumor with
enhanced efficacy and reduced toxicity are needed to improve
patient outcomes.
Calcium channel blockers (CCB) have been implicated
as anti-cancer molecules in several types of human cancers. For
example, amlodipine, a dihydropyridine CCB, has been shown to
induce apoptosis, resulting in cell cycle arrest, and suppress the
proliferation of cancerous cells in several studies (11-13).
In addition, Ji et al (14)
showed that p-glycoprotein-mediated multidrug resistance could be
ameliorated in leukemic cells when an amlodipine derivative was
used, which in turn prevented doxorubicin efflux and thus enhanced
its efficacy. Moreover, in vitro and in vivo studies
on human epidermoid cancerous cells have shown that several CCBs
can inhibit cancer cell growth including amlodipine, nicardipine,
and nimodipine (15). However, the
exact cellular and molecular anticancer mechanisms of amlodipine
have not been studied in breast cancer cells. In the present study,
the effects of amlodipine treatment on breast cancer cell
proliferation, apoptosis, colony formation, and invasion were
evaluated, and the protein expression levels of the downstream
targets were determined as well.
Materials and methods
Cell culture and drug treatment
Triple-negative MDA-MB-231 and luminal MCF-7 breast
cell lines were purchased from the American Type Culture Collection
(ATCC). Cells were cultured in RPMI-1640 media supplemented with
10% FBS and 1% penicillin/streptomycin in a humidified incubator
with 5% CO2 at 37˚C. DMSO was used as a solvent to
prepare amlodipine stocks (Tocris Bioscience), with a final DMSO
concentration of <0.1% in all experiments.
Cell viability assay
To evaluate the effects of amlodipine on breast
cancer cell viability, the colorimetric MTT cell proliferation
assay (ATCC) was performed as described previously (16). Briefly, 1x104 cells/well
were cultured in a 96-well plate and incubated overnight. Then,
cells were treated with several concentrations of amlodipine or
with DMSO as a control. After 48 h of treatment, cells were
incubated at 37˚C for 4 h with MTT solution at a final
concentration of 500 µg/ml. To solubilize formazan crystals, 100 µl
DMSO was added to each well. The optical density was measured at
490 nm on a microplate reader (BioTek Instruments, Inc.). The
results are expressed as a percentage of viable cells normalized to
vehicle-treated cells using the following equations: % Of viable
cells in each well=(Absorbance treatment/Average of
Absorbance vehicle in 4 replicates)x100; and % of viable
cells for each treatment concentration=Average of normalized % of
viable cells in 4 treatment replicates.
Caspase-3/7 assay
An Apo-ONE® homogeneous caspase-3/7 assay
(Promega Corporation) was used to assess the effects of amlodipine
on the induction of caspase-3/7 activities in MDA-MB-231 cells as
described previously (17).
Briefly, cells were plated at a density of 1x104
cells/well in a 96-well black plate. After attachment, cells were
treated with several concentrations of amlodipine or with DMSO as a
control. After 48 h of treatment, the caspase-3/7 reagent was added
to each well in a 1:1 ratio with the sample volume at room
temperature. After 3 h of incubation, enzyme activity was analyzed
using a synergy 2 multi-mode microplate reader (Biotek Instruments,
Inc.) at excitation and emission wavelengths of 499 nm and 521 nm,
respectively.
Colony formation assay
Assessing anchorage-dependent growth of breast
cancer cells was performed using colony formation assays as
previously described (18,19). MCF-7 cells were plated at a low
density (2x103 cells/flask) in T25 flasks and incubated
for 24 h, then treated with amlodipine or DMSO as a control.
Culture media was replaced every 3 days. Following 3 weeks of
incubation, PBS was used to wash the cells before fixing them with
pre-cooled (1:1) methanol/acetone at -20˚C for 15 min. After
staining with 0.1% crystal violet for 5 min at room temperature,
the colonies that had formed were visualized using a light
microscope (x4 magnification).
Invasion assay
Invasion assays were performed using Corning BioCoat
Matrigel Invasion Chambers (Corning Inc.) as described previously
(20,21). Cells were resuspended in serum-free
media with various concentrations of amlodipine (0, 5 or 10 µM),
and a chemotactic serum gradient was generated by placing media
supplemented with 10% FBS in the bottom chambers. After 24 h, the
invading cells were fixed with ice-cold ethanol at -20˚C for 15
min, stained with 0.1% crystal violet for 5 min at room
temperature, visualized using a light microscope (x4 magnification)
and counted using ImageJ (version 1.53q; National Institutes of
Health). The results are expressed as a percentage of invading
cells in treatment groups relative to the control group.
Western blotting
To assess the effects of amlodipine on the protein
expression levels of downstream targets, western blotting was
performed as previously described (21). Briefly, cells were plated at a
density of 5x104 cells/well into a 6-well plate. The
following day, the cells were treated with amlodipine (1-25 µM) or
DMSO as a control for 48 h. After cell washing with ice-cold PBS,
cells were lysed in RIPA buffer (Thermo Fisher Scientific, Inc.)
containing protease inhibitor (150 µl/well for 30 min on ice). Cell
lysates were transferred into Eppendorf tubes and then centrifuged
at 13,000 x g for 5 min at 4˚C. Proteins were quantified in all
collected supernatants using a BCA assay. Protein samples were
loaded in equal amounts (35 µg per lane) with one lane for 10 µl of
the protein ladder into 10% polyacrylamide gels in tris-glycine
buffer and run at 200 mv for 40 min at room temperature. After the
proteins had been resolved, they were transferred to nitrocellulose
membranes, incubated with primary antibodies (all 1:1,000 dilution)
for 2 h at room temperature against phospho (p-)ERK1/2 (Cell
Signaling Technology, Inc.; cat. no. 5726), ERK1/2 (Cell Signaling
Technology, Inc.; cat. no. 9102), Bcl-2 (Cell Signaling Technology,
Inc.; cat. no. 3498), and integrin β1 (Cell Signaling Technology,
Inc.; cat. no. 4706). GAPDH was used as the loading control (Cell
Signaling Technology, Inc.; cat. no. 5174). After washing with TBST
buffer, membranes were incubated with the secondary horseradish
peroxidase-conjugated antibodies (1:1,000) for 1 h at room
temperature [anti-rabbit IgG (cat. no. 7074) or anti-mouse IgG
(cat. no. 7076)]. An enhanced chemiluminescent detection kit was
used to visualize the immunoreactive protein bands using the
Montreal Biotech Fusion Pulse 6 imaging system (Montreal Biotech
Inc.). All experiments were repeated three times.
Statistical analysis
Data were analyzed using GraphPad Prism version 9
(GraphPad Software, Inc.). A one-way ANOVA followed by a Tukey's
multiple comparison test was used to compare the difference between
multiple groups. The half-maximal inhibitory concentration
(IC50) values were obtained by applying a nonlinear
regression curve fit analysis. P<0.05 was considered to indicate
a statistically significant difference. Data are presented as the
mean ± SEM.
Results
Cytotoxic effects of amlodipine on
breast cancer cells
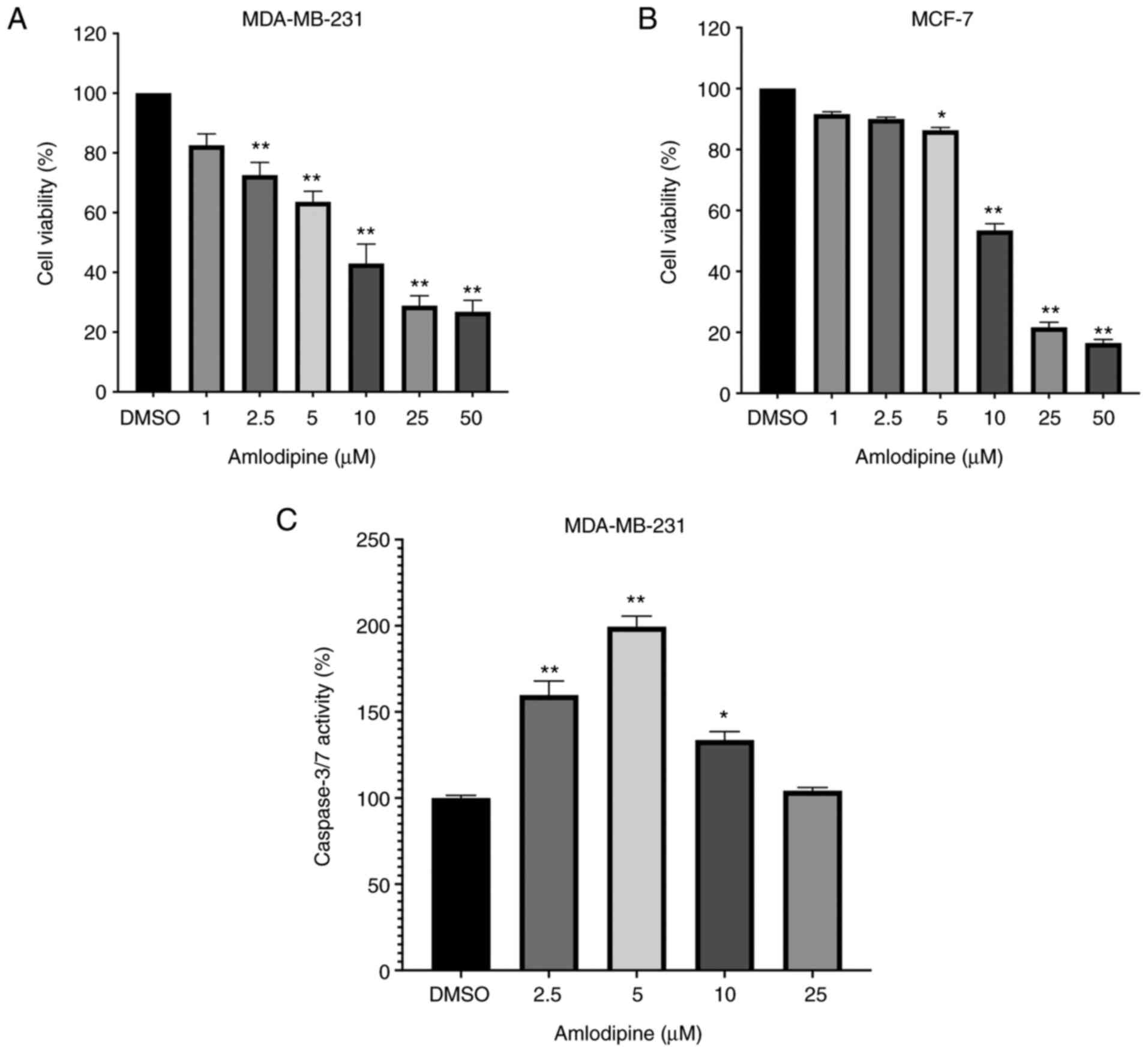
The in vitro biological effects of amlodipine
treatment on MDA-MB-231 and MCF-7 cell proliferation are shown in
Fig. 1. Amlodipine treatment
reduced MDA-MB-231 and MCF-7 cell viability in a dose-dependent
manner. In MDA-MB-231 cells, treatment with 2.5-50 µM amlodipine
significantly reduced cell viability compared with the
control-treated cells (P<0.05, Fig.
1A). In MCF-7 cells, 5-50 µM amlodipine significantly reduced
cell viability compared with the control-treated cells (P<0.05,
Fig. 1B). The IC50
values for amlodipine in MDA-MB-231 and MCF-7 cells were 8.66 and
12.60 µM, respectively. These findings suggest a cytotoxic effect
of amlodipine on breast cancer cells.
Since amlodipine reduced breast cancer cell
viability, the potential underlying mechanisms of the growth
suppression were assessed by analyzing caspase-3/7 activity, which
is a well-established marker of apoptosis (22). The results revealed that amlodipine
treatment (2.5-10 µM) significantly increased caspase-3/7 activity
compared with the control treatment in MDA-MB-231 cells (P<0.05,
Fig. 1C) and thus highlighted
caspase activation as a potential mechanism underlying the
cytotoxic effects of amlodipine. However, caspase-3/7 activity was
not assessed in the MCF-7 cells since they do not express
caspase-3, and thus caspase activation may be underestimated
(23,24).
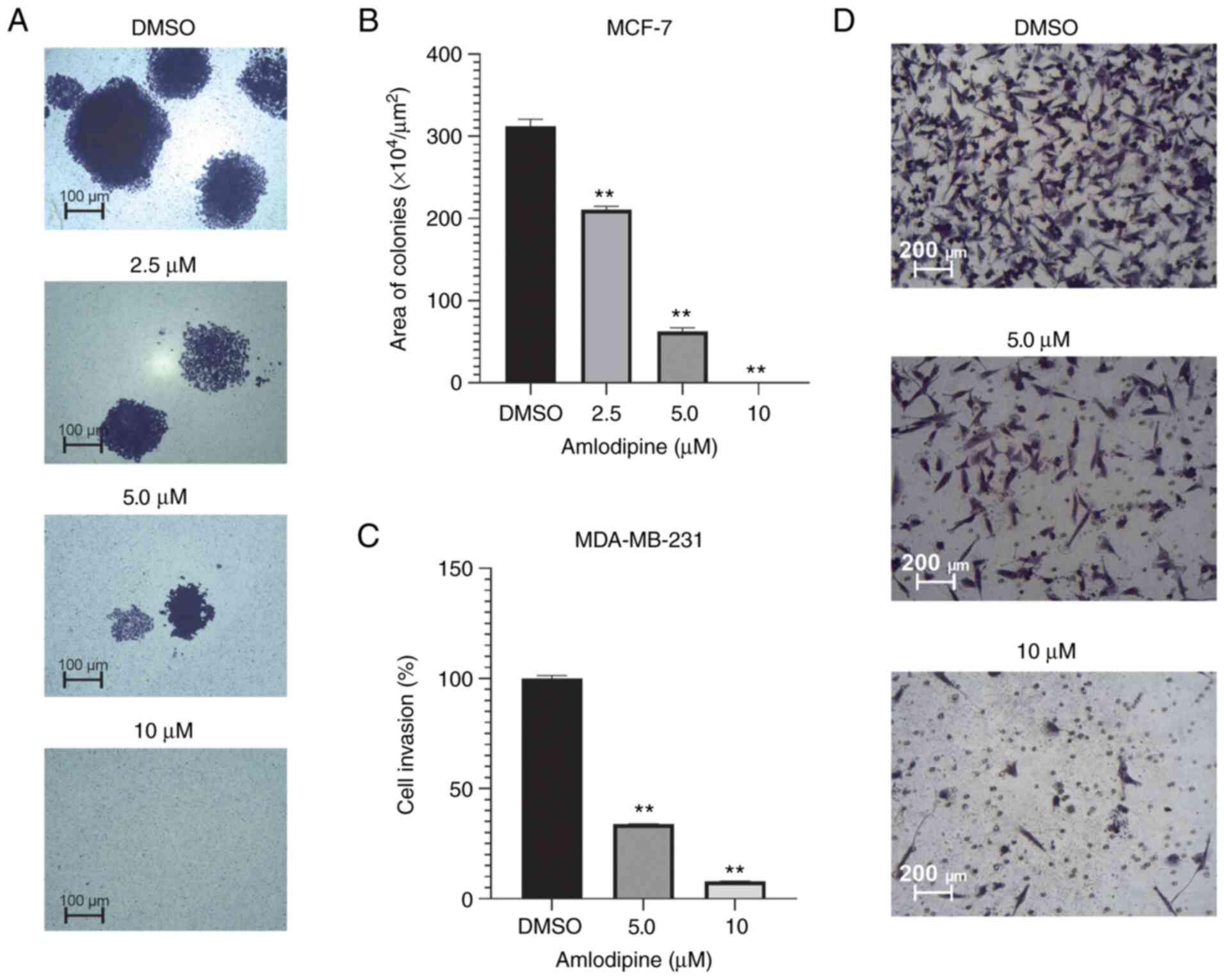
To further determine the anticancer effects of
amlodipine in cell proliferation, whether amlodipine could modulate
anchorage-dependent growth was assessed using colony formation
assays. Although it may be considered a limitation of the present
study for MCF-7 cells to have a high capacity to form colonies
compared to MDA-MB-231 cells (25),
the MCF-7 clonogenic ability was still assessed following
amlodipine treatment. The effect of amlodipine on colony formation
of MCF-7 cells is shown in Fig. 2A
and B. Amlodipine markedly
inhibited colony formation in a dose-dependent manner in MCF-7
cells. Treatment with 2.5-10 µM amlodipine significantly decreased
the colony size compared with the control (P<0.05). These
findings indicate the ability of amlodipine to suppress the
clonogenic proliferation of breast cancer cells over a prolonged
period of time.
Effect of amlodipine on the invasion
of breast cancer cells
To mimic the in vivo process of cell invasion
through the extracellular matrix, the effects of amlodipine on the
invasive abilities of MDA-MB-231 cells were evaluated using
Matrigel invasion chambers. As shown in Fig. 2C and D, amlodipine significantly suppressed
MDA-MB-231 cell invasion in a dose-dependent manner. Treatment with
5 and 10 µM amlodipine significantly reduced MDA-MB-231
invasiveness by >60 and 90% compared with the control-treated
cells, respectively. Since MCF-7 cells are not highly invasive
cells (25), invasion assays were
not performed using these cells, which is considered a limitation
of our study. These results provide robust evidence of the
anti-invasive effects of amlodipine on breast cancer cells in
vitro.
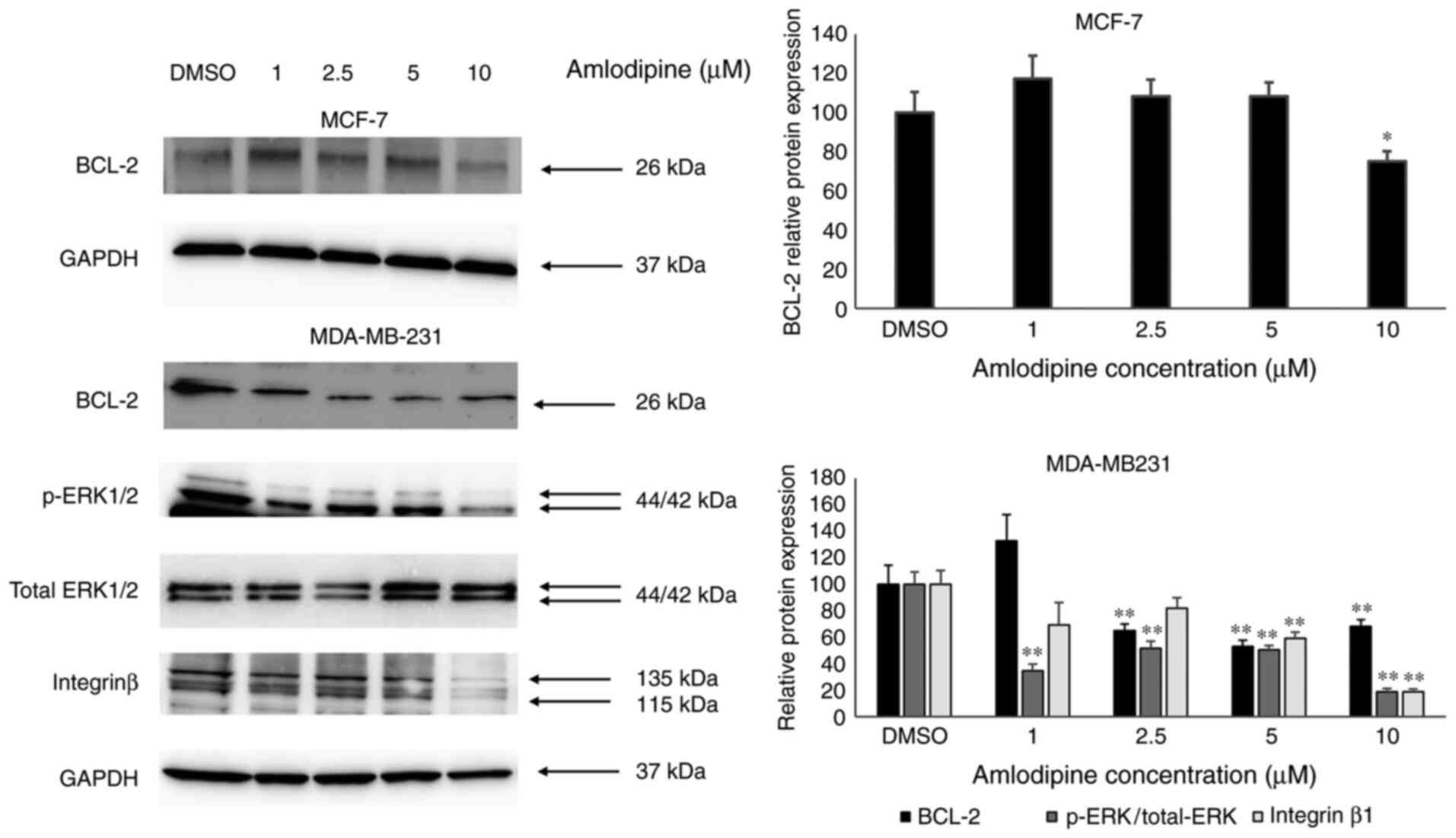
Anticancer effects of amlodipine may
be mediated via ERK1/2, integrin β1, and Bcl-2 inhibition
To further shed light on the potential signaling
molecules driving the anticancer effects of amlodipine on breast
cancer cells, the expression levels of key proteins involved in
cell proliferation, apoptosis, and invasion were evaluated using
western blotting. The results indicated that amlodipine reduced the
protein expression levels of the anti-apoptotic protein Bcl-2, in
both MDA-MB-231 and MCF-7 cells compared with the control (Fig. 3). As MCF-7 cells are not highly
invasive cells, p-ERK1/2 and integrin β1 protein expression levels
were only assessed in MDA-MB-231 cells. Amlodipine treatment
reduced ERK1/2 phosphorylation in MDA-MB-231 cells compared with
the control treatment. Moreover, amlodipine reduced the protein
expression levels of integrin-β1 in MDA-MB-231 cells compared with
the control treatment. These findings may partially explain the
possible molecular drivers of the anti-cancer effects of
amlodipine.
Discussion
Voltage-activated calcium channels are widely
distributed in all types of human cells (26). Several studies have shown that
calcium channel expression is altered as an adaptive mechanism in
human cancers such as in breast, prostate, and colorectal cancer
(26-28).
Interestingly, recent studies have implicated calcium channels in
cancer cell proliferation, invasion, and metastasis (29-31).
Recent studies have also shown that calcium channel expression is
upregulated in breast cancer cells (31,32).
In the present study, treatment of breast cancer cells with the
CCB, amlodipine, resulted in a dose-dependent reduction in breast
cancer cell viability. Similarly, recent studies have shown that
silencing calcium channel expression inhibited breast cancer cell
growth both in vitro and in vivo (31,32).
To ascertain the underlying mechanism(s) of
amlodipine-induced growth suppression, breast cancer cellular
apoptosis was assessed by measuring caspase-3/7 activity. The
results showed that amlodipine induced caspase-3/7 activity in
MDA-MB-231 cells, which may contribute to caspase-dependent
apoptosis. Although this finding was limited by a lack of flow
cytometry analysis to confirm the occurrence of apoptosis,
activation of caspase-3/7 pathways was accompanied by
downregulation of the anti-apoptotic protein Bcl-2, which strongly
indicated that caspase-dependent apoptosis occurred in the breast
cancer cells following amlodipine treatment. The Bcl-2 gene
promotes cell survival and protects cells against apoptosis. High
expression of Bcl-2 is associated with lower apoptosis-mediated
death and contributes to resistance to chemotherapy. Moreover,
Bcl-2 protein expression is typically altered in breast cancer
cells (33,34). In agreement with the findings of the
present study, a previous study demonstrated that amlodipine
treatment induced apoptosis in MDA-MB-231 cells via downregulation
of Bcl-2 protein expression (35).
In addition, activation of caspase-dependent apoptosis has been
reported with other dihydropyridine CCBs (36). Moreover, Wong et al (36) reported that treating cancer cells
with calcium channel inhibitors may also lead to
caspase-independent apoptosis. In the present study, amlodipine
treatment of breast cancer cells resulted in caspase-dependent
apoptosis as shown by the activation of caspase3/7. However, the
increase in caspase3/7 activity appeared to decrease at higher
concentrations (10-25 µM), which could be due to the dominance of
caspase-independent apoptosis at higher concentrations.
To further illustrate the antiproliferative effect
of amlodipine, the tumorigenic ability of breast cancer cells was
assessed using colony formation assays whilst being treated with
amlodipine. The inhibitory effects of amlodipine on colony
formation were notable in MCF-7 cells and in agreement with
previous findings in gastric cancer (37). To the best of our knowledge, this is
the first study to provide proof of the inhibitory effects of
amlodipine on breast cancer colony formation. In the present study,
the effects of amlodipine on breast cancer cell proliferation and
colony formation were accompanied by a reduction in ERK1/2
phosphorylation. Recent studies have also shown the inhibitory
effects of amlodipine and other CCBs on the ERK1/2 pathway in
gastric cancer (38), hepatic
cancer (39), ovarian cancer
(40), and melanoma (41). Taken together, the current and
previous studies highlight the suppressive effects of amlodipine on
cell proliferation, resistance to apoptosis, and tumorigenic
potential via inhibition of major signaling proteins such as ERK1/2
and Bcl-2.
Previous studies have implicated calcium channels in
breast cancer cell adhesion and invasion (30,31,42).
For example, silencing calcium channel expression in breast cancer
cells has been associated with a reduction in cell motility and
adhesion (31). In the present
study, amlodipine significantly reduced MDA-MB-231 breast cancer
cell invasion. Filopodia structures are finger-like cytoplasmic
projections that extend beyond the cell's edge and promote cancer
cell invasion (42,43). The results of the present study are
consistent with a recent study that showed the ability of several
CCBs, including amlodipine, in inhibit filopodia formation and thus
impairing breast cancer cell invasion (30). In the present study, the
anti-invasive effects of amlodipine were accompanied by
downregulation in p-ERK1/2 and integrin β1 protein expression.
These proteins are well-established key players in breast cancer
cell migration, invasion, and metastasis (44-46).
Together, these findings suggest that the anti-invasive effects of
amlodipine are mediated via at least the inhibition of p-ERK1/2 and
integrin β1 expression.
In conclusion, the results of the present study
showed that amlodipine exerted anticancer effects on cell
proliferation, colony formation, and invasion, and they were, at
least in part, achieved by the inhibition of p-ERK1/2, integrin β1,
and Bcl-2 expression and activation of caspase-3/7, indicating the
induction of caspase-dependent apoptosis. This study highlights
amlodipine as a potential therapeutic agent for the management of
breast cancer and may provide novel insights for future research on
the effects of amlodipine in the sensitization of breast cancer
cells to chemotherapy.
Acknowledgements
We would like to acknowledge Jordan University of
Science and Technology for providing sabbatical leave to Dr.
Mohammad A. Y. Alqudah. We are grateful to Dr. Moh'd Shara for his
kind revision of the language proficiency of this manuscript and to
Prof. Omar Khabour for providing us with the total ERK1/2 antibody
(Jordan University of Science and Technology, Jordan).
Funding
Funding: This project was funded by Jordan University of Science
and Technology (Deanship of Research, grant no. 238/2019).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
MAYA was involved in the study conception and
design. MAYA, RAS and MA performed the experiments. MAYA, RAS, and
KHA conducted the data analysis. MAYA and RAS wrote the first draft
of the manuscript. All authors revised the manuscript. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249.
2021.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Siegel RL, Miller KD, Fuchs HE and Jemal
A: Cancer statistics, 2021. CA Cancer J Clin. 71:7–33.
2021.PubMed/NCBI View Article : Google Scholar
|
|
3
|
World Health Organization. Jordan-Global
Cancer Observatory [Fact sheet] 2020, December. Available from:
https://gco.iarc.fr/today/data/factsheets/populations/400-jordan-fact-sheets.pdf.
|
|
4
|
Seyfried TN and Huysentruyt LC: On the
origin of cancer metastasis. Crit Rev Oncog. 18:43–73.
2013.PubMed/NCBI View Article : Google Scholar
|
|
5
|
van Zijl F, Krupitza G and Mikulits W:
Initial steps of metastasis: Cell invasion and endothelial
transmigration. Mutat Res. 728:23–34. 2011.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Howlader N, Noone AM, Krapcho M, Miller D,
Brest A, Yu M, Ruhl J, Tatalovich Z, Mariotto A, Lewis DR (eds), et
al: SEER cancer statistics review, 1975-2017, National Cancer
Institute. Bethesda, MD, 2020. Available from: https://seer.cancer.gov/csr/1975_2017/.
|
|
7
|
American Cancer Society. Breast cancer
facts and figures 2019-2020: American Cancer Society, 2020.
Available from: https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/breast-cancer-facts-and-figures/breast-cancer-facts-and-figures-2019-2020.pdf.
|
|
8
|
Carrick S, Parker S, Thornton CE, Ghersi
D, Simes J and Wilcken N: Single agent versus combination
chemotherapy for metastatic breast cancer. Cochrane Database Syst
Rev: Apr 15, 2009 (Epub ahead of print). doi:
10.1002/14651858.CD003372.pub3.
|
|
9
|
Boster BL, Patel NK and Michaud LB: Breast
cancer. In: Pharmacotherapy: A Pathophysiologic Approach, 11e.
DiPiro JT, Yee GC, Posey LM, Haines ST, Nolin TD and Ellingrod V
(eds). McGraw-Hill Education, New York, NY, 2020.
|
|
10
|
Velaei K, Samadi N, Barazvan B and
Soleimani Rad J: Tumor microenvironment-mediated chemoresistance in
breast cancer. Breast. 30:92–100. 2016.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Wilson LE, D'Aloisio AA, Sandler DP and
Taylor JA: Long-term use of calcium channel blocking drugs and
breast cancer risk in a prospective cohort of US and Puerto Rican
women. Breast Cancer Res. 18(61)2016.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Lee AR, Seo MJ, Kim J, Lee DM, Kim IY,
Yoon MJ, Hoon H and Choi KS: Lercanidipine synergistically enhances
bortezomib cytotoxicity in cancer cells via enhanced endoplasmic
reticulum stress and mitochondrial Ca2+ overload. Int J
Mol Sci. 20(6112)2019.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Alqudah MAY, Alrababah BA and Mhaidat NM:
Amlodipine inhibits cell proliferation and induces cell cycle
arrest in colorectal cancer cells. Jordan J Pharm Sci. 10:189–197.
2017.
|
|
14
|
Ji BS, He L and Liu GQ: Reversal of
p-glycoprotein-mediated multidrug resistance by CJX1, an amlodipine
derivative, in doxorubicin-resistant human myelogenous leukemia
(K562/DOX) cells. Life Sci. 77:2221–2232. 2005.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Yoshida J, Ishibashi T and Nishio M:
Antitumor effects of amlodipine, a Ca2+ channel blocker,
on human epidermoid carcinoma A431 cells in vitro and in vivo. Eur
J Pharmacol. 492:103–112. 2004.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Alqudah MA, Agarwal S, Al-Keilani MS,
Sibenaller ZA, Ryken TC and Assem M: NOTCH3 is a prognostic factor
that promotes glioma cell proliferation, migration and invasion via
activation of CCND1 and EGFR. PLoS One. 8(e77299)2013.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Al-Oudat BA, Alqudah MA, Audat SA,
Al-Balas QA, El-Elimat T, Hassan MA, Frhat IN and Azaizeh MM:
Design, synthesis, and biologic evaluation of novel chrysin
derivatives as cytotoxic agents and caspase-3/7 activators. Drug
Des Devel Ther. 13:423–433. 2019.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Siragusa M, Dall'Olio S, Fredericia PM,
Jensen M and Groesser T: Cell colony counter called CoCoNut. PLoS
One. 13(e0205823)2018.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Ayoub NM, Alkhalifa AE, Ibrahim DR and
Alhusban A: Combined crizotinib and endocrine drugs inhibit
proliferation, migration, and colony formation of breast cancer
cells via downregulation of MET and estrogen receptor. Med Oncol.
38(8)2021.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Ayoub NM, Al-Shami KM, Alqudah MA and
Mhaidat NM: Crizotinib, a MET inhibitor, inhibits growth,
migration, and invasion of breast cancer cells in vitro and
synergizes with chemotherapeutic agents. Onco Targets.
10:4869–4883. 2017.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Alqudah MAY, Azaizeh M, Zayed A and Asaad
L: Calcium-sensing receptor antagonist NPS-2143 inhibits breast
cancer cell proliferation, migration and invasion via
downregulation of p-ERK1/2, Bcl-2 and integrin β1 and induces
caspase 3/7 activation. Adv Pharm Bull. 12:383–388. 2022.
|
|
22
|
Brentnall M, Rodriguez-Menocal L, De
Guevara RL, Cepero E and Boise LH: Caspase-9, caspase-3 and
caspase-7 have distinct roles during intrinsic apoptosis. BMC Cell
Biol. 14(32)2013.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Jänicke RU: MCF-7 breast carcinoma cells
do not express caspase-3. Breast Cancer Res Treat. 117:219–221.
2009.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Kottke TJ, Blajeski AL, Meng XW, Svingen
PA, Ruchaud S, Mesner PW Jr, Boerner SA, Samejima K, Henriquez NV,
Chilcote TJ, et al: Lack of correlation between caspase activation
and caspase activity assays in paclitaxel-treated MCF-7 breast
cancer cells. J Biol Chem. 277:804–815. 2002.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Comşa Ş, Cîmpean AM and Raica M: The story
of MCF-7 breast cancer cell line: 40 Years of experience in
research. Anticancer Res. 35:3147–3154. 2015.PubMed/NCBI
|
|
26
|
Yamakage M and Namiki A: Calcium
channels-basic aspects of their structure, function and gene
encoding; anesthetic action on the channels-a review. Can J
Anaesth. 49:151–164. 2002.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Phan NN, Wang CY, Chen CF, Sun Z, Lai MD
and Lin YC: Voltage-gated calcium channels: Novel targets for
cancer therapy. Oncol Lett. 14:2059–2074. 2017.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Prevarskaya N, Skryma R and Shuba Y: Ion
channels and the hallmarks of cancer. Trends Mol Med. 16:107–121.
2010.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Varghese E, Samuel SM, Sadiq Z, Kubatka P,
Liskova A, Benacka J, Pazinka P, Kruzliak P and Büsselberg D:
Anti-cancer agents in proliferation and cell death: The calcium
connection. Int J Mol Sci. 20(3017)2019.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Jacquemet G, Baghirov H, Georgiadou M,
Sihto H, Peuhu E, Cettour-Janet P, He T, Perälä M, Kronqvist P,
Joensuu H and Ivaska J: L-type calcium channels regulate filopodia
stability and cancer cell invasion downstream of integrin
signalling. Nat Commun. 7(13297)2016.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Kanwar N, Carmine-Simmen K, Nair R, Wang
C, Moghadas-Jafari S, Blaser H, Tran-Thanh D, Wang D, Wang P, Wang
J, et al: Amplification of a calcium channel subunit CACNG4
increases breast cancer metastasis. EBioMedicine.
52(102646)2020.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Ji Y, Han Z, Shao L and Zhao Y:
Ultrasound-targeted microbubble destruction of calcium channel
subunit α 1D siRNA inhibits breast cancer via G protein-coupled
receptor 30. Oncol Rep. 36:1886–1892. 2016.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Zhang GJ, Kimijima I, Tsuchiya A and Abe
R: The role of bcl-2 expression in breast carcinomas (Review).
Oncol Rep. 5:1211–1216. 1998.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Pratt MA, Niu M and White D: Differential
regulation of protein expression, growth and apoptosis by natural
and synthetic retinoids. J Cell Biochem. 90:692–708.
2003.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Lan L, Xinghua X, Wenjuan S and Liying D:
Effect of amlodipine on apoptosis of human breast carcinoma
MDA-MB-231 cells. J Med Coll PLA. 23:358–363. 2008.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Wong BS, Chiu LY, Tu DG, Sheu GT and Chan
TT: Anticancer effects of antihypertensive L-type calcium channel
blockers on chemoresistant lung cancer cells via autophagy and
apoptosis. Cancer Manag Res. 12:1913–1927. 2020.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Shiozaki A, Katsurahara K, Kudou M,
Shimizu H, Kosuga T, Ito H, Arita T, Konishi H, Komatsu S, Kubota
T, et al: Amlodipine and verapamil, voltage-gated Ca2+
channel inhibitors, suppressed the growth of gastric cancer stem
cells. Ann Surg Oncol. 28:5400–5411. 2021.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Panneerpandian P, Rao DB and Ganesan K:
Calcium channel blockers lercanidipine and amlodipine inhibit
YY1/ERK/TGF-β mediated transcription and sensitize the gastric
cancer cells to doxorubicin. Toxicol In Vitro.
74(105152)2021.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Li Y, Liu S, Lu F, Zhang T, Chen H, Wu S
and Zhuang H: A role of functional T-type Ca2+ channel
in hepatocellular carcinoma cell proliferation. Oncol Rep.
22:1229–1235. 2009.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Lee H, Kim JW, Kim DK, Choi DK, Lee S, Yu
JH, Kwon OB, Lee J, Lee DS, Kim JH and Min SH: Calcium channels as
novel therapeutic targets for ovarian cancer stem cells. Int J Mol
Sci. 21(2327)2020.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Granados K, Hüser L, Federico A, Sachindra
S, Wolff G, Hielscher T, Novak D, Madrigal-Gamboa V, Sun Q,
Vierthaler M, et al: T-type calcium channel inhibition restores
sensitivity to MAPK inhibitors in de-differentiated and adaptive
melanoma cells. Br J Cancer. 122:1023–1036. 2020.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Jacquemet G, Hamidi H and Ivaska J:
Filopodia in cell adhesion, 3D migration and cancer cell invasion.
Curr Opin Cell Biol. 36:23–31. 2015.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Jacquemet G, Green DM, Bridgewater RE, von
Kriegsheim A, Humphries MJ, Norman JC and Caswell PT: RCP-driven
α5β1 recycling suppresses Rac and promotes RhoA activity via the
RacGAP1-IQGAP1 complex. J Cell Biol. 202:917–935. 2013.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Hou S, Isaji T, Hang Q, Im S, Fukuda T and
Gu J: Distinct effects of β1 integrin on cell proliferation and
cellular signaling in MDA-MB-231 breast cancer cells. Sci Rep.
6(18430)2016.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Yin HL, Wu CC, Lin CH, Chai CY, Hou MF,
Chang SJ, Tsai HP, Hung WC, Pan MR and Luo CW: β1 integrin as a
prognostic and predictive marker in triple-negative breast cancer.
Int J Mol Sci. 17(1432)2016.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Thibaudeau L, Taubenberger AV,
Theodoropoulos C, Holzapfel BM, Ramuz O, Straub M and Hutmacher DW:
New mechanistic insights of integrin β1 in breast cancer bone
colonization. Oncotarget. 6:332–344. 2015.PubMed/NCBI View Article : Google Scholar
|