Introduction
Community-acquired pneumonia (CAP) is a common and
potentially fatal infection of the lungs caused by bacteria or
other pathogens (1). Accumulating
studies demonstrate that patients who survive pneumonia have up to
a 4-fold greater risk of cardiovascular disease (CVD) (i.e.,
myocardial infarction or stroke) in the first 30 days post
infection; however, the risk of CVD complications can remain
elevated ~2-fold for up to 10 years post-infection (2-5).
While the mechanisms for the increased risk of CVD observed
following CAP are unclear, it is also not known if pre-existing CVD
(such as atherosclerosis) can modify the response of an individual
to pneumonia infection. Studies indicate that dysregulated
inflammatory activity related to CAP could be associated with the
progression of atherosclerosis and its CVD complications (2,6,7).
Recent data suggest that pre-existing atherosclerotic inflammation
can modulate pulmonary immunity or the immunopathogenesis of
respiratory infections (8,9). Fang et al demonstrated that
hypercholesterolemia induced by 12-16 weeks of a high fat diet
(HFD) in wild-type C57BL/6J mice caused low grade pulmonary
inflammation mediated by the activation of the toll-like
receptor/nuclear factor kappa-light-chain-enhancer of activated B
cells (TLR/NF-κB) pathways (8).
Similarly, Ouyang et al showed that
ApoE-/- mice on a HFD have increased pulmonary
immune cell infiltration at 4 weeks, and increased lung cholesterol
content and production of inflammatory mediators at 12 weeks
(9). Compared to wild-type mice,
the lungs of ApoE-/- mice infected with
Chlamydophilia pneumoniae (C. pneumoniae) were revealed to
have increased levels of interleukin (IL)-10, IL-6 and IL-4 and
reduced cellular infiltration, whereas serum C.
pneumoniae-specific IgG and IgM levels were increased (10).
The present study was conceived with the objective
of investigating the longitudinal immune responses and changes in
lung morphology that occur following induction of pneumonia with
Streptococcus pneumoniae (S. pneumoniae) serotype 4
strain (TIGR4) in ApoE-/- mice and monitoring for
up to 28 days post inoculation (PI). S. pneumoniae was
selected because it is the most prominent cause of CAP in humans
and is associated with significant morbidity and mortality
worldwide (11).
ApoE-/- mice were selected because this is an
already well-established animal model of pre-clinical
atherosclerosis in humans.
Materials and methods
Animals
Two groups of ApoE-/- male mice
aged 6-7 weeks were obtained from the Animal Resources Centre (West
Australia, Australia) and maintained under pathogen-free
conditions. A total of 75 male ApoE-/- mice, aged 6-7
weeks were obtained from the Animal Resources Centre (West
Australia, Australia), housed in pairs and maintained under
pathogen-free conditions. Mice were housed at 21˚C, 41% humidity
and on a standard 12-h light/dark cycle. Animals were acclimated
for 7 days with standard rodent chow before they were transitioned
to a HFD containing 21% fat and 0.15% cholesterol (Fig. 1A and B) to accelerate development of
atherosclerosis (Specialty Feeds). After 8 weeks of a HFD,
atherosclerotic plaques were evident in the aortic arch of
ApoE-/- mice (Fig.
S1), as previously described (12). All animal experiments and procedures
were approved by the local Ethics Committees of Harry Perkins
Institute of Medical Research, Perth, Australia (approval no.
AE114) and the University of Western Australia, Perth, Australia
(approval no. F71731).
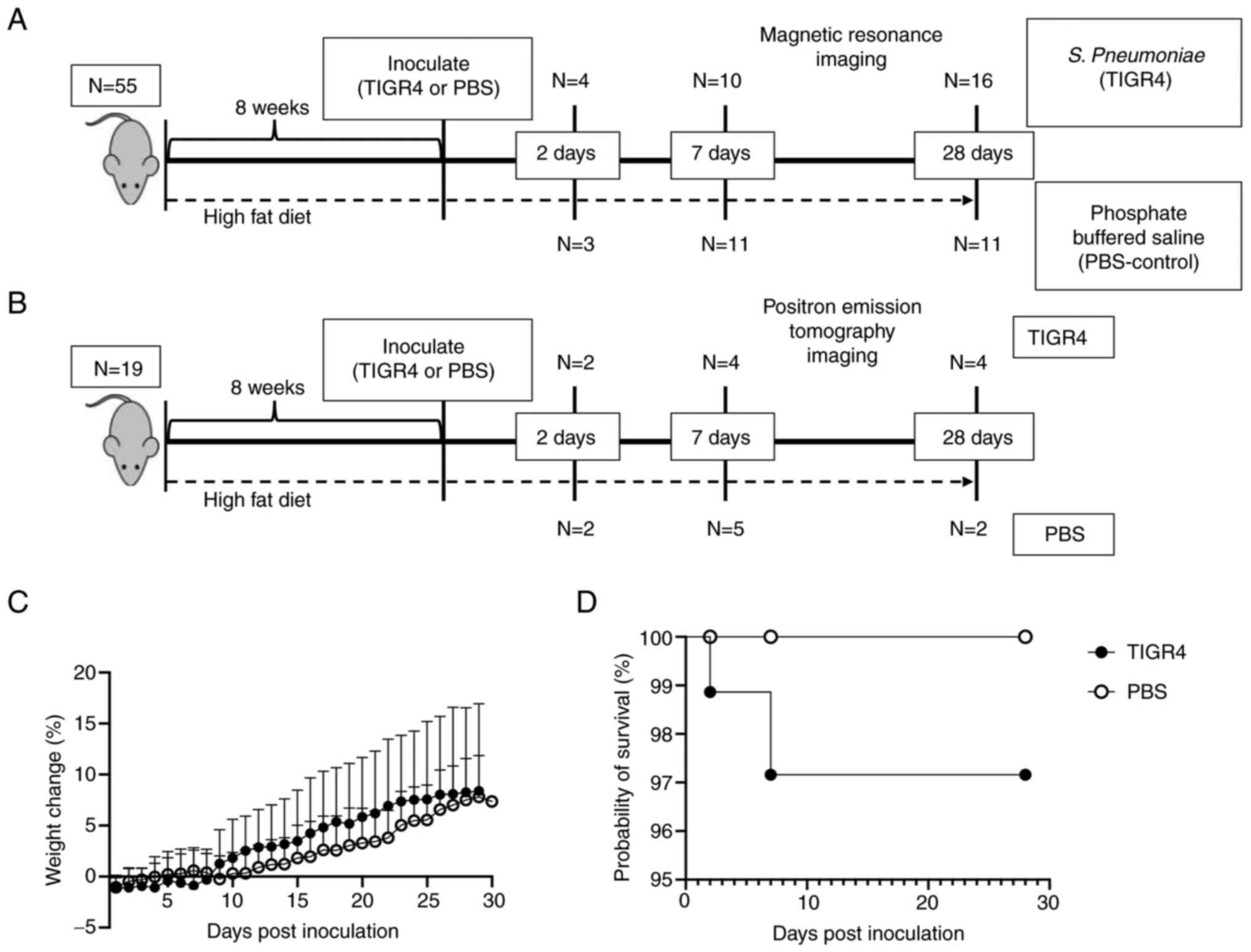 | Figure 1Body weight gain and survival rate.
(A) Experimental flow chart. Mice underwent MRI at specific time
points (2, 7 or 28 days PI). Blood was also collected before the
switch to a HFD, 5 weeks into a HFD, pre-inoculation and at the
study end-point (2, 7 or 28 days PI). (B) In parallel, a group of
19 mice were similarly treated but assigned for PET imaging. Due to
the presence of radiotracer, only blood was collected prior to
inoculation and imaging otherwise no tissues were harvested from
these mice. (C) Weight loss of mice intranasally inoculated with
105 TIGR4 or PBS. (D) Survival curve of mice
intranasally inoculated with TIGR4 or PBS. MRI, magnetic resonance
imaging; PI, post inoculation; HFD, high fat diet; PET, positron
emission tomography; PBS, phosphate-buffered saline. |
Bacterial culture
S. pneumoniae strain TIGR4 (gifted by
Professor Gary Lee, University of Western Australia), was grown
overnight on blood agar plates (with 5% sheep blood) or in brain
heart infusion broth (both from Thermo Fisher Scientific, Inc.) at
37˚C in 5% CO2 until OD600=0.4. Aliquots of
stock cultures in logarithmic growth were frozen in 10% glycerol
(Sigma-Aldrich; Merck KGaA) and stored at -80˚C. Colony forming
units (CFU) were determined by plating on blood agar plates and
measuring the optical density. Bacteria were washed twice and
diluted in phosphate-buffered saline (PBS; Thermo Fisher
Scientific, Inc.) to obtain the appropriate concentrations for the
mouse intranasal inoculation.
Intranasal inoculation
After 8 weeks of a HFD, mice were lightly
anesthetized by inhalation with 3% isofluorane (Provet NZ Pty,
Ltd.) and inoculated intranasally with 105 CFU of
bacteria in a total volume of 40 µl. To determine the final dose,
three factors were considered: i) The dose had to be sufficient to
impose weight loss while allowing the mice the opportunity to
recover from the infection; ii) the survival rate had to be
>80%; and iii) pneumonia had to be detectable by magnetic
resonance imaging (MRI) or positron emission tomography/computed
tomography (PET/CT) imaging. The final dose was within the range of
CFUs from similar mouse studies (104-106 CFU)
(13-17),
with comparable infection and survival rates and without antibiotic
intervention. The dose was initially confirmed in two independent
experiments of 10 mice to confirm the mortality rate and
consistency in MRI imaging. Bacterial inoculation titres were
confirmed by serial dilution and plating on blood agar plates.
Control mice were challenged intranasally with 40 µl PBS. For the
final experiment, a total of 75 mice were utilised: 55 mice were
assigned for MRI imaging (Fig. 1A;
TIGR4, n=30; PBS n=25) and the remaining 20 were assigned to PET
imaging (Fig. 1B; TIGR4, n=11,
PBS=9). Following inoculation, accommodations in temperature (heat
pads) and supplementary fluids (hydration gel) were provided as
necessary. The health of mice was monitored twice daily for the
first 48 h PI and once daily thereafter up until a final time point
of 28-days PI (total maximum experiment duration of 13 weeks).
Health and disease severity were assessed via weight loss and
scoring in any of the following areas: Respiration, body posture,
eye condition, social interaction, and activity. Based on these
criteria and a scoring system developed by the Animal Ethics
Committee at Harry Perkins Institute of Medical Research, the mice
were given a clinical score of healthy (0) to moribund (3). Any mice displaying a score above 3
were immediately humanely euthanized via cervical dislocation in
accordance with our protocol. In total, 3 mice were euthanised due
to severe S. pneumoniae TIGR4 infection and are further
reported in the section Intranasal inoculation and mice
mortality. Euthanasia was confirmed by loss of heartbeat and no
response to foot pinch test.
Bacterial quantification in blood and
lungs
Blood was collected from the tail vein, serially
diluted in PBS and plated on blood agar plates. To determine
bacterial burden in the lungs, supernatants of homogenised lungs
were serially diluted in PBS and plated on blood agar plates.
Plates were incubated for 18-24 h at 37˚C with 5% CO2
and colonies were manually counted.
PET/CT imaging
Mice were fasted from food for 4-6 h (water
available). The animals were warmed for 30 min (at approximately
30˚C) prior to administration of
2-deoxy-2-[fluorine-18]fluoro-D-glucose (18F-FDG). Anaesthesia with
3-5% isoflurane was administered until visible loss of
consciousness and administration of ~20 MBq of 18F-FDG by either
intravenous (IV) or intraperitoneal (IP) injection (volume <200
µl for IV injection). After completion of 1 h uptake, the mice were
sacrificed via cervical dislocation, and PET/CT scan was completed
using Bioscan BioPET/CT 105 camera (Bioscan, Inc.). Mice were
sacrificed prior to imaging to reduce motion artifact from mouse
orientation and movements and to overall improve image quality, and
semi-quantitation. 18F-FDG-PET/CT scans were analysed by Syngio.via
software (VB40; Siemens Healthineers).
MRI imaging
Upon reaching the assigned time point, mice were
sequentially MRI imaged and euthanised. Mice were placed under
anaesthesia with 3-5% isoflurane under warming conditions (~30˚C).
Once the depth of anaesthesia was adequate, the mouse was moved to
the imaging bed where the animal was imaged using the MR Solution
MRI 3T scanner (MR Solutions). Based on the signal and image
quality, a gadolinium-based contrast agent was administered to
improve imaging quality if deemed required by the imaging
technologist. Anaesthesia with isoflurane was maintained during the
scan while respiration and vital signs were monitored remotely.
InVivoScope software (Bioscan, Inc.) was used for image capture
according to the Harry Perkins Cancer Imaging Facility licence.
Measurement of IgG levels by
ELISA
S. pneumoniae TIGR4 was cultured in brain
heart infusion broth, harvested at mid-log phase and washed twice
with PBS. Nunc MaxiSorp 96 well plates (Thermo Fisher Scientific,
Inc.) were coated overnight at 4˚C with 105 CFU per well of whole
TIGR4 pneumococci in 100 µl of PBS. Plates were washed three times
with 0.05% Tween/PBS and blocked for 1 h in blocking buffer (PBS
with 5% skim milk) at room temperature. Serial 2-fold dilutions of
serum in 2% skim milk/PBS were added to the well and incubated for
2 h at room temperature. After washing, pneumococcal-specific
antibodies were detected by incubating with HRP-conjugated goat
anti-mouse IgG Ab (cat. no. PA1-7259; Thermo Fisher Scientific,
Inc.) diluted 1:10,000 in 2% skim milk/PBS for 1 h in the dark at
room temperature. After washing five times with wash buffer,
3,3',5,5'-tetramethylbenzidine substrate (Sigma-Aldrich; Merck
KGaA) was added and colour development was stopped after 15 min by
the addition of H2SO4. Plates were read at
450 nm using an Omega plate reader (BMG Labtech). The cut-off
values were defined using average plus three standard
deviations.
Histological analysis
Whole lungs were harvested and embedded in
Tissue-Tek OCT (ProScitech) and immediately frozen to prevent
tissue damage. Samples were stored at -80˚C. Subsequently, 10-µm
sections were cut, air-dried and stained at room temperature unless
otherwise described. Hematoxylin and eosin (H&E; cat. no.
ab245880; Abcam) was completed as per the manufacturer's
instructions. Briefly, sections were fixed in 100% MeOH for 3 min
before staining with hematoxylin for 5 min, 2X ddH2O
wash, ~12 sec in bluing reagent, 2X ddH2O wash, dipped
in 100% EtOH, counterstained for 3 min in eosin and rinsed in 100%
EtOH. Trichrome staining (cat. no. ab150686; Abcam) of the frozen
sections was also performed according to the manufacturer's
instructions with slight modification. The collagen stain, aniline
blue, was replaced with 0.5% fast green in 70% EtOH (Sigma-Aldrich;
Merck KGaA). This allowed for red, green and blue colour staining
and subsequent colour deconvolution using ImageJ software (v1.53k;
National Institutes of Health, Inc.) (18). Briefly, trichrome staining was
completed following 3 min of MeOH fixing. First, the slides were
incubated for 1 h in Bouin's fluid (included in trichrome staining
kit, aforementioned) pre-heated to 54-64˚C, air cooled for 10 min,
washed in ddH2O until clear and incubated in Weigert's
Iron Hematoxylin (included in H&E kit, aforementioned) for 5
min. Slides were rinsed in ddH2O, stained with Biebrich
Scarlet (included in trichrome staining kit, aforementioned) for 15
min, rinsed in ddH2O, differentiated in phosphomolybdic
acid for ~12 min, incubated in fast green for ~7 min, rinsed in
ddH2O and incubated in acetic acid for 3-5 min. H&E
and trichrome-stained sections were mounted in aqueous mounting
medium (Fronine Pty, Ltd.). Sections were imaged using a Nikon
Eclipse TE2000-U microscope (Nikon Corporation).
Quantification of gene expression by
reverse transcription-quantitative polymerase chain reaction
(RT-qPCR)
Ribonucleic acid (RNA) from lungs was extracted
using TRIzol reagent (Thermo Fisher Scientific, Inc.) and reverse
transcribed to complementary deoxyribonucleic acid (cDNA) using the
Tetro cDNA Synthesis Kit (Meridian Bioscience) according to the
manufacturer's recommendations. Messenger RNA (mRNA) levels of
GAPDH (Mm99999915_g1), HPRT (Mm00446968_m1),
IL-6 (Mm00446190_m1), tumor necrosis factor-α (TNFA)
(Mm00443258_m1), IL-1β (Mm00434228_m1), interferon-γ
(IFN-γ) (Mm00801778_m1), IL-10 (Mm00439614_m1),
IL-17A (Mm00439618_m1), IL-18 (Mm00434225_m1), NLR
family pyrin domain containing 3 (NLRP3) (Mm00840904_m1),
chemokine (C-C motif) ligand 2 (CCL2) (Mm00441242_m1),
chemokine (C-C motif) receptor 2 (CCR2) (Mm00438270_01),
SMAD family member 7 (SMAD7) (Mm00484742_m1), P2X
purinoreceptor 7 (P2X7) (Mm00440582_m1) and tumor growth
factor-β (TGF-β) (Mm01178820_m1) were determined by
quantitative PCR using Taqman primers and probe and Taqman Gene
Expression Master Mix (all from Applied Biosystems; Thermo Fisher
Scientific, Inc.) on a Rotorgene 6000 (Qiagen, Inc.). Cycling
conditions for TaqMan PCR were 2 min at 50˚C and 10 min at 95˚C
followed by 45 cycles of 15 sec at 95˚C and 1 min at 60˚C. Samples
were performed in triplicate. Data were analysed on the basis of
the relative expression method with the formula relative expression
2-ΔΔCq, where the amount of the target gene was
normalized first to the endogenous reference (HPRT and GAPDH) and
then relative to a calibrator (control animal) (19).
Determination of the serum levels of
soluble proteins by multiplex Luminex Assay
Blood was collected from the tail vein and
centrifuged at 541 x g for 15 min at room temperature. Serum was
collected and stored at -80˚C until use. Levels of the following
soluble proteins: TNF-α, IFN-γ, IL-6, IL-1β, IL-5, IL-10, IL-17,
CCL3, dickkopf (Dkk)-1 and matrix metalloproteinase (MMP)-12 were
assayed using the multiplex Mouse Magnetic Luminex Assay (cat. no.
LXSAMSM; R&D Systems, Inc.) according to the manufacturer's
instructions. Quantification of proteins were determined using the
Luminex 200™ System (Thermo Fisher Scientific Inc.) via xPONENT
software 4.3 (Luminex, A DiaSorin Company). The levels of detection
for each analyte are: CCL3, 0.45 pg/ml; Dkk-1, 31.8 pg/ml; IFN-γ,
1.85 pg/ml; IL-1β, 41.8 pg/ml; IL-5, 0.24 pg/ml; IL-6, 2.30 pg/ml;
IL-10, 8.20 pg/ml; IL-17, 7.08 pg/ml; MMP-12, 0.42 pg/ml; and
TNF-α, 1.47 pg/ml.
Statistical analysis
Data are expressed as the median (range). All
statistical tests were performed with GraphPad Prism 7 (GraphPad
Software, Inc.). The Kaplan-Meir method was used to compare
survival rates. Mann-Whitney was used to compare two groups.
Differences with P-values <0.05 were considered statistically
significant.
Results
Intranasal inoculation and mice
mortality
To investigate the impact of the selected inoculum
of S. pneumoniae on the mortality of mice with
atherosclerosis, ApoE-/- mice (n=74) were
intranasally inoculated with either 105 CFU of S.
pneumoniae (n=40) or PBS (control, n=34 after 8 weeks on a HFD
(Fig. 1A and B). Most TIGR4-inoculated mice developed
minor symptoms of infection including ruffled fur, reduced
activity, enlarged nasal vestibule, arched backs and squinted eyes
during the first 48-72 h PI. Despite a subtle change in weight loss
(Fig. 1C), there was no significant
evidence of higher mortality (Fig.
1D) in infected compared to uninfected
ApoE-/- mice. A total of three mice succumbed to
pneumococcal infection. The first mouse succumbed on day 2 PI and
cultures of its blood and liver tissue yielded 8.0x105
CFU/ml and 5.3x105 CFU/ml of S. pneumoniae,
respectively. The second succumbed on day 7 PI and while blood
culture yielded no bacterial growth, cultures of lung and liver
tissues yielded 3.7x105 CFU/ml and 3.3x105
CFU/ml of S. pneumoniae, respectively. The third mouse
succumbed 2 days PI, yielding 4.3x105 CFU/ml growth in
the lung and 3.7x105 CFU/ml growth in the liver.
Macroscopic pathological features (e.g., hemorrhagic and mottling)
of the lungs and spleen are shown in Fig. S1B and C. Bacterial growth and morphology are
represented in Fig. S1D. These
results confirmed that intranasal inoculation of 105 CFU
bacteria produced mild mortality in ApoE-/- mice,
with >95% survival.
Intranasal inoculation with S.
pneumonia and bacterial burden in tissues and blood
Blood and lung tissues were collected at 2, 7 and 28
days PI. No bacteria were recovered from the lungs and there was no
evidence of bacteraemia in infected ApoE-/- mice
that survived until the endpoints of the study. This observation is
in line with previous research of low dose pneumonia in C57Bl/6
mice. Sender et al recovered only 2% of bacteria via
bronchoalveolar lavage 6 h post-intratracheal injection of
105 TIGR4 bacteria in C57Bl/6 mice (20).
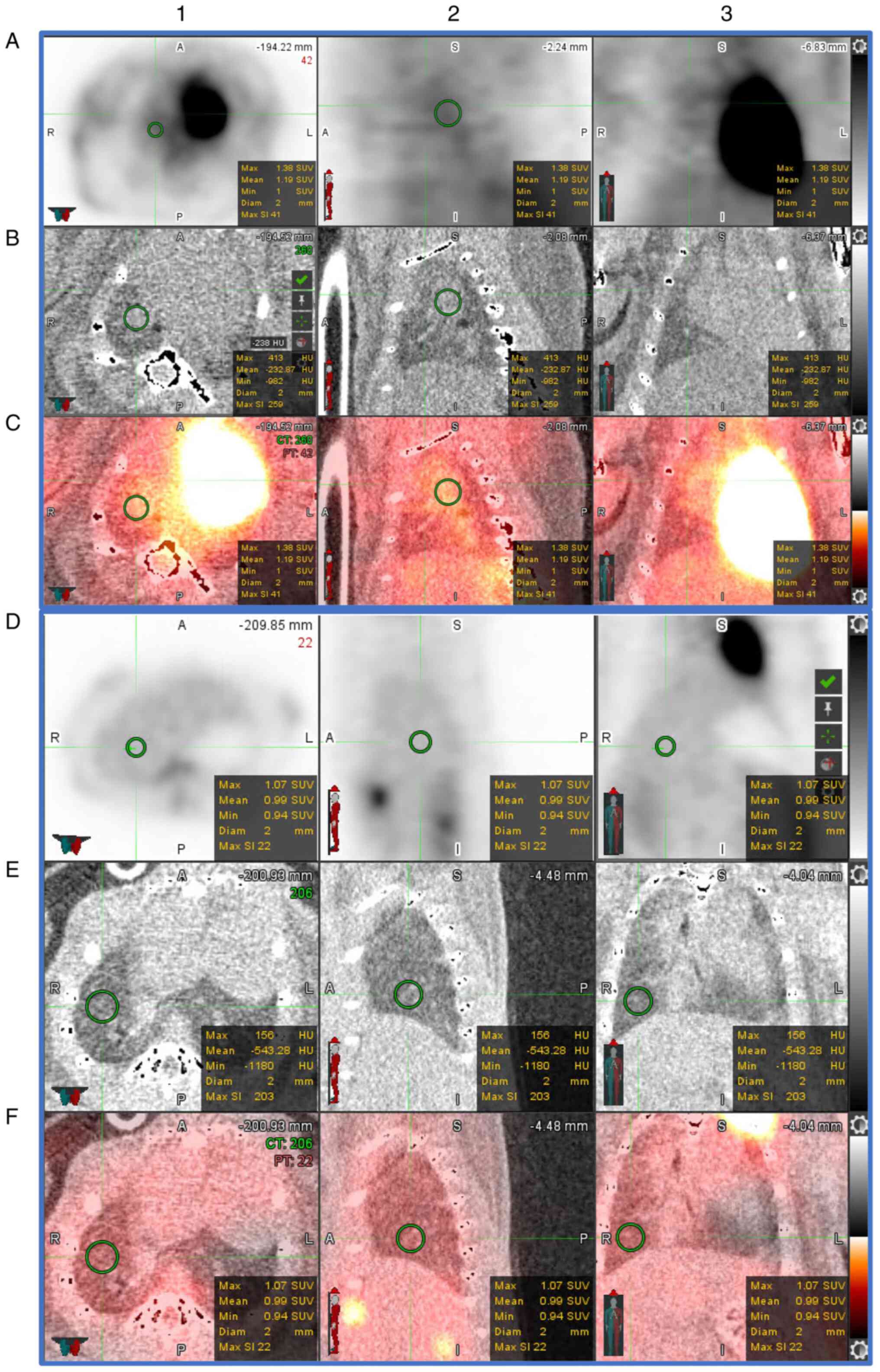
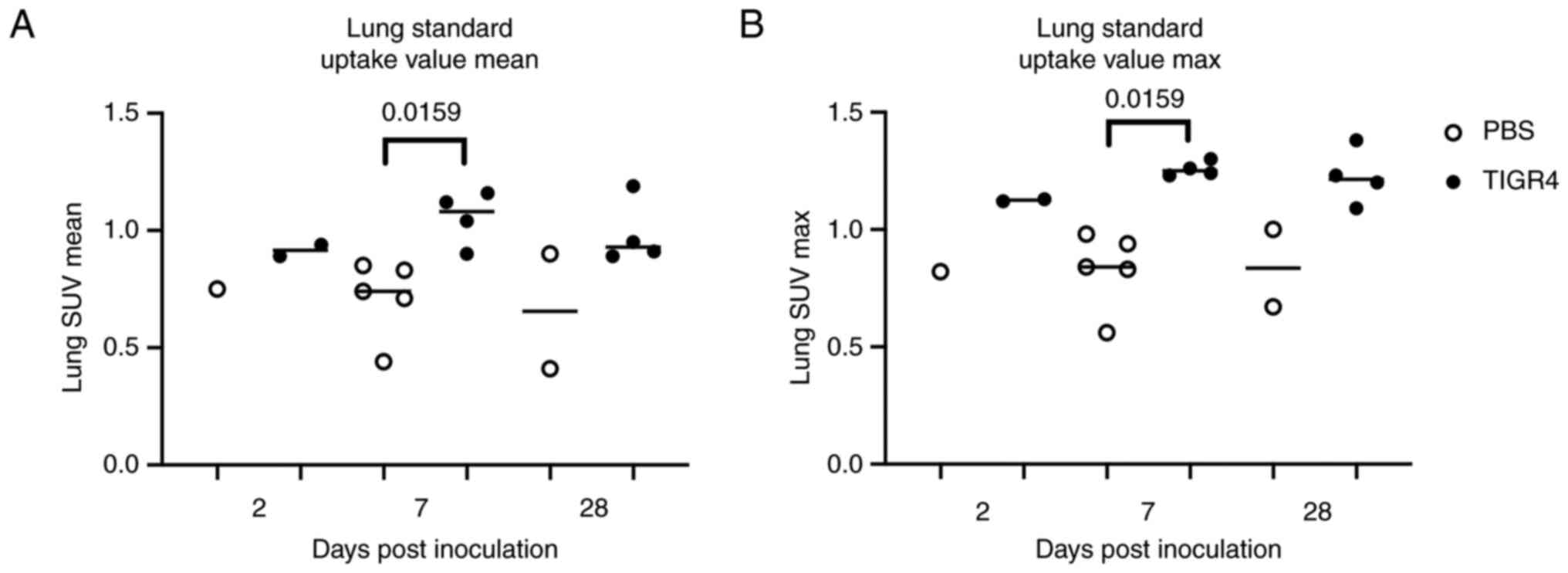
Ongoing inflammation on FDG PET/CT
imaging
Evaluation of mouse lung PET/CT scans was performed
by a level 3 trained nuclear medicine specialist (SV), who was
blinded to the study groups and designations. Representative PET/CT
scans are presented in Fig. 2. In
line with previous PET imaging in patients with pneumonia (21), of the 20 mice assigned to PET
imaging, all 11 mice that received an intranasal dose of TIGR4
bacteria presented with increased FDG uptake [standardised uptake
value (SUV)] in the lungs compared to PBS-inoculated mice (n=8) at
7 days PI (Fig. 3A and B). Of note, at 7-days PI there was a
significant increase (P=0.0159 at 7 days) in both the average and
maximum lung SUVs in TIGR4-inoculated mice compared to PBS. In
addition, one PBS mouse was excluded from the study due to the
presence of a genetic heart defect.
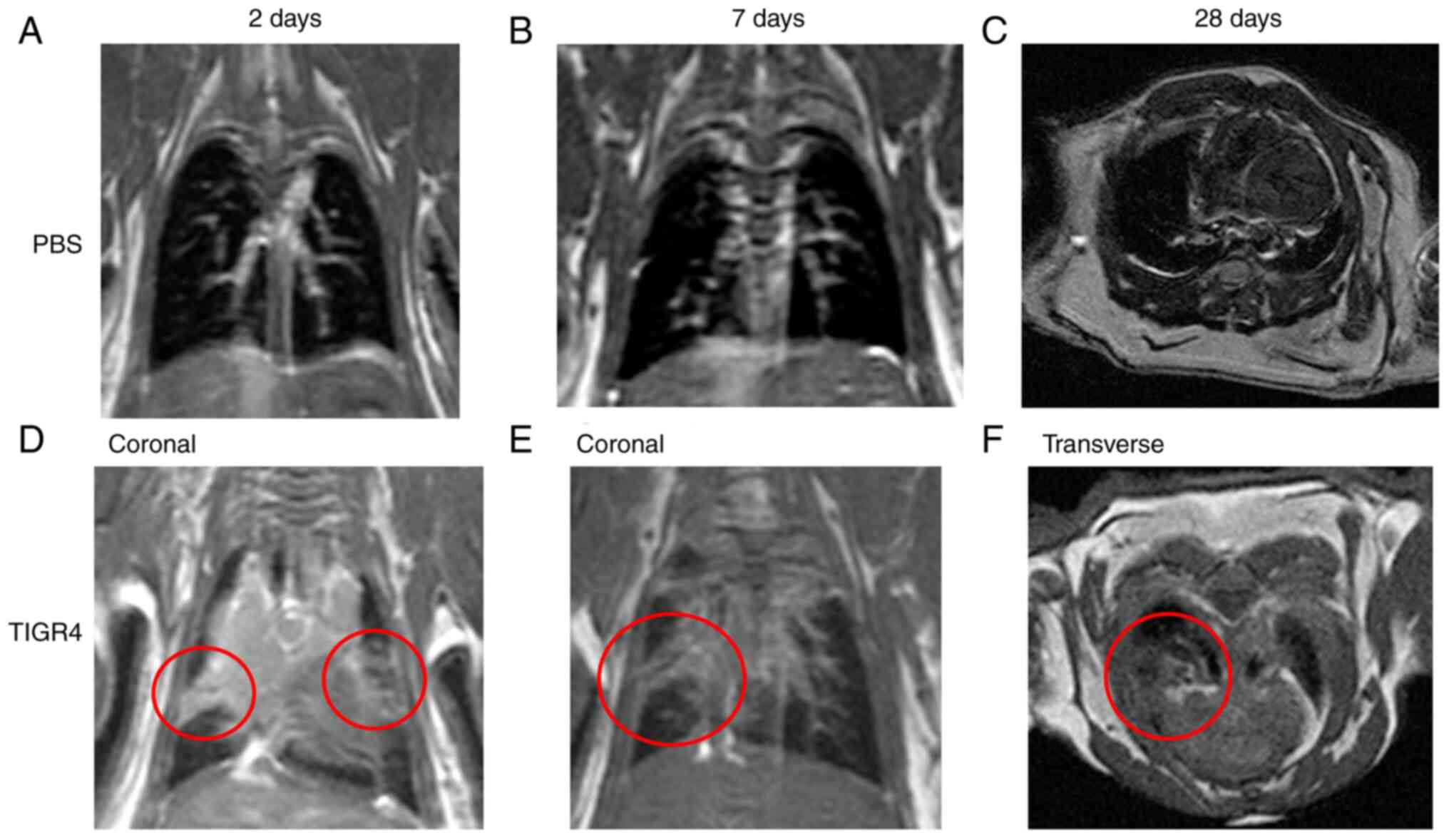
Intranasal inoculation of S.
pneumoniae and lung changes on MRI
Evaluation of mouse lungs was performed by a level 3
trained radiologist (TS), who was blinded to the study groups and
designations. The two mice that succumbed to the TIGR4 infection
were not imaged. Of the four TIGR4-inoculated mice that underwent
MRI at 2 days PI, three presented radiological findings consistent
with lung infection including pleural effusion, consolidation, and
various degrees of lung infiltration. In total, 4/8 (50%)
TIGR4-inoculated mice scanned at 7 days PI, and 6/15 (40%)
TIGR4-inoculated mice scanned at 28 days PI presented with the
characteristics described above. None of the PBS-inoculated mice
presented with any lung radiological abnormalities that would
suggest a respiratory infection. Hence, across all time points, 13
of the 27 mice inoculated with S. pneumoniae presented with
radiological findings suggestive of pulmonary infection (Fig. 4). The weight the mice that were
positive for lung infection were compared to the weights of the
remaining TIGR4 mice (Fig.
S2K).
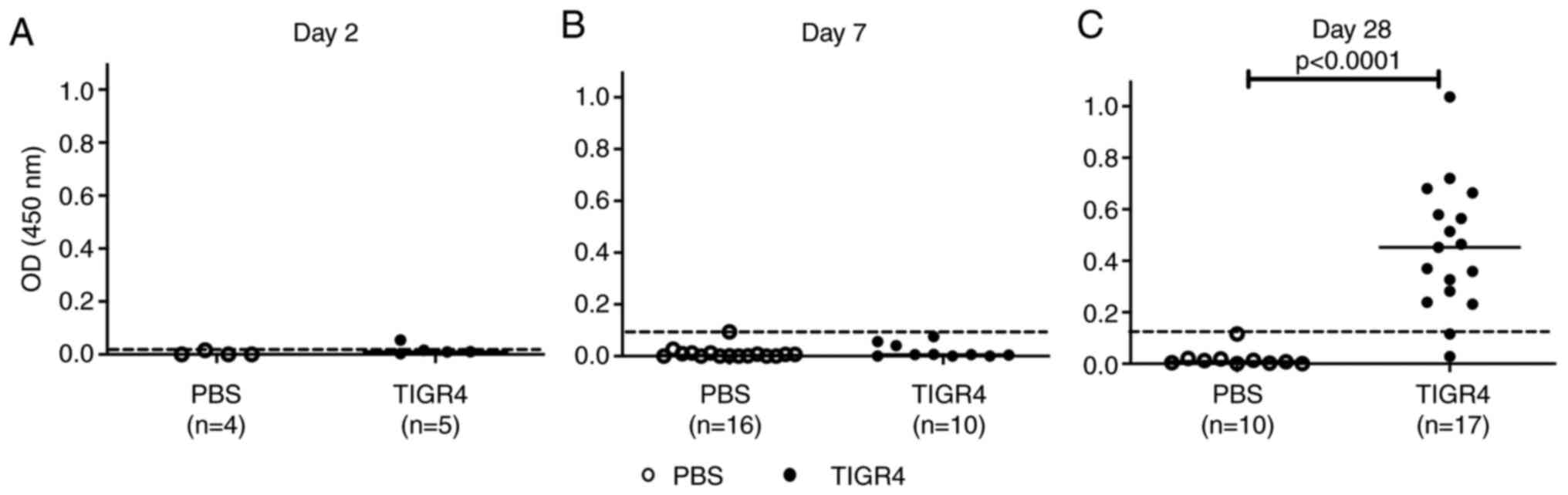
Pneumococcal-specific IgG antibody
responses
At 2 days PI, one TIGR4-inoculated mouse produced a
low level of pneumococcal-specific IgG antibody (Fig. 5A). No antibody responses were
recorded in TIGR4-inoculated mice at 7 days PI (Fig. 5B). By contrast, 15/17 (88%)
TIGR4-inoculated mice developed an antibody response (Fig. 5C; P<0.0001) at 28 days PI.
Pneumococcal-specific IgG antibodies were not detected in any of
the PBS-inoculated animals. These results are consistent with IgG
antibody kinetics to S. pneumonia infection (22) and demonstrated that the inoculating
dose selected for this study was sufficient to elicit
antigen-specific antibody responses.
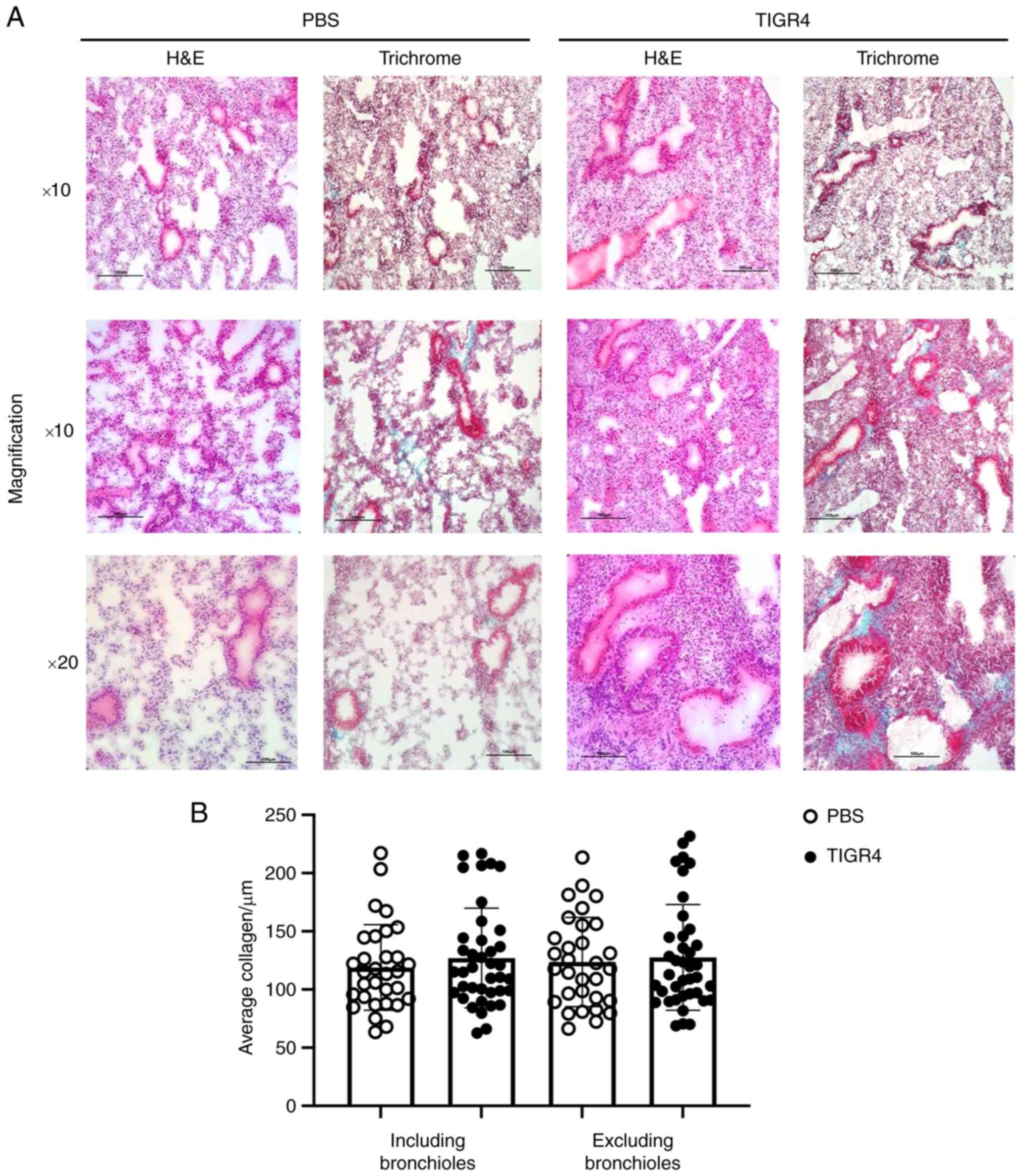
Lung pathology
H&E staining revealed lung remodelling
consistent with previous studies in ApoE-/- mice
(23,24), however this appeared exacerbated in
TIGR4-inoculated mice. Progressively, alveolar space and nuclei
density increased 2-, 7- and 28-days PI, with the greatest
difference observed at 28 days PI (Fig.
6A). Compared to PBS-inoculated animals, TIGR4-inoculated mice
demonstrated increased alveolar septal thickness, suggestive of
increased immune cell infiltration. Despite increased lung density
and infiltration, there was no significant difference in collagen
presence detected in the lungs of TIGR4- and PBS-inoculated mice
(Fig. 6B).
Increased IL-1β and IL-6 gene
expression in the lungs of ApoE-/- mice infected with S.
pneumoniae
IL-1β gene expression was significantly
increased in the lungs of TIGR4-inoculated mice compared to control
mice at 7 days PI (P=0.002; Fig.
7A). IL-6 gene expression was also significantly higher
in TIGR4-inoculated mice at 28 days PI (P=0.01; Fig. 7B). There was also a trend for
elevated IL-1β (P=0.09) and TNF-α (P=0.11) gene
expression in TIGR4-inoculated mice at 28 days PI (Fig. 7A and D). By contrast, a trend towards decreased
IFN-γ gene expression (P=0.09) was evident in
TIGR4-inoculated mice at 7 days (Fig.
7E). There was no difference in TGF-β gene expression in
lung tissues between TIGR4- and PBS-inoculated mice at any of the
time points (Fig. 7C), confirming
the immunohistochemistry collagen results. Gene expression for
CCR2, CCL2, IL-10, IL-17, IL-18, NLRP3, P2X7 and
SMAD7 were similar in TIGR4- and PBS-inoculated mice
(Fig. S3). Mice positive for
respiratory infection confirmed by MRI were compared to the
remaining TIGR4 mice, and exhibited an increasing trend for
IL-6, TNF-α, IL-1, NLRP3 and CCL2 at 7 days before
decreasing at 28 days PI (Fig.
S2). The results indicated residual local inflammatory
dysregulation in mice inoculated with S. pneumoniae despite
no bacterial burden in the lungs.
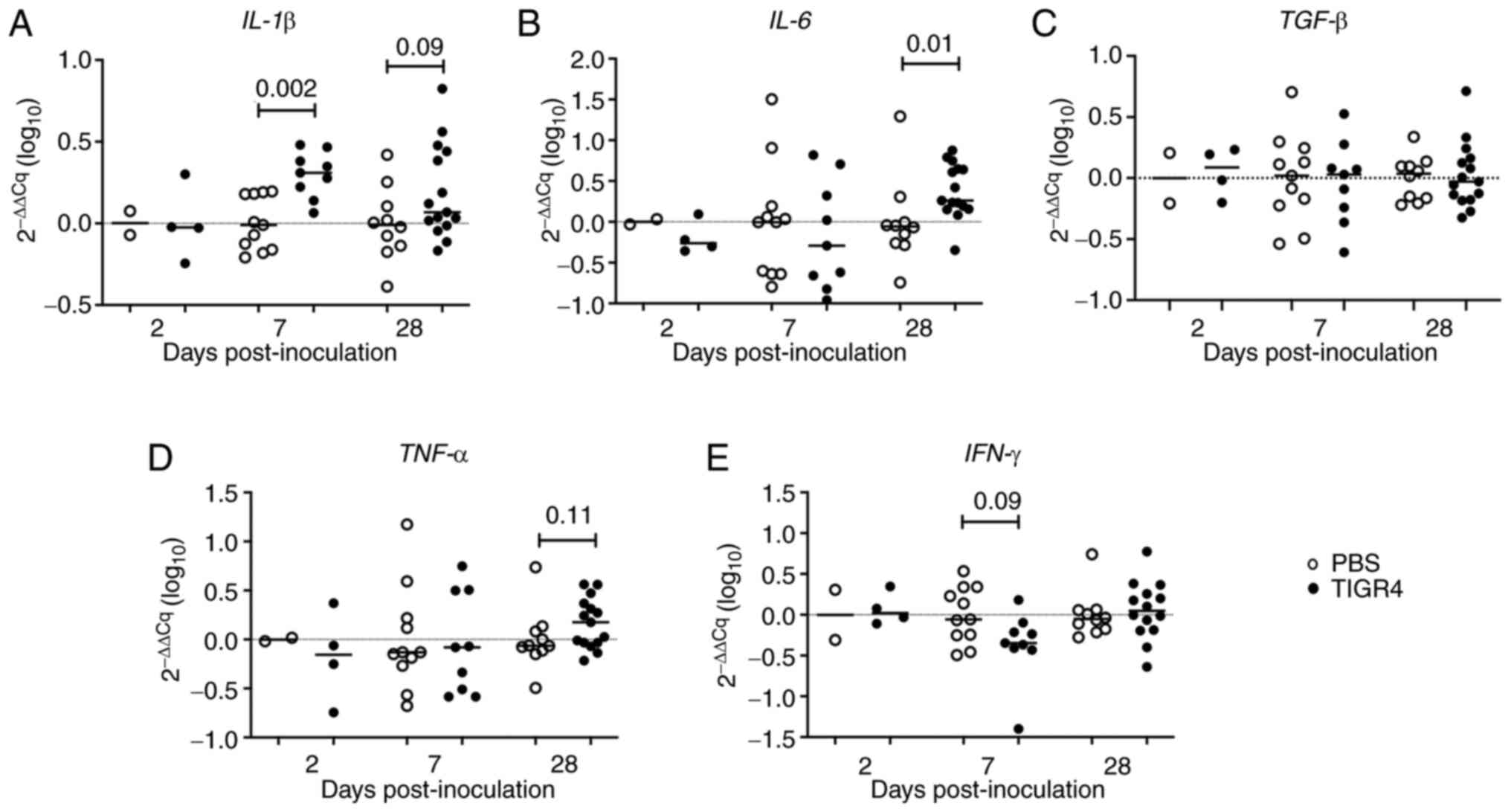 | Figure 7Gene expression of inflammatory
mediators in the lungs. Phosphate-buffered saline control or
TIGR4-inoculated mice were sacrificed at specific time points and
RNA was extracted from lung tissues. mRNA expression levels of (A)
IL-1β, (B) IL-6, (C) TGF-β, (D) TNF-α,
and (E) IFN-γ were quantified by real-time polymerised chain
recation PCR and normalised against two housekeeping genes (GAPDH
and HPRT). The solid horizontal line represents the median and the
dotted line represents no change in gene expression. RNA,
ribonucleic acid; PBS, phosphate-buffered saline; IL-1β,
interleukin-1β; IL-6, interleukin-6; TGF-β, tumor
growth factor-β; TNF-α, tumor necrosis factor; IFN-γ,
interferon-γ. |
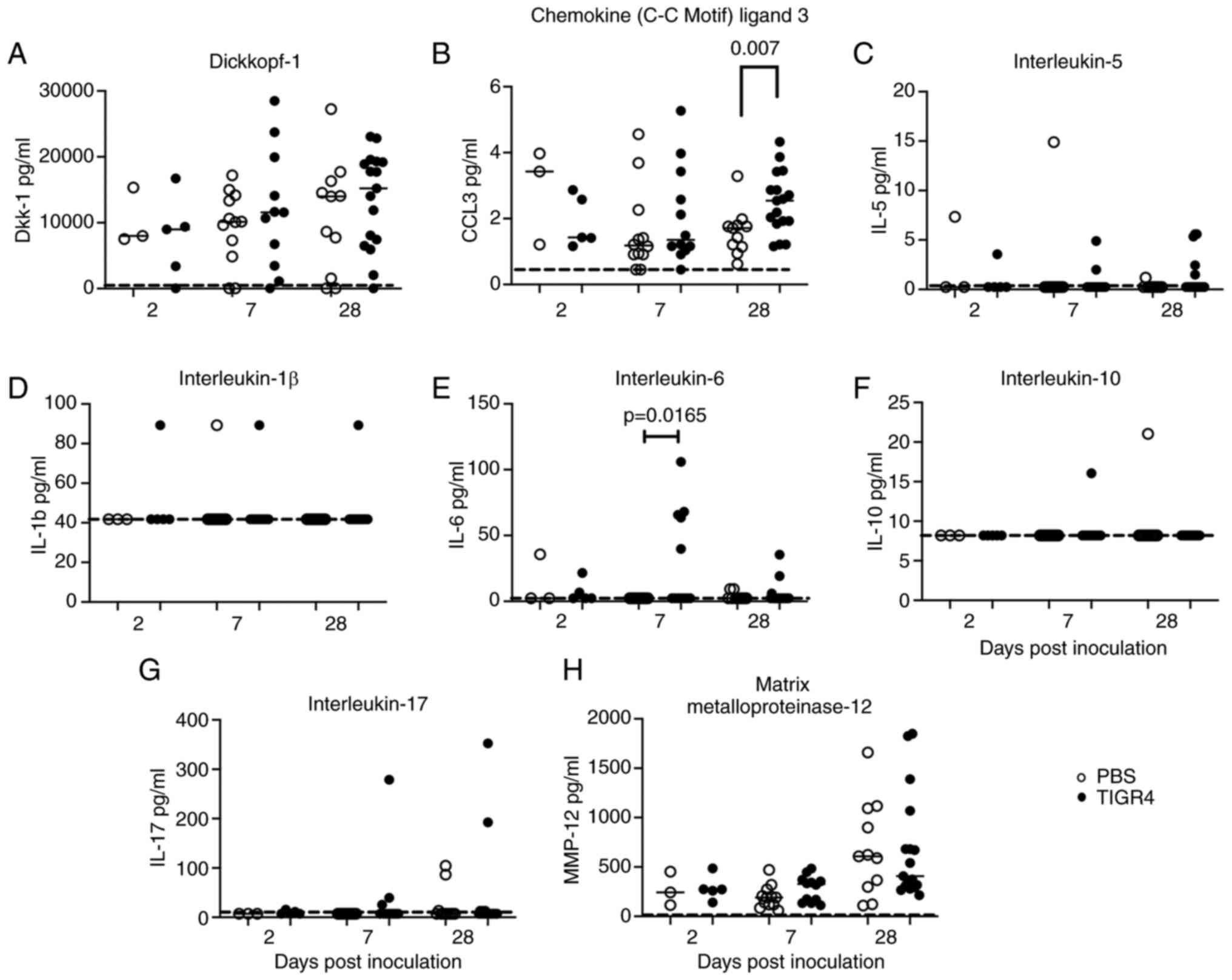
Increased circulating levels of IL-6
and CCL3 in ApoE-/- mice infected with S.
pneumoniae
There were no significant differences observed in
the serum levels for any of the soluble proteins at 2 days PI
between TIGR4- and PBS-inoculated mice. At 7 days PI, levels of
IL-6 were significantly higher in TIGR4-inoculated mice compared to
mice inoculated with PBS (Fig. 8E;
P=0.0165). At 28 days PI, CCL3 levels were significantly higher in
TIGR4-inoculated mice compared to mice inoculated with PBS
(Fig. 8B; P=0.007). No differences
were observed for levels of Dkk-1 and MMP-12 between TIGR4- and
PBS-inoculated mice at 7- and 28-days PI (Fig. 8A and H). In most samples, IL-5, IL-1β, IL-10 and
IL-17 were below the levels of detection (Fig. 8C, D,
F and G), whereas TNF-α and IFN-γ were
undetectable for all the mice (data not shown). The results
indicated ongoing heightened systemic inflammatory activity in mice
inoculated with S. pneumoniae despite clearance of bacterial
infection.
Discussion
To the best of our knowledge, this is the first
study to establish a longitudinal model of S. pneumoniae
infection without antibiotic rescue to explore the pulmonary and
systemic pathology in ApoE-/- mice beyond the
acute phase of the infection and well into the clinical recovery of
surviving animals.
It has been proposed that the introduction of a
respiratory infection leads to a mounting synergistic inflammatory
response that could lead to adverse cardiovascular events. In CAP,
exposure of alveolar epithelial cells and resident macrophages to
S. pneumoniae stimulates the production of inflammatory
cytokines such as IL-1β, IL-6 and TNF-α, as well as chemokines
(25-28).
The duration and magnitude of the inflammatory response have been
linked to disease severity and clinical outcome. In a longitudinal
study of 247 hospitalised patients with CAP, cytokine and chemokine
levels were highest at hospital admission and while most decreased
at 6-week follow-up, some markers remained elevated (29), suggesting ongoing residual
inflammation. This was confirmed in a recent study of 22 CAP
survivors, with 68% of subjects displaying increased
18FDG uptake in the lungs, 30 to 45 days after their
hospital discharge (21).
In agreement with these observations,
TIGR4-inoculated mice displayed delayed elevated inflammatory
response in the lungs (e.g., IL-6 at 28 days PI) and systemically
(e.g., CCL3 at 28 days PI). Interestingly, systemic levels of IL-6
peaked at 7 days PI. IL-6 plays an important role in linking innate
to an acquired immune response by promoting differentiation of
naïve CD4+ T cells (30). IL-6 can serve as an activator for
other pro-inflammatory cytokines including IL-1 and TNF-α (31).
In response to an infection, IL-6 stimulates a range
of signalling pathways including NF-κB, enhancing the transcription
of the mRNA of inflammatory cytokines including IL-6, TNF-α, and
IL-1β. TNF-α and IL-1β in turn also activate transcription factors
to produce more IL-6(32).
A study published by Bacci et al correlated
increased IL-6 and TNF-α with worse outcomes in patients with
pneumonia (31). While TNF-α and
IL-6 gene expression in the lungs of TIGR4-inoculated mice at 28
days was not statistically significant, results do appear to show
an upward trend. This could be attributed to the low TIGR4 dose,
delivery and/or efficacy of the immune system response. Regardless,
patients discharged with pneumonia leave hospital with ongoing
subclinical inflammation (33), and
in an ApoE-/- model of bacterial tuberculosis
(34), there is a delayed adaptive
immune response. Therefore, it is possible in the present study
that not enough time PI had lapsed and a later time point may be
worth investigating to elucidate long-term pneumonia-mediated
inflammatory changes.
Neutrophils play a key role in response to
controlling a pneumococcal pneumonia infection (35). CCL3 acts as a chemotactic factor for
different leukocyte subsets and has also been shown to increase
during development of atherosclerotic lesions (36,37).
It has been shown that during acute inflammation triggered by
lipopolysaccharide stimulation, CCL3 induces chemotaxis for
neutrophils to atherosclerotic lesions (37). The data in the present study
revealed the induction of CCL3 in response to an acute infection
that may support atherosclerosis disease progression (Fig. 8B). Altogether there is great overlap
in immune response to atherosclerosis and pneumonia, sharing a lot
of the same immune pathways that could be pathologically relevant
for atherosclerotic progression.
Previously associated with lung fibrosis, epithelial
cell proliferation, acute lung inflammation, increased
atherosclerotic apoptosis and enlarged and destabilized plaques,
Dkk-1 has been identified in both a respiratory infection and
atherosclerosis setting (38,39,40).
Due to the majority of Dkk-1 being platelet-produced and its
involvement in atherosclerosis, it has been recently suggested as
an independent risk factor for major adverse cardiovascular events
(40). A progressively increasing
median of Dkk-1 was measured in TIGR4-inoculated mice compared to
PBS (Fig. 8A).
In terms of lung fibrosis, a HFD and deletion of the
ApoE gene can induce morphological changes to the lungs
including lipidosis, increased pulmonary inflammation and an
increase in alveolar septal thickness (9,24,41).
These are consistent with findings in this model, with altered lung
morphology in PBS-inoculated mice in response to
hypercholesterolemia, and TIGR4-inoculated mice presenting with
more severe remodelling as a result of inflammatory infiltrate
caused by TIGR4 inoculation. The lungs of TIGR4-inoculated mice
exhibited increased nuclei and inflammatory gene expression
indicating an elevated sublethal amount of inflammation. While no
difference in systemic Dkk-1 or lung collagen was detected, it
could be argued that S. pneumoniae affects specific collagen
groups and overall staining of the classical fibrillar and
network-forming collagen may not be entirely representative of the
disease state. Moreover, the novel Dkk-1 biomarker requires further
investigation to validate its clinical relevance in pneumonia and
role in prediction of adverse events.
To date, few alternative multiple comorbidities
animal models of acute pneumonia infection and atherosclerosis have
been described (13,17,42-45),
and a model of unstable plaque (46). In contrast to the model in the
present study, these other models utilise surgical intervention
(46), viral pathogens (e.g.,
influenza virus) (43), alternative
routes of infection (e.g., intraperitoneal) that are more relevant
to sepsis than pneumonia (44),
intervene with antibiotics to improve mortality (13,17),
or do not describe the lung pathology associated with the
infectious challenge (13,17). The model of the present study
utilises a low infectious dose to establish infection with S.
pneumoniae, the most important bacterial cause of pneumonia in
humans (25,47,48),
via intranasal inoculation; hence, being representative of the
pneumococcal infection that is acquired via the respiratory route
in humans. Additionally, the respiratory infection was
characterised via MRI and PET imaging modalities. The method used
in the present study produces a model with a range of pathology
that includes no detectable illness, subclinical disease, clinical
infection, and infection-induced death thus resembling the full
scope of pathology produced by exposure to respiratory pathogens in
humans. Finally, by foregoing antibiotics, no putative additional
confounder was introduced from such bioactive compounds (for
example, some antibiotics such as macrolides, tetracyclines and
fluoroquinolones can exert important anti-inflammatory activity)
(49-51).
There are some limitations associated with the
present study. Firstly, the cellular immune responses in the lungs
that may have shed light on the lack of bacterial recovery from the
lung, were not investigated. As a low dose of TIGR4 bacteria was
administered to achieve low mortality in the animal model, it is
likely that the bacteria were cleared quickly by the immune system
precluding us from bacterial recovery from lungs. Indeed, the
pre-existing inflammatory response activated by atherosclerosis may
have helped to clear the low intranasal dose of S.
pneumoniae TIGR4. The significant increase in
pneumococcal-specific antibody response observed at 28 days PI is
in agreement with the strong systemic CCL3 response indicative of
exposure to S. pneumoniae supporting this hypothesis,
however this will require further investigation with wild-type mice
used as a control. The absence of the use of wild-type mice as a
control is a limitation of the present study and should be
addressed in future investigations.
Overall, the TIGR4 strain, used in studies by the
authors, is an invasive strain that has been shown to cause
pneumonia and lethal systemic disease following intranasal
challenge (13-15,17,52).
The utility of MRI and PET imaging modalities to diagnose
respiratory infection, while somewhat limited with resolution in
murine studies, are sufficient and valuable in supporting the
diagnosis and investigation of respiratory and vascular disease
(53).
In summary, the ApoE-/- mouse
model of the present study utilising an intranasal inoculation of
S. pneumoniae offers a representation of pneumonia infection
in humans with subclinical atherosclerotic disease. The model is
relevant to the study of pneumonia infections and characterisation
of lung and systemic changes that occur following an episode of
pneumonia in the context pre-existing atherosclerosis. This
multiple comorbidity murine model will be useful in assessing
cardiovascular risk following exposure to S.
pneumoniae-caused pneumonia (12).
Supplementary Material
Characteristics of TIGR4-inoculated
ApoE-/- mice. (A) Representative images of
atherosclerotic plaques in the aortic arch of
ApoE-/- mice after 8 weeks of a high fat diet.
(B) Morphological changes in the lungs of mice inoculated with
TIGR4 bacteria. (C) Morphological abnormalities of black and white
patches on the spleen of TIGR4-inoculated mice. (D)
Streptococcus pneumoniae serotype 4 (TIGR4) growth on blood
agar plate.
Gene expression of inflammatory
mediators in lungs from TIGR4-inoculated animals stratified based
on radiological findings. TIGR4-inoculated mice with evidence of
respiratory infection confirmed by MRI were compared to mice with
no radiological abnormalities at respective timepoints (2, 7 and 28
days PI). Ribonucleic acid (mRNA) expression levels of (A)
IL-6, (B) TNF-α, (C) IL-1, (D) IFN-γ,
(E) IL-10, (F) IL-17A, (G) NLPR3, (H)
IL-18, (I) CCL2 and (J) P2X7 were quantified
by real-time polymerase chain reaction and normalised against two
housekeeping genes (GAPDH and HPRT). Solid horizontal line
represents the median. (K) Weight loss of TIGR4-inoculated mice
stratified according to the absence or presence of respiratory
infection confirmed by MRI. MRI, magnetic resonance imaging;
IL-6, interleukin-6; TNF-α, tumor necrosis factor-α;
IL-1, interleukin 1; IFN-γ, interferon-γ;
IL-10, interleukin-10; IL-17A, interleukin-17A;
NLPR3, NLR family pyrin domain containing 3; IL-18,
interleukin-18; CCL2, chemokine (C-C motif) ligand 2;
P2X7, P2X purinoceptor 7.
Gene expression of inflammatory
mediators in the lungs. Phosphate-buffered saline (control) or
TIGR4-inoculated mice were sacrificed at specific time points and
RNA was extracted from lung tissues. mRNA expression levels of (A)
CCR2, (B) CCL2, (C) NLPR3, (D) P2X7, (E) SMAD7, (F) IL-17A, (G)
IL-18 and (H) IL-10 were quantified by real-time polymerase chain
reaction and normalised against two housekeeping genes (GAPDH and
HPRT). Solid horizontal line represents the median. RNA,
ribonucleic acid; CCR2, chemokine (C-C motif) receptor 2; CCL2,
chemokine (C-C motif) ligand 2; NLPR3, NLR family pyrin domain
containing 3; P2X7, P2X purinoceptor 7; SMAD7, SMAD family member
7; IL-17A, interleukin-17A; IL-18, interleukin-18; IL-10,
interleukin-10.
Acknowledgements
The authors gratefully acknowledge Mr Lincoln Codd
(Charles Gairdner Hospital, Perth, Australia), Mr Brenton O'Mara
(Charles Gairdner Hospital), Ms Kirsty Richardson (Harry Perkins
Institute of Medical Research, Perth, Australia), Dr Liesl Celliers
(Harry Perkins Institute of Medical Research), Dr Penny Maton
(University of Western Australia, Perth, Australia) and Professor
Roslyn J. Francis (University of Western Australia) for technical
assistance. The authors acknowledge the facilities, and the
scientific and technical assistance of Microscopy Australia at the
Centre for Microscopy, Characterisation and Analysis, The Cancer
Imaging Facility at Harry Perkins Institute of Medical Research,
The University of Western Australia, a facility funded by the
University, State and Commonwealth Governments.
Funding
Funding: The present study was funded by the Harry Perkins
Institute of Medical Research. BB received funding from the
Australia-India Strategic Research Fund (AISRF; grant no.
AIRXIIICO000068) held by GD.
Availability of data and materials
The datasets used and/or analysed during the
current study are available from the corresponding author on
reasonable request.
Authors' contributions
The study was initially conceived by GW, VCM and
GD. The study was developed from a combined effort of BB, SL, HPL,
GW, VCM and GD. All animal work and subsequent experiments were
completed by BB, SL and HPL. MRI and FDG-PET imaging analysis was
completed by TS, SV, GW, VCM and GD. Manuscript preparation was
performed by BB. Revisions and assessment for intellectual content
was completed by SL, HPL, TS, SV, GW, VCM and GD. SL, HPL, TS and
SV confirm the authenticity of all the raw data. All authors read
and approved the final manuscript and agree to be accountable for
all aspects of the research in ensuring that the accuracy or
integrity of any part of the work are appropriately investigated
and resolved.
Ethics approval and consent to
participate
All animal procedures were carried out in
accordance with the Western Australian Animal Welfare Act, National
Institute of Health guidelines and ARRIVE guidelines. The present
study and its procedures were approved by the Animal Ethics
Committee of the Harry Perkins Institute for Medical Research,
Perth, Australia (approval no. AE114) and the University of Western
Australia, Perth, Australia (approval no. F71731). All handling,
procedure and assistance techniques training was provided by the
Bioresources team at the Harry Perkins Institute for Medical
Research centre.
Patient consent for publication
Not applicable.
Competing interests
GD is Wesfarmers Chair in Cardiology at the
University of Western Australia with an Adjunct Professor
appointment at UOHI. GD reports 3 paid lectures from AstraZeneca,
Pfizer, and Amgen not related to the topic in the manuscript. GD
provides consultancy services and also has an equity interest
Artrya Pty Ltd. The other authors (BB, SL, HPL, TS, SV, VFCM and
GW) have nothing to disclose and declare that they have no
competing interests.
References
|
1
|
Asthma chronic obstructive pulmonary
disease and other respiratory diseases in Australia, Summary.
Australian Institute of Health and Welfare https://www.aihw.gov.au/reports/chronic-respiratory-conditions/asthma-chronic-obstructive-pulmonary-disease-and/summary.
|
|
2
|
Corrales-Medina VF, Alvarez KN, Weissfeld
LA, Angus DC, Chirinos JA, Chang CCH, Newman A, Loehr L, Folsom AR,
Elkind MS, et al: Association between hospitalization for pneumonia
and subsequent risk of cardiovascular disease. JAMA. 313:264–274.
2015.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Corrales-Medina VF, Taljaard M, Yende S,
Kronmal R, Dwivedi G, Newman AB, Elkind MSV, Lyles MF and Chirinos
JA: Intermediate and long-term risk of new-onset heart failure
after hospitalization for pneumonia in elderly adults. Am Heart J.
170:306–312. 2015.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Corrales-Medina VF, Taljaard M, Fine MJ,
Dwivedi G, Perry JJ, Musher DM and Chirinos JA: Risk stratification
for cardiac complications in patients hospitalized for
community-acquired pneumonia. Mayo Clinic Proc. 89:60–68.
2014.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Corrales-Medina VF, Musher DM, Shachkina S
and Chirinos JA: Acute pneumonia and the cardiovascular system.
Lancet. 381:496–505. 2013.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Corrales-Medina VF, Musher DM, Wells GA,
Chirinos JA, Chen L and Fine MJ: Cardiac complications in patients
with community-acquired pneumonia clinical perspective: Incidence,
timing, risk factors, and association with short-term mortality.
Circulation. 125:773–781. 2012.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Bartlett B, Ludewick HP, Lee S and Dwivedi
G: Cardiovascular complications following pneumonia: Focus on
pneumococcus and heart failure. Curr Opin Cardiol. 34:233–239.
2019.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Fang Y, Wang S, Zhu T, Zhang Y and Lian X:
Atherogenic high cholesterol/high fat diet induces TLRs-associated
pulmonary inflammation in C57BL/6J mice. Inflamm Res. 66:39–47.
2017.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Ouyang Q, Huang Z, Lin H, Ni J, Lu H, Chen
X, Wang Z and Lin L: Apolipoprotein E deficiency and high-fat diet
cooperate to trigger lipidosis and inflammation in the lung via the
toll-like receptor 4 pathway. Mol Med Rep. 12:2589–2597.
2015.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Nazzal D, Therville N, Yacoub-Youssef H,
Garcia V, Thomsen M, Levade T, Segui B and Benoist H:
Apolipoprotein E-deficient mice develop an anti-chlamydophila
pneumoniae T helper 2 response and resist vascular infection. J
Infect Dis. 202:782–790. 2010.PubMed/NCBI View
Article : Google Scholar
|
|
11
|
Ramos-Sevillano E, Ercoli G and Brown JS:
Mechanisms of naturally acquired immunity to streptococcus
pneumoniae. Front Immunol. 10(358)2019.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Bartlett B, Ludewick HP, Verma S,
Corrales-Medina VF, Waterer G, Lee S and Dwivedi G: Cardiovascular
changes after pneumonia in a dual disease mouse model. Sci Rep.
12(11124)2022.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Bazaz R, Francis S and Dockrell D: 215
increased atherosclerotic plaque macrophage content following
streptococcus pneumoniae pneumonia. Heart. 101:A117–A118. 2015.
|
|
14
|
Ghanem ENB, Maung NHT, Siwapornchai N,
Goodwin AE, Clark S, Muñoz-Elías EJ, Camilli A, Gerstein RM and
Leong JM: Nasopharyngeal exposure to streptococcus pneumoniae
induces extended age-dependent protection against pulmonary
infection mediated by antibodies and CD138+ cells. J Immunol.
200:3739–3751. 2018.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Ritchie ND, Ritchie R, Bayes HK, Mitchell
TJ and Evans TJ: IL-17 can be protective or deleterious in murine
pneumococcal pneumonia. PLoS Pathog. 14(e1007099)2018.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Dallaire F, Ouellet N, Bergeron Y, Turmel
V, Gauthier MC, Simard M and Bergeron MG: Microbiological and
inflammatory factors associated with the development of
pneumococcal pneumonia. J Infect Dis. 184:292–300. 2001.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Bazaz R: The effect of Streptococcus
pneumoniae pneumonia on atherosclerosis (University of Sheffield,
2016).
|
|
18
|
Chen Y, Yu Q and Xu CB: A convenient
method for quantifying collagen fibers in atherosclerotic lesions
by ImageJ software. Int J Clin Exp Med. 10:14904–14910. 2017.
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Sender V, Hentrich K, Pathak A, Ler ATQ,
Embaie BT, Lundström SL, Gaetani M, Bergstrand J, Nakamoto R, Sham
LT, et al: Capillary leakage provides nutrients and antioxidants
for rapid pneumococcal proliferation in influenza-infected lower
airways. Proc Natl Acad Sci USA. 117:31386–31397. 2020.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Corrales-Medina VF, deKemp RA, Chirinos
JA, Zeng W, Wang J, Waterer G, Beanlands RSB and Dwivedi G:
Persistent lung inflammation after clinical resolution of
community-acquired pneumonia as measured by 18FDG-PET/CT imaging.
Chest. 160:446–453. 2021.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Dommaschk A, Ding N, Tarres MT, Bittersohl
LF, Maus R, Stolper J, Jonigk D, Braubach P, Lippmann T, Welte T
and Maus UA: Nasopharyngeal colonization with Streptococcus
pneumoniae triggers dendritic cell dependent antibody responses
against invasive disease in mice. Eur J Immunol. 47:540–551.
2017.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Naura AS, Hans CP, Zerfaoui M, Errami Y,
Ju J, Kim H, Matrougui K, Kim JG and Boulares AH: High-fat diet
induces lung remodeling in ApoE-deficient mice: An association with
an increase in circulatory and lung inflammatory factors. Lab
Invest. 89:1243–1251. 2009.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Massaro D and Massaro GD: Apoetm1Unc mice
have impaired alveologenesis, low lung function, and rapid loss of
lung function. Am J Physiol Lung Cell Mol Physiol. 294:L991–L997.
2008.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Torres A, Cilloniz C, Niederman MS,
Menéndez R, Chalmers JD, Wunderink RG and van der Poll T:
Pneumonia. Nat Rev Dis Primers. 7:1–28. 2021.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Brooks LRK and Mias GI: Streptococcus
pneumoniae's virulence and host immunity: Aging, diagnostics, and
prevention. Front Immunol. 9(1366)2018.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Lagousi T, Basdeki P, De Jonge MI and
Spoulou V: Understanding host immune responses to pneumococcal
proteins in the upper respiratory tract to develop
serotype-independent pneumococcal vaccines. Expert Rev Vaccines.
19:959–972. 2020.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Bartlett B, Ludewick HP, Misra A, Lee S
and Dwivedi G: Macrophages and T cells in atherosclerosis: A
translational perspective. Am J Physiol Heart Circ Physiol.
317:H375–H386. 2019.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Siljan WW, Holter JC, Nymo SH, Husebye E,
Ueland T, Aukrust P, Mollnes TE and Heggelund L: Cytokine
responses, microbial aetiology and short-term outcome in
community-acquired pneumonia. Eur J Clin Invest.
48(e12865)2018.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Dienz O and Rincon M: The effects of IL-6
on CD4 T cell responses. Clin Immunol. 130:27–33. 2009.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Bacci MR, Leme RCP, Zing NPC, Murad N,
Adami F, Hinnig PF, Feder D, Chagas ACP and Fonseca FLA: IL-6 and
TNF-α serum levels are associated with early death in
community-acquired pneumonia patients. Brazi J Med Biol Res.
48:427–432. 2015.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Tanaka T, Narazaki M and Kishimoto T: IL-6
in inflammation, immunity, and disease. Cold Spring Harb Perspect
Biol. 6(a016295)2014.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Yende S, D'Angelo G, Kellum JA, Weissfeld
L, Fine J, Welch RD, Kong L, Carter M and Angus DC: GenIMS
Investigators. Inflammatory markers at hospital discharge predict
subsequent mortality after pneumonia and sepsis. Am J Respir Crit
Care Med. 177:1242–1247. 2008.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Martens GW, Arikan MC, Lee J, Ren F,
Vallerskog T and Kornfeld H: Hypercholesterolemia impairs immunity
to tuberculosis. Infect Immun. 76:3464–3472. 2008.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Domon H and Terao Y: The role of
neutrophils and neutrophil elastase in pneumococcal pneumonia.
Front Cell Infect Microbiol. 11(615959)2021.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Zernecke A and Weber C: Chemokines in
atherosclerosis. Arterioscler Thromb Vasc Biol. 34:742–750.
2014.PubMed/NCBI View Article : Google Scholar
|
|
37
|
de Jager Saskia CA, Bot I, Kraaijeveld AO,
Korporaal SJA, Bot M, van Santbrink PJ, van Berkel TJC, Kuiper J
and Biessen EAL: Leukocyte-specific CCL3 deficiency inhibits
atherosclerotic lesion development by affecting neutrophil
accumulation. Arterioscler Thromb Vas Biol. 33:e75–e83.
2013.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Pfaff EM, Becker S, Günther A and
Königshoff M: Dickkopf proteins influence lung epithelial cell
proliferation in idiopathic pulmonary fibrosis. Eur Res J.
37:79–87. 2011.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Guo Y, Mishra A, Howland E, Zhao C, Shukla
D, Weng T and Liu L: Platelet-derived Wnt antagonist Dickkopf-1 is
implicated in ICAM-1/VCAM-1–mediated neutrophilic acute lung
inflammation. Blood. 126:2220–2229. 2015.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Kim KI, Park KU, Chun EJ, Choi SI, Cho YS,
Youn TJ, Cho GY, Chae IH, Song J, Choi DJ and Kim CH: A novel
biomarker of coronary atherosclerosis: Serum DKK1 concentration
correlates with coronary artery calcification and atherosclerotic
plaques. J Korean Med Sci. 26:1178–1184. 2011.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Yao X, Gordon EM, Figueroa DM, Barochia AV
and Levine SJ: Emerging roles of apolipoprotein E and
apolipoprotein A-I in the pathogenesis and treatment of lung
disease. Am J Respir Cell Mol Biol. 55:159–169. 2016.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Bazaz R, Francis S and Dockrell D: 407.
The effect of streptococcus pneumoniae pneumonia on
atherosclerosis. Open Forum Infect Dis. 6(S207)2019.
|
|
43
|
Naghavi M, Wyde P, Litovsky S, Madjid M,
Akhtar A, Naguib S, Siadaty MS, Sanati S and Casscells W: Influenza
infection exerts prominent inflammatory and thrombotic effects on
the atherosclerotic plaques of apolipoprotein E-deficient mice.
Circulation. 107:762–768. 2003.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Kaynar AM, Yende S, Zhu L, Frederick DR,
Chambers R, Burton CL, Carter M, Stolz DB, Agostini B, Gregory AD,
et al: Effects of intra-abdominal sepsis on atherosclerosis in
mice. Crit Care. 18(469)2014.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Brown AO, Mann B, Gao G, Hankins JS,
Humann J, Giardina J, Faverio P, Restrepo MI, Halade GV, Mortensen
EM, et al: Streptococcus pneumoniae Translocates into the
Myocardium and forms unique microlesions that disrupt cardiac
function. PLoS Pathog. 10(e1004383)2014.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Chen YC, Bui AV, Diesch J, Manasseh R,
Hausding C, Rivera J, Haviv I, Agrotis A, Htun NM, Jowett J, et al:
A novel mouse model of atherosclerotic plaque instability for drug
testing and mechanistic/therapeutic discoveries using gene and
microRNA expression profiling. Circ Res. 113:252–265.
2013.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Zivich PN, Grabenstein JD, Becker-Dreps SI
and Weber DJ: Streptococcus pneumoniae outbreaks and implications
for transmission and control: A systematic review. Pneumonia
(Nathan). 10(11)2018.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Dion CF and Ashurst JV: Streptococcus
pneumoniae. in StatPearls (StatPearls Publishing, 2021).
|
|
49
|
Ogino H, Fujii M, Ono M, Maezawa K, Hori S
and Kizu J: In vivo and in vitro effects of fluoroquinolones on
lipopolysaccharide-induced pro-inflammatory cytokine production. J
Infect Chemother. 15:168–173. 2009.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Tilakaratne A and Soory M:
Anti-inflammatory actions of adjunctive tetracyclines and other
agents in periodontitis and associated comorbidities. Open Dent J.
8:109–124. 2014.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Steel HC, Theron AJ, Cockeran R, Anderson
R and Feldman C: Pathogen- and host-directed anti-inflammatory
activities of macrolide antibiotics. Mediators Inflamm.
2012(584262)2012.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Sandgren A, Albiger B, Orihuela CJ,
Tuomanen E, Normark S and Henriques-Normark B: Virulence in mice of
pneumococcal clonal types with known invasive disease potential in
humans. J Infect Dis. 192:791–800. 2005.PubMed/NCBI View
Article : Google Scholar
|
|
53
|
Bartlett B, Ludewick HP, Lee S, Verma S,
Francis RJ and Dwivedi G: Imaging inflammation in patients and
animals: Focus on PET imaging the vulnerable plaque. Cells.
10(2573)2021.PubMed/NCBI View Article : Google Scholar
|