Introduction
The molecular-targeted drug ramucirumab (RAM) is a
recombinant humanized monoclonal antibody against human vascular
endothelial growth factor (VEGF) receptor 2 (VEGFR-2) (1,2). VEGF
is a cytokine that promotes angiogenesis by increasing the
proliferation rate of endothelial cells and functions as a survival
factor (3). RAM binds specifically
to VEGFR-2 and interferes with its downstream intracellular signal
cascades, inhibiting angiogenesis in tumor tissues and exerting an
antitumor effect (1,2). RAM is effective when used in
combination with standard chemotherapy for patients with advanced
or recurrent gastric cancer in which curative resection is not
possible, those with unresectable advanced or recurrent colorectal
cancer, and those with inoperable or recurrent non-small-cell lung
cancer (4,5). In particular, amongst the VEGF
inhibitors used in Japan including bevacizumab, aflibercept, and
RAM, only RAM has the therapeutic indication for unresectable
advanced or recurrent gastric cancer, and it is typically combined
with paclitaxel (PTX). The typical adverse effects of VEGF
inhibitors include hypertension, proteinuria, and bleeding
(6,7). In particular, the development of
proteinuria causes renal failure and requires discontinuation of
VEGF inhibitors (8-10).
A previous clinical study conducted on cancer patients receiving
the VEGF inhibitor bevacizumab showed that there was a close
association between elevated blood pressure and the occurrence of
proteinuria (11), indicating that
appropriate management of blood pressure during treatment with VEGF
inhibitors is important for the reduction of proteinuria.
Additionally, we previously reported that management of blood
pressure using renin-angiotensin system inhibitors (RAS-I), such as
angiotensin-converting enzyme inhibitors and angiotensin II (AII)
receptor blockers, may reduce bevacizumab-induced proteinuria
occurrence (12). However, this
study did not investigate whether RAS-I had an advantage over
calcium channel blockers (CCB), which are potent vasodilators, in
terms of proteinuria reduction. Furthermore, to the best of our
knowledge, there are no studies on RAS-I vs. CCB for gastric cancer
patients treated with RAM. This retrospective study aimed to
compare the effectiveness of RAS-I and CCB in the reduction of
proteinuria in patients with gastric cancer treated with
combination therapy with RAM and PTX.
Materials and methods
Patients
Adult Japanese patients with gastric cancer who were
outpatients at Asahikawa Medical University Hospital, National
Hospital Organization Hokkaido Cancer Center, and Iwate Medical
University Hospital between July 1, 2015, and March 31, 2021, were
selected for inclusion in this study. Of these patients, those who
had received first-time RAM treatment (as concomitant therapy with
RAM and PTX), and those treated with hypertension therapy with
antihypertensive agents including RAS-I or CCB at initial RAM
administration were included. Patients with metastatic pancreatic
cancer, diabetes, prior proteinuria, liver and kidney dysfunction,
or inadequate laboratory data at the start of RAM administration
were excluded from the analysis. In addition, patients who had
changed their chemotherapeutic regimen, those with changes in types
or dosage of antihypertensive agents, those who had taken any
additional antihypertensive agents, those who had taken both RAS-I
and CCB, and those who had discontinued RAS-I and CCB
administration within 12 weeks of the start of RAM treatment were
also excluded. This study was reviewed and approved by the ethics
committee of each participating institution. The requirement for
informed consent was waived due to the retrospective nature of the
study. However, the study was described on the websites of all
participating medical institutes, and the patients orally confirmed
their agreement or refusal to participate in the study.
Retrospective survey
Data on sex, age, height, weight, doses of RAM and
PTX, type of concomitant drugs, the occurrence of proteinuria,
laboratory test results [including the levels of alanine
aminotransferase (ALT), aspartate aminotransferase (AST), serum
creatinine (Scr), and blood pressure (systolic and diastolic)] at
initial RAM administration, and at 12 weeks following initial RAM
administration were retrieved from the medical records. The
estimated glomerular filtration rate (eGFR) was calculated using
the sex, Scr, and age of each patient using the estimation equation
proposed by the Japanese Society of Nephrology (13). Additionally, proteinuria was
considered present if the urinary albumin dipstick test results
were positive (1+). The progression of proteinuria was evaluated
according to the Common Terminology Criteria for Adverse Events
version 5.0 (https://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_8.5x11.pdf).
Analysis of the association between
the occurrence of proteinuria and the type of antihypertensive
drug
Patients were classified into a RAS-I or a CCB group
based on their use of antihypertensive agents over 12 weeks
following initial RAM administration. The prevalence of
proteinuria, cumulative doses of RAM and PTX, and laboratory data
at 12 weeks following initial RAM administration between the two
groups were compared. Additionally, changes in blood pressure and
cumulative occurrence of proteinuria over 12 weeks after initial
RAM administration between the two groups were compared.
Blood concentration simulation of
ramucirumab
RAM concentrations were simulated based on the
estimates of O'Brien's model and well-characterized by a
two-compartment model (14). The
analyses were performed using Phoenix NLME version 8.3 (Certara)
and R version 4.1.2 (15,16).
Statistical analysis
The two groups were compared using a χ2
test, Fisher's exact test, or Student's t-test. The change in blood
pressure between groups over the 12 weeks following initial RAM
administration was assessed using a two-way ANOVA followed by the
Tukey-Kramer post-hoc test. The incidence of proteinuria over 12
weeks after initial RAM administration was analyzed using
Kaplan-Meier curves and compared using a log-rank test. Data were
analyzed using SPSS version 25.0 (IBM Corp). P<0.05 was
considered to indicate a statistically significant difference.
Results
Patients
A total of 78 patients were reviewed in this study;
excluded patients were 2 patients who experienced an onset of
proteinuria at the onset of RAM administration, 20 who had a change
in type or dosage of antihypertensive agents, 16 who had additional
hypertensive agents, and 4 who had inadequate liver and renal
function from the analysis. Finally, data from 36 patients were
analyzed.
Comparison of the occurrence of
proteinuria and the changes in blood pressure between RAS-I and CCB
groups
Of the 31 patients, 15 were classified into the
RAS-I group and the remaining 16 into the CCB group. At the time of
commencing RAM administration, age, body mass index (BMI), ALT,
AST, Scr, blood pressure (systolic and diastolic), and initial
doses of RAM and PTX did not differ significantly between the two
groups (Table I).
 | Table IComparison of patient characteristics
between the RAS-I and CCB groups at the onset of ramucirumab
treatment. |
Table I
Comparison of patient characteristics
between the RAS-I and CCB groups at the onset of ramucirumab
treatment.
| Parameter | RAS-I group,
n=17 | CCB group, n=19 | P-value |
|---|
| Sex, male/female | 10/7 | 10/9 | 0.709a |
| Age | 64.2±2.1 | 65.6±1.5 | 0.589b |
| BMI | 20.5±0.6 | 20.2±0.5 | 0.758b |
| ALT, IU/l | 24.5±1.7 | 21.8±1.7 | 0.300b |
| AST, IU/l | 26.7±1.9 | 27.0±1.7 | 0.911b |
| Scr, mg/dl | 0.69±0.03 | 0.72±0.02 | 0.387b |
| eGFR, ml/min/1.73
m2 | 80.0±3.1 | 73.5±2.4 | 0.114b |
| Blood pressure,
mmHg | | | |
|
Systolic | 125.1±1.6 | 128.0±2.4 | 0.347b |
|
Diastolic | 74.8±1.3 | 72.6±1.9 | 0.390b |
| RAM dose,
mg/body | 435.6±14.0 | 415.8±15.0 | 0.358b |
| PTX dose,
mg/body | 126.2±2.4 | 121.9±2.6 | 0.258b |
| RAS-I | | | - |
|
Azilsartane | 4 | | |
|
Candesartan
cilexetil | 4 | | |
|
Enalapril | 2 | | |
|
Olmesartan | 5 | | |
|
Telmisartan | 2 | | |
| CCB | | | - |
|
Amlodipine | | 13 | |
|
Azelnidipine | | 3 | |
|
Cilnidipine | | 1 | |
|
Nifedipine | | 2 | |
| Diuretics | 2 | 3 | - |
| Concomitant
drugs | | | 0.973a |
|
Antilipidemic | 2 | 3 | |
|
Antipodagric | 3 | 3 | |
|
Peptic
ulcer | 11 | 10 | |
|
Diabetes | 2 | 3 | |
|
Antithyroid | 2 | 4 | |
|
Bone-building | 2 | 5 | |
|
Antithrombogenic
and anticoagulant | 2 | 2 | |
|
Antipyretic
and analgesic | 2 | 8 | |
|
Hypnotics | 2 | 5 | |
|
Anti-anxiety | 2 | 4 | |
|
Laxative | 3 | 5 | |
|
Stomach and
intestinal | 4 | 7 | |
|
Others | 5 | 7 | |
At 12 weeks after RAM administration, ALT, AST, Scr,
blood pressure (systolic and diastolic), and cumulative doses of
RAM and PTX were not significantly different between the two groups
(Table II). In addition, the
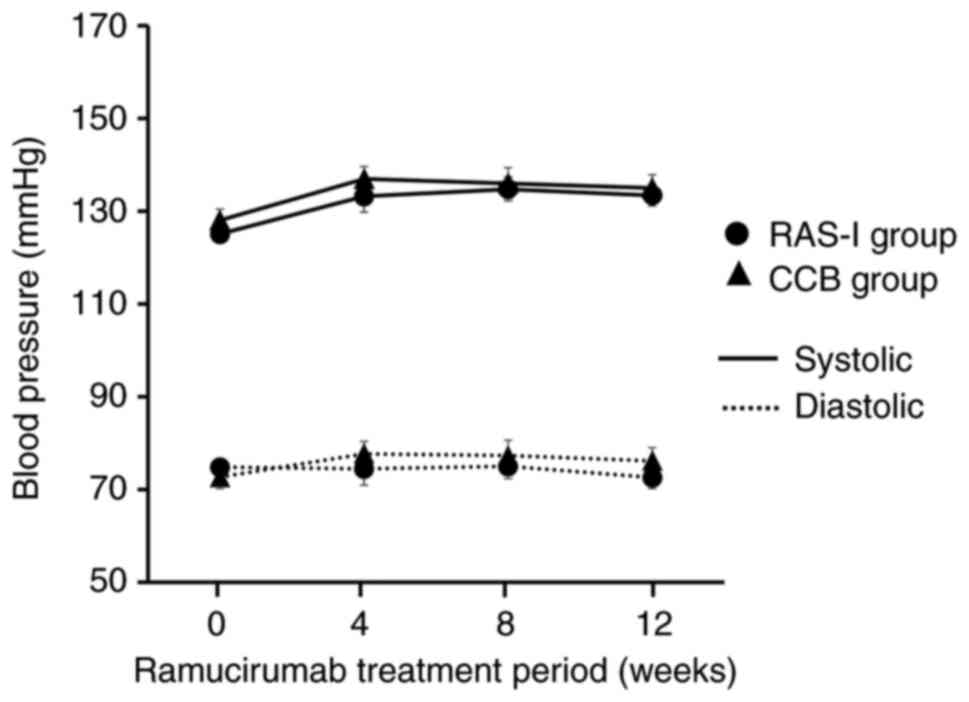
changes in systolic and diastolic blood pressure were not
significantly different between the two groups (Fig. 1). However, the prevalence of
proteinuria in the RAS-I group was significantly lower than that in
the CCB group (Table II).
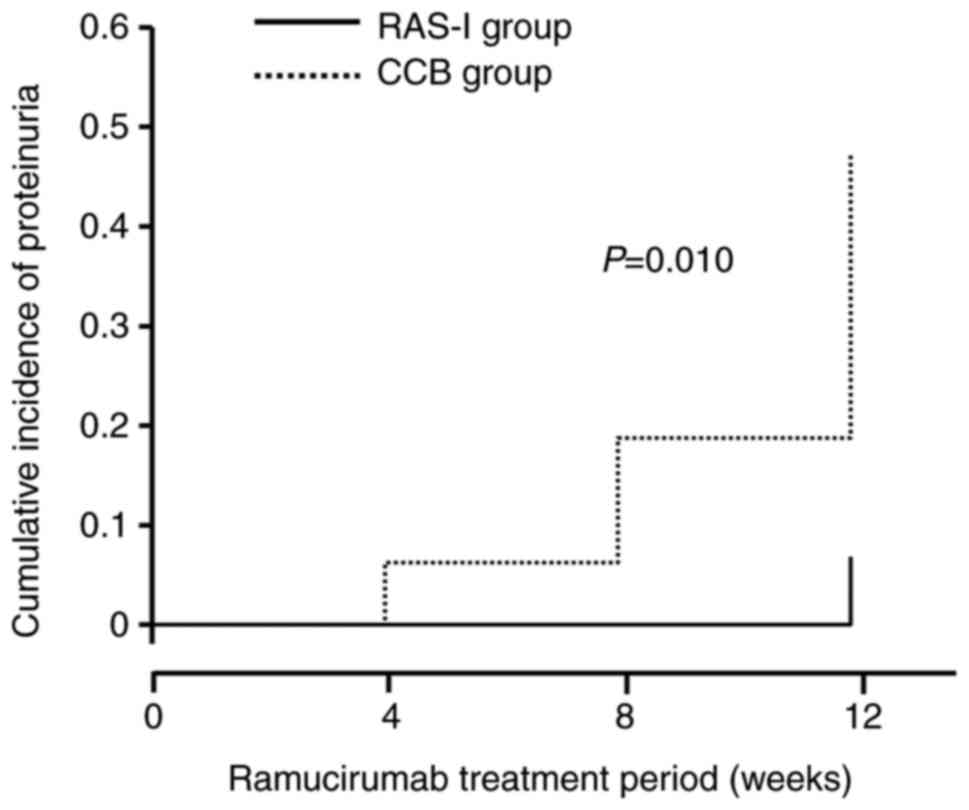
Additionally, Kaplan-Meier analysis showed that the cumulative
occurrence of proteinuria in the RAS-I group over 12 weeks after
RAM administration was significantly lower than that in the CCB
group (P=0.01, Fig. 2).
 | Table IIComparison of patient characteristics
between the RAS-I and CCB groups at 12 weeks following initial
ramucirumab treatment. |
Table II
Comparison of patient characteristics
between the RAS-I and CCB groups at 12 weeks following initial
ramucirumab treatment.
| Parameter | RAS-I group,
n=17 | CCB group,
n=19 | P-value |
|---|
| ALT, IU/l | 23.9±2.0 | 23.5±2.2 | 0.896b |
| AST, IU/l | 30.3±1.7 | 28.1±1.7 | 0.362b |
| Scr, mg/dl | 0.76±0.02 | 0.80±0.03 | 0.322b |
| eGFR, ml/min/1.73
m2 | 71.7±2.3 | 65.9±2.5 | 0.111b |
| Blood pressure,
mmHg | | | |
|
Systolic | 133.4±2.3 | 135.0±2.8 | 0.676b |
|
Diastolic | 72.5±1.6 | 76.2±2.6 | 0.260b |
| Cumulative RAM
dose, mg/body | 1,298.1±45.1 | 1,245.9±44.6 | 0.432b |
| Cumulative PTX
dose, mg/body | 371.0±10.3 | 354.2±10.7 | 0.279b |
| Proteinuria | | | 0.008a,c |
|
Yes | 1 (Grade 1) | 9 (Grade 1, 6;
Grade 2, 3) | |
|
No | 16 | 10 | |
Comparison of the patients'
characteristics classified into proteinuria and non-proteinuria
groups
Of the 26 patients, 9 were classified into the
proteinuria group and the remaining 17 patients were classified
into the non-proteinuria group. At the time of commencing RAM
administration, the sex ratio, BMI, ALT, AST, Scr, eGFR, blood
pressure, RAM dose, PTX dose, and use of concomitant drugs were not
significantly different between the two groups (Table III). At 12 weeks after RAM
administration, there remained no significant difference in AST,
ALT, Scr, eGFR, blood pressure, and cumulative dose of RAM and PTX
between the two groups (Table
IV).
 | Table IIIComparison of patient characteristics
in the proteinuria and non-proteinuria groups at the onset of
ramucirumab treatment. |
Table III
Comparison of patient characteristics
in the proteinuria and non-proteinuria groups at the onset of
ramucirumab treatment.
| Parameter | Proteinuria,
n=10 | Non-proteinuria,
n=26 | P-value |
|---|
| Sex,
male/female | 7/3 | 13/13 | 0.456a |
| Age | 64.2±2.1 | 65.8±1.7 | 0.602b |
| BMI | 20.8±0.7 | 20.6±0.5 | 0.836b |
| ALT, IU/l | 25.0±2.1 | 23.9±1.6 | 0.720b |
| AST, IU/l | 26.8±3.2 | 27.6±1.4 | 0.803b |
| Scr, mg/dl | 0.74±0.03 | 0.75±0.03 | 0.871b |
| eGFR, ml/min/1.73
m2 | 76.3±3.7 | 71.3±2.3 | 0.265b |
| Blood pressure,
mmHg | | | |
|
Systolic | 127.3±2.1 | 125.9±2.8 | 0.776b |
|
Diastolic | 72.8±1.8 | 74.3±1.7 | 0.625b |
| RAM dose,
mg/body | 443.5±19.6 | 427.7±12.4 | 0.358b |
| PTX dose,
mg/body | 127.1±3.2 | 123.9±2.3 | 0.459b |
| RAS-I | | | - |
|
Azilsartane | 0 | 4 | |
|
Candesartan
cilexetil | 0 | 4 | |
|
Enalapril | 0 | 2 | |
|
Olmesartan | 1 | 4 | |
|
Telmisartan | 1 | 1 | |
| CCB | | | - |
|
Amlodipine | 7 | 6 | |
|
Azelnidipine | 0 | 3 | |
|
Cilnidipine | 0 | 1 | |
|
Nifedipine | 1 | 1 | |
| Diuretics | 1 | 4 | - |
| Concomitant
drugs | | | 0.970c |
|
Antilipidemic | 2 | 3 | |
|
Antipodagric | 2 | 4 | |
|
Peptic
ulcer | 4 | 17 | |
|
Diabetes | 1 | 4 | |
|
Antithyroid | 2 | 4 | |
|
Bone-building | 3 | 4 | |
|
Antithrombogenic
and anticoagulant | 2 | 2 | |
|
Antipyretic
and analgesic | 3 | 7 | |
|
Hypnotics | 1 | 6 | |
|
Anti-anxiety | 2 | 4 | |
|
Laxative | 3 | 5 | |
|
Stomachic
and intestinal | 4 | 7 | |
|
Others | 4 | 8 | |
 | Table IVComparison of patient characteristics
in the proteinuria and non-proteinuria groups at 12 weeks following
initial ramucirumab treatment. |
Table IV
Comparison of patient characteristics
in the proteinuria and non-proteinuria groups at 12 weeks following
initial ramucirumab treatment.
| Parameter | Proteinuria,
n=10 | Non-proteinuria,
n=26 | P-value |
|---|
| ALT, IU/l | 19.5±1.9 | 23.2±1.9 | 0.296a |
| AST, IU/l | 25.6±2.7 | 29.8±1.3 | 0.131a |
| Scr, mg/dl | 0.85±0.06 | 0.77±0.02 | 0.256a |
| eGFR, ml/min/1.73
m2 | 67.8±3.8 | 68.3±2.2 | 0.911a |
| Blood pressure,
mmHg | | | |
|
Systolic | 132.4±2.7 | 135.0±2.3 | 0.549a |
|
Diastolic | 72.1±1.6 | 75.4±2.1 | 0.368a |
| Cumulative RAM
dose, mg/body | 1327.1±57.9 | 1274.0±40.0 | 0.489a |
| Cumulative PTX
dose, mg/body | 358.5±29.0 | 349.4±14.1 | 0.762a |
Comparison of simulated RAM blood
concentrations between the CCB and RAS-I groups
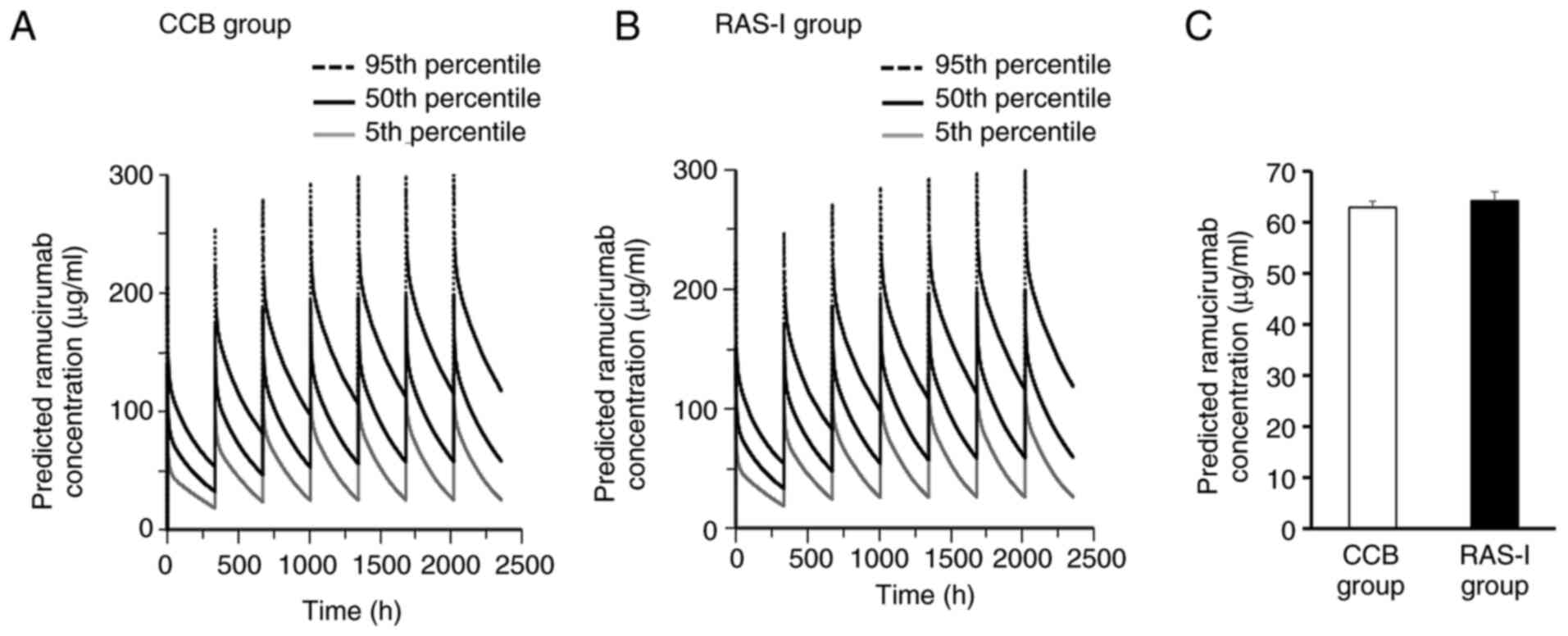
Blood concentration profiles of RAM simulated based
on the O'Brien model were compared between CCB and RAS-I groups.
There was little difference in the predicted concentration curve of
the 5th, 50th and 95th percentile over 12 weeks (2,016 h) after RAM
administration between the two groups (Fig. 3A and B). Additionally, there was no significant
difference in the predicted minimum steady-state RAM concentrations
(trough concentration) at 12 weeks (2,016 h) after RAM
administration between CCB (62.9±1.2 µg/ml) and RAS-I (64.2±1.8
µg/ml) groups (Fig. 3C).
Discussion
The objective of this study was to investigate
whether RAS-I has an advantage over CCB in proteinuria suppression
in hypertensive patients with gastric cancer receiving RAM
treatment. To evaluate this advantage properly, we excluded
patients with possible risk factors that may have influenced the
evaluation of proteinuria occurrence between the two groups,
including a history of VEGF inhibitor use, diabetes, metastatic
pancreatic cancer, elevated blood pressure (>160 mmHg), type of
cancer, and duration of RAM administration.
Management of blood pressure in cancer patients
receiving VEGF inhibitors is closely associated with the prevalence
of proteinuria (11). According to
The Japanese Society of Hypertension guidelines for the management
of hypertension 2019, monotherapy with RAS-I, CCB, or a diuretic is
recommended as the first-line therapy for hypertensive patients
without complications such as renal or cardiac disease (17). If the effect of monotherapy is
insufficient, concomitant use of two of the aforementioned three
agents (RAS-I + CCB, RAS-I + diuretic, or CCB + diuretic) is
recommended as a second-line therapy (17). In this study, participants used only
diuretics as a concomitant drug with CCB or RAS-I, and there was no
significant difference in the use of diuretics between the two
groups (Table I, RAS-I group, 2;
CCB group, 3). Additionally, we confirmed that the changes in
systolic and diastolic blood pressures were not significantly
different between the two groups (Fig.
1). These factors suggest that there might not have been a
significant difference in the severity of hypertension between the
two groups. Moreover, in this study, although the changes in blood
pressure over 12 weeks after initial RAM administration were not
significantly different between the two groups (Fig. 2), there was a significant difference
in both proteinuria occurrence at 12 weeks after RAM administration
and cumulative occurrence of proteinuria over 12 weeks after RAM
administration between the two groups (Table II, Fig.
1). Additionally, in this study, the mean blood pressure in
patients with proteinuria and without proteinuria did not differ
(Table IV), indicating that
proteinuria in RAM-treated patients was not related to blood
pressure. Furthermore, simulation of the time course of RAM blood
concentrations based on the O'Brien model showed that there may not
be a difference in RAM blood concentration profiles over 12 weeks
between the two groups (Fig. 3A-C).
These results show that RAS-I may be preferable to CCB when
selecting hypertensive agents in hypertensive patients with gastric
cancer receiving RAM treatment. Additionally, the difference in
proteinuria prevalence between the two groups may be due to the
difference in pharmacological action between RAS-I and CCB.
VEGF accelerates endothelial nitric oxidase (NO)
synthase (eNOS) phosphorylation and NO production, leading to
vasodilatation and vascularization in glomerular endothelial cells
(18,19). VEGF inhibitors, including RAM, cause
vasoconstriction by suppressing VEGF/VEGFR-2 signaling (20). Proteinuria is closely associated
with glomerular disintegration due to podocyte and glomerular
endothelial cells (21). A
meta-analysis of patients with hypertension showed that CCB
resulted in a significant decrease in albuminuria and proteinuria
(22). Additionally, Batova et
al (23) reported that CCB
treatment potentiated VEGF-induced activation of eNOS downstream of
VEGFR-2 in bovine aortic endothelial cells. Furthermore, Yuen et
al (24) showed that acute
podocytopathy and heavy proteinuria occurred in eNOS-deficient mice
as early as 2 weeks after induction of diabetes with
streptozotocin, compared with that in non-eNOS-deficient mice.
These results show that the decrease in eNOS activation leads to an
increase in proteinuria prevalence and suggest that suppression of
proteinuria by CCB may be involved in eNOS activation.
Nakamura et al (25) reported that the prevalence of
proteinuria in hypertensive patients with chronic kidney disease
receiving telmisartan, a RAS-I, was significantly lower than that
in patients receiving amlodipine, a CCB, which was consistent with
our results. It is well established that AII is a mediator of
proteinuria (26). AII increases
blood pressure via a decrease in eNOS levels in vascular
endothelial cells in various tissues (26,27).
Additionally, AII decreases nephrin expression, which is a molecule
that constitutes the slit diaphragm in podocytes and suppresses the
ultrafiltration barrier in the renal glomerulus (26). These results show that RAS-I may
suppress proteinuria via inhibition of eNOS reduction by
suppressing the action of AII. Furthermore, AII also induces
transforming growth factor-β (TGF-β) expression in tubular
endothelial cells (26,28). TGF-β is related to apoptosis in
cultured mouse podocytes via upregulation of mitochondrial NADPH
oxidase 4, a major inducer of oxidative stress in podocytes
(29). This suggests that RAS-I may
suppress proteinuria and inhibit eNOS reduction via another
mechanism. These multiple mechanisms of RAS-I in suppressing
proteinuria may have resulted in the advantage of RAS-I over CCB
observed in this study.
In this study, patients in the CCB group had taken
amlodipine, azelnidipine, cilnidipine, or nifedipine, and patients
in the RAS-I group had taken azilsartan, candesartan cilexetil,
enalapril, Olmesartan, or telmisartan (Table I). For the CCB group, an animal
study by Nagasu et al (30)
reported that azelnidipine improved proteinuria compared with
amlodipine. For RAS-I, Suehiro et al (31) showed that azilsartane had potent
antiproteinuric effects compared with candesartan cilexetil. These
results imply that RAS-I and CCB have different anti-proteinuria
effects. Further studies, including investigating the differences
in the anti-proteinuria effect between RAS-I or CCB, should be
performed to provide more useful information for medical
settings.
In this study, we aimed to investigate which
mechanism (inhibition of the renin-angiotensin system or a CCB
mechanism) had the superior anti-proteinuria effect in gastric
cancer patients with hypertension receiving RAM. To compare the
anti-proteinuria effects between RAS-I and CCB groups accurately,
we included patients with gastric cancer receiving RAM treatment
only to equalize the background characteristics of participants in
the two groups as much as possible. We consider that this study
bears significant value in the prevalence of proteinuria in the
RAS-I group was lower than that in the CCB group, and there was no
significant difference in blood pressure control between the two
groups during RAM treatment. However, since our study had a small
sample size, further studies including more patients should be
conducted to verify our results.
This study had several limitations. Firstly, it was
a small retrospective investigation conducted in several
institutions in a single country. Secondly, the cohort size was
small, which constitutes a major limitation of the study. Thirdly,
patients with a single type of cancer were selected. Yen et
al (32) showed that Asian and
non-Asian patients with advanced hepatocellular carcinoma showed
slightly different incidence rates of hypertension (Asian, 18.5%;
non-Asian, 14.9%) and proteinuria (Asian, 20.2%; non-Asian, 23.6%)
as adverse effects of RAM. This suggests that race may affect the
anti-hypertensive and anti-proteinuria effects of CCB and RAS-I.
However, since race is not selected as a significant covariate of
RAM pharmacokinetics parameters in population pharmacokinetics
analysis, it is possible that race may have little effect on RAM
disposition (14). Further studies
are required to investigate the racial differences in the
anti-proteinuria effects of CCB and RAS-I. Finally, the prevalence
of proteinuria was evaluated using a simple urine dipstick method.
Evaluating the spot urine albumin-to-creatinine ratio may provide a
better assessment of proteinuria than simple urine dipstick
measurements. Therefore, our findings require validation in a
prospective study using patients with different types of cancer, in
which proteinuria and renal function are evaluated with greater
reliability and precision.
In conclusion, RAS-I may have an advantage over CCB
for suppressing proteinuria in hypertensive patients with gastric
cancer who are treated with anti-hypertensive agents for blood
pressure management. Our results provide useful information to
healthcare professionals involved in the administration of RAM
treatment.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Author contributions
TC, HU, YY, KU, ST, KO, KK, and YT collected the
patients' data. KS, YN, and TM conducted simulations of ramucirumab
blood concentrations. TC performed all data analyses and wrote the
manuscript. TM and HS confirmed the authenticity of all the raw
data. All authors have read and approved the final manuscript.
Ethics approval and consent to
participate
This research was conducted according to the
Declaration of Helsinki and was reviewed and approved by the Ethics
Committee of each participating institution (Hokkaido University of
Science, Approval No. 19-06-022; Iwate Medical University Hospital,
Approval No. MH2020-153; Asahikawa university hospital, Approval
No. 20119; National Hospital Organization Hokkaido Cancer Center,
Approval No. 02-24). Patient consent was obtained though the
opt-out method.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Lu D, Jimenez X, Zhang H, Bohlen P, Witte
L and Zhu Z: Selection of high affinity human neutralizing
antibodies to VEGFR2 from a large antibody phage display library
for antiangiogenesis therapy. Int J Cancer. 97:393–399.
2002.PubMed/NCBI View
Article : Google Scholar
|
|
2
|
Lu D, Shen J, Vil MD, Zhang H, Jimenez X,
Bohlen P, Witte L and Zhu Z: Tailoring in vitro selection for a
picomolar affinity human antibody directed against vascular
endothelial growth factor receptor 2 for enhanced neutralizing
activity. J Biol Chem. 278:43496–43507. 2003.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Ferrara N: Vascular endothelial growth
factor. Arterioscler Thromb Vasc Biol. 29:789–791. 2009.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Suzuki T, Shinozaki E, Osumi H, Nakayama
I, Ota Y, Ichimura T, Ogura M, Wakatsuki T, Ooki A, Takahari D, et
al: Second-line FOLFIRI plus ramucirumab with or without prior
bevacizumab for patients with metastatic colorectal cancer. Cancer
Chemother Pharmacol. 84:307–313. 2019.PubMed/NCBI View Article : Google Scholar
|
|
5
|
De Vita F, Borg C, Farina G, Geva R,
Carton I, Cuku H, Wei R and Muro K: Ramucirumab and paclitaxel in
patients with gastric cancer and prior trastuzumab: Subgroup
analysis from RAINBOW study. Future Oncol. 15:2723–2731.
2019.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Arnold D, Fuchs CS, Tabernero J, Ohtsu A,
Zhu AX, Garon EB, Mackey JR, Paz-Ares L, Baron AD, Okusaka T, et
al: Meta-analysis of individual patient safety data from six
randomized, placebo-controlled trials with the antiangiogenic
VEGFR2-binding monoclonal antibody ramucirumab. Ann Oncol.
28:2932–2942. 2017.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Yamaguchi K, Fujitani K, Nagashima F,
Omuro Y, Machida N, Nishina T, Koue T, Tsujimoto M, Maeda K and
Satoh T: Ramucirumab for the treatment of metastatic gastric or
gastroesophageal junction adenocarcinoma following disease
progression on first-line platinum- or fluoropyrimidine-containing
combination therapy in Japanese patients: A phase 2, open-label
study. Gastric Cancer. 21:1041–1049. 2018.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Abdel-Rahman O and ElHalawani H:
Proteinuria in patients with solid tumors treated with ramucirumab:
A systematic review and meta-analysis. Chemotherapy. 60:325–333.
2014.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Zhao T, Wang X, Xu T, Xu X and Liu Z:
Bevacizumab significantly increases the risks of hypertension and
proteinuria in cancer patients: A systematic review and
comprehensive meta-analysis. Oncotarget. 8:51492–51506.
2017.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Gordon MS and Cunningham D: Managing
patients treated with bevacizumab combination therapy. Oncology. 69
(Suppl 3):S25–S33. 2005.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Feliu J, Salud A, Safont MJ, García-Girón
C, Aparicio J, Losa F, Bosch C, Escudero P, Casado E, Jorge M, et
al: Correlation of hypertension and proteinuria with outcome in
elderly bevacizumab-treated patients with metastatic colorectal
cancer. PLoS One. 10(e0116527)2015.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Nihei S, Sato J, Harada T, Kuyama S,
Suzuki T, Waga N, Saito Y, Kisara S, Yokota A, Okada K, et al:
Antiproteinuric effects of renin-angiotensin inhibitors in lung
cancer patients receiving bevacizumab. Cancer Chemother Pharmacol.
81:1051–1059. 2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Matsuo S, Imai E, Horio M, Yasuda Y,
Tomita K, Nitta K, Yamagata K, Tomino Y, Yokoyama H and Hishida A:
Collaborators developing the Japanese equation for estimated GFR.
Revised equations for estimated GFR from serum creatinine in Japan.
Am J Kidney Dis. 53:982–992. 2009.PubMed/NCBI View Article : Google Scholar
|
|
14
|
O'Brien L, Westwood P, Gao L and Heathman
M: Population pharmacokinetic meta-analysis of ramucirumab in
cancer patients. Br J Clin Pharmacol. 83:2741–2751. 2017.PubMed/NCBI View Article : Google Scholar
|
|
15
|
R Core Team: R: A language and environment
for statistical computing. R Foundation for Statistical Computing,
Vienna, 2012. http://www.R-project.org/.
|
|
16
|
Carneiro T, Nobrega RVMD, Nepomuceno T,
Bian GB, Albuquerque VHCD and Filho APP: Performance Analysis of
Google Colaboratory as a Tool for Accelerating Deep Learning
Applications. IEEE Access. 6:61677–61685. 2018.
|
|
17
|
Umemura S, Arima H, Arima S, Asayama K,
Dohi Y, Hirooka Y, Horio T, Hosihde S, Ikeda S, Ishimitsu T, et al:
The Japanese society of hypertension guidelines for the management
of hypertension (JSH 2019). Hypertens Res. 42:1235–1481.
2019.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Zhang Z, Meszaros G, He WT, Xu Y, de
Fatima Magliarelli H, Mailly L, Mihlan M, Liu Y, Puig Gámez M,
Goginashvili A, et al: Protein kinase D at the Golgi controls NLRP3
inflammasome activation. J Exp Med. 214:2671–2693. 2017.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Kanesaki Y, Suzuki D, Uehara G, Toyoda M,
Katoh T, Sakai H and Watanabe T: Vascular endothelial growth factor
gene expression is correlated with glomerular neovascularization in
human diabetic nephropathy. Am J Kidney Dis. 45:288–294.
2005.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Pandey AK, Singhi EK, Arroyo JP, Ikizler
TA, Gould ER, Brown J, Beckman JA, Harrison DG and Moslehi J:
Mechanisms of VEGF (vascular endothelial growth factor)
inhibitor-associated hypertension and vascular disease.
Hypertension. 71:e1–e8. 2018.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Haraldsson B, Nyström J and Deen WM:
Properties of the glomerular barrier and mechanisms of proteinuria.
Physiol Rev. 88:451–487. 2008.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Thamcharoen N, Susantitaphong P,
Wongrakpanich S, Chongsathidkiet P, Tantrachoti P, Pitukweerakul S,
Avihingsanon Y, Praditpornsilpa K, Jaber BL and Eiam-Ong S: Effect
of N- and T-type calcium channel blocker on proteinuria, blood
pressure and kidney function in hypertensive patients: A
meta-analysis. Hypertens Res. 38:847–855. 2015.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Batova S, DeWever J, Godfraind T,
Balligand JL, Dessy C and Feron O: The calcium channel blocker
amlodipine promotes the unclamping of eNOS from caveolin in
endothelial cells. Cardiovasc Res. 71:478–485. 2006.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Yuen DA, Stead BE, Zhang Y, White KE,
Kabir MG, Thai K, Advani SL, Connelly KA, Takano T, Zhu L, et al:
eNOS deficiency predisposes podocytes to injury in diabetes. J Am
Soc Nephrol. 23:1810–1823. 2012.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Nakamura T, Inoue T, Suzuki T, Kawagoe Y,
Ueda Y, Koide H and Node K: Comparison of renal and vascular
protective effects between telmisartan and amlodipine in
hypertensive patients with chronic kidney disease with mild renal
insufficiency. Hypertens Res. 31:841–850. 2008.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Rüster C and Wolf G:
Renin-angiotensin-aldosterone system and progression of renal
disease. J Am Soc Nephrol. 17:2985–2991. 2006.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Huang PL: eNOS, metabolic syndrome and
cardiovascular disease. Trends Endocrinol Metab. 20:295–302.
2009.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Goumenos DS, Tsakas S, El Nahas AM,
Alexandri S, Oldroyd S, Kalliakmani P and Vlachojannis JG:
Transforming growth factor-beta(1) in the kidney and urine of
patients with glomerular disease and proteinuria. Nephrol Dial
Transplant. 17:2145–2152. 2002.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Das R, Xu S, Quan X, Nguyen TT, Kong ID,
Chung CH, Lee EY, Cha SK and Park KS: Upregulation of mitochondrial
Nox4 mediates TGF-β-induced apoptosis in cultured mouse podocytes.
Am J Physiol Renal Physiol. 306:F155–F167. 2014.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Nagasu H, Satoh M, Fujimoto S, Tomita N,
Sasaki T and Kashihara N: Azelnidipine attenuates glomerular damage
in Dahl salt-sensitive rats by suppressing sympathetic nerve
activity. Hypertens Res. 35:348–355. 2012.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Suehiro T, Tsuruya K, Yoshida H, Tsujikawa
H, Yamada S, Tanaka S, Tsuchimoto A, Eriguchi M, Fujisaki K, Torisu
K, et al: Stronger effect of azilsartan on reduction of proteinuria
compared to candesartan in patients with CKD: A randomized
crossover trial. Kidney Blood Press Res. 46:173–184.
2021.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Yen CJ, Kudo M, Lim HY, Hsu CH, Vogel A,
Brandi G, Cheng R, Nitu IS, Abada P, Hsu Y, et al: Efficacy and
safety of ramucirumab in Asian and non-Asian patients with advanced
hepatocellular carcinoma and elevated alpha-fetoprotein: Pooled
individual data analysis of two randomized studies. Liver Cancer.
9:440–454. 2020.PubMed/NCBI View Article : Google Scholar
|