Introduction
Inflammation is a protective mechanism against
harmful stimuli, which is necessary for cells to maintain
physiological conditions (1). The
inflammatory response is essential in protecting the body; however,
continuous inflammation can cause diseases such as cancer, high
blood pressure, arteriosclerosis, allergies, and asthma (2,3).
Macrophages play a role in cytokine production, antigen
presentation, phagocytosis, and immune regulation in inflammation.
Macrophages produce IL-6, TNF-α, IFN-γ, and nitric oxide (NO)
following LPS stimulation (4,5). NO
protects cells from pathogenically induced DNA damage; however,
excessive production of iNOS exacerbates inflammation by promoting
the production of inflammatory mediators (6). COX-2 induced by LPS and cytokines is
involved in prostaglandin production in acute inflammatory
responses (7). High levels of NO,
COX-2, and cytokines in the blood are typical features of chronic
inflammation, leading to cancer, rheumatic diseases, and endocrine
diseases (8-10).
Therefore, the control of these inflammatory mediators is a key
topic in the treatment of chronic inflammation. Drugs such as
Aspirin, Ibuprofen, and Dexibuprofen are used to suppress excessive
inflammation (11,12). However, anti-inflammatory drugs
cause hypotension, decreased gastrointestinal function, and mucosal
damage bleeding due to a decrease in cortisol levels (13,14).
Therefore, studies on inflammation control through natural products
with relatively fewer side effects are being increasingly studied
(15). Red ginseng has been widely
used as a traditional medicine in Korea, China, and Japan (16). It contains saponins, ginsenosides,
polysaccharides, and fatty acids, which have anti-inflammatory,
anti-cancer, and antioxidant effects (17).
Red ginseng (steamed and dried panax ginseng
C.A. Meyer) marc, a by-product of processed red ginseng products,
is usually thrown away or used as feed for livestock. However, red
ginseng marc contains the active ingredients of red ginseng, thus
it has potential use (18).
Paeonia japonica Miyabe & Takeda is a plant of the
Angelica family, known for its antioxidant and immunomodulatory
activities, and is widely used in oriental medicine (19). The paeoniflorin and paeonol
contained in P. japonica possess analgesic, antipyretic,
anti-inflammatory and anti-ulcer effects (20). Angelica gigas Nakai belongs
to the Umbelliferae family and is a traditional medicinal plant
distributed throughout North Asia, and it has been reported to be
efficacious in the treatment of anemia and cancer, and possess
anti-inflammatory properties (21).
Artemisia scoparia Waldst.et Kit, a perennial plant in the
Asteraceae family, grows on sandy beaches and is known to possess
anti-inflammatory and antioxidant effects (22-24).
Although natural products each have their own health benefits,
several studies have been reported that show synergistic effects
when they are used as a mixture (25,26).
Therefore, the aim of this study is to evaluate the
anti-inflammatory effects of red ginseng marc, P.
japonica, A. gigas, and A.
scoparia complexes in LPS-stimulated RAW 264.7 cells.
Materials and methods
Materials
Antibodies against JNK (cat. no. sc-7345), p-JNK
(cat. no. sc-293136), ERK (cat. no. sc-514302), p-ERK (cat. no.
sc-81492), NF-κB (cat. no. sc-8008), p-NF-κB (cat. no. sc-136548),
p-IκB (cat. no. sc-8404), and β-actin (cat. no. sc-8432) were
purchased from Santa Cruz Biotechnology, Inc. Antibodies against
iNOS (cat. no. 13120) and COX-2 (cat. no. 4842) were acquired from
Cell Signaling Technology, Inc. The Qunati-MAX™ WST-8 Cell
Viability Assay Kit and WestGlow™ Chemiluminescent substrate were
obtained from Biomax FBS, RIPA buffer and DMEM were purchased from
Gibco; Thermo Fisher Scientific, Inc. Penicillin/streptomycin
antibiotics, carboxy-H2DCFDA and Goat anti-Mouse IgG
Alexa Fluor 488 (cat. no. A-11001) were purchased from Invitrogen;
Thermo Fisher Scientific, Inc. (Thermo Fisher Scientific, Inc.).
Griess reagent and lipopolysaccharide (LPS, cat. no. L2630) were
procured from Sigma-Aldrich; Merck KGaA. ELISA kits for IL-6 and
TNF-α were obtained from R&D Systems, Inc. The Bradford assay
reagent and SDS-PAGE sample loading buffer were purchased from
Bio-Rad Laboratories, Inc.
Plant extract preparation
Red ginseng marc, A. scoparia, P.
japonica and A. gigas were purchased from Jinandang
Farming Association Corporation (Jeollabuk-do). The plants were
authenticated by Prof Kim Hong-Jun at the College of Oriental
Medicine, Woosuk University. Red ginseng marc, A. scoparia,
P. japonica and A. gigas (100 g each) were boiled in
distilled water (2l) for 2 h at 121˚C, 15 psi. After incubation,
these extracts were filtered thrice using an 7 µm filter paper
(cat. no. AD.01511110; Advantec) concentrated, dried and further
stored at -20˚C. Each extract was mixed in a ratio of red ginseng
marc 50: A. scoparia 20: P. japonica 20: A.
gigas 10. The mixture was termed RAPA.
Cell culture
RAW 264.7 cells derived from mice were purchased
from ATCC and cultured in DMEM containing 10% FBS and 1% penicillin
& streptomycin in a CO2 incubator (5% CO2
and 95% atmosphere) with sufficient humidity at 37˚C.
Cell cytotoxicity
RAW 264.7 cells were aliquoted to a final
concentration of 2x105 cells/ml in a 96-well plate and
cultured for 24 h at 37˚C in an incubator under 5% CO2
conditions. Cultured cells were treated with RAPA extract at a
concentration of 40-400 µg/ml, and after 24 h of incubation,
cytotoxicity was measured using Qunati-MAX™ WST-8 Cell Viability
Assay Kit (Biomax).
NO assay
NO assays were performed as described previously
(25).
ELISA
ELISA for IL-6 and TNF-α cytokines were performed
using the ELISA Kits on the collected culture medium according to
the manufacturer's protocol.
Western blot
A total of 2x105 cells/ml RAW264.7 cells
were inoculated into a 60 mm dish for 24 h, pretreated with RAPA
mixture (0, 100, or 400 µg/ml) for 1 h, and stimulated with LPS (1
µg/ml) for 30 min or 24 h. To collect the cells, 1 ml PBS was added
to the cells, collected with a scraper, and centrifuged twice using
PBS. RIPA buffer (100 µl) was added, the cells were incubated on
ice for 15 min and the total protein was extracted by
centrifugation at 14,000 x g. The protein content was quantified
using a Bradford assay, and the extracted proteins from the cell
lysate were resolved using a 10% SDS-gel and SDS-PAGE. Resolved
proteins were transferred to PVDF membranes, which were
subsequently blocked with 5% skimmed milk [0.01 M Tris-HCL buffer
(TBST)] for 60 min at room temperature. Next, the PVDF membranes
were washed 5 times with TBST and then incubated with the
antibodies (iNOS 1:1,000; COX-2 1:1,000; p-JNK 1:200; JNK 1:200;
p-ERK 1:200; ERK 1:200; p-NF-κB 1:200; NF-κB 1:200; p-IκB 1:200;
IκB 1:200; and β-actin 1:1,000) for 16 h at 4˚C. After 16 h of
incubation, the membranes were washed 5 times for 5 min with TBST,
incubated with horseradish peroxidase-conjugated secondary primary
antibodies for 2 h at room temperature, washed 5 times for 5 min
with TBST, and examined using a UVItec Chemiluminescence Imaging
System. The protein band densities were analyzed using ImageJ
version 1.53 (National Institutes of Health).
Intracellular ROS measurements
A total of 2x105 cells/ml RAW 264.7 were
inoculated into a 60 mm dish, and treated with 400 µg/ml RAPA
mixture after 24 h. After 1 h, the cells were stimulated with LPS
(1 µg/ml) and cultured for 24 h. The cells were treated with
carboxy-H2DCFDA (5 µM) for 30 min, harvested, and
counted at a FITC wavelength (488 nm) in a flow cytometer (Cyto
FLEX LX; Beckman Coulter, Inc.).
Immunofluorescence assay
RAW264.7 cells were aliquoted in 4-well cell culture
slides at a concentration of 2x105 cells/ml and cultured
for 24 h. After 24 h of incubation, the cells were mixed with RAPA
(0, 100, or 400 µg/ml) for 30 min and stimulated with 1 µg/ml LPS
for 24 h. The cells were fixed with methanol, blocked with PBS
containing 1% BSA, and then incubated at 4˚C for 16 h after
addition of the p65 NF-κB antibody (1:50). After washing 3 times
for 10 min with PBST, the secondary antibody was reacted with goat
anti-Rabbit IgG Alexa Fluor 488, followed by mounting with a
ProLong® Gold Antifade Reagent (cat. no. #9071; Cell
Signaling Technology Inc.). After drying the slides overnight,
images were captured using a fluorescence microscope (Carl Zeiss
GmbH; x100 magnification).
Statistical analysis
Data are presented as the mean ± SD. Differences
between groups were compared using a one-way ANOVA followed by a
Tukey's post-hoc test. P<0.05 was considered to indicate a
statistically significant difference.
Results and discussion
Effect of RAPA on NO production in
LPS-treated RAW 264.7 cells
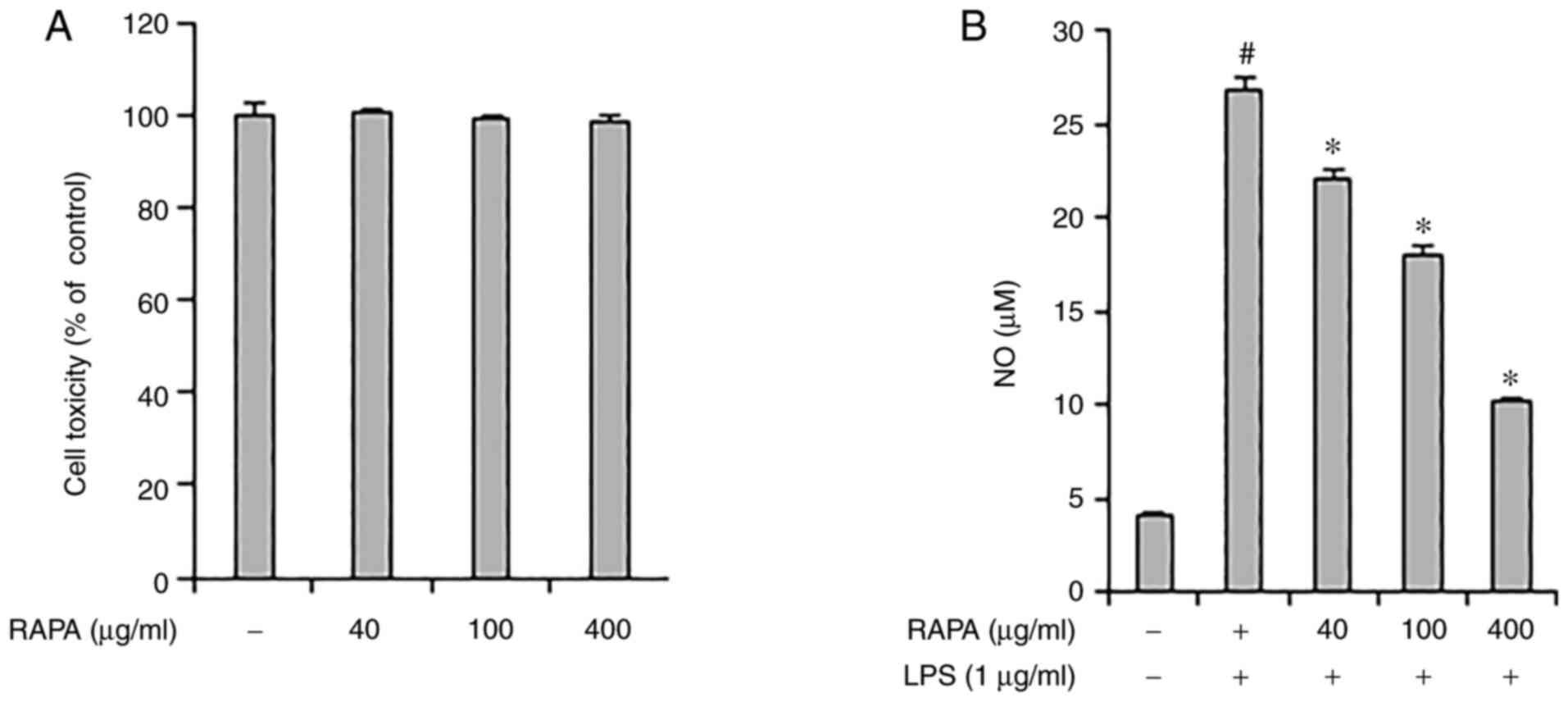
The effect of RAPA on the viability of RAW 264.7
cells was investigated. RAPA did not show any notable toxicity
between 40-400 µg/ml (Fig. 1A).
Therefore, in this study, cells were treated with <400 µg/ml
RAPA. Next, the effect of RAPA on NO production by LPS was
investigated using an NO assay. The results showed that treatment
with RAPA suppressed NO production. In particular, 400 µg/ml RAPA
treatment reduced NO production by 62% compared with the positive
control group (Fig. 1B). NO
protects cells from pathogens; however, excessive NO production has
a cytotoxic effect on the surrounding tissues (27). Therefore, regulation of NO
production is essential in the control of chronic inflammation.
Based on the above results, it is suggested that RAPA may be useful
in managing chronic inflammation as it effectively suppressed NO
production in LPS-treated RAW 264.7 cells.
Effect of RAPA on iNOS and COX-2
production in LPS-treated RAW 264.7 cells
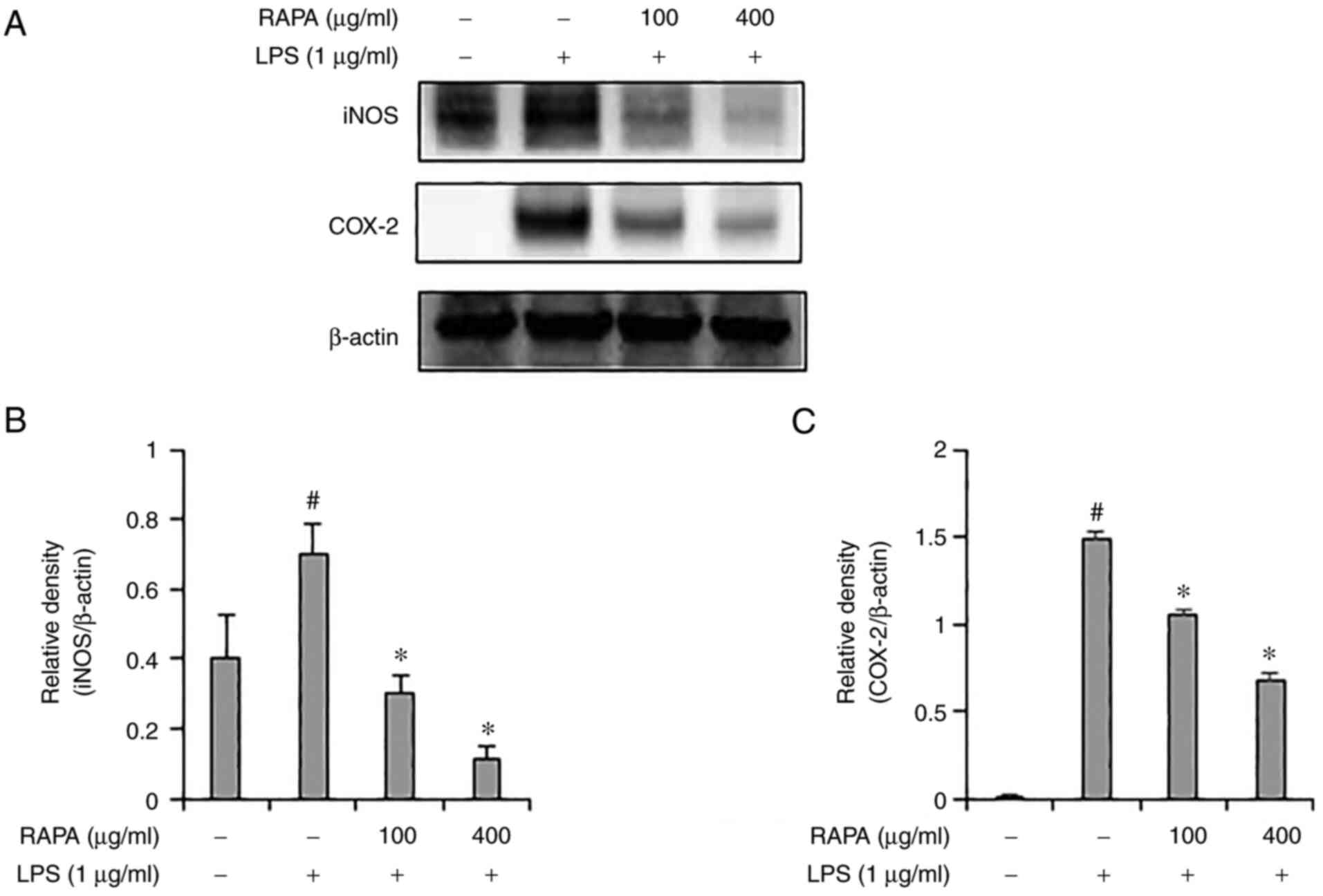
iNOS and COX-2 induce inflammation through the
production of NO and PGE2. According to previous
studies, natural products such as red ginseng marc, A.
scoparia, and P. japonica, A. gigas have been
reported to exert an anti-inflammatory effect via regulation of
iNOS and COX-2 expression. (25,28).
Therefore, in this study, the expression of iNOS and COX-2 were
determined in the LPS-treated RAW 264.7 cells. iNOS and COX-2
expression levels were significantly increased in the group treated
with LPS alone, but their expression was suppressed in the cells
pretreated with RAPA (Fig. 2).
These results suggest that RAPA suppresses inflammation by
suppressing iNOS and COX-2 expression.
Effect of RAPA on IL-6 and TNF-α
production in LPS-treated RAW 264.7 cells
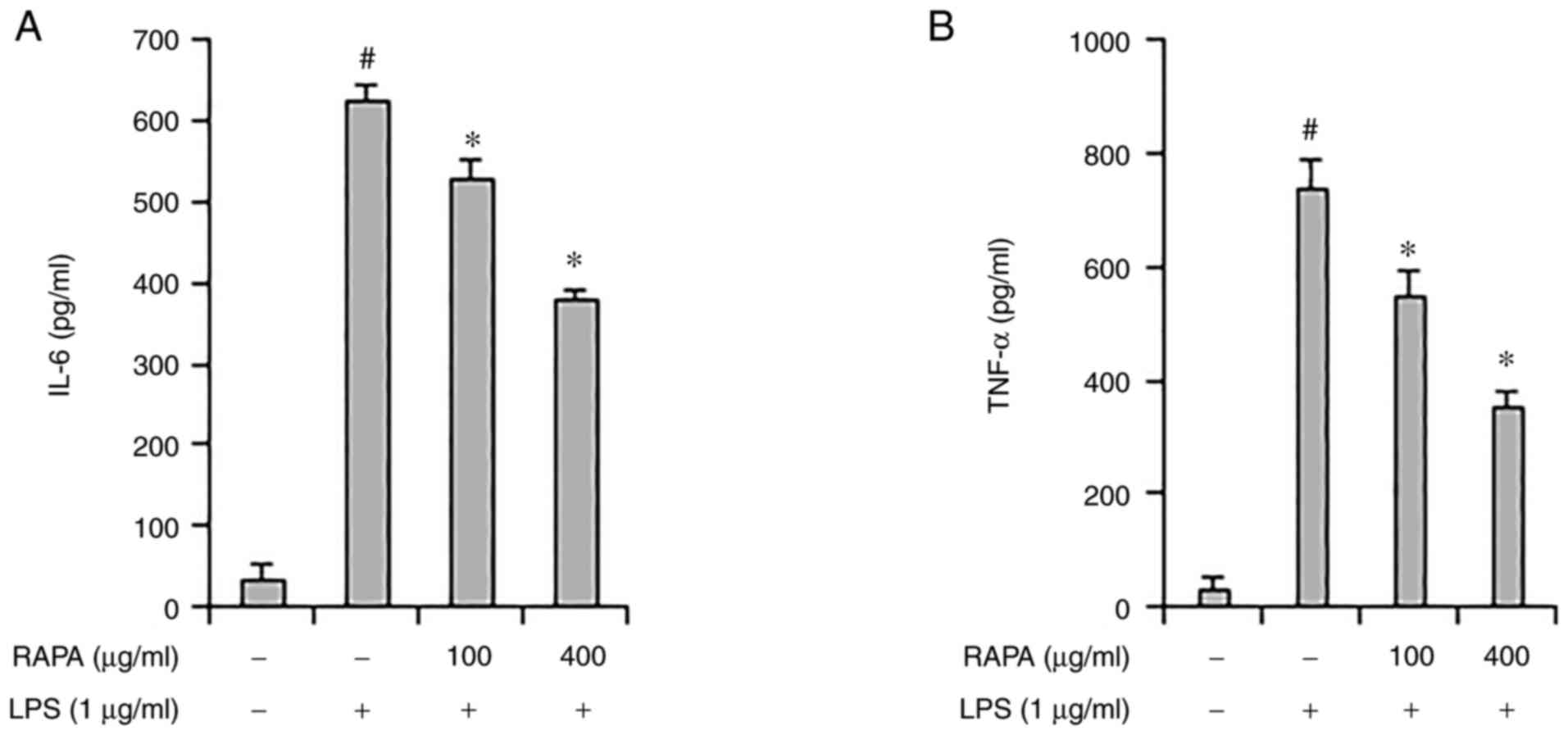
The role of cytokines in inflammation is very
important, and TNF-α and IL-6 are considered representative
pro-inflammatory cytokines, as they both regulate the inflammatory
response (29). Herbs such as
Rehmannia glutinosa and Suaeda japonica are known to
inhibit inflammation by regulating IL-6 and TNF-α expression
(30,31). ELISA was performed to investigate
the effect of RAPA on IL-6 and TNF-α expression LPS-treated RAW
264.7 cells. IL-6 and TNF-α expression increased significantly in
the positive control group, and their expression decreased in the
RAPA-pretreatment group (Fig. 3A
and B). These results show that
RAPA suppresses inflammation by suppressing cytokine
expression.
Effect of RAPA on the MAPK signaling
pathway in LPS-treated RAW 264.7 cells
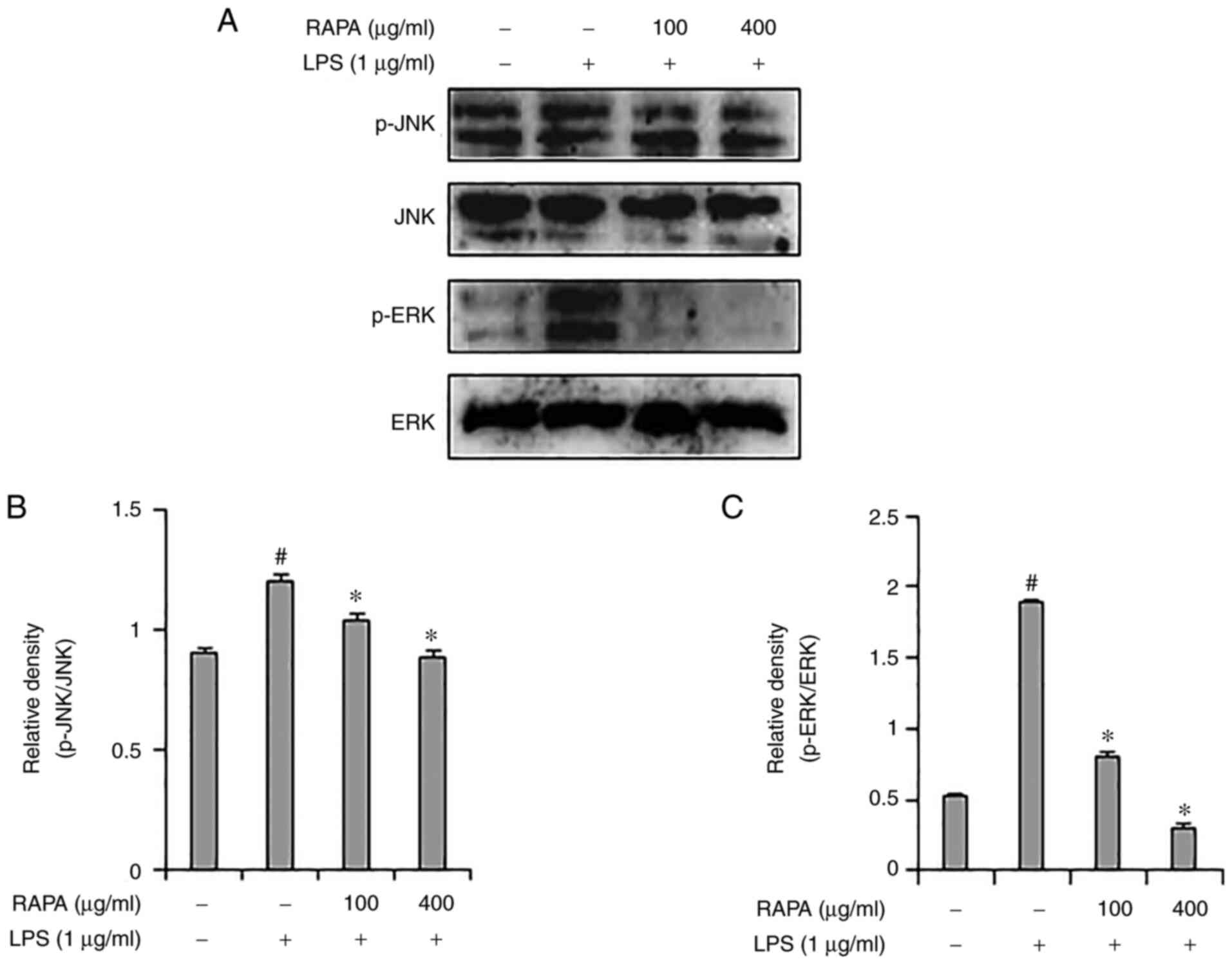
Representative members of the MAPK signaling pathway
include JNK, p38 and ERK1/2, which are important signal
transmitters in the inflammatory response. MAPKs are associated
with COX-2 expression in iNOS and play an important role in NF-κB
activation (32,33). Western blotting was used to assess
whether the anti-inflammatory effects of RAPA were mediated through
the MAPK signaling pathway. Increased ERK1/2 and JNK
phosphorylation were observed in the LPS-treated group (Fig. 4), and RAPA pretreatment inhibited
ERK1/2 and JNK phosphorylation. Of note, p38 activation was not
affected (data not shown). These results suggest that RAPA is
involved in anti-inflammatory activity through inhibition of the
ERK1/2 and JNK pathways.
Effect of RAPA on the activity of
NF-κB signaling pathway in LPS-treated RAW 264.7 cells
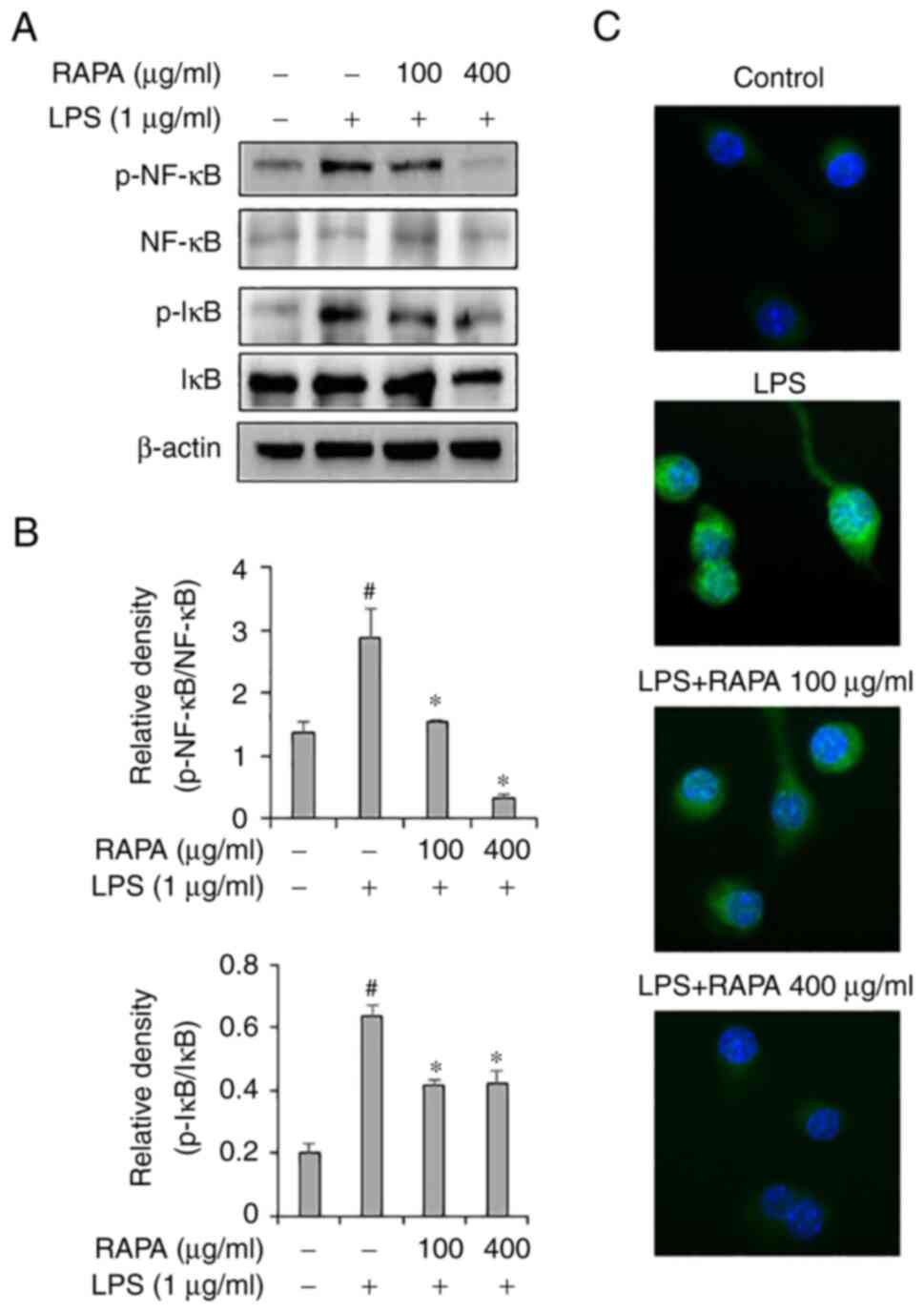
To investigate the effects of RAPA on the NF-κB
signaling pathway, western blotting and immunofluorescence staining
were performed. Western blot results demonstrated that RAPA
treatment effectively inhibited the activity of NF-κB and IκB in
LPS-treated RAW 264.7 cells (Fig.
5A). Immunofluorescence staining results also showed that RAPA
treatment inhibited the translocation of NF-κB to the nucleus
(Fig. 5B). The NF-κB signaling
pathway plays a key role in LPS-induced inflammation models. In the
resting state, NF-κB is present in the cytoplasm and is bound to
the repressor protein IκB, which has no transcriptional activity.
Stimulation by LPS liberates and activates NF-κB from IκB, and it
then translocates to the nucleus to induce transcription of
inflammatory genes (34,35). In this study, the activation of
NF-κB and IκB was significantly increased in the LPS-treated RAW
264.7 cells, and this was decreased by RAPA pretreatment.
Therefore, in the LPS inflammation model, it was hypothesized that
RAPA may suppress excessive inflammatory responses by inhibiting
the NF-κB signaling pathway. Medicinal plants with
anti-inflammatory activity contain substances such as alkaloids,
flavonoids, saponins, condensed tannins, terpenoids,
phenylpropanoids and hydrolysable tannins (36). Whilst RAPA was shown to exhibit an
anti-inflammatory effect, it is not known which specific
component(s) exerted the anti-inflammatory effects.
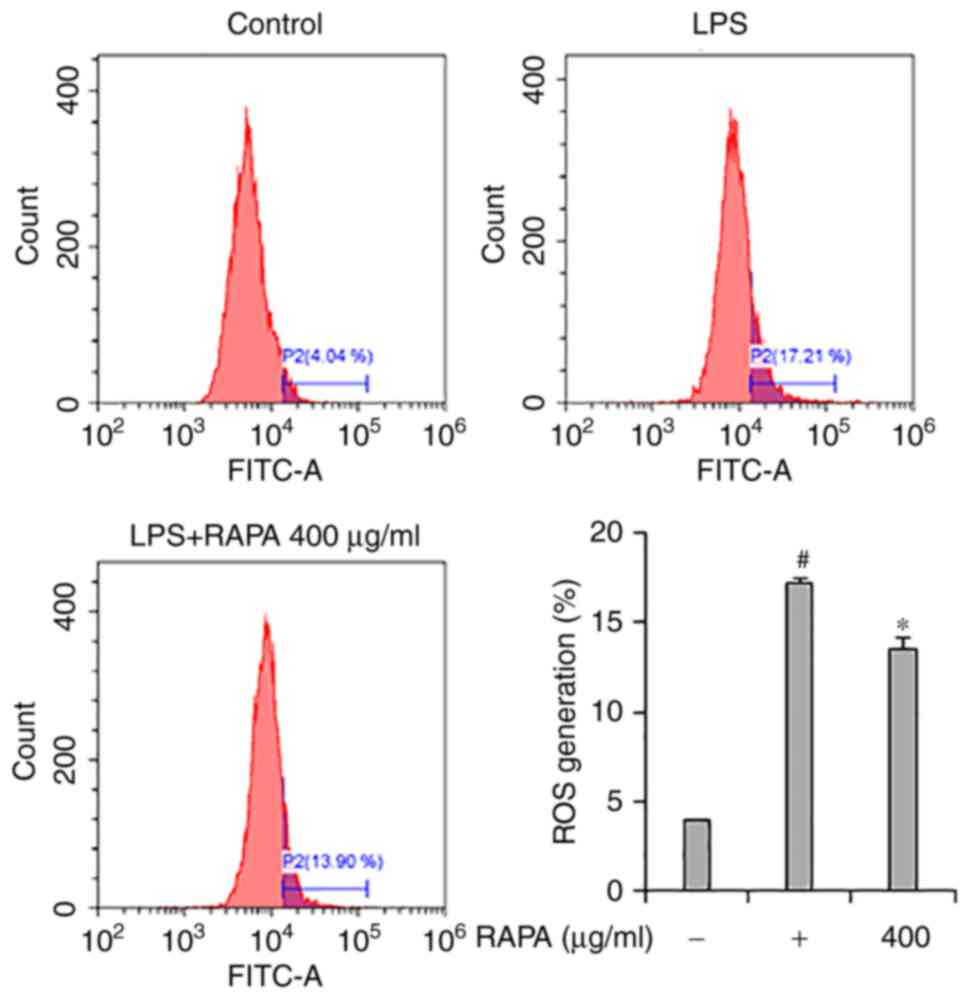
Effect of RAPA on ROS generation in
LPS-treated RAW 264.7 cells
Flow cytometry was performed to investigate the
effects of RAPA on ROS generation in LPS-treated RAW 264.7 cells.
The results showed that LPS treatment significantly increased ROS
production in cells (Fig. 6).
However, pretreatment with RAPA inhibited the production of
LPS-induced ROS. LPS-induced ROS participates in the regulation of
NF-κB activation, and activated NF-κB is involved in the production
of COX-2, iNOS, and cytokines in macrophages (37). Thus, it is hypothesized that the
inhibition of NF-κB activity in RAPA pretreated LPS-treated cells
may have contributed to the inhibition of ROS production.
In conclusion, the anti-inflammatory effects of RAPA
in LPS-treated cells were demonstrated. It is hypothesized that the
anti-inflammatory effects of RAPA are achieved though the induction
of production of inflammatory mediators such as IL-6, COX-2, iNOS,
and TNF-α via inhibition of the MAPK and NF-κB signaling pathway.
These results suggest that RAPA may be used as an anti-inflammatory
agent derived from natural products.
Acknowledgements
Not applicable.
Funding
Funding: This research was financially supported by the Ministry
of Small and Medium-sized Enterprises and Startups, under the
‘Regional Specialized Industry Development Plus Program (R&D,
Project no. S3088018)’ supervised by the Korea Technology and
Information Promotion Agency.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
JYS and BOC conceived and designed the experiments.
JYS and ESK participated in the design of the study and drafting of
the manuscript. JYS and ESK performed the majority of the
experiments. JHP participated in the cell culture and western
blotting. JYS and SIJ confirm the authenticity of all the raw data.
All authors have read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests. The data included in this study were previously
published in the ‘Korean Society and Food Science Nutrition 2021
International Symposium and Annual meeting’.
References
|
1
|
Lawrence T, Willoughby DA and Gilroy DW:
Anti-inflammatory lipid mediators and insights into the resolution
of inflammation. Nat Rev Immunol. 2:787–795. 2002.PubMed/NCBI View
Article : Google Scholar
|
|
2
|
Shacter E and Weitzman SA: Chronic
inflammation and cancer. Oncology (Williston Park). 16:217–226,
229; discussion 230-2. 2002.PubMed/NCBI
|
|
3
|
Pahwa R, Goyal A, Bansal P and Jialal I:
Chronic inflammation. 2018.
|
|
4
|
Fujiwara N and Kobayashi K: Macrophages in
inflammation. Curr Drug Targets Inflamm Allergy. 4:281–286.
2005.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Echizen K, Hirose O, Maeda Y and Oshima M:
Inflammation in gastric cancer: Interplay of the
COX-2/prostaglandin E2 and Toll-like receptor/MyD88 pathways.
Cancer Sci. 107:391–397. 2016.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Goldsby R, Kindt TJ, Osborne BA and Kuby
J: In: Immunology (5: e uppl.). 5th Edition. W. H. Freeman and
Company, New York, 2003.
|
|
7
|
Hume DA, Wells CA and Ravasi T:
Transcriptional regulatory networks in macrophages. Novartis Found
Symp. 281:2–18. 2007.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Sharma JN, Al-Omran A and Parvathy SS:
Role of nitric oxide in inflammatory diseases.
Inflammopharmacology. 15:252–259. 2007.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Zelová H and Hošek J: TNF-α signalling and
inflammation: Interactions between old acquaintances. Inflamm Res.
62:641–651. 2013.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Balkwill F: Tumour necrosis factor and
cancer. Nat Rev Cancer. 9:361–371. 2009.PubMed/NCBI View
Article : Google Scholar
|
|
11
|
Farinelli I and Martelletti P: Aspirin and
tension-type headache. J Headache Pain. 8:49–55. 2007.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Bonabello A, Galmozzi MR, Canaparo R,
Isaia GC, Serpe L, Muntoni E and Zara GP: Dexibuprofen (S+-isomer
ibuprofen) reduces gastric damage and improves analgesic and
antiinflammatory effects in rodents. Anesth Analg. 97:402–408.
2003.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Wiechert R: Modern steroid problems. Angew
Chem Int Ed Engl. 9:321–332. 1970.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Grennan D and Wang S: Steroid side
effects. JAMA. 322(282)2019.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Kumar S, Bajwa BS, Kuldeep S and Kalia AN:
Anti-inflammatory activity of herbal plants: A review. Int J Adv
Pharm Biol Chem. 2:272–281. 2013.
|
|
16
|
Baeg IH and So SH: The world ginseng
market and the ginseng (Korea). J Ginseng Res. 37:1–7.
2013.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Yang Y, Yang WS, Yu T, Sung GH, Park KW,
Yoon K, Son YJ, Hwang H, Kwak YS, Lee CM, et al:
ATF-2/CREB/IRF-3-targeted anti-inflammatory activity of Korean red
ginseng water extract. J Ethnopharmacol. 154:218–228.
2014.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Bak MJ, Hong SG, Lee JW and Jeong WS: Red
ginseng marc oil inhibits iNOS and COX-2 via NFκB and p38 pathways
in LPS-stimulated RAW 264.7 macrophages. Molecules. 17:13769–13786.
2012.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Lee SB, Lee JS, Moon SO, Lee HD, Yoon YS
and Son CG: A standardized herbal combination of Astragalus
membranaceus and Paeonia japonica, protects against muscle atrophy
in a C26 colon cancer cachexia mouse model. J Ethnopharmacol.
267(113470)2021.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Lim SY, Jang JH, Lee HJ, Park SS, Kim SR,
Lee KM, Kim JK, Park H and Jung HK: Characteristics and
phylogenetic analysis of the complete chloroplast genome of Paeonia
japonica (Paeoniaceae). Mitochondrial DNA B Resour. 6:734–735.
2021.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Sowndhararajan K and Kim S:
Neuroprotective and cognitive enhancement potentials of Angelica
gigas Nakai Root: A Review. Sci Pharm. 85(21)2017.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Ding J, Wang L, He C, Zhao J, Si L and
Huang H: Artemisia scoparia: Traditional uses, active constituents
and pharmacological effects. J Ethnopharmacol.
273(113960)2021.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Boudreau A, Burke SJ, Collier JJ, Richard
AJ, Ribnicky DM and Stephens JM: Mechanisms of Artemisia scoparia's
anti-inflammatory activity in cultured adipocytes, macrophages, and
pancreatic β-cells. Obesity (Silver Spring). 28:1726–1735.
2020.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Ahn JH, Park YL, Song AY, Kim WG, Je CY,
Jung DH, Kim YJ, Oh J, Cho JY, Kim DJ and Park JH: Water extract of
Artemisia scoparia Waldst. & Kitam suppresses LPS-induced
cytokine production and NLRP3 inflammasome activation in
macrophages and alleviates carrageenan-induced acute inflammation
in mice. J Ethnopharmacol. 268(113606)2021.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Cho BO, Shin JY, Kang HJ, Park JH, Hao S,
Wang F and Jang SI: Anti-inflammatory effect of Chrysanthemum
zawadskii, peppermint, Glycyrrhiza glabra herbal mixture in
lipopolysaccharide-stimulated RAW264. 7 macrophages. Mol Med Rep.
24(532)2021.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Nayak SS, Ghosh AK, Debnath B, Vishnoi SP
and Jha T: Synergistic effect of methanol extract of Abies webbiana
leaves on sleeping time induced by standard sedatives in mice and
anti-inflammatory activity of extracts in rats. J Ethnopharmacol.
93:397–402. 2004.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Stoclet J, Muller B, Andriantsitohaina R
and Kleschyov A: Overproduction of nitric oxide in pathophysiology
of blood vessels. Biochemistry (Mosc). 63:826–832. 1998.PubMed/NCBI
|
|
28
|
Shin JY, Park JH, Che DN, Kang HJ, Cho BO,
Lim YT and Jang SI: Protective effects of halophyte complex extract
against UVB-induced damage in human keratinocytes and the skin of
hairless mice. Exp Ther Med. 22(682)2021.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Fernandez-Escobar R, Moreno R and
Garcıa-Creus M: Seasonal changes of mineral nutrients in olive
leaves during the alternate-bearing cycle. Scientia Horticulturae.
82:25–45. 1999.
|
|
30
|
Bastidas-Coral AP, Bakker AD,
Zandieh-Doulabi B, Kleverlaan CJ, Bravenboer N, Forouzanfar T and
Klein-Nulend J: Cytokines TNF-α, IL-6, IL-17F, and IL-4
differentially affect osteogenic differentiation of human adipose
stem cells. Stem Cells Int. 2016(1318256)2016.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Yi L, Zhou Z, Zheng Y, Chang M, Huang X,
Guo F, Zhao Q and Huan J: Suppressive effects of GSS on
lipopolysaccharide-induced endothelial cell injury and ALI via
TNF-α and IL-6. Mediators Inflamm. 2019(4251394)2019.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Sugiura R, Satoh R, Ishiwata S, Umeda N
and Kita A: Role of RNA-Binding Proteins in MAPK signal
transduction pathway. J Signal Transduct.
2011(109746)2011.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Du W, Hu H, Zhang J, Bao G, Chen R and
Quan R: The mechanism of MAPK signal transduction pathway involved
with electroacupuncture treatment for different diseases. Evid
Based Complement Alternat Med. 2019(8138017)2019.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Xia YF, Liu LP, Zhong CP and Geng JG:
NF-kappaB activation for constitutive expression of VCAM-1 and
ICAM-1 on B lymphocytes and plasma cells. Biochem Biophys Res
Commun. 289:851–856. 2001.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Li L, Chen J, Lin L, Pan G, Zhang S, Chen
H, Zhang M, Xuan Y, Wang Y and You Z: Quzhou Fructus Aurantii
Extract suppresses inflammation via regulation of MAPK, NF-κB, and
AMPK signaling pathway. Sci Rep. 10(1593)2020.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Nunes CDR, Barreto Arantes M, Menezes de
Faria Pereira S, Leandro da Cruz L, de Souza Passos M, Pereira de
Moraes L, Vieira IJC and Barros de Oliveira D: Plants as sources of
anti-inflammatory agents. Molecules. 25(3726)2020.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Xie QW, Kashiwabara Y and Nathan C: Role
of transcription factor NF-kappa B/Rel in induction of nitric oxide
synthase. J Biol Chem. 269:4705–4708. 1994.PubMed/NCBI
|