Introduction
The economic burden and the rapidly increasing
prevalence of diabetes mellitus (DM) render it one of the major
health care challenges of the 21st century. In 2015, ~415 million
individuals were diagnosed with DM, with the majority of these
patients living in low to middle-income regions, including
countries of the Middle East and North Africa (MENA) region
(1). Jordan is a developing country
in the MENA region, and health statistics have revealed that DM is
indeed a growing health problem in the country (2,3). The
reasons behind the increase in DM prevalence in Jordan are not
entirely understood. However, evidence refers to a major role
played by a rapidly changing social structure in the country
accompanied by Western-influenced dietary habits and a sedentary
lifestyle (2). Regardless, the DM
magnitude emphasizes the need for a national healthcare policy that
aims to dissect the environmental and genetic causes behind the DM
epidemic in Jordan and the MENA region.
DM represents a heterogeneous mixture of metabolic
diseases which present themselves by a chronic elevation in blood
glucose (4). The two most common
types of DM include; type 1 (T1DM), caused by a near-complete
absence of insulin resulting from an autoimmune attack on the
β-cells of the islets of Langerhans (4); and the more frequent type 2 (T2DM),
caused by failure of peripheral target tissues to elicit a response
to circulating blood insulin (4). A
high percentage of individuals affected with T2DM are obese
(5), and an increase in total fat
percentage of the body remains a major predisposing factor for
insulin resistance (6). In addition
to the role of diet and lifestyle, the genetic background in
determining the risk of DM is receiving attention as of late
(7), and several loci were found to
be associated with the risk of T2DM in several patient cohorts
(7-9).
Vitamin D, cholecalciferol, is a fat-soluble vitamin
and a hormone (10). Vitamin D is
synthesized in skin cells as a result of exposure to UV light
(11). Vitamin D is mainly known
for its traditional role in maintaining calcium homeostasis and
bone health (12). Vitamin D
deficiency is associated with the development of metabolic bone
diseases, i.e., rickets in children and osteomalacia in adults
(13). In addition to its
traditional role in maintaining bone health, vitamin D plays a
vital role in several extra-skeletal processes (14). For example, vitamin D is
increasingly recognized for its anti-proliferative (15), pro-differentiative (15), and immunomodulatory (16) activities. In the context of T2DM,
several studies have revealed that vitamin D regulates the effect
of insulin on target tissues (17,18).
Vitamin D elicits its action through binding to the
vitamin D receptor (VDR), a member of the superfamily of
transcription factors known as nuclear receptors (19). Nuclear receptors mediate their
effect through transcriptional regulation of target genes (20). Generally, the activity of the VDR is
governed by (i) the levels of the active form of vitamin D
(1,25-dihydroxycholecalciferol) and/or (ii) the expression level of
the VDR itself or its attendant co-factors (21). Moreover, several research groups
have determined that VDR expression/activity is affected by genetic
variation in the sequence of the VDR locus (22,23).
In that regard, four single nucleotide polymorphisms (SNPs) in the
VDR gene (rs2228570, rs1544410, rs7975232, and rs731236)
were heavily investigated for their role in modulating the activity
of VDR on its target tissues (24-26).
SNPs in the VDR gene have been revealed to be associated
with male infertility (27,28), psoriasis (29), and prostate cancer (30), all of which are conditions where
vitamin D was demonstrated to lower the risk of the disease
(31). Herein, it was investigated
whether the serum levels of vitamin D are associated with the risk
of T2DM in a Jordanian population. The same population was also
used to assess whether VDR SNPs are associated with
T2DM.
Materials and methods
Study design
This was a prospective case-control study. The study
was approved by the Institutional Review Board (approval ID
92/118/2018) of Jordan University of Science and Technology (JUST;
Irbid, Jordan). Study participants were required to sign a consent
form prior to their enrollment. Subject recruitment and blood
sample collection was performed from December 2018 to March
2019.
Subject description
A total of 250 subjects were enrolled in this study.
A total of 125 subjects were already diagnosed with T2DM according
to the American Diabetes Association (ADA) guidelines (32). Subjects with diabetes were patients
actively treated for T2DM at the Endocrinology clinic of King
Abdullah University Hospital (KAUH). A total of 125 non-diabetic
subjects were recruited during their visit to the Family Medicine
clinic of KAUH. The control subjects were matched to T2DM patients
according to sex and Body Mass Index (BMI). Following a short
interview, it was confirmed that the non-diabetic subjects did not
complain of any of the usual symptoms associated with T2DM at the
time of their recruitment. Moreover, non-diabetic subjects were
requested to assess their fasting blood glucose (FBG) levels on two
separate occasions to confirm the absence of T2DM. Pre-diabetes
individuals with a repeated FBG of 100-125 mg/dl were excluded from
participating in this study. Subjects with chronic kidney or liver
disease which may interfere with vitamin D metabolism were excluded
from the study. Subjects with Cushing's syndrome, polycystic
ovarian syndrome, thyroid dysfunction or hyperprolactinemia, and
subjects who indicated receiving any of the vitamin D
pharmacological preparations (dihydrotachysterol, calcitriol,
ergocalciferol, cholecalciferol) by mouth or topically
(calcipotriene) for supplemental or therapeutic purposes (including
the treatment of chronic skin conditions such as psoriasis) were
also excluded from the study. All recruited subjects were of
Jordanian descent.
Anthropometric measurements
During the visit of the subjects to KAUH, the height
[measured in centimeters (cm)], weight [measured in kilograms
(kg)], and waist circumference (WC; measured in cm) of the subjects
were recorded. The height and weight were then used to calculate
the BMI according to the following equation: BMI=weight
(kg)/height2 (m2).
Blood sampling
Following a 12-h fast, 10 ml of blood was collected
into an evacuated EDTA tube (AFCO), and 5 ml of blood was collected
in a plain tube with a clot activator (AFCO). Blood in the EDTA
tube was stored at 4˚C and was later used for DNA extraction, as
explained below. Blood samples in plain tubes were centrifuged at
4,000 x g for 5 min at room temperature to separate the serum.
Serum samples were stored at -80˚C for later use to measure
glucose, total cholesterol, triglycerides, and 25-hydroxyvitamin D
[25(OH)D] levels.
Biochemical measurements
Measurements of serum glucose, total cholesterol,
and triglycerides were performed at the laboratories of KAUH. A
delayed, one-step immunoassay (ARCHITECT 25-OH Vitamin D) was used
to measure serum 25(OH)D levels. The kit was purchased from Abbott
Laboratories (cat. no. 3L52). Measurements were performed as per
the manufacturer's guidelines (33).
DNA extraction and genotyping
Whole blood stored in EDTA tubes was used for the
extraction of genomic DNA. The procedure used QIAamp DNA Blood Mini
Kits (cat. no. 51104; Qiagen GmbH). Following DNA extraction, the
purity of DNA was evaluated spectrophotometrically using an ND-2000
Nanodrop (Thermo Fisher Scientific, Inc.). Four SNPs in the
VDR gene (rs2228570, rs1544410, rs7975232, and rs731236)
were evaluated for their association with T2DM. Genotyping of the
SNPs was performed using polymerase chain reaction-restriction
fragment length polymorphism (PCR-RFLP). The concentrations of the
reagents used in the PCR reaction and the final reaction volume
were as previously described (34).
Specifically, the reaction mixture contained GoTaq®
Green Master Mix (Promega Corporation), 5 ng of template genomic
DNA and 0.4 µM primers (forward and reverse) in a final reaction
volume of 20 µl. The following thermocycling conditions were used
to run the PCR reactions: Initial denaturation at 95˚C for 2 min,
followed by 30 cycles of denaturation at 95˚C for 2 min, annealing
at 65˚C for 30 sec and extension at 72˚C for 30 sec and a final
extension at 72˚C for 5 min. The sequence of the primers used to
genotype each SNP are listed in Table
I. The location of the SNP on the VDR gene, the size of
the PCR product, the restriction enzyme used for genotyping, and
the size of the fragments that resulted from restriction enzyme
digestion are also listed in Table
I. Restriction enzymes were all purchased from New England
Biolabs. Following restriction enzyme treatment, the reaction
mixture was run on a 3% agarose gel. The agarose gel was prepared
directly in SYBR™ Safe DNA gel stain (cat. no. S33102; Thermo
Fisher Scientific, Inc.) according to the manufacturer's
instructions. In brief, 10 µl of 10,000X SYBR™ Safe
stain concentrate was added to 100 ml of 1XTAE buffer
(Sigma-Aldrich; Merck KGaA). The aforementioned solution was then
added to 3 g of powdered agarose and the mixture was heated in a
microwave. Molten agarose was then used to cast gels. The gel was
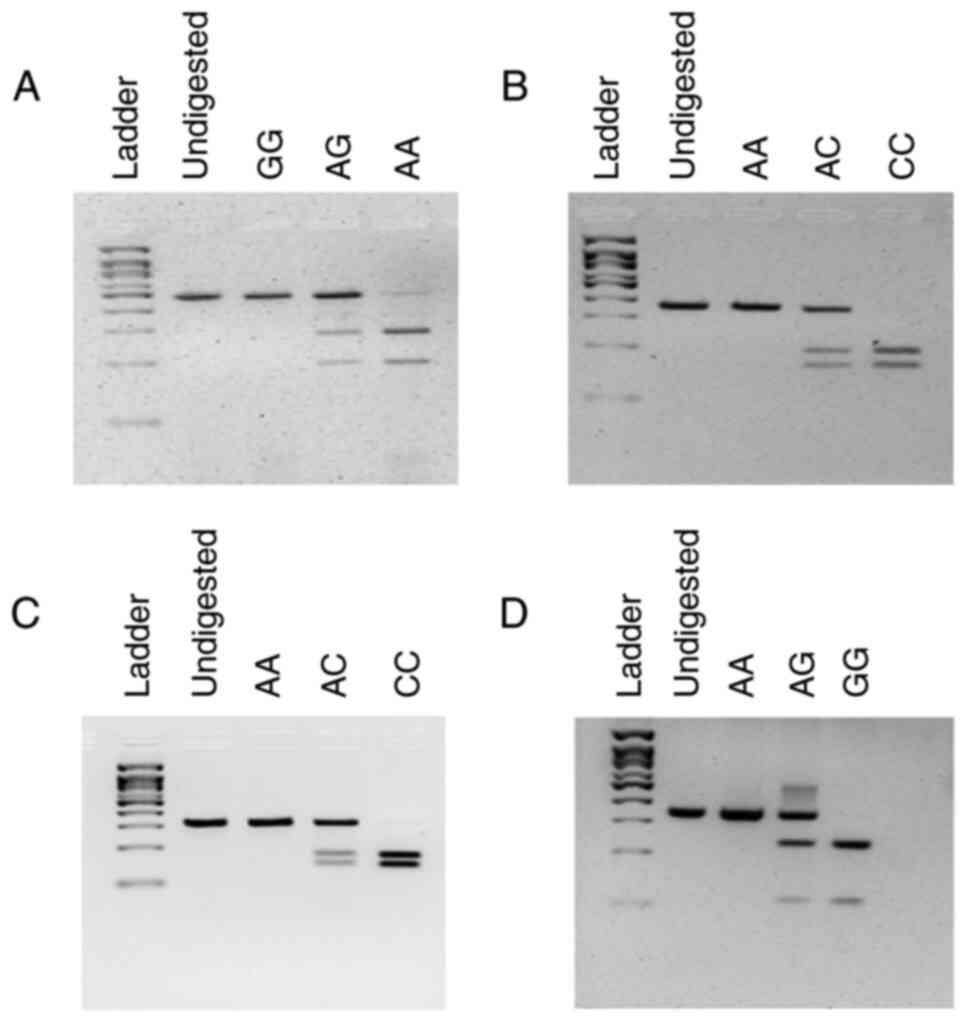
visualized under blue light. A representative gel image
illustrating the genotyping strategy of each SNP is presented in
Fig. 1.
 | Table IGenotyping strategy of VDR
SNPs. |
Table I
Genotyping strategy of VDR
SNPs.
| SNP ID | Location | Forward primer 5'
to 3' Reverse primer 5' to 3' | PCR Program | PCR product size
(bp) | Restriction enzyme,
incubation temperature and time | RFLP product
(bp) |
|---|
| rs2228570 | Exon 2 |
TTCTTCTCCCTCCCTTTCCA
TGCAGAGGTGAACCACTAAC | 95˚C, 30 sec at
61.1˚C and 1 min at 72˚C | 487 bp | BccI, 25˚C
for 60 min | GG: 487 bp AG: 487,
288, 199 bp AA: 288, 199 bp |
| rs1544410 | Intron 8 |
TCTCCCTCTTCTCACCTCTAAC
GGAAATACCTACTTTGCTGGTTTG | 95˚C, 30 sec at
61.1˚C and 1 min at 72˚C | 357 bp | BsmI, 65˚C
for 90 min | CC: 106, 230 bp AC:
336, 106, 230 bp AA: 336 bp |
| rs7975232 | Intron 8 |
GGATCATCTTGGCATAGAGCAG
GGATCCTAAATGCACGGAGAAG | 95˚C, 30 sec at
65˚C and 1 min at 72˚C | 322 bp | ApaI, 25˚C
for 60 min | AA: 322 bp AC: 322,
178, 144 bp CC: 178, 144 bp |
| rs731236 | Exon 9 |
GGCTAGCTTCTGGATCATCTT
CCTAGGTCTGGATCCTAAATGC | 95˚C, 30 sec at
65˚C and 1 min at 72˚C | 342 bp | TaqαI, 65˚C
for 60 min | AA: 342 bp AG: 342,
235, 107 bp GG: 235, 107 bp |
Statistical analysis
Statistical analysis was performed using the
Statistical Package for Social Studies (SPSS) software (version 23;
IBM Corp.). Continuous variables were assessed using an unpaired
Student's t-test. Discrete variables were evaluated using Pearson's
chi-squared test. The association between allele or genotype
categories of each SNP with the risk of T2DM was assessed using
Pearson's chi-squared test. Differences in serum 25(OH)D levels
among the genotype classes of each of the VDR SNPs were
examined using one-way ANOVA test followed by Tukey's post hoc
analysis. SHEsis software was used to run haplotype analysis
(35). Multivariate regression
analysis included the following variables: Age, BMI, WC, 25(OH)D,
cholesterol, triglycerides, and genotype category of rs2228570
(under a dominant inheritance model). For all analyses, P<0.05
was considered to indicate a statistically significant
difference.
Results
Serum 25(OH)D is decreased in patients
with T2DM
Baseline characteristics of the study subjects are
presented in Table II. The
analysis revealed that patients with T2DM were significantly older
than the control subjects (P<0.05). However, no significant
differences were revealed between patients with T2DM and controls
with regard to sex distribution, BMI, or WC (P>0.05).
Measurement of several analytes in the serum collected from the
study subjects revealed that patients with T2DM had significantly
higher levels of serum glucose (P<0.0001) but significantly
lower levels of serum 25(OH)D (P=0.0306). Notably, in this
population, both patients with T2DM and controls had a mean value
of serum 25(OH)D below 20 ng/ml, a widely used cut-off value for
vitamin D deficiency.
 | Table IIBaseline characteristics of study
subjects. |
Table II
Baseline characteristics of study
subjects.
| Variables | Controls n=125 | T2DM n=125 | P-value |
|---|
| Age (years) | 49.33±7.75 | 58.60±9.62 | <0.0001 |
| Sex, n (%) | | | |
|
Male | 57 (45.6%) | 57 (45.6%) | NS |
|
Female | 68 (54.4%) | 68 (54.4%) | |
| BMI
(kg/m2) | 31.95±5.40 | 31.35±6.02 | 0.4108 |
| WC (cm) | 111.10±11.34 | 109.08±11.82 | 0.1668 |
| Triglycerides
(mg/dl) | 172.04±109.08 | 151.83±103.71 | 0.1347 |
| Cholesterol
(mg/dl) | 196.19±61.12 | 196.84±56.58 | 0.9311 |
| Glucose
(mg/dl) | 93.90±9.62 | 213.45±100.70 | <0.0001 |
| 25(OH)D
(ng/ml) | 10.11±11.93 | 7.49±7.59 | 0.0306 |
Association of rs2228570 in VDR with
the risk of T2DM
Considering that serum 25(OH)D was significantly
lower in patients with T2DM and that vitamin D elicits its response
in target tissues via binding and activating VDR, the association
of several SNPs in the VDR gene was assessed with regard to
the risk of T2DM. A PCR-RFLP-based approach was used to determine
the genotype of the study subjects for the following SNPs
(rs2228570, rs1544410 rs7975232, and rs731236). The association of
the alleles of each of the abovementioned SNPs with the risk of
T2DM was first assessed. The results of this analysis are presented
in Table III. The findings
indicated that the frequency of the G allele of rs2228570 was
higher in patients with T2DM than in controls, while the frequency
of the A allele of rs2228570 was lower (P=0.0392).
 | Table IIIAllele frequencies of rs2228570,
rs1544410, rs7975232, and rs731236 VDR SNPs in controls and
patients with T2DM. |
Table III
Allele frequencies of rs2228570,
rs1544410, rs7975232, and rs731236 VDR SNPs in controls and
patients with T2DM.
| SNP ID | Allele | Controls n (%) | T2DM n (%) | P-value |
|---|
| rs2228570 | G | 166 (66.0) | 187 (75.0) | 0.0392 |
| | A | 84 (34.0) | 63 (25.0) | |
| rs1544410 | C | 148 (59.0) | 131 (52.0) | 0.1258 |
| | A | 102 (41.0) | 119 (48.0) | |
| rs7975232 | A | 151 (60.0) | 162 (65.0) | 0.3093 |
| | C | 99 (40.0) | 88 (35.0) | |
| rs731236 | A | 162 (65.0) | 146 (58.0) | 0.1412 |
| | G | 88 (35.0) | 104 (42.0) | |
Given this result, the association of genotype
categories of each of the VDR SNPs with the risk of T2DM was
then assessed. In this analysis, where only a co-dominant model of
inheritance was evaluated, there was no significant (P>0.05)
association between any of the genotypes of the tested SNPs with
the risk of T2DM (Table IV).
 | Table IVGenotype frequencies of rs2228570,
rs1544410, rs7975232 and rs731236 VDR SNPs in controls and
patients with T2DM. |
Table IV
Genotype frequencies of rs2228570,
rs1544410, rs7975232 and rs731236 VDR SNPs in controls and
patients with T2DM.
| SNP ID | Genotype | Controls (n=125)
(%) | T2DM (n=125)
(%) | P-value |
|---|
| rs2228570 | GG | 58 (46.4) | 74 (59.2) | 0.1248 |
| | AG | 50 (40.0) | 39 (31.2) | |
| | AA | 17 (13.6) | 12 (9.6) | |
| rs1544410 | CC | 41 (32.8) | 36 (28.8) | 0.1559 |
| | AC | 66 (52.8) | 59 (47.2) | |
| | AA | 18 (14.4) | 30 (24.0) | |
| rs7975232 | AA | 47 (37.6) | 56 (44.8) | 0.5106 |
| | AC | 57 (45.6) | 50 (40.0) | |
| | CC | 21 (16.8) | 19 (15.2) | |
| rs731236 | AA | 51 (40.8) | 44 (35.2) | 0.2542 |
| | AG | 60 (48.0) | 58 (46.4) | |
| | GG | 14 (11.2) | 23 (18.4) | |
The association of each of the SNPs with the risk of
T2DM under three other inheritance models (dominant, recessive, and
overdominant), was then examined. Using this approach, it was
revealed that rs2228570 in the VDR gene was associated with
the risk of T2DM under a dominant model of inheritance (P=0.0432;
Table V). Specifically, under this
inheritance model, the frequencies of AG-AA genotypes were lower in
patients with T2DM compared with the control subjects (40.8% vs.
53.6%). Therefore, in this model, AG-AA genotypes reduced the risk
of T2DM relative to the GG genotype (OR=0.597; CI: 0.362-0.984;
P=0.0432; Table V).
 | Table VAssociation of rs2228570 with risk of
T2DM under different inheritance models. |
Table V
Association of rs2228570 with risk of
T2DM under different inheritance models.
| Model | Genotype | Controls (%) | T2DM (%) | OR (95% CI) | P-value |
|---|
| Codominant | GG | 58 (46.4) | 74 (59.2) | 1 | 0.1248 |
| | AG | 50 (40.0) | 39 (31.2) | 0.611
(0.356-1.051) | |
| | AA | 17 (13.6) | 12 (9.6) | 0.553
(0.245-1.250) | |
| Dominant | GG | 58 (46.4) | 74 (59.2) | 1 | 0.0432 |
| | AG-AA | 67 (53.6) | 51 (40.8) | 0.597
(0.362-0.984) | |
| Recessive | GG-AG | 108 (86.4) | 113 (90.4) | 1 | 0.3256 |
| | AA | 17 (13.6) | 12 (9.6) | 1.482
(0.676-3.249) | |
| Overdominant | GG-AA | 75 (60.0) | 86 (68.8) | 1 | 0.1470 |
| | AG | 50 (40.0) | 39 (31.2) | 0.680
(0.404-1.145) | |
In order to examine whether differences in the
VDR genotype could be linked with differences in serum
25(OH)D, the study subjects were categorized according to their
genotype class for each of the VDR SNPs. An ANOVA test was
then performed to examine whether there were statistically
significant differences in serum 25(OH)D between different genotype
categories. This analysis was performed on control subjects only,
case subjects only or both groups. None of the analyses revealed a
significant difference in 25(OH)D between the different genotype
classes for each of the VDR SNPs (P>0.05) (data not
shown).
It was then examined whether genetic variation in
rs2228570 SNP was associated with differences in serum glucose
levels. To achieve this aim, study subjects were categorized
according to their genotype class of rs2228570, and then it was
determined whether there were significant differences in serum
glucose levels between the different genotype classes. Since
association of rs2228570 with T2DM was under a dominant model of
inheritance, the study subjects were categorized according to their
genotype class into two categories instead of three; AA or AG
genotype in one category and the GG genotype in the second
category. It was then determined whether there were significant
differences in the serum glucose levels between the study subjects
of each category. No significant differences were observed between
the two groups aforementioned (P>0.05) (data not shown). The
same analysis was performed only on patients with T2DM, or only on
the control subjects. No significant differences were observed in
either analysis (P>0.05) (data not shown).
Age, sex, BMI, serum cholesterol, and triglycerides
are confounding variables that could modify the association of low
serum 25(OH)D or rs2228570 with T2DM. To adjust for these
variables, a multivariate regression analysis was performed
(Table VI). The results revealed
that serum 25(OH)D remained associated with T2DM and reduced its
risk (OR=0.997; CI: 0.994-0.998; P=0.0390). It was also
demonstrated by this analysis that the rs2228570 SNP in the
VDR gene remained associated with T2DM, where the AG-AA
genotypes reduced the risk of T2DM relative to the GG genotype
(OR=0.548; CI: 0.307-0.977; P=0.0410).
 | Table VIRegression analysis of the effect of
study variables with the risk of T2DM. |
Table VI
Regression analysis of the effect of
study variables with the risk of T2DM.
| Variables | OR | 95% CI | P-value |
|---|
| Age | 1.131 | 1.091-1.173 | <0.0001 |
| 25(OH)D
rs2228570 | 0.997 | 0.994-0.998 | 0.0390 |
| GG (Reference) | 1.000 | - | - |
| AG-AA | 0.548 | 0.307-0.977 | 0.0410 |
Next, to determine the presence of a significant
interaction between any of the following environmental variables
(age, BMI and WC) with rs2228570 that could modify the risk of T2DM
in the study population, three interaction terms with rs2228570
were included in the regression model (one for each variable). The
analysis demonstrated the absence of any significant interaction
between rs2228570 with any of the aforementioned variables
(P>0.05), with only a trend for the presence of a significant
role for an interaction between rs2228570 with age in determining
the risk of T2DM (P=0.06) (data not shown).
Association of two haplotypes in the
VDR with risk of T2DM
Finally, the genotype data of all four SNPs were
examined to explore the presence of any haplotype in the VDR
gene associated with the risk of T2DM. Herein, two haplotypes were
revealed to significantly (P<0.05) modify the risk of T2DM
(Table VII). The first haplotype,
ACAA, was less frequent in patients with T2DM and significantly
reduced its risk (OR=0.346; CI: 0.147-0.812; P=0.0112), while the
second haplotype, GAAG, was more frequent in cases with T2DM and
significantly increased the risk of T2DM (OR=1.909; CI:
1.260-2.891; P=0.0021).
 | Table VIIHaplotype frequencies of VDR
SNPs, rs2228570, rs1544410, rs7975232 and rs731236 in controls and
patients with T2DM. |
Table VII
Haplotype frequencies of VDR
SNPs, rs2228570, rs1544410, rs7975232 and rs731236 in controls and
patients with T2DM.
| rs2228570 | rs1544410 | rs7975232 | rs731236 | Frequency in
control subjects | Frequency in
patients with T2DM | OR (95% CI) | P-value |
|---|
| A | A | A | G | 0.133 | 0.095 | 0.658
(0.376-1.152) | 0.1407 |
| A | C | A | A | 0.081 | 0.031 | 0.346
(0.147-0.812) | 0.0112 |
| A | C | C | A | 0.117 | 0.112 | 0.925
(0.533-1.606) | 0.7821 |
| G | A | A | A | 0.053 | 0.049 | 0.881
(0.397-1.955) | 0.7558 |
| G | A | A | G | 0.191 | 0.317 | 1.90
(1.260-2.891) | 0.0021 |
| G | C | A | A | 0.128 | 0.151 | 1.172
(0.705-1.948) | 0.5400 |
| G | C | C | A | 0.248 | 0.225 | 0.845
(0.558-1.280) | 0.4265 |
Discussion
The present study supports the theory that serum
25(OH)D or one of its direct or indirect metabolites or an effector
downstream of the VDR modifies the risk of T2DM. Additionally, the
findings of the present study indicated that genetic variations in
the VDR gene itself were associated with the risk of T2DM.
These findings aid in improving comprehension of the factors that
modulate the risk of T2DM, in a Jordanian population. This is
particularly important considering the magnitude of the pressure
that T2DM places on the health and economic sectors of this
developing country and the requirement for a national health policy
plan to help manage the disease and its life-threatening
complications.
One of the alarming findings of the present
investigation was the low level of vitamin D among the individuals
recruited to participate in the study. In fact, the mean value of
serum 25(OH)D in both study groups was <20 ng/ml, a widely used
cut-off value for vitamin D deficiency (36). There is an ongoing debate regarding
the cut-off value for vitamin D deficiency. Several groups have
recommended a new definition of vitamin D deficiency, where the
cut-off value is lower than the widely used value of 20 ng/ml
(37-39).
Nonetheless, regardless of the cut-off, the existing evidence
supports a relatively high prevalence rate of vitamin D deficiency
in Jordan.
Although the sample size and geographic distribution
of the subjects included in the present study were not
representative of the population in Jordan, the results are in
agreement with other investigations that assessed the levels of
25(OH)D across different age groups. For example, in a
representative sample that included 4,056 subjects aged >17
years, El-Khateeb et al reported an overall prevalence of
vitamin D deficiency of 89.7% (40). Moreover, Abdul-Razzak et al
demonstrated a prevalence of vitamin D deficiency of 29% in a
cross-sectional sample of 275 healthy infants and toddlers between
6 to 36 months (41).
Out of a possible 4,383 h, there are 3,602 h of
sunlight per year in Jordan (42).
Despite this value, the findings of the present study as well as
those from the study by El-Khateeb et al (40) refer to a high prevalence of vitamin
D deficiency in this Middle Eastern country. The exact reason
behind this observation is currently not understood but may be
explained by several factors. The dressing style in Jordan is
largely conservative with a considerable percentage of women
wearing either the veil or the niqab (40), a factor that reduces the skin area
exposed to the sun. Furthermore, recent epidemiological data
indicates that the current rate of obesity in Jordan is alarmingly
high and is increasing (43).
Obesity itself is associated with low levels of vitamin D (44) and may be contributing to the high
prevalence of vitamin D deficiency in Jordan.
The high prevalence rate of vitamin D deficiency in
Jordan and the conclusions of the present study linking vitamin D
deficiency with T2DM strongly highlight the need to address this
issue by the public health authorities. In addition to the
association with T2DM, vitamin D deficiency has been linked with
several diseases, including cancer (45), infertility (46), and metabolic syndrome (47). There is an eminent need to initiate
nationwide awareness campaigns that explain the dietary and
environmental sources of vitamin D, the link between vitamin D
deficiency and chronic diseases, and the relative safety and
cost-effectiveness of vitamin D supplementation protocols in
preventing vitamin D deficiency and its numerous public health
implications. In this context, it is of note that a growing body of
evidence indicates that vitamin D supplementation may be of utility
in achieving better glycemic control in patients with T2DM. For
example, in a previous study, it was recently reported that
normalizing serum vitamin D levels in patients with T2DM decreases
their HbA1c and serum glucose levels (48). These results are in agreement with a
study by Alqudah et al which reported a similar observation
(49).
Vitamin D elicits its response in target tissues
through binding and transcriptional activation of its receptor
(VDR). Genetic variation in the sequence of the VDR gene was
reported to influence its activity. Considering the findings of the
present study, associating vitamin D levels with the risk of T2DM,
the association of several SNPs in the VDR gene with the
risk of T2DM was investigated. The results of the genetic analysis
revealed that the frequency of the major G allele of rs2228570 was
higher in patients with T2DM than in control subjects. Furthermore,
it was determined that the GG genotype of rs2228570 increased the
risk of T2DM in a dominant model of inheritance in both univariate
and multivariate analyses. Finally, the haplotype frequencies of
all four VDR SNPs genotyped in the present investigation
revealed that a specific haplotype containing the G allele of
rs2228570 was more frequent in patients with T2DM and increased its
risk. These results indicate that the major G allele of rs2228570
may be a high-risk allele for T2DM, in Jordan. This is consistent
with the conclusion of a study by Angel et al, which also
demonstrated using a case-control design, that the G allele of
rs2228570 was a high-risk allele for T2DM in a Chilean population
(50). However, the abovementioned
study only included older adults with an age range of 60-79 years.
This finding is also comparable to a study by Safar et al,
which revealed that the G allele of rs2228570 was significantly
associated with the risk of T2DM in a population of the UAE, a
Middle Eastern country with T2DM trends similar to Jordan (51).
Rs2228570 is a genetic variant found in the coding
sequence of the VDR gene (22,52).
Upon the translation of the resulting cDNA, this polymorphism
alters the length of the VDR (22).
Specifically, the presence of the G allele abolishes a translation
initiation codon causing translation to start 9 bp downstream from
the original initiation site (22).
This results in a 424 amino acid protein instead of the 427 VDR
(22). Previous in vitro
data using cell reporter assays in transfected HeLa or COS-7 cells
indicated that the shorter VDR protein, containing the G allele,
has higher transcriptional activity (22). Notably, these findings were never
replicated in any other cell line system. Additionally, there is no
conclusive literature describing whether there are differences in
the affinity of the shorter protein to its ligand [1,25(OH)D]
(50).
The present investigation indicated that the G
allele of rs2228570 is a high-risk allele of T2DM. This is in
agreement with observations in an Emirati (51) and a Chilean (50) population as well as in a
meta-analysis of 14 studies on Asian populations (53). However, the role of the G allele as
a high-risk allele appears counterintuitive to the previously
published data above, demonstrating the enhanced transcriptional
activity of the resulting VDR upon the presence of the G allele.
This may be explained by the presence of another genetic variant in
linkage disequilibrium with the G allele which modulates its effect
specially in Asian populations. This should also be an invitation
to evaluate the activity of the shorter 424 amino acid VDR in
relevant pancreatic cell line models or conclusively determine its
affinity to 1,25(OH)D.
The results of the present investigation as well as
those of others clearly demonstrate that multiple factors play a
role in determining the risk of T2DM. The risk of T2DM appears to
be influenced by the complex interaction of a group of
environmental (54), behavioral
(55) and/or genetic factors
(56). Consequently, the prevention
of developing this disease extends beyond the simple recommendation
of a better diet and lifestyle. Accordingly, given the complicated
nature of this issue and the magnitude of the T2DM problem, public
health policy decision-makers should adopt a well-rounded, holistic
approach to reduce the risk of T2DM, including dietary, behavioral,
and genetic counseling components.
In the present study, subjects recruited to the
control group were matched to patients with T2DM as regards age and
BMI. Considering that obesity is a well-established risk factor for
T2DM (57), this resulted in
subjects of the control group having a mean BMI value of 31 which
indicates that numerous control subjects were obese. Recognizing
that obesity itself is linked with lower vitamin D levels (44), this may explain the low levels of
vitamin D observed in the control group. In fact, failure to
include more control subjects with a normal BMI is a limitation of
the present study.
The serum levels of vitamin D are affected by a
plethora of factors, including age, dress style, latitude, activity
of metabolizing enzymes, body fat distribution, and eating behavior
(54,58). Data which reflect these factors were
not collected. For example, another limitation of the present study
was the failure to collect parameters that correspond to body fat
distribution, such as waist-hip or visceral fat ratios. A third
limitation was the inability to collect information on the eating
behavior of the participants. These factors were demonstrated in
previous studies to affect vitamin D serum levels (58,59).
In conclusion, the present case-control study
demonstrated that decreased levels of vitamin D and genetic
variation in the VDR gene were associated with the risk of
T2DM (Fig. 2). Although several
investigations across multiple populations have explored the
association of rs2228570 with T2DM with inconsistent results, the
present study is of significance as it reinforces the growing body
of evidence of a possible ethnic variation of the role of rs2228570
or the VDR itself in the pathophysiology of T2DM. This is of
particular interest considering that rs2228570 is a functional
variant of the VDR gene with an established effect on VDR
activity. Given the high prevalence of vitamin D deficiency in
Jordan, the initiation of awareness campaigns that explain the
sources of vitamin D and the implications of its deficiency on
health and disease are strongly recommended.
Acknowledgements
The authors would like to thank Ms Khawla Mhedat
(Department of Physiology and Biochemistry, Jordan University of
Science and Technology, Irbid, Jordan) for the technical
support.
Funding
Funding: The present study was supported by a grant provided to
MAA from the Deanship of Research at Jordan University of Science
and Technology (grant no. 93/2017). Article processing charges were
provided by a grant to MZA from the College of Medicine and Health
Sciences at the United Arab Emirates University (grant no.
G00003632).
Availability of data and materials
The datasets generated and/or analysed during the
current study are available from the corresponding author on
reasonable request.
Authors' contributions
MAA and OAS conceived the study. MAA, AA and MK
contributed to the methodology of the study. MAA and AA contributed
to the validation of the results. MAA, AA and RS performed the
formal analysis. MAA, AA and MK performed the experiments. MAA, ZA,
RS and OAS interpreted the data. MAA, ZA and MZA obtained the
resources required for the study. MAA, AA and MK performed the data
curation. MAA, AA and MZA wrote the original draft of the
manuscript. MAA and MZA wrote, reviewed and edited the manuscript.
MAA, OAS and MZA supervised the study. MAA contributed to the
project administration. MAA and MZA contributed to the funding
acquisition. MAA, OAS and MZA confirm the authenticity of the raw
data. All authors have read and approve the published version of
the manuscript.
Ethics approval and consent to
participate
All procedures performed in studies involving human
participants were in accordance with the ethical standards of
Jordan University of Science and Technology and King Abdullah
University Hospital Institutional Review Board (approval ID
92/118/2018) and with the 1964 Declaration of Helsinki and its
later amendments. Informed consent was obtained from all individual
participants included in the study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ogurtsova K, da Rocha Fernandes JD, Huang
Y, Linnenkamp U, Guariguata L, Cho NH, Cavan D, Shaw JE and
Makaroff LE: IDF diabetes atlas: Global estimates for the
prevalence of diabetes for 2015 and 2040. Diabetes Res Clin Pract.
128:40–50. 2017.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Ajlouni K, Khader YS, Batieha A, Ajlouni H
and El-Khateeb M: An increase in prevalence of diabetes mellitus in
Jordan over 10 years. J Diabetes Complications. 22:317–324.
2008.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Alfaqih MA, Abu-Khdair Z, Saadeh R, Saadeh
N, Al-Dwairi A and Al-Shboul O: Serum branched chain amino acids
are associated with type 2 diabetes mellitus in Jordan. Korean J
Fam Med. 39:313–317. 2018.PubMed/NCBI View Article : Google Scholar
|
|
4
|
American Diabetes Association. Diagnosis
and classification of diabetes mellitus. Diabetes Care. 37 (Suppl
1):S81–S90. 2014.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Roth A: Diabetes mellitus and obesity.
Prim Care. 29:279–295. 2002.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Kahn BB and Flier JS: Obesity and insulin
resistance. J Clin Invest. 106:473–481. 2000.PubMed/NCBI View
Article : Google Scholar
|
|
7
|
Sladek R, Rocheleau G, Rung J, Dina C,
Shen L, Serre D, Boutin P, Vincent D, Belisle A, Hadjadj S, et al:
A genome-wide association study identifies novel risk loci for type
2 diabetes. Nature. 445:881–885. 2007.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Duggirala R, Blangero J, Almasy L, Dyer
TD, Williams KL, Leach RJ, O'Connell P and Stern MP: Linkage of
type 2 diabetes mellitus and of age at onset to a genetic location
on chromosome 10q in Mexican Americans. Am J Hum Genet.
64:1127–1140. 1999.PubMed/NCBI View
Article : Google Scholar
|
|
9
|
Knowler WC, Williams RC, Pettitt DJ and
Steinberg AG: Gm3;5,13,14 and type 2 diabetes mellitus: An
association in American Indians with genetic admixture. Am J Hum
Genet. 43:520–526. 1988.PubMed/NCBI
|
|
10
|
Müller DN, Kleinewietfeld M and Kvakan H:
Vitamin D review. J Renin Angiotensin Aldosterone Syst. 12:125–128.
2011.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Holick MF, Binkley NC, Bischoff-Ferrari
HA, Gordon CM, Hanley DA, Heaney RP, Murad MH and Weaver CM:
Endocrine Society. Evaluation, treatment, and prevention of vitamin
D deficiency: An Endocrine Society clinical practice guideline. J
Clin Endocrinol Metab. 96:1911–1930. 2011.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Norman AW, Coburn JW and Hartenbower D:
Letter: Vitamin D and calcium homeostasis. West J Med.
121(508)1974.PubMed/NCBI
|
|
13
|
Lips P, van Schoor NM and Bravenboer N:
Vitamin D-related disorders. In: Primer on the Metabolic Bone
Diseases and Disorders of Mineral Metabolism. Rosen CJ (ed). The
American Society for Bone and Mineral Research, Wiley Blackwell,
pp613-623, 2013.
|
|
14
|
Wolden-Kirk H, Gysemans C, Verstuyf A and
Mathieu C: Extraskeletal effects of vitamin D. Endocrinol Metab
Clin North Am. 41:571–594. 2012.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Bernardi RJ, Johnson CS, Modzelewski RA
and Trump DL: Antiproliferative effects of
1alpha,25-dihydroxyvitamin D(3) and vitamin D analogs on
tumor-derived endothelial cells. Endocrinology. 143:2508–2514.
2002.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Adorini L: Immunomodulatory effects of
vitamin D receptor ligands in autoimmune diseases. Int
Immunopharmacol. 2:1017–1028. 2002.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Alvarez JA and Ashraf A: Role of vitamin d
in insulin secretion and insulin sensitivity for glucose
homeostasis. Int J Endocrinol. 2010(351385)2010.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Zeitz U, Weber K, Soegiarto DW, Wolf E,
Balling R and Erben RG: Impaired insulin secretory capacity in mice
lacking a functional vitamin D receptor. FASEB J. 17:509–511.
2003.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Haussler MR, Whitfield GK, Haussler CA,
Hsieh JC, Thompson PD, Selznick SH, Dominguez CE and Jurutka PW:
The nuclear vitamin D receptor: Biological and molecular regulatory
properties revealed. J Bone Miner Res. 13:325–349. 1998.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Green S and Chambon P: Nuclear receptors
enhance our understanding of transcription regulation. Trends
Genet. 4:309–314. 1988.PubMed/NCBI View Article : Google Scholar
|
|
21
|
McKenna NJ and O'Malley BW: Combinatorial
control of gene expression by nuclear receptors and coregulators.
Cell. 108:465–474. 2002.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Arai H, Miyamoto K, Taketani Y, Yamamoto
H, Iemori Y, Morita K, Tonai T, Nishisho T, Mori S and Takeda E: A
vitamin D receptor gene polymorphism in the translation initiation
codon: Effect on protein activity and relation to bone mineral
density in Japanese women. J Bone Miner Res. 12:915–921.
1997.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Jurutka PW, Remus LS, Whitfield GK,
Thompson PD, Hsieh JC, Zitzer H, Tavakkoli P, Galligan MA, Dang HT,
Haussler CA and Haussler MR: The polymorphic N terminus in human
vitamin D receptor isoforms influences transcriptional activity by
modulating interaction with transcription factor IIB. Mol
Endocrinol. 14:401–420. 2000.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Lisker R, López MA, Jasqui S, Ponce De
León Rosales S, Correa-Rotter R, Sánchez S and Mutchinick OM:
Association of vitamin D receptor polymorphisms with osteoporosis
in mexican postmenopausal women. Hum Biol. 75:399–403.
2003.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Taylor JA, Hirvonen A, Watson M, Pittman
G, Mohler JL and Bell DA: Association of prostate cancer with
vitamin D receptor gene polymorphism. Cancer Res. 56:4108–4110.
1996.PubMed/NCBI
|
|
26
|
Uitterlinden AG, Fang Y, van Meurs JB, van
Leeuwen H and Pols HA: Vitamin D receptor gene polymorphisms in
relation to vitamin D related disease states. J Steroid Biochem Mol
Biol. 89-90:187–193. 2004.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Valdivielso JM and Fernandez E: Vitamin D
receptor polymorphisms and diseases. Clin Chim Acta. 371:1–12.
2006.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Vladoiu S, Dinu Draganescu D, Botezatu A,
Anton G, Oros S, Paun DL, Ianas O, Rosca R and Badiu C:
Correlations between polymorphisms of estrogen 1, vitamin D
receptors and hormonal profile in infertile men. Acta Endocrinol
(Buchar). 12:137–144. 2016.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Park BS, Park JS, Lee DY, Youn JI and Kim
IG: Vitamin D receptor polymorphism is associated with psoriasis. J
Invest Dermatol. 112:113–116. 1999.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Blazer DG III, Umbach DM, Bostick RM and
Taylor JA: Vitamin D receptor polymorphisms and prostate cancer.
Mol Carcinog. 27:18–23. 2000.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Holick MF: Vitamin D: Important for
prevention of osteoporosis, cardiovascular heart disease, type 1
diabetes, autoimmune diseases, and some cancers. South Med J.
98:1024–1027. 2005.PubMed/NCBI View Article : Google Scholar
|
|
32
|
American Diabetes Association.
Introduction: Standards of medical care in diabetes-2022. Diabetes
Care. 45 (Suppl 1):S1–S2. 2022.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Avci E, Demir S, Aslan D, Nar R and Şenol
H: Assessment of abbott architect 25-OH vitamin D assay in
different levels of vitamin D. J Med Biochem. 39:100–107.
2020.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Alfaqih MA, Khader YS, Al-Dwairi AN,
Alzoubi A, Al-Shboul O and Hatim A: Lower levels of serum
adiponectin and the T allele of rs1501299 of the ADIPOQ gene are
protective against polycystic ovarian syndrome in Jordan. Korean J
Fam Med. 39:108–113. 2018.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Shi YY and He L: SHEsis, a powerful
software platform for analyses of linkage disequilibrium, haplotype
construction, and genetic association at polymorphism loci. Cell
Res. 15:97–98. 2005.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Sharifi F, Mousavinasab N and Mellati AA:
Defining a cutoff point for vitamin D deficiency based on insulin
resistance in children. Diabetes Metab Syndr. 7:210–213.
2013.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Manson JE, Brannon PM, Rosen CJ and Taylor
CL: Vitamin D deficiency-is there really a pandemic? N Engl J Med.
375:1817–1820. 2016.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Schramm S, Lahner H, Jöckel KH, Erbel R,
Führer D and Moebus S: Heinz Nixdorf Recall Study Group. Impact of
season and different vitamin D thresholds on prevalence of vitamin
D deficiency in epidemiological cohorts-a note of caution.
Endocrine. 56:658–666. 2017.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Shah D and Gupta P: Vitamin D deficiency:
Is the pandemic for real? Indian J Community Med. 40:215–217.
2015.PubMed/NCBI View Article : Google Scholar
|
|
40
|
El-Khateeb M, Khader Y, Batieha A, Jaddou
H, Hyassat D, Khawaja N, Abujbara M and Ajlouni K: Vitamin D
deficiency and associated factors in Jordan. SAGE Open Med.
7(2050312119876151)2019.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Abdul-Razzak KK, Ajlony MJ, Khoursheed AM
and Obeidat BA: Vitamin D deficiency among healthy infants and
toddlers: A prospective study from Irbid, Jordan. Pediatr Int.
53:839–845. 2011.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Al-Qarqaz F, Marji M, Bodoor K, Almomani
R, Al Gargaz W, Alshiyab D, Muhaidat J and Alqudah M: Clinical and
demographic features of basal cell carcinoma in North Jordan. J
Skin Cancer. 2018(2624054)2018.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Ajlouni K, Khader Y, Batieha A, Jaddou H
and El-Khateeb M: An alarmingly high and increasing prevalence of
obesity in Jordan. Epidemiol Health. 42(e2020040)2020.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Vranić L, Mikolašević I and Milić S:
Vitamin D deficiency: Consequence or cause of obesity? Medicina
(Kaunas). 55(541)2019.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Garland CF, Garland FC, Gorham ED, Lipkin
M, Newmark H, Mohr SB and Holick MF: The role of vitamin D in
cancer prevention. Am J Public Health. 96:252–261. 2006.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Alzoubi A, Mahdi H, Al Bashir S, Halalsheh
O, Al Ebbini M, Alzarir M, Al-Ahmar K, Alfaqih M and Al-Hadidi AH:
Normalization of serum vitamin D improves semen motility parameters
in patients with idiopathic male infertility. Acta Endocrinol
(Buchar). 13:180–187. 2017.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Lee SH, Kim SM, Park HS, Choi KM, Cho GJ,
Ko BJ and Kim JH: Serum 25-hydroxyvitamin D levels, obesity and the
metabolic syndrome among Korean children. Nutr Metab Cardiovasc
Dis. 23:785–791. 2013.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Alfaqih MA, Melhem NY, O FK, Al-Dwairi A,
Elsalem L, Alsaqer TG and Allouh MZ: Normalization of vitamin D
serum levels in patients with type two diabetes mellitus reduces
levels of branched chain amino acids. Medicina (Kaunas).
58(1267)2022.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Alqudah M, Khanfar M, Alfaqih MA,
Al-Shboul O, Ghazi Al-U'Datt D, Al-Dwairi A and Allouh M:
Correlation between vitamin D and serum brain derived neurotropic
factor levels in type 2 diabetes mellitus patients. Biomed Rep.
16(54)2022.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Angel B, Lera L, Márquez C and Albala C:
The association of VDR polymorphisms and type 2 diabetes in older
people living in community in Santiago de Chile. Nutr Diabetes.
8(31)2018.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Safar HA, Chehadeh SEH, Abdel-Wareth L,
Haq A, Jelinek HF, ElGhazali G and Anouti FA: Vitamin D receptor
gene polymorphisms among Emirati patients with type 2 diabetes
mellitus. J Steroid Biochem Mol Biol. 175:119–124. 2018.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Sazci A, Uren N, Idrisoglu HA and Ergul E:
The rs2228570 variant of the vitamin D receptor gene is associated
with essential tremor. Neurosci Bull. 35:362–364. 2019.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Li L, Wu B, Liu JY and Yang LB: Vitamin D
receptor gene polymorphisms and type 2 diabetes: A meta-analysis.
Arch Med Res. 44:235–241. 2013.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Dendup T, Feng X, Clingan S and
Astell-Burt T: Environmental risk factors for developing type 2
diabetes mellitus: A systematic review. Int J Environ Res Public
Health. 15(78)2018.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Iwasaki T, Hirose A, Azuma T, Ohashi T,
Watanabe K, Obora A, Deguchi F, Kojima T, Isozaki A and Tomofuji T:
Association between eating behavior and poor glycemic control in
Japanese adults. Sci Rep. 9(3418)2019.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Ali O: Genetics of type 2 diabetes. World
J Diabetes. 4:114–123. 2013.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Smyth S and Heron A: Diabetes and obesity:
The twin epidemics. Nat Med. 12:75–80. 2006.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Mithal A, Wahl DA, Bonjour JP, Burckhardt
P, Dawson-Hughes B, Eisman JA, El-Hajj Fuleihan G, Josse RG, Lips P
and Morales-Torres J: IOF Committee of Scientific Advisors (CSA)
Nutrition Working Group. Global vitamin D status and determinants
of hypovitaminosis D. Osteoporos Int. 20:1807–1820. 2009.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Zhang M, Li P, Zhu Y, Chang H, Wang X, Liu
W, Zhang Y and Huang G: Higher visceral fat area increases the risk
of vitamin D insufficiency and deficiency in Chinese adults. Nutr
Metab (Lond). 12(50)2015.PubMed/NCBI View Article : Google Scholar
|