Introduction
The cholinergic system refers to the constituents
for enzymatic synthesis, degradation, transport and receptors for
acetylcholine (ACh), a neurotransmitter in neurons and non-neuronal
cells (1). ACh is i) synthesized by
choline acetyltransferase from choline and acetyl coenzyme A in the
cytoplasm; ii) degraded by butyrylcholinesterase and
acetylcholinesterase into choline and acetic acid; and iii) stored
by the vesicular ACh transporter in neurons or organic cation
transporters in non-neuronal cells (1,2). The
actions of ACh are mediated via ligation with cholinergic
receptors, including muscarinic ACh (mAChRs) and nicotinic ACh
(nAChRs) receptors (2). mAChRs are
G-protein coupled receptors, including inhibitory receptors (M2 and
M4) that block adenylate cyclase activity, and excitatory receptors
(M1, M3 and M5) that trigger the mobilization of intracellular
calcium ions (3). nAChRs are
ligand-gated channels of homo- or hetero-pentamers of several
subunits, namely α1-α10, β1-β4, γ and δ (2). The interaction of ACh with nAChR
evokes the change in subunit conformation, resulting in the opening
of a non-selective cation channel to form a central pore that
enables the influx of cations (1).
The cholinergic system drives the cholinergic
anti-inflammatory pathway (CAIP) in the intestine through α7nAChRs
expressed on immune cells, specifically macrophages. ACh is
released by vagal efferent terminal fibers (4). Unlike CAIP, which has been extensively
documented in several animal models, the role of ACh and AChRs in
the modulation of anti-inflammatory immunoglobulin A (IgA) remains
unknown.
IgA, along with the polymeric immunoglobulin
receptor (pIgR), are regarded as markers of intestinal homeostasis
(5,6). Experimental assays using perfused
porcine colonic explants and rat intestinal loops indicated that
luminal IgA secretion is increased by mAChR agonists (carbachol,
pilocarpine and bethanechol) or decreased by mAChR antagonists
(atropine) (7,8). During vagotomy, neuronal inputs of the
cholinergic system include downmodulation, as found in secretions
of the human jejunum (9,10) and in the small intestines of mice
(11,12). Vagotomy effects on IgA plasma cells
also include downmodulation, as described in the lamina propria of
mice (11), and increased
modulation documented in the lamina propria of the rat jejunum
(13).
To the best of our knowledge, the specific role of
ACh in the anti-inflammatory IgA response remains unknown. Thus,
the present study aimed to evaluate the outcomes of nAChR
modulation in the IgA response. The present study may act as a
novel theoretical basis for the development of strategies that
contribute to the maintenance of IgA responses in diseases, such as
inflammatory bowel diseases, in which the cholinergic system may be
involved (1).
Materials and methods
Animals
BALB/c mice (age, 8 weeks; male; weight, 25-30 g)
were obtained from Unidad de Production y Experimentation de
Animales de Laboratorio, Universidad Autónoma Metropolitana, Unidad
Xochimilco, and individually housed in acrylic cages on a 12-h
light/dark cycle, with a room temperature of 20˚C and relative
humidity of 55%. Water and a controlled diet, including >23%
crude protein, >4.5% crude fat, <6% crude fiber, <2%
moisture and <8% ash (Laboratory Rodent Diet 5001; LabDiet) were
provided ad libitum. A 1-week period preceded any
experimental intervention. All experiments were performed by a
single handler and always took place prior to 10:00 a.m. The use of
animals in the experimental protocols was approved (approval no.
ESM-CICUAL-01/09-10-2018) by the Comité Interno para Uso y Cuidado
de Animales de Laboratorio (CICUAL) of the Escuela Superior de
Medicina (ESM), following the Mexican Federal regulations for
animal care (NOM-062-ZOO-1999; Ministry of Agriculture, Mexico
City, Mexico) and in accordance with the ARRIVE guidelines for
reporting animal research (14).
Drugs
Drugs were purchased from MilliporeSigma. The
vehicle for drug dissolution was sterile isotonic saline solution,
and drugs were used in the following doses: GTS-21 dihydrochloride,
a selective α7nAChR agonist (cat. no. 505277) at a dose of 4
mg/kg/day, and mecamylamine hydrochloride (MLA), a non-selective
nAChR antagonist (cat. no. M9020) at a dose of 1 mg/kg/day
(15,16). Drugs were administered once a day
for 7 consecutive days via intraperitoneal injection. Isoflurane
(Sofloran, Lab Pisa) was used for animal sacrifice.
Experimental model and sampling
A total of 10 mice were included in each group, and
intraperitoneally injected daily with GTS-21 or MLA for 7
consecutive days. A group of mice without drug treatment was
included as the control. At 24 h after the final dose, each mouse
was euthanized using 5% isoflurane for 3 min, mice were
subsequently exsanguinated using cardiac puncture. The whole small
intestine was resected from the pyloric to the cecal junction and
washed with a phosphate-buffered saline solution (PBS; pH 7.4)
containing a protease inhibitor cocktail (cOmplete™; cat. no.
11836153001; Roche Diagnostics GmbH). Intestinal fluids were
individually centrifuged at 10,000 x g for 20 min at 4˚C, and the
supernatants were aliquoted and stored at -70˚C until further ELISA
assays. Peyer's patches, the lamina propria and epithelial cells
were collected and processed as previously described (17,18).
Single cell suspension from Peyer's patches and the lamina propria
were used for flow cytometric analysis, and epithelial cells were
used for reverse transcription-quantitative polymerase chain
reaction (RT-qPCR).
ELISA
Sandwich ELISA was used to quantify the antibody
concentrations using 96-microwell polystyrene plates (cat. no.
8590; Corning, Inc.). A total of 200 µl PBS containing 0.05%
Tween-20 (PBST) was used for washing both before and after the
incubation of plates at 4˚C or at 37˚C. A total of 100 µl PBST was
used for washing of reactants and samples. Total protein was
quantified using Bradford assay with commercial dye reagent (cat.
no. 500-0006; Bio-Rad Laboratories, Inc.). The 96-microwell
polystyrene plates were coated with 0.1 µg/ml goat anti-mouse IgA
(cat. no. 626700; Invitrogen; Thermo Fisher Scientific, Inc.) or
with 2.0 µg/ml goat anti-mouse IgM (cat. no. 115-001-020; Jackson
ImmunoResearch Laboratories, Inc.) in carbonate-bicarbonate buffer
(pH 9.6). Following overnight incubation at 4˚C and washing as
previously described, non-specific binding sites were blocked with
5%/volume blotto (cat. no. 37530; Thermo Fisher Scientific, Inc.)
in carbonate-bicarbonate buffer (pH 9.6). Following incubation for
2 h at 37˚C and washing, the plates were treated with intestinal
fluid samples, tested in triplicate and normalized at various
concentrations of total protein. Samples were dissolved in
5%/volume blotto in PBST. Following overnight incubation at 4˚C and
washing, the horseradish (HRP) conjugates (both from Invitrogen;
Thermo Fisher Scientific, Inc.) dissolved in 5% blotto/PBST,
1:5,000 goat anti-mouse IgA-HRP (cat. no. 626720) or 1:5,000
goat-anti mouse IgM-HRP (cat. no. PA184383) were added and
incubated for 1 h at 37˚C. Subsequently, the plates were washed and
the substrate solution [hydrogen peroxide and o-phenylenediamine in
citrate phosphate buffer (pH 5.0)] was added, followed by
incubation for 20 min in the dark at room temperature. The
enzymatic reaction was terminated using 2.5 M sulfuric acid and the
absorbance was measured at 492 nm using a microplate reader
(EpochTM; BioTek Instruments, Inc.). Standard curves with purified
α chain of mouse IgA (cat. no. M1421; MilliporeSigma) or mouse IgM
(cat. no. CLCMGM00; Cedar Lane Labs) were prepared to quantify
total IgA and IgM in µg per 100 mg of total protein.
Flow cytometry
A single suspension procedure was achieved according
to a previously described protocol (17,18).
Cell suspensions including Peyer's patches and the lamina propria
cells were adjusted to a final concentration of 1x106/ml
in PBS. Lymphocytes were stained at 4˚C for 25 min in PBS and 0.01%
sodium azide. Fluorochrome-tagged antibodies for surface markers
used [all from BD Biosciences, except the transforming growth
factor (TGF)-β-APC from BioLegend, Inc.] were as follows: Anti-CD4
PERCP (1:20) (cat. no 553052), anti-CD8 FITC (1:10) (cat. no.
553030), anti-CD3 APC (1:20) (T-cells; cat. no 565643), anti-CD19
PE (1:20) (cat. no 557399), anti-CD138 APC (1:10) (cat. no 558626),
anti-IgM PeCy7 (1:20) (cat. no. 552867) and anti-IgA FITC (1:20)
(B-cells and plasma cells; cat. no. 559354). To detect
intracellular cytokines, fixation and permeabilization were carried
out using a BD Cytofix/Cytoperm kit (cat. no. 554714; BD
Biosciences) and the following antibodies: IL-4 PE (1:20) (cat. no.
554389), IL-5 PE (1:20) (cat. no. 554395), IL-6 APC (1:20) (cat.
no. 561367), IL-10 FITC (1:20) (cat. no. 554466) and TGF-β-APC
(1:10) (cat. no. 1406). The fluorescent signal intensity was
recorded and analyzed using a FACSCalibur flow cytometer (BD
Biosciences). Events were collected from the lymphocyte gate on the
forward scatter area/side scatter area dot plot. A total of 20,000
gated events were acquired from each sample using BD FACSDIVA
software version 6.1 (BD Biosciences). Data were analyzed using
Summit software version 4.3 (Dako; Agilent Technologies, Inc.).
RT-qPCR for pIgR
The mRNA expression of pIgR was analyzed in
epithelial cells isolated following discontinuous 20% percoll
gradient, as previously described (17,18).
RT-qPCR was performed to assess the expression of pIgR. Briefly,
total RNA was extracted from epithelial samples using
TRIzol® reagent (50 mg/1 ml) according to the
manufacturer's protocol (cat. no. 15596018; Invitrogen; Thermo
Fisher Scientific, Inc.). The purity and concentration of RNA were
determined spectrophotometrically using Nanodrop Lite (Thermo
Fisher Scientific, Inc.). Samples with an A260/280 ratio of 1.8-2.0
were included. RNA samples were loaded onto 1.5% gels for
electrophoresis and stained with ethidium bromide to visualize RNA
integrity on a transilluminator (Fusion SL; Vilber Lourmat
Deutschland GmbH). Reverse transcription using RNA as the template
was performed using a cDNA synthesis kit Transcriptor First Strand
cDNA for RT-qPCR (cat. no. 04896866001; Roche Diagnostics GmbH) as
per the manufacturer's instructions. For cDNA amplification, TaqMan
Master reaction mix (cat. no. 04535286001; Roche Diagnostics GmbH)
was used as per the manufacturer's instructions. Amplification was
conducted on a Techne Prime Pro48 Real-Time qPCR system (Thermo
Fisher Scientific, Inc.) using the following conditions: Initial
denaturation for 10 min at 95˚C, followed by 50 cycles of 10 sec at
94˚C, 20 sec at 90˚C and 5 sec at 72˚C. The nucleotide sequences of
primers used for RT-qPCR were as follows: forward,
5'-CTGTGCCCGAAACTGGAT-3' and reverse, 5'-TCAGGTTGGCTTCTTGTATGAG-3',
and these were designed using Probe Finder version 4.5 (http://www.universalprobelibrary.com).
The samples were analyzed in quintuplets, and the relative gene
expression levels were calculated using the 2-ΔΔCq
method and normalized to the level of the 18S ribosomal RNA subunit
(forward, 5'-GCCGCTAGAGGTGAAATTCTT-3' and reverse,
5'-CGTCTTCGAACCTCCGACT-3') (19).
Statistical analysis
A total of three independent experiments were
performed (10 mice per group for each experiment), and data are
presented as the mean ± standard deviation. Data were analyzed
using one-way ANOVA followed by Dunnett's post hoc test. All
analyses were carried out using GraphPad Prism version 8.0.2
(GraphPad Software, Inc.). P<0.05 was considered to indicate a
statistically significant difference.
Results
Antibody and plasma cell
determination
In the present study, total IgA and
antibody-secreting cells were evaluated as markers of intestinal
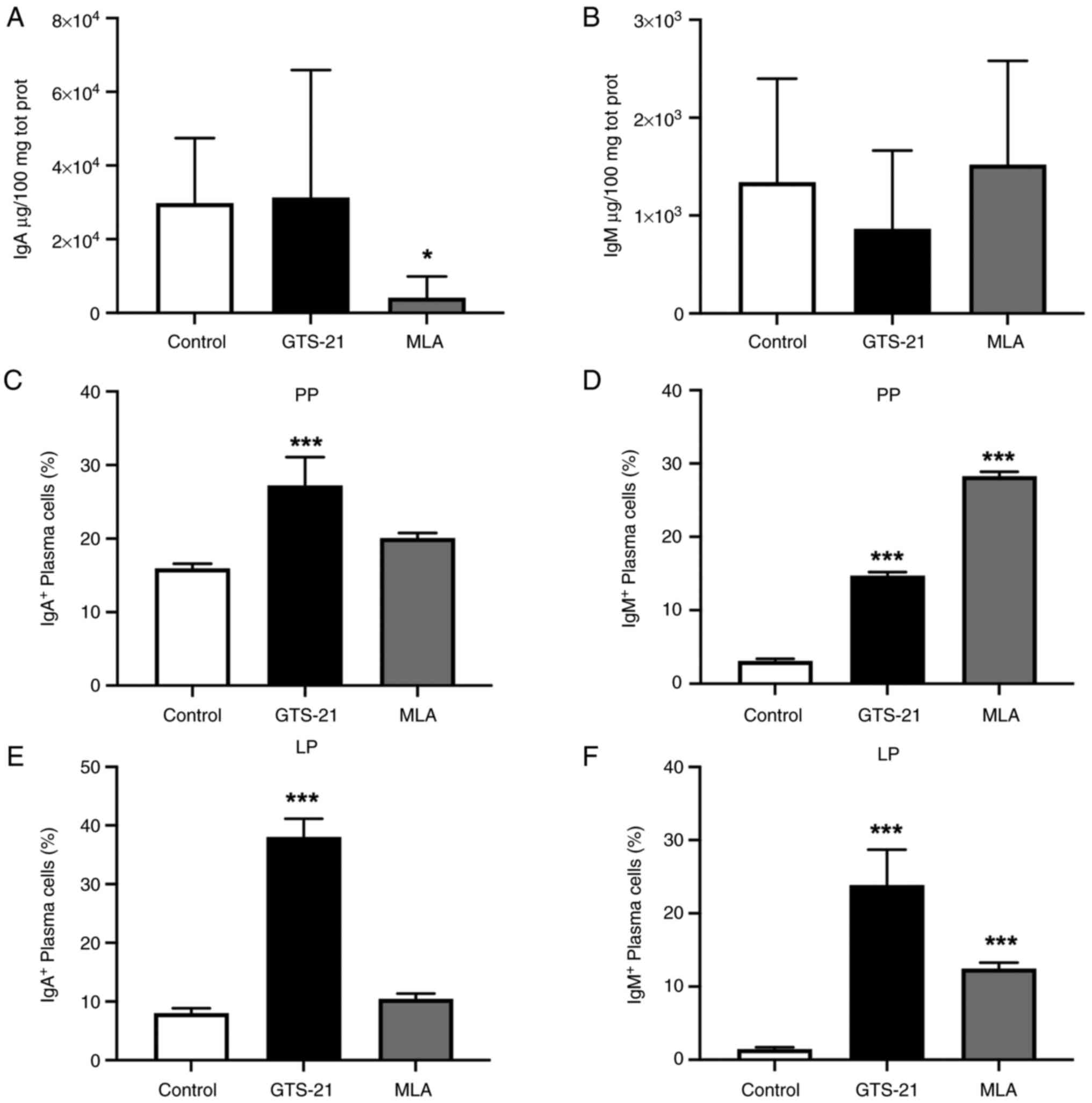
homeostasis (5,6). Compared with the control group, the
concentration of IgA was not significatly different following
treatment with GTS-21, and decreased (P<0.05) following
treatment with MLA (Fig. 1A). On
the other hand, IgM concentration was not significatily different
following treatment with either drug (Fig. 1B).
Plasma cell responses were analyzed in Peyer's
patches as the main compartment of the gut-associated lymphoid
tissue (GALT), and in the lamina propria as the effector site of
the intestine (5). Compared with
the control group, the percentage of IgA+ plasma cells
were significantly increased in Peyer's patches and the lamina
propria following treament with GTS-21 (P<0.001) (Fig. 1C and E). Moreover, the percentage of
IgM+ plasma cells was significantly increased in these
compartments, following treatment with either drug (P<0.001)
(Fig. 1D and F).
Intracellular IL assessment in
CD4+ T-lymphocytes
IgA generation requires TGF-β, which determines the
IgM+ B to IgA+ B cell class switch. It also
requires IL-4, -5, -6 and -10, which reinforce the class switch,
and favor the clonal expansion of IgA+ B cells and their
maturation in IgA+ plasma cells (5,20). In
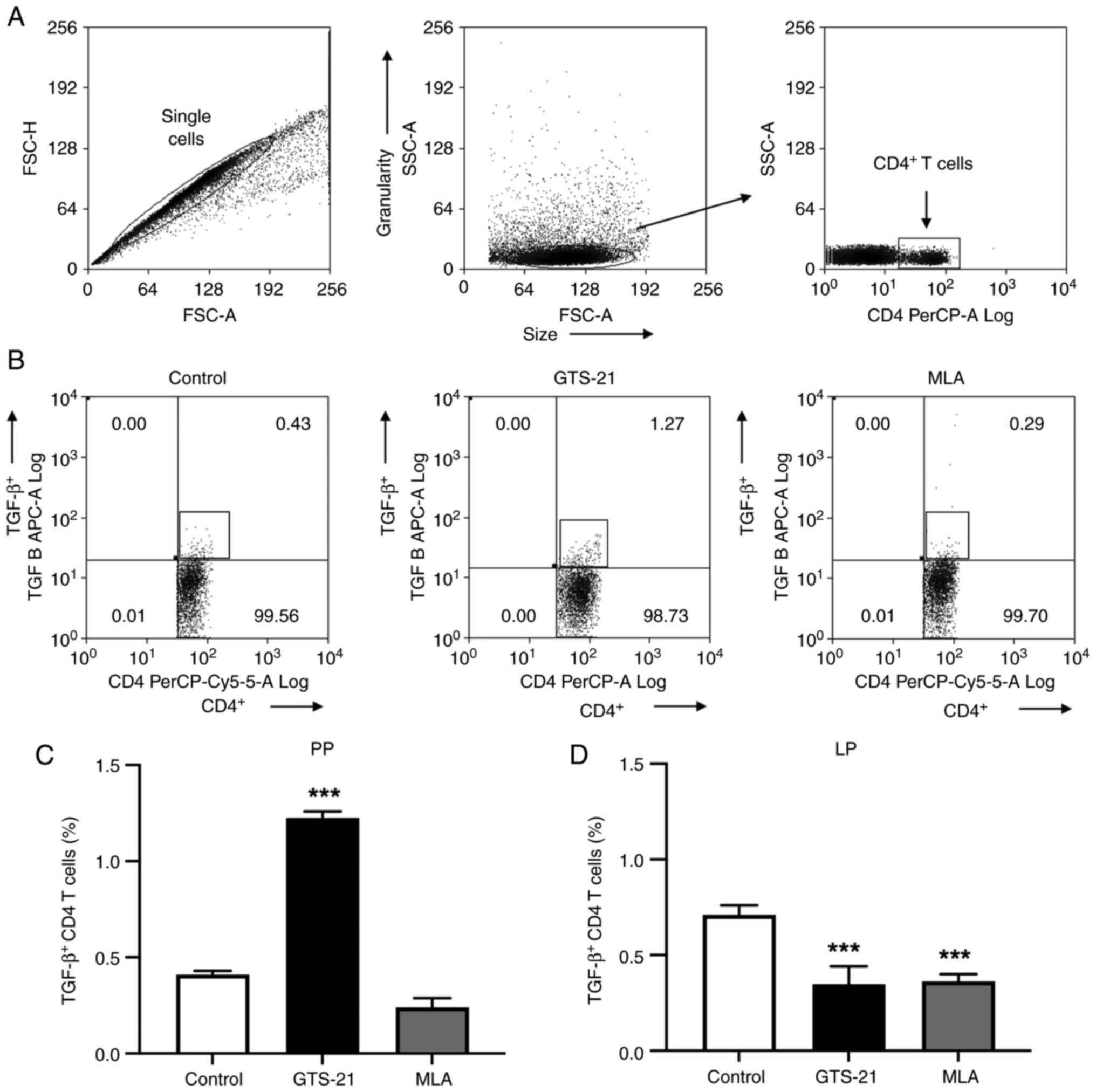
the present study, flow cytometry was performed to determine the
number of CD4+T lymphocytes. The flow cytometry gating
strategy is illustrated in Fig. 2A
and a representative dot-plot from Peyer's patches is presented in
Fig. 2B. Compared with the control
group, intracellular TGF-β in CD4+T lymphocytes was
significantly increased (P<0.001) in Peyer's patches following
treatment with GTS-21 (Fig. 2C),
and significantly decreased (P<0.001) in the lamina propria
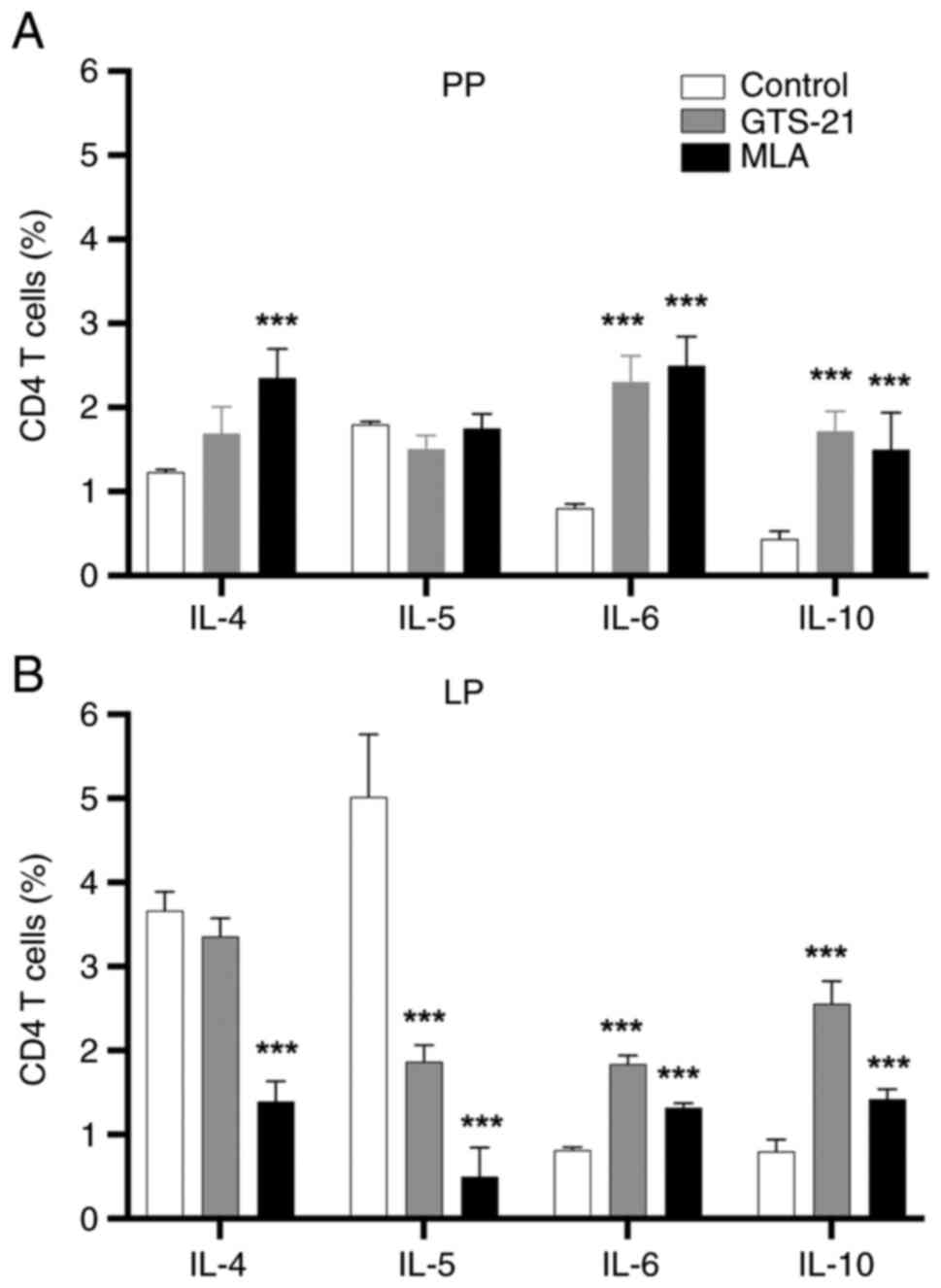
following treatment with MLA or GTS-21 (Fig. 2D). Compared with the control, IL-6
and -10 CD4+ T-cell responses were significantly
increased (P<0.001) in Peyer's patches (Fig. 3A) and the lamina propria (Fig. 3B) following treatment with MLA or
GTS-21. Compared with the control, the IL-4 CD4+ T-cell
response was increased (P<0.001) in Peyer's patches (Fig. 3A) and decreased (P<0.001) in the
lamina propria following treatment with MLA (Fig. 3B). On the other hand, the IL-5
CD4+ T-cell response was not significatly changed in
Peyer's patches (Fig. 3A), and
decreased (P<0.001) in the lamina propria following treatment
with MLA or GTS-21 (Fig. 3B).
mRNA expression of pIgR
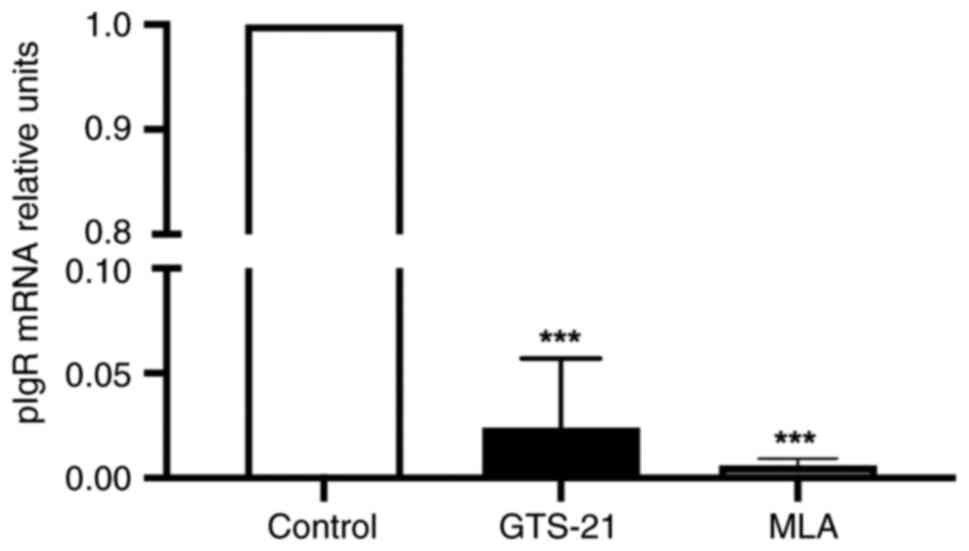
pIgR involved in IgA-transcytosis acts as a marker
of homeostasis (6,21), and was therefore analyzed in the
present study. The results indicated that compared with the control
group, the mRNA expression of pIgR was significantly decreased in
both drug-treated groups (P<0.001) (Fig. 4).
Discussion
The impact of the cholinergic system on the IgA
response has previously been addressed through analyzing mAChR
modulation in terms of IgA secretion (7,8,22,23).
Moreover, the role of the cholinergic system on IgA secretion
and/or generation has been addressed in a limited number of
vagotomy settings in humans (9,10),
mice (11,12) and rats (13). To the best of our knowledge, the
present study was the first to analyze the outcome of nAChR
modulation on the IgA response and IgA-associated parameters in
Peyer's patches and the lamina propria. The IgA generation via
T-cell dependent mechanisms occurs at Peyer's patches as the main
compartment of the GALT, whereas T-independent generation occurs in
the lamina propria, a prominent intestinal effector site (5,20).
The present study demonstrated that IgA
concentration was decreased following treatment with MLA (a
non-selective nAChR antagonist). The down-modulatory effect on IgA
secretion via nAChRs has also been reported through blocking mAChRs
using atropine, a non-selective mAChR antagonist, as documented in
perfused porcine colonic explants and intestinal loops of rats
(7,8). In murine models of vagotomy, a
decreased IgA concentration supports the neuronal inputs of the
cholinergic system for IgA generation (11,12).
In the present study, GTS-21, a partial α7nAChR
agonist, did not significantly alter IgA concentration, suggesting
that other α-nAChR subunits may be required to provide inputs for
eliciting IgA secretion. The complete role of the cholinergic
system is yet to be fully established; however, it appears to
control IgA output through a complex interplay with enteric
peptides, such as somatostatin, as described in isolated perfused
porcine ileum (22) and
cholecystokinin, as found in the intestinal mucosa of rats
sensitized to ovalbumin (23). Data
analysis evidenced no significant changes in IgM concentration were
observed following treatment with MLA or GTS-21. These findings are
comparable with those of a previous study, in which the unaltered
IgM response in intestinal secretions was observed in the whole
small intestine of mice that underwent posterior vagotomy (12). Although nAChR signals did not affect
IgM secretion, the results of a previous study using regionalized
analysis demonstrated that IgM concentration was triggered in the
proximal and distal segments of the small intestine of mice that
underwent anterior vagotomy (11).
Thus, neuronal cholinergic inputs may differentially drive the IgM
response in each region of the small intestine.
The analysis of antibody-secreting cells in both
compartments indicated that the percentage of IgA+
plasma cells was elicited following treatment with GTS-21, whereas
the percentage of IgM+ plasma cells was triggered
following treatment with MLA or GTS-21. The results of a previous
study demonstrated that the increase in the number of
IgA+ plasma cells in the jejunal lamina propria was
ascribed to an impaired intestinal motility in rats that underwent
both proximal gastric vagotomy and pyloroplasty (13). Moreover, cholinergic modulation on
antibody-secreting cells encompassed both up- or downmodulating
effects, according to the compartment (Peyer's patches or the
lamina propria) and the region of the small intestine (proximal or
distal), as documented in vagotomized mice (11,12).
Thus, cholinergic inputs may differentially drive the plasma cell
response in each region of the small intestine.
In the present experimental setting, flow cytometric
analysis of intracellular ILs demonstrated that the percentage of
CD4+ T-cells expressing TGF-β was increased in Peyer's
patches following treatment with GTS-21, or decreased in the lamina
propria following treatment with either drug. The results of a
previous study demonstrated that the percentage of
TGF-β+/CD4+ T-cells was significantly reduced
in the proximal and distal small intestine of mice that underwent
anterior vagotomy (11). The
present study, for the first time to the best of our knowledge,
demonstrated that nAChRs provide signals at Peyer's patches to
modulate the cells expressing TGF-β, a critical factor that
determines the class switch of IgM+ B-cells to
IgA+ B-cells (5,20). In contrast to CD4+ T-cell
responses expressing TGF-β, the impact of GTS-21 or MLA on
CD4+ T-cells expressing IL-4, -5, -6 and -10 involved in
IgA-generation was not observed (Fig.
3A and B). The mechanisms
underlying these findings are unknown; however, they may mirror the
complex interplay of non-neuronal and neuronal nAChR signals that
drive divergent outcomes in inductive or effector sites in each
region of the small intestine. The latter premise is based on
findings derived from regionalized analysis in mice that underwent
anterior vagotomy, in which the percentage of CD4+
T-cells expressing IgA-producing ILs was modulated divergently in
each region of the small intestine (11).
In the current assay, pIgR mRNA expression levels
were suppressed following treatment with MLA or GTS-21. The effects
of the cholinergic system on pIgR expression via nAChR regulation
has not yet been fully elucidated. However, the results of a
previous study demonstrated that carbachol, a non-selective mAChR
agonist, increased both anion transport and pIgR-mediated IgA
transcytosis. Notably, these events are independent of each other
(7). To the best of our knowledge,
the regulation of IgA transport by assessing pIgR expression with
nAChR agonist is unknown. Thus, the role of Ca2+
intracellular pathway signaling after nAChR activation on IgA
transport by determining pIgR expression needs further addressing
in future assays.
The results of the present study demonstrated that
the inhibition of nAChRs following treatment with MLA reduces both
IgA secretion and pIgR mRNA expression, which suggests that
mechanisms of IgA-transport are dependent on pIgR-mediated
transcytosis. These data suggested that
IL4+/CD4+ T cells downmodulation may result
in a blunted response of secreted IL-4, an important cytokine
involved in PIGR gene modulation for de novo
synthesis of pIgR protein (24).
Thus, the effect of MLA may be associated with the reduction of
IL-4+/CD4+ T cells in the lamina propria,
resulting in the decrease in pIgR mRNA expression.
Moreover, GTS-21 treatment did not alter IgA
concentration; however, it did decrease pIgR mRNA expression.
GTS-21 is a partial α7 nAChR agonist, which suggests that other
nAChR subunits may play a role in the regulation of IgA transport
via pIgR. As has been described in in vitro assays on
different cell types including monocytes, macrophages and
endothelial cells, α7 nAChR mediated-activation inhibits the
nuclear translocation of NF-κB (25). It has been revealed that NF-κB
activation via classical and alternative pathways is involved in
PIGR gene transcription (24). These findings may provide a
potential mechanism for supporting the role of α7 nAChR on
downmodulation of PIGR gene transcription by impeding NF-κB
translocation.
There are a number of limitations to the present
study. Namely, immunohistochemical analysis of pIgR to gain insight
into the effects of nAChR on IgA-transcytosis was not included.
Therefore, experimental settings focused to assess the protein
expression of pIgR, via western blotting and/or immunohistochemical
analysis, as well as secretory component and IgA concentration in
intestinal fluids, by means of immunoenzymatic assays, may address
presumable mechanisms of the participation of nicotinic subunits
other than α7 and their role on cholinergic modulation on IgA
transcytosis via pIgR.
In conclusion, the selective activation of α7nAChR
elicited IgA+ plasma cells in both compartments, and the
percentage of CD4+ T-cells expressing TGF-β in Peyer's
patches. Notably, the non-selective inhibition of nAChRs decreased
IgA levels and mRNA pIgR expression, which may highlight their
involvement in pIgR-mediated IgA transcytosis. The aforementioned
aspects require further examination. This basic study may be an
experimental reference for clinical trials that address the role of
the nicotinic system in intestinal dysfunctions as postoperative
ileus (26).
Acknowledgements
The authors would like to thank Mariazell Yépez
Ortega (Laboratorio de Inmunonutricion) for her collaboration with
figure editing.
Funding
Funding: The present study was supported by Consejo Nacional de
Ciencia y Tecnología (CONACyT; grant no. 254501).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
RCR proposed the original idea and experimental
design. JECL, MEDS and JPY were involved in the conceptualization
of the study. JECL, IMAM, AARA and MGL were involved in the study
methodology. JECL, IMAM and MGL were involved in the formal
analysis. JECL and MEDS were involved in data curation. MEDS was
involved in the writing and preparation of the original draft. JECL
and JPY were involved in the writing, reviewing and editing of the
manuscript. JPY supervised the study and was involved in project
administration. All authors confirm the authenticity of all the raw
data. All authors have read and approved the final manuscript.
Ethics approval and consent to
participate
Experiments using animals were approved (approval
no. ESM-CICUAL-01/09-10-2018) by the Institutional Review Board of
Instituto Politécnico Nacional (Ciudad de México, México).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Beckmann J and Lips KS: The non-neuronal
cholinergic system in health and disease. Pharmacology. 92:286–302.
2013.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Halder N and Lal G: Cholinergic system and
its therapeutic importance in inflammation and autoimmunity. Front
Immunol. 12(660342)2021.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Felder C: Acetylcholine multiple
receptors: Signal transduction through multiple effectors. Faseb J.
9:619–625. 1995.PubMed/NCBI
|
|
4
|
Goverse G, Stakenborg M and Matteoli G:
The intestinal cholinergic anti-inflammatory pathway. J Physiol.
594:5771–5780. 2016.PubMed/NCBI View
Article : Google Scholar
|
|
5
|
Li Y, Jin L and Chen T: The effects of
secretory IgA in the mucosal immune system. Biomed Res Int.
2020(2032057)2020.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Wells JM, Brummer RJ, Derrien M, MacDonald
TT, Troost F, Cani PD, Theodorou V, Dekker J, Méheust A, de Vos WM,
et al: Homeostasis of the gut barrier and potential biomarkers. Am
J Physiol Gastrointest Liver Physiol. 312:G171–G193.
2017.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Schmidt LD, Xie Y, Lyte M, Vulchanova L
and Brown DR: Autonomic neurotransmitters modulate immunoglobulin A
secretion in porcine colonic mucosa. J Neuroimmunol. 185:20–28.
2007.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Wilson ID, Soltis RD, Olson RE and
Erlandsen SL: Cholinergic stimulation of immunoglobulin A secretion
in rat intestine. Gastroenterology. 83:881–888. 1982.PubMed/NCBI
|
|
9
|
McLoughlin GA, Bradley J, Chapman DM,
Temple JG, Hede JE and McFarland J: IgA deficiency and severe
post-vagotomy diarrhoea. Lancet. 24:168–170. 1976.PubMed/NCBI View Article : Google Scholar
|
|
10
|
McLoughlin GA, Hede JE, Temple JG, Bradley
J, Chapman DM and McFarland J: The role of IgA in the prevention of
bacterial colonization of the jejunum in the vagotomized subject.
Br J Surg. 6:435–437. 1978.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Arciniega-Martínez IM, Drago-Serrano ME,
Salas-Pimentel M, Ventura-Juárez J, Reséndiz-Albor AA and
Campos-Rodríguez R: Anterior subdiaphragmatic vagotomy decreases
the IgA antibody response in the small intestines of BALB/c mice. J
Neuroimmunol. 337(577072)2019.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Pérez-López JA, Rojas-Hernández S,
Campos-Rodríguez R, Arciniega-Martínez IM, Cruz-Hernández TR,
Reséndiz-Albor AA and Drago-Serrano ME: Posterior subdiaphragmatic
vagotomy downmodulates the IgA levels in the small intestine of
BALB/c mice. Neuroimmunomodulation. 26:292–300. 2019.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Gottwald T, Lhoták Š and Stead RH: Effect
of truncal vagotomy and capsaicin on mast cells and IgA-positive
plasma cells in rat jejunal mucosa. Neurogastroenterol Motil.
9:25–32. 1997.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Kilkenny C, Browne WJ, Cuthill IC, Emerson
M and Altman DG: Improving bioscience research reporting: The
ARRIVE guidelines for reporting animal research. Osteoarthr Cartil.
20:256–260. 2012.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Sitapara RA, Gauthier AG, Valdés-Ferrer
SI, Lin M, Patel V, Wang M, Martino AT, Perron JC, Ashby CR Jr,
Tracey KJ, et al: The α7 nicotinic acetylcholine receptor agonist,
GTS-21, attenuates hyperoxia-induced acute inflammatory lung injury
by alleviating the accumulation of HMGB1 in the airways and the
circulation. Mol Med. 26(63)2020.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Mina-Osorio P, Rosas-Ballina M,
Valdes-Ferrer SI, Al-Abed Y, Tracey KJ and Diamond B: Neural
signaling in the spleen controls B-cell responses to blood-borne
antigen. Mol Med. 18:618–627. 2012.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Reséndiz-Albor AA, Esquivel R,
López-Revilla R, Verdín L and Moreno-Fierros L: Striking phenotypic
and functional differences in lamina propria lymphocytes from the
large and small intestine of mice. Life Sci. 76:2783–2803.
2005.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Reséndiz-Albor AA, Reina-Garfias H,
Rojas-Hernández S, Jarillo-Luna A, Rivera-Aguilar V, Miliar-García
A and Campos-Rodríguez R: Regionalization of pIgR expression in the
mucosa of mouse small intestine. Immunol Lett. 128:59–67.
2010.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Cerutti A: The regulation of IgA class
switching. Nat Rev Immunol. 8:421–434. 2008.PubMed/NCBI View
Article : Google Scholar
|
|
21
|
Wei H and Wang JY: Role of polymeric
immunoglobulin receptor in IgA and IgM transcytosis. Int J Mol Sci.
22:1–20. 2021.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Schmidt PT, Eriksen L, Loftager M,
Rasmussen TN and Holst JJ: Fast acting nervous regulation of
immunoglobulin A secretion from isolated perfused porcine ileum.
Gut. 45:679–685. 1999.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Freier S, Eran M and Faber J: Effect of
cholecystokinin and of its antagonist, of atropine, and of food on
the release of immunoglobulin A and immunoglobulin G specific
antibodies in the rat intestine. Gastroenterology. 93:1242–1246.
1987.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Johansen FE and Kaetzel CS: Regulation of
the polymeric immunoglobulin receptor and IgA transport: New
advances in environmental factors that stimulate pIgR expression
and its role in mucosal immunity. Mucosal Immunol. 4:598–602.
2011.PubMed/NCBI View Article : Google Scholar
|
|
25
|
de Jonge WJ and Ulloa L: The alpha7
nicotinic acetylcholine receptor as a pharmacological target for
inflammation. Br J Pharmacol. 151:915–929. 2007.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Lambrichts DPV, Boersema GSA, Tas B, Wu Z,
Vrijland WW, Kleinrensink GJ, Jeekel J, Lange JF and Menon AG:
Nicotine chewing gum for the prevention of postoperative ileus
after colorectal surgery: A multicenter, double-blind, randomised,
controlled pilot study: Int J Colorectal. Dis. 32:1267–1275.
2017.PubMed/NCBI View Article : Google Scholar
|