Introduction
Diabetes Mellitus (DM) currently ranks as the most
common endocrine disorder. Current opinion views DM as a group of
heterogenous metabolic diseases characterized by hyperglycemia,
which is triggered by defects in the ability of the body to produce
or use insulin in type 1 and type 2 DM, respectively (1,2).
Several predisposing factors have been linked to the
pathogenesis of type 2 diabetes mellitus (T2DM), such as obesity,
western lifestyles (especially diets), lack of physical activity,
genetic predispositions, ethnicity, and inflammation (3). Low-grade inflammation is closely
involved in the development of diabetes and its microvascular
complications (4). There is a
considerable body of evidence supporting the etiological role of
inflammatory cytokines in the pathogenesis and prognosis of T2DM
(4-7).
Brain-derived neurotrophic factor (BDNF), one of the
neurotrophin family of growth factors, is essential for synaptic
transmission and plasticity, along with neuronal integrity,
survival, growth, and differentiation (8). Shi et al (9) reported a rapid increase in the levels
of BDNF following exposure of rats to stress. Similarly, Jiang
et al (10) studied the role
of BDNF in rats following an ischemic stroke. Their results showed
increases in the levels of BDNF, which subsequently promoted the
expression of anti-inflammatory cytokines and inhibited the levels
of pro-inflammatory cytokines. These observations reflect the roles
of BDNF as a part of a neuronal protective response.
Subsequently, several studies have linked BDNF to
systemic inflammatory conditions such as coronary diseases,
atherosclerosis, insulin resistance, inflammatory bowel diseases,
and the development of diabetes (11-15).
In vivo studies reported BDNF modulation and changes in its
expression levels as a response to inflammatory conditions
(16-18).
Vitamin D, collectively referring to ergocalciferol
(vitamin D2) and cholecalciferol (vitamin D3), was initially
recognized for its role in calcium homeostasis and bone health
(19). Subsequently, the
extraskeletal roles of vitamin D were studied; studies demonstrated
the crucial role of vitamin D in immunoregulation and inflammation.
A review by Calton et al (20) highlighted the body of evidence on
the anti-inflammatory role of vitamin D through its ability to
inhibit the proliferation of pro-inflammatory cells and in
modulating the production of inflammatory cytokines.
Ojaimi et al (21) first reported the role of vitamin D
in regulating the levels of pro-inflammatory markers during
inflammation in a dose-dependent manner. In their study,
supplementation with the optimal dose of vitamin D significantly
reduced the levels of inflammatory markers, including that of IL6,
TNF, and IFN. Subsequent studies demonstrated similar outcomes in
several inflammatory and low-grade inflammation conditions, such as
insulin resistance, obesity, and diabetes (20-23).
Recently, Nadimi et al (24) investigated the effects of vitamin D
supplementation on BDNF levels in diabetic rats. BDNF levels were
shown to be positively correlated with vitamin D supplementation in
aged rats (25). However, in
primary cultures of astrocytes, BDNF levels were not altered by
vitamin D supplementation. The interactions between vitamin D and
BDNF have never been investigated in humans, to the best of our
knowledge. Herein, the alterations in serum BDNF and vitamin D
levels in T2DM patients in Jordan were investigated, prior to and
following vitamin D supplementation.
Materials and methods
Study design and patient
characteristics
The present study was a combination of a
non-experimental case-controlled study and an experimentally
designed study. The study was approved by the Institutional Review
Board of the Jordan University of Science and Technology (approval
no. 2019/121/7). The study was conducted at the clinics of King
Abdullah University Hospital (KAUH; Irbid, Jordan) between November
2018 and March 2019.
This study included 150 individuals with T2DM and
150 healthy controls. In the T2DM group, 66 of the recruited
patients were male with an age range of 39-63 years and a median
age of 51 years, and 84 were female, with an age range of 33-69
years and a median age of 53 years. In the control group, 78 of the
recruited participants were female with an age range of 39-96 years
and a median age of 51, and 72 were male with an age range of 36-64
years and a median age of 50 years. T2DM participants were
diagnosed according to the American Diabetes Association guidelines
(26) and were recruited during
their visit to the Endocrinology and Diabetic clinic of KAUH, and
the healthy control group were recruited from other clinics at
KAUH. The healthy controls had no signs or symptoms associated with
T2DM during their recruitment. Subjects in the study were matched
by age and body mass index (BMI) and were required to sign a
consent form prior to their enrollment.
For further confirmation of the presence or absence
of T2DM, repeated fasting blood glucose (FBG) analysis was
performed on all subjects. Pre-diabetic individuals with a repeated
FBG of 100-125 mg/dl were excluded from this study. Subjects with
chronic kidney or liver diseases that may interfere with vitamin D
metabolism were excluded from the study. Subjects with Cushing's
syndrome, polycystic ovarian syndrome, thyroid dysfunction, or
hyperprolactinemia and subjects who indicated receiving any of the
vitamin D pharmacological preparations by mouth or topically were
also excluded from the study. Height (cm), weight (kg), and waist
circumference (WC) were measured for all subjects during their
visit to the hospital. The BMI was calculated using the formula:
weight/height2 (kg/m2).
Collection of blood and serum
samples
Following overnight fasting, a blood sample (5 ml)
was withdrawn using a sterile plain tube with a clot activator and
gel (AFCO) from each participant. Another sample (5 ml) was
withdrawn into an EDTA tube (AFCO). Within 60 min of collection,
the blood-containing plain tubes were centrifuged at 4,500 x g for
5 min to separate the serum. Serum samples were aliquoted in three
Eppendorf tubes (1.5 ml) and stored at -80˚C until required for
measuring the BDNF, vitamin D, and fasting glucose levels.
Blood-containing EDTA tubes were used for HbA1c measurements.
Therapeutic interventions
A total of 26 subjects (21 males and 5 females) from
the 150 T2DM patients aged between 40 and 77 years old, were
confirmed as having low serum levels of vitamin D (<30 ng/ml).
These subjects were included for the assessment of vitamin D
supplementation.
Vitamin D3 tablets (weekly dose: 50,000
IU; Biodal) were given for 3 consecutive months (from June to
September) for each patient. Before and after the intervention
period, plain and EDTA tubes were withdrawn in the morning after
overnight fasting. EDTA tubes were used for HbA1c analysis. Serum
from the plain tube was used for measuring the levels of BDNF,
vitamin D, as well as fasting glucose.
All subjects were instructed not to change their
lifestyle during this period, including their dietary habits,
medications, daily exposure to the sun as well as daily exercise.
Weekly sun exposure and exercise hours were recorded during the
intervention period.
Biochemical measurements
Collected serum samples were used for BDNF, 25(OH)
vitamin D as well as FBG measurements. Whole blood samples
collected in EDTA tubes were used for HbA1c measurements. FBG and
HbA1c were measured on a Roche automated clinical analyzer system
(Roche Diagnostics GmbH).
The concentrations of 25(OH) vitamin D in serum were
measured quantitatively using specific ELISA kits purchased from
Abcam (cat. no. ab213966) according to the manufacturer's
instructions. For accurate measurement, a 1:10 dilution of samples
with Dissociation Buffer was used as described in the assay
procedure. Serum BDNF concentrations were measured using an ELISA
kit purchased from R&D systems (cat. no. DY248) according to
the manufacturer's instructions. Optical density was read
spectrophotometrically using an 800TM TS Microplate reader (BioTek
Instruments, Inc.) at 405 nm for 25(OH) vitamin D and at 450 nm for
BDNF.
Statistical analysis
All statistical analyses were performed using SPSS
version 22 (IBM Corp). Figures were generated using GraphPad Prism
version 8.0.2 (GraphPad Software, Inc.). Differences in serum
25(OH) vitamin D and BDNF levels between the healthy controls and
T2DM cases, in addition to biochemical parameters and indices for
the pre- and post-supplementation subjects were evaluated using a
paired Student's t-test. P<0.05 was considered to indicate a
statistically significant difference. Moreover, linear regression
analysis was performed to determine the association between 25(OH)
vitamin D levels and BDNF levels.
Results
Subject characteristics and the
biochemical profile
The baseline characteristics and biochemical
profiles of the study subjects are presented in Table I. Data are presented as the mean ±
SD. There were no significant differences observed with regard to
age, BMI, and WC between the two groups (P=1.000, 0.7008, and
0.3248, respectively). The T2DM cases had significantly higher
levels of FBG (P<0.0001) as well as HbA1c levels (P<0.0001)
compared with the healthy controls.
 | Table IPatient characteristics and the
biochemical profile. |
Table I
Patient characteristics and the
biochemical profile.
| Variable | Controls, n=150 | Type 2 diabetes
mellitus, n=150 | P-valueb |
|---|
| Age, years n (%) | | | 1.0000 |
|
<40 | 7 (4.66%) | 7 (4.66%) | |
|
40-49 | 54 (36.00%) | 54 (36.00%) | |
|
50-59 | 73 (48.67%) | 73 (48.67%) | |
|
>60 | 16 (10.67%) | 16 (10.67%) | |
| Body mass index,
kg/m2c | 31.69±6.28 | 31.42±5.84 | 0.7008 |
| Waist
circumference, cmc | 109.86±10.03 | 108.59±12.22 | 0.3248 |
| Glucose,
mg/dlc | 93.20±10.43 | 205.20±95.76 |
<0.0001a |
| HbA1c
(%)c | 5.05±1.28 | 7.54±1.87 |
<0.0001a |
Serum 25(OH) vitamin D and BDNF levels
between healthy controls and T2DM cases
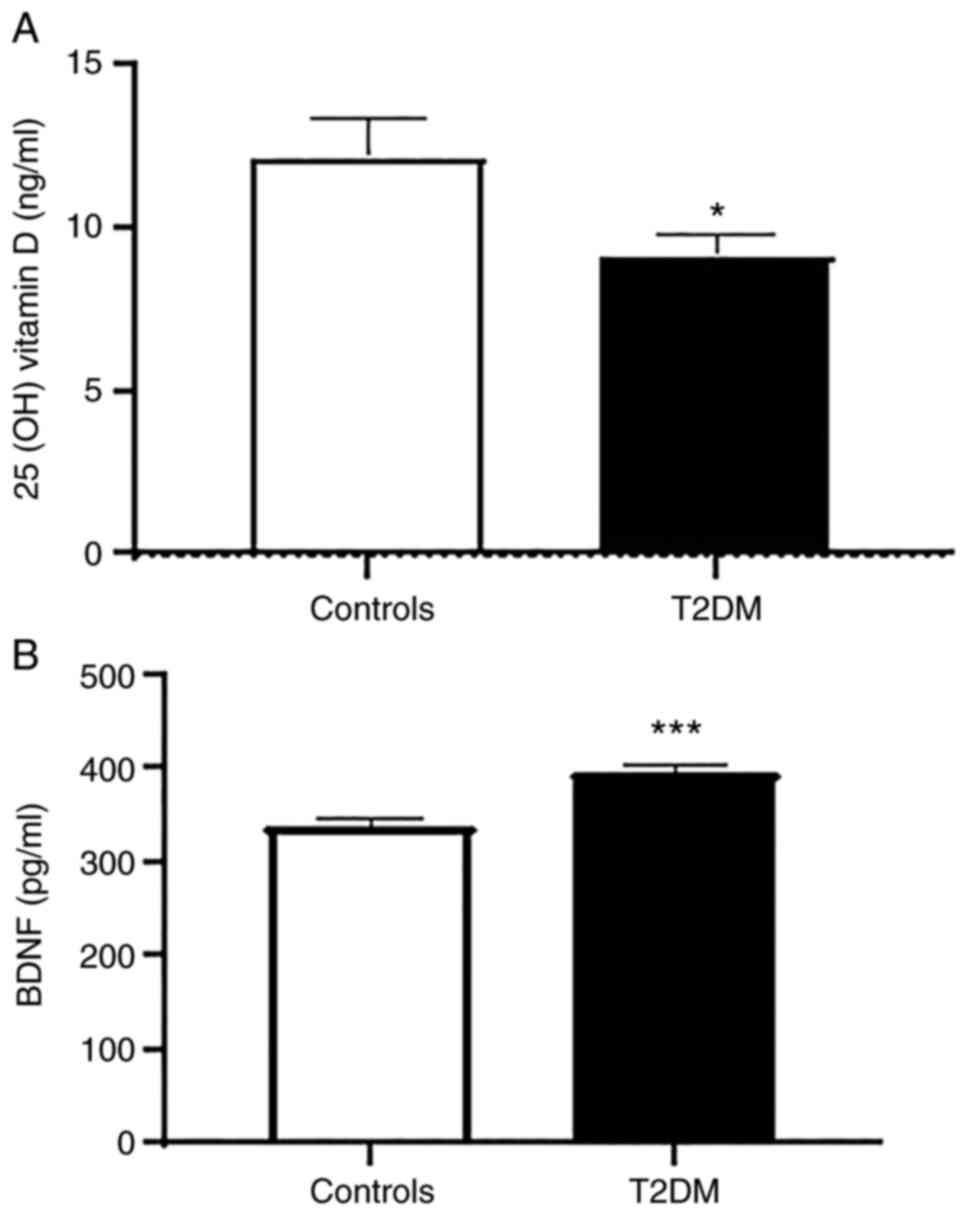
T2DM cases had significantly lower levels of serum
25(OH) vitamin D compared with the healthy controls with mean ± SEM
values of 9.14±0.69 and 12.17±12.5 ng/ml, respectively (P=0.0349,
Fig. 1A). Moreover, a significant
difference was observed in the BDNF concentrations between the two
groups (P<0.0001, Fig. 1B).
Specifically, the T2DM cases had a higher BDNF level (390.8+10.4
pg/ml) compared with the healthy controls (329.7+9.9 pg/ml).
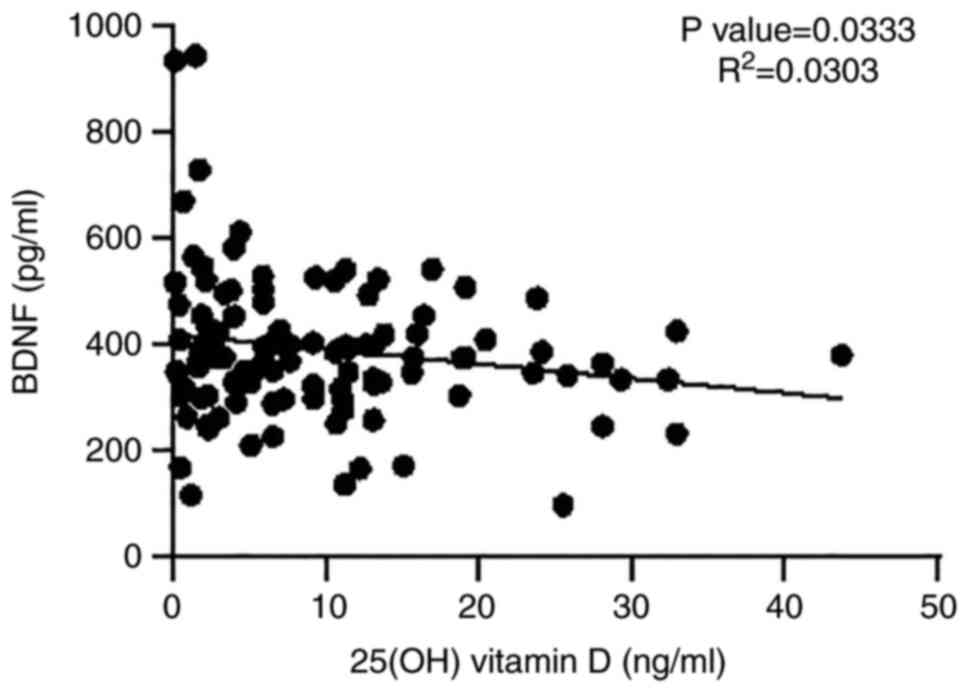
Correlation between BDNF and 25(OH)
vitamin D levels in the serum
Fig. 2 shows the
significant negative correlation observed between the BDNF levels
and 25(OH) vitamin D levels in the serum (P=0.0333, based on linear
regression analysis).
Vitamin D intervention
Based on the above results showing significantly
lower levels of 25(OH) vitamin D and higher BDNF levels in the
serum of T2DM cases, along with the significant negative
correlation between BDNF levels and 25(OH) vitamin D levels in the
serum, it was hypothesized that vitamin D supplementation may be
helpful in improving BDNF levels.
Following the end of the administration period, the
levels of 25(OH) vitamin D were significantly increased in the
post-supplementation measurements compared with the
pre-supplementation measurements (P<0.0001), highlighting the
adequacy of the treatment. Interestingly, supplementation resulted
in a significant reduction in the BDNF levels (P<0.0001), FBG
levels (P=0.0324), and HbA1c levels (P=0.0195, Table II).
 | Table IIBiochemical parameters before and
after vitamin D supplementation. |
Table II
Biochemical parameters before and
after vitamin D supplementation.
| Variable |
Pre-supplementationd |
Post-supplementationd |
P-valuec |
|---|
| 25(OH) vitamin D
(ng/ml) | 1.35±0.82 | 34.82±16.98 |
<0.0001b |
| Glucose, mg/dl | 214.68±78.00 | 182.79±65.76 | 0.0324 |
| HbA1c (%) | 8.29±1.90 | 7.54±1.42 | 0.0195a |
| Brain derived
neurotrophic factor, pg/ml | 383.65±91.35 | 247.22±113.66 |
<0.0001b |
Discussion
In the present study, it was demonstrated that
diabetic patients had lower levels of serum 25(OH) vitamin D and
higher levels of BDNF compared with the healthy controls. Moreover,
linear regression analysis indicated that BDNF levels were
inversely correlated with serum 25(OH) vitamin D levels.
Furthermore, vitamin D supplementation significantly improved
25(OH) vitamin D serum levels and decreased BDNF serum levels in
diabetic patients. Intriguingly, FBG and HbA1c levels were also
significantly improved by vitamin D supplementation.
The relationship between diabetes and inflammation
is well-established. Inflammatory cytokines such as TNF-α and IL-6,
amongst several others, are elevated in T2DM patients (27). Diabetes predisposing factors such as
obesity and a sedentary lifestyle result in the continuous presence
of a low level of inflammation that affects insulin sensitivity and
contributes to the pathogenesis of T2DM (27,28).
Vitamin D exerts an essential role in modulating and regulating the
immune system and body inflammation. A strong body of evidence
indicates that 25(OH) vitamin D exerts regulatory effects on innate
and specific immunity (22).
Vitamin D is essential for anti-inflammatory responses produced by
human immune monocytes (20).
Inadequate vitamin D levels are commonly associated with
obesity-related continuous diseases that in turn are associated
with chronic low-grade inflammation (20,22,23).
Here, it was shown that individuals with a confirmed T2DM diagnosis
had lower levels of serum 25(OH) vitamin D, and interestingly
treating these patients for 3 consecutive months with oral vitamin
D supplements improved the blood glucose lab results. These results
suggest that monitoring and adjusting vitamin D levels play a
crucial role in treating T2DM. Several studies have highlighted the
importance of vitamin D in the pathogenesis of T2DM. Vitamin D
stimulates insulin release from pancreatic β-cells and low levels
of vitamin D as seen in hyperthyroidism have been implicated in the
impairment of insulin release from pancreatic cells (23).
Chronic inflammation has been linked to disruption
of the neurotrophin family of growth factors, particularly BDNF. In
the present study, BDNF levels were higher in T2DM serum samples,
suggesting that BDNF is involved in the pathogenesis of T2DM. This
contribution may be explained by the chronic inflammation that
accompanies T2DM and is considered one of the main pathological
factors to T2DM. Alterations in BDNF levels are associated with a
myriad of systemic inflammatory conditions such as coronary
diseases, atherosclerosis, insulin resistance, inflammatory bowel
diseases, airway inflammation, and the development of diabetes
(10,29,30).
Contrary to the results of the present study, lower serum BDNF
levels in T2DM patients have been reported previously (30). There is a large body of evidence
that supports the role of BDNF in pathologies that involve
inflammation and neuronal impairment such as diabetes (31,32).
However, the alterations in BDNF levels and how the body manages
the changes in such conditions are not completely understood. For
example, BDNF serum levels have been reported to be reduced during
neurodegenerative diseases according to several reports (32,33),
whilst other reports have reported increases in its levels
(31). The increased levels of
serum BDNF reported in the present may reflect the differences in
the inclusion criteria of diabetic patients and the extent of
neuronal involvement. The severity of neuronal impairment accounts
for differences in BDNF serum levels (32,34).
T2DM is strongly associated with a reduction in cognition (33,35).
Cognition impairment in T2DM may occur during the early stages of
development and may then be further aggravated with time. Thus, the
increased serum BDNF levels could represent a compensatory
mechanism to rescue neuronal damage and to ameliorate cognitive
impairment. Further studies should be performed to classify
diabetic patients according to the neuronal involvement and to
assess the role of BDNF during the different stages of the
disease.
Vitamin D supplementation resulted in a significant
reduction in BDNF serum levels in the T2DM patients. The available
data regarding the effect of vitamin D on BDNF levels are
contradictory; some reports indicate a positive effect, others have
indicated a negative effect and others yet have reported no effect
(24,25,36).
In support of the results of the present study, BDNF levels were
altered by vitamin D treatment in several reports (35,37,38).
Babaei et al (35) showed
that serum BDNF levels were significantly higher in vitamin D
deficient rats and were increased after 25(OH) vitamin D
intervention. The results of the present study are in accordance
with a report describing a reduction in plasma nerve growth factor
and BDNF levels subsequent to vitamin D supplementation in healthy
postmenopausal females (39). The
increase in serum BDNF levels in vitamin D deficient diabetic
patients may reflect a BDNF compensatory mechanism to counteract
neuropathies that are associated with the disease; however,
additional studies are required to test this theory.
In conclusion, vitamin D insufficiency/deficiency
aggravates metabolic syndrome components in diabetic patients and
supplementation significantly ameliorates aberrant FBG and HbA1c
levels parallel with a reduction in circulating BDNF levels. These
data demonstrate a positive effect of vitamin D supplementation in
diabetic patients, suggesting the implementation of vitamin D as
part of a standard T2DM treatment plan. However, additional studies
are required to investigate the direct link between vitamin D,
inflammation, BDNF, and T2DM.
Acknowledgements
Not applicable.
Funding
Funding: This work was supported by a grant from the Jordan
University of Science and Technology (Irbid, Jordan, Grant no.
2019/0110) and a grant from the United Arab Emirates University, Al
Ain, UAE (grant no. G00003289).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author on reasonable request.
Authors' contributions
MAlqudah, MK, MAA and AAD designed the study and
analyzed the data. MK and MAA performed the experiments. OAS, DGAU
and MK reviewed the analysis and formatted the manuscript.
MAlqudah, DGAU, MAllouh and MK wrote the manuscript. MAlqudah
performed the statistical analysis. MAlqudah, MAllouh and MAA
confirm the authenticity of all the raw data. All authors read and
approved the final manuscript.
Ethical approval consent to participate
The study was approved by the Institutional Review
Board of the Jordan University of Science and Technology (approval
no. 2019/121/7) and informed consent was obtained from all
participants.
Patient consent for publication
Informed consent was obtained from all participants
for publication of their data.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
American Diabetes Association. 2.
Classification and diagnosis of diabetes: Standards of medical care
in diabetes-2019. Diabetes Care. 42 (Suppl 1):S13–S28.
2019.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Elmarakby AA and Sullivan JC: Relationship
between oxidative stress and inflammatory cytokines in diabetic
nephropathy. Cardiovasc Ther. 30:49–59. 2012.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Galicia-Garcia U, Benito-Vicente A, Jebari
S, Larrea-Sebal A, Siddiqi H, Uribe KB, Ostolaza H and Martín C:
Pathophysiology of type 2 diabetes mellitus. Int J Mol Sci.
21(6275)2020.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Scheithauer TPM, Rampanelli E, Nieuwdorp
M, Vallance BA, Verchere CB, van Raalte DH and Herrema H: Gut
microbiota as a trigger for metabolic inflammation in obesity and
type 2 diabetes. Front Immunol. 11(571731)2020.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Xiao J, Li J, Cai L, Chakrabarti S and Li
X: Cytokines and diabetes research. J Diabetes Res.
2014(920613)2014.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Oguntibeju OO: Type 2 diabetes mellitus,
oxidative stress and inflammation: Examining the links. Int J
Physiol Pathophysiol Pharmacol. 11:45–63. 2019.PubMed/NCBI
|
|
7
|
Cho NH, Ku EJ, Jung KY, Oh TJ, Kwak SH,
Moon JH, Park KS, Jang HC, Kim YJ and Choi SH: Estimated
association between cytokines and the progression to diabetes:
10-year follow-up from a community-based cohort. J Clin Endocrinol
Metab. 105:e381–e389. 2020.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Marie C, Pedard M, Quirie A, Tessier A,
Garnier P, Totoson P and Demougeot C: Brain-derived neurotrophic
factor secreted by the cerebral endothelium: A new actor of brain
function? J Cereb Blood Flow Metab. 38:935–949. 2018.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Shi SS, Shao SH, Yuan BP, Pan F and Li ZL:
Acute stress and chronic stress change brain-derived neurotrophic
factor (BDNF) and tyrosine kinase-coupled receptor (TrkB)
expression in both young and aged rat hippocampus. Yonsei Med J.
51:661–671. 2010.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Jiang Y, Wei N, Zhu J, Lu T, Chen Z, Xu G
and Liu X: Effects of brain-derived neurotrophic factor on local
inflammation in experimental stroke of rat. Mediators Inflamm.
2010(372423)2010.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Manni L, Nikolova V, Vyagova D, Chaldakov
GN and Aloe L: Reduced plasma levels of NGF and BDNF in patients
with acute coronary syndromes. Int J Cardiol. 102:169–171.
2005.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Li B, Lang N and Cheng ZF: Serum levels of
brain-derived neurotrophic factor are associated with diabetes
risk, complications, and obesity: A cohort study from Chinese
patients with type 2 diabetes. Mol Neurobiol. 53:5492–5499.
2016.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Al-Qudah M, Shammala DA, Al-Dwairi A,
Al-Shboul O and Mustafa AG: Dextran sodium sulphate (DSS)-induced
colitis alters the expression of neurotrophins in smooth muscle
cells of rat colon. Physiol Res. 66:1009–1020. 2017.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Al Qudah M, Alfaqih M, Al-Shboul O, Saadeh
R and Al-Dwairi A: Effect of cytokine treatment on the expression
and secretion of brain derived neurotrophic factor in the smooth
muscle of the rat colon. Biomed Rep. 13:55–60. 2020.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Krabbe KS, Nielsen AR, Krogh-Madsen R,
Plomgaard P, Rasmussen P, Erikstrup C, Fischer CP, Lindegaard B,
Petersen AM, Taudorf S, et al: Brain-derived neurotrophic factor
(BDNF) and type 2 diabetes. Diabetologia. 50:431–438.
2007.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Jin H, Chen Y, Wang B, Zhu Y, Chen L, Han
X, Ma G and Liu N: Association between brain-derived neurotrophic
factor and von Willebrand factor levels in patients with stable
coronary artery disease. BMC Cardiovasc Disord.
18(23)2018.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Kermani P and Hempstead B: BDNF actions in
the cardiovascular system: Roles in development, adulthood and
response to injury. Front Physiol. 10(455)2019.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Cho HJ, Kim SY, Park MJ, Kim DS, Kim JK
and Chu MY: Expression of mRNA for brain-derived neurotrophic
factor in the dorsal root ganglion following peripheral
inflammation. Brain Res. 749:358–362. 1997.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Giustina A, Adler RA, Binkley N,
Bollerslev J, Bouillon R, Dawson-Hughes B, Ebeling PR, Feldman D,
Formenti AM, Lazaretti-Castro M, et al: Consensus statement from
2nd International conference on controversies in vitamin D. Rev
Endocr Metab Disord. 21:89–116. 2020.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Calton EK, Keane KN, Newsholme P and
Soares MJ: The impact of vitamin D levels on inflammatory status: A
systematic review of immune cell studies. PLoS One.
10(e0141770)2015.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Ojaimi S, Skinner NA, Strauss BJ,
Sundararajan V, Woolley I and Visvanathan K: Vitamin D deficiency
impacts on expression of toll-like receptor-2 and cytokine profile:
A pilot study. J Transl Med. 11(176)2013.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Krishnan AV and Feldman D: Mechanisms of
the anti-cancer and anti-inflammatory actions of vitamin D. Annu
Rev Pharmacol Toxicol. 51:311–336. 2011.PubMed/NCBI View Article : Google Scholar
|
|
23
|
George PS, Pearson ER and Witham MD:
Effect of vitamin D supplementation on glycaemic control and
insulin resistance: A systematic review and meta-analysis. Diabet
Med. 29:e142–e150. 2012.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Nadimi H, Djazayery A, Javanbakht MH,
Dehpour A, Ghaedi E, Derakhshanian H, Mohammadi H, Mousavi SN and
Djalali M: Effect of vitamin D supplementation on CREB-TrkB-BDNF
pathway in the hippocampus of diabetic rats. Iran J Basic Med Sci.
23:117–123. 2020.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Khairy EY and Attia MM: Protective effects
of vitamin D on neurophysiologic alterations in brain aging: Role
of brain-derived neurotrophic factor (BDNF). Nutr Neurosci.
24:650–659. 2021.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Chamberlain JJ, Johnson EL, Leal S,
Rhinehart AS, Shubrook JH and Peterson L: Cardiovascular disease
and risk management: Review of the American diabetes association
standards of medical care in diabetes 2018. Ann Intern Med.
168:640–650. 2018.PubMed/NCBI View
Article : Google Scholar
|
|
27
|
Tsalamandris S, Antonopoulos AS, Oikonomou
E, Papamikroulis GA, Vogiatzi G, Papaioannou S, Deftereos S and
Tousoulis D: The role of inflammation in diabetes: Current concepts
and future perspectives. Eur Cardiol. 14:50–59. 2019.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Kolb H and Martin S:
Environmental/lifestyle factors in the pathogenesis and prevention
of type 2 diabetes. BMC Med. 15(131)2017.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Al-Qudah M, Shammala DA, Al-Dwairi A and
Al-Shboul O: Differential expression of neurotrophins in
(DSS)-induced colitis in smooth muscle of rat colon. J Teknologi.
78 (5-5):2016.
|
|
30
|
Rozanska O, Uruska A and
Zozulinska-Ziolkiewicz D: Brain-derived neurotrophic factor and
diabetes. Int J Mol Sci. 21(841)2020.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Faria MC, Gonçalves GS, Rocha NP, Moraes
EN, Bicalho MA, Gualberto Cintra MT, Jardim de Paula J, José Ravic
de Miranda LF, Clayton de Souza Ferreira A, Teixeira AL, et al:
Increased plasma levels of BDNF and inflammatory markers in
Alzheimer's disease. J Psychiatr Res. 53:166–172. 2014.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Borba EM: Association of two levels of
BDNF (brain derived neurotrophic factor) no mild cognitive
impairment and Alzheimer's disease (unpublished PhD thesis).
Federal University of Rio Grande do Sul, 2012.
|
|
33
|
Zilliox LA, Chadrasekaran K, Kwan JY and
Russell JW: Diabetes and cognitive impairment. Curr Diab Rep.
16(87)2016.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Angelucci F, Spalletta G, di Iulio F,
Ciaramella A, Salani F, Colantoni L, Varsi AE, Gianni W, Sancesario
G, Caltagirone C and Bossù P: Alzheimer'S disease (AD) and mild
cognitive impairment (MCI) patients are characterized by increased
BDNF serum levels. Curr Alzheimer Res. 7:15–20. 2010.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Babaei P, Shirkouhi SG, Hosseini R and
Soltani Tehrani B: Vitamin D is associated with metabotropic but
not neurotrophic effects of exercise in ovariectomized rats.
Diabetol Metab Syndr. 9(91)2017.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Neveu I, Naveilhan P, Baudet C, Brachet P
and Metsis M: 1,25-dihydroxyvitamin D3 regulates NT-3, NT-4 but not
BDNF mRNA in astrocytes. Neuroreport. 6:124–126. 1994.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Kulaksizoglu S and Kulaksizoglu B: The
relationship between metabolic syndrome, BDNF, and vitamin D in
patients with schizophrenia. Neurochem. J. 11:104–111. 2017.
|
|
38
|
Seyedi M, Gholami F, Samadi M, Djalali M,
Effatpanah M, Yekaninejad MS, Hashemi R, Abdolahi M, Chamari M and
Honarvar NM: The effect of vitamin D3 supplementation on serum
BDNF, dopamine, and serotonin in children with
attention-deficit/hyperactivity disorder. CNS Neurol Disord Drug
Targets. 18:496–501. 2019.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Pozzi F, Frajese GV and Frajese G: Vitamin
D (Calcifediol) supplementation modulates NGF and BDNF and improves
memory function in postmenopausal women: A pilot study.
Endocrinology. 2013:1–11. 2013.
|