Introduction
Chronic diabetic wounds are characterized by a
prolonged inflammation stage, impaired proliferation stage and
long-lasting remodeling stage. During the impaired proliferation
stage, VEGF protein expression, angiogenesis and
re-epithelialization (RE) are impaired due to prolonged
inflammation and hyperglycemia-induced excessive oxidative stress
(1). Therefore, the resolution of
prolonged inflammation is a key point in the transition to the
impaired proliferation stage that may improve wound healing
(1-3).
Notably, promoting angiogenesis is one of the gold standard
treatments for diabetic foot ulcers (1-3).
Bitto et al (4) demonstrated
that topical application of simvastatin (SIM) enhanced wound
healing in diabetic mice. Recently, pleiotropic effects of SIM on
wound healing have been discovered, in addition to its classic
lipid-lowering effect (4-8).
The possible pleiotropic effects of SIM on wound healing have been
reported to be induced by reducing inflammation, and upregulating
VEGF production, angiogenesis and RE (4-8).
Phyllanthus emblica Linn. (PE) is a medicinal plant of the
Phyllanthus genus, which is distributed in tropical and
subtropical areas, and is widely used in Ayurvedic medicine
(9). PE extracts have been shown to
exert wound-healing effects in vitro and in rat wound models
(9-11).
Based on the effective chemical constituents in PE used in various
models, the wound-healing benefit of PE has been reported to be
associated with its antioxidant and anti-inflammatory effects
(9-14).
To the best of our knowledge, no research has yet
been conducted on the combined treatment of diabetic wounds with
topical PE and SIM. Therefore, the present study aimed to determine
the effect and the potential associated mechanisms of topical
application of PE and SIM in a mouse model of diabetic wounds.
Materials and methods
Animal preparation
Male BALB/C mice (total number of mice, 75; number
of mice/group, 5); age, 7-8 weeks; weight, 20-25 g) were purchased
from Nomura Siam International Co. Ltd. The experimental procedures
and daily care of the mice were approved by the Animal Care and Use
Ethics Committee, Faculty of Medicine, Chulalongkorn University
(Bangkok, Thailand; IRB no. 007/2562). The procedures were
conducted according to the experimental animal guidelines of The
National Research Council of Thailand (15). All mice were housed at 25±3˚C and
55+5% humidity, with ad libitum access to standard chow and
sterilized water under a 12-h light/dark cycle.
For the induction of diabetes, the mice were
intraperitoneally injected with streptozotocin (STZ;
MilliporeSigma) in citrate buffer (pH 4.5; MilliporeSigma), at a
dose of 45 mg/kg daily for 5 consecutive days (16). The control mice received an equal
volume of citrate buffer (pH 4.5) intraperitoneally for 5
consecutive days (16). A total of
2 weeks after the first day of STZ injections, the fasting blood
glucose (FBG) level was detected in 1 µl tail-vein blood from each
mouse. The diabetic mouse model was established successfully when
the FBG level was ≥200 mg/dl (14).
The diabetic mice were divided into four subgroups: Mice treated
daily with vehicle [diabetes mellitus (DM) + Vehicle (PMA2 cream)],
mice treated daily with 100% PE cream (DM + PE), mice treated daily
with 5% SIM cream (DM + SIM) and mice treated daily with a
combination of 100% PE + 5% SIM cream (DM + Combination). The
diabetic mice in each group were further divided into three minor
subgroups according to experimental period, as follows: Days 4, 7
and 14. The mice in the early and intermediate groups (day 4 and 7)
were used to assess the inflammation and proliferation phases
(3). The parameters measured on
days 4 and 7 included the number of infiltrated neutrophils, and
the tissue levels of IL-6 and VEGF proteins. On day 14, the
percentages of wound closure (%WC), capillary vascularity (%CV) and
RE (%RE) were measured.
PE extraction
A total of 10.0 kg fresh PE fruits were purchased
from Kru-La-Or Farm (Sai Yok District, Kanchanaburi, Thailand). PE
fruits were squeezed to obtain 3.4 kg minced pulp. Subsequently,
the pulp was extracted with distilled ethanol (1:2, w/v) three
times (72 h each time) at room temperature (10). Subsequently, the extracted solutions
were collected and pooled together. The pooled alcoholic extraction
solution was passed through filter paper (pore size, 11 µm) and
dried under vacuum rotary evaporation (Rotavapor R-114; Buchi AG)
below 45˚C to yield 219.81 g PE ethanol extract. The dried PE
ethanol extract was kept in an airtight and light-protecting
container at 4˚C in a refrigerator.
Combination drug preparation
For the combined PE and SIM cream, the exact amount
of dried PE extract and SIM powder [lot no. 116M4716 V;
MilliporeSigma; >97% purity (HPLC grade)], were weighed
according to the calculation of 100% PE cream and 5% SIM (w/v)
cream. PMA2 cream (Paragon Aesthetic Co., Ltd.) was used as a base
cream [10 µl PMA2 base cream contained 100% (w/v) PE, and 5% (w/v)
SIM] (17). The well-mixed combined
cream was kept in tightly closed light-proof brown glass bottle at
4˚C.
Wound model
After 4 weeks of successful diabetic mouse model
establishment, the mice were anesthetized by an intraperitoneal
injection of sodium pentobarbital (55 mg/kg). Subsequently, the fur
on the dorsal area of the mice was sheared using electric clippers.
To minimize the number of sacrificed mice, two 6x6 mm2
full-thickness wounds were made on the dorsal skin of both sides of
the vertebral column along the spine, 30 mm from the middle of the
ears and 15 mm from the spine (16,17).
The wound skin collected from the left side of mice was used for
the measurement of MDA content, IL-6 and VEGF protein levels,
whereas the wound skin collected from the right side of the mice
was used for measurement of re-epithelialization and neutrophil
infiltration. The number of animals in each group was 4-5, whereas
the number of wounds in each group was 3-5; the number of wounds in
each group was sometimes <5 if some of the wounds were scratched
and could therefore not be used to assess wound area and other
parameters. A Tegaderm™ frame was sutured to each wound to minimize
the confounding factor of mouse skin contraction (16,17).
Subsequently, 10 µl PMA2 base cream, 100% PE, 5% SIM (w/v) or a
combination of PE and SIM was gently applied to the wound area once
a day every day until the end date of the experiment. At the end of
the experiment, the mice euthanized by intraperitoneal injection of
an overdose of sodium pentobarbital (100 mg/kg). After euthanasia
was confirmed by the absence of cardiovascular function and no
vital signs (IRB no. 007/2562), the wound tissues were collected
for histological analysis and ELISA.
Determination of %WC
On days 7 and 14 post-wounding, after the mice were
anesthetized by an intraperitoneal injection of sodium
pentobarbital (55 mg/kg), images of each wound area were captured
using a stereoscopic zoom microscope (Nikon SMZ800; Nikon
Corporation). The unhealed wound area was determined from each
microscopic image using digital imaging software (Image-Pro II 6.1
software; Media Cybernetics, Inc.). %WC was calculated using the
following equation: %WC=[(area of the original wound-area of actual
wound)/area of the original wound] x100 (16,17).
Determination of %CV
On days 7 and 14 post-wounding, after the mice were
anesthetized by an intraperitoneal injection of sodium
pentobarbital (55 mg/kg), the jugular vein was cannulated for
injection of 0.1 ml 5% FITC-labeled dextran (molecular weight,
250,000; MilliporeSigma). By using confocal fluorescence microscopy
at x100 magnification (Nikon Eclipse E800; Nikon Corporation), the
surrounding capillary vasculature in the wound area was recorded.
To avoid tissue damage from fluorescent light, each image was
captured in a few seconds. From the fluorescent images of the
capillaries (diameter <15 µm), %CV was calculated using
Image-Pro II 6.1 software (Media Cybernetics, Inc.) (16,17).
%CV located in each area of interest (40x40 pixels) was determined
using the following equation: %CV=(number of pixels within
capillaries/total number of pixels in the entire frame) x100
(16,17).
Determination of neutrophil
infiltration
Wound tissues were collected from each mouse and
fixed in 10% formaldehyde at room temperature (25±2˚C) for 24 h.
The centers of the wound specimens were cut and embedded in
paraffin. Sections (5 µm) were then stained with hematoxylin
[Modified Hematoxylin Solution (progressive stain); C.V.
Laboratories Co., Ltd.] for 5 min and eosin [Eosin Solution
(Working Solution); C.V. Laboratories Co., Ltd.] (H&E) for 3
min at room temperature (25±2˚C) at the Department of Pathology,
Faculty of Medicine, Chulalongkorn University. The H&E-stained
slides were used to measure neutrophil infiltration at x400
magnification under a stereoscopic zoom microscope (Nikon SMZ800).
The x400 magnification wound images were captured using a digital
camera (Nikon DS-L2; Nikon Corporation). The number of neutrophils
per 1,000 cells counted from the wound edge was recorded. Digital
imaging software (Image-Pro II 6.1 software) was used to count the
numbers of infiltrated neutrophils. The results were confirmed by
blinded assessment (15,17).
Determination of %RE
The H&E-stained slides were also used for the
measurement of RE. The wound edge and RE tip judgment were
conducted in images at x400 magnification, which were captured
using a digital camera (Nikon DS-L2). The lengths of the curved
lines from both wound edges to the RE tips and the wound gap
between the RE tips were also measured. The %RE of the wound was
calculated using the following equation: %RE=distance covered by
epithelium/distance between wound edges) x100 (5,16).
Determination of VEGF and IL-6 protein
levels
Wound tissue was collected from each mouse and kept
at -80˚C until processing. The wound samples were weighed and
homogenized in RIPA buffer (Cell Signaling Technology, Inc.) and
phosphatase inhibitor cocktails (MilliporeSigma). The samples were
sonicated (20 kHz; Model VCX750; Sonics & Materials Inc., USA)
three times (each time, 15 sec; interval time, of 10 sec), and
centrifuged (Sorvall™ Legend™ X1R; Thermo Fisher Scientific, Inc.)
at 13,416 x g for 10 min at 4˚C. The supernatants of each sample
were used to determine the levels of total protein using the BCA
protein assay kit (Thermo Fisher Scientific, Inc.), VEGF using the
VEGF ELISA kit (mouse VEGF quantikine ELISA kit; cat. no. MMV00;
R&D Systems, Inc.), and IL-6 using the IL-6 ELISA kit (mouse
IL-6 quantikine ELISA kit; cat. no. M6000B; R&D Systems, Inc).
Subsequently, the OD value of the solution in each well was
measured using a colorimetric microplate reader (Model 860; Bio-Rad
Laboratories, Inc.), setting the measured wavelength at 450 nm and
the reference wavelength at 570 nm. The VEGF and IL-6 protein
levels were expressed in units of pg/mg total protein. All standard
solutions and samples were duplicated in a second plate (5,16).
Determination of tissue MDA
After the wound tissue was sonicated (20 kHz; Model
VCX750) three times (each time, 15 sec; interval time, 10 sec) and
centrifuged (Sorvall Legend X1R) at 13,416 x g for 10 min at 4˚C,
the MDA content was determined using the TBARS assay kit (cat. no.
10009055, Cayman Chemical Company). The OD value was measured using
a colorimetric microplate reader (Model 860), setting the
wavelength at 540 nm. The MDA content was expressed in nmol/mg
total protein. All standard solutions and samples were run in
duplicate (16).
Statistical analysis
The results are presented as the mean ± standard
error of the mean. The significance of differences between groups
was determined by one-way analysis of variance followed by the
Tukey's post hoc test. Correlation analysis was performed using the
Pearson's correlation coefficient. Statistical analyses were
conducted using SPSS (version 22; IBM, Corp.) and GraphPad Prism 6
software (GraphPad Software; Dotmatics). P<0.05 was considered
to indicate a statistically significant difference.
Results
Effects of topical application of
combined PE and SIM on wound oxidative stress
The MDA content (nmol/mg total protein) of the wound
site on days 4, 7 and 14 is shown in Fig. 1A. The results indicated that the
tissue MDA content seemed to increase during the progression of
diabetes. However, in the DM + Combination group, the tissue MDA
content was demonstrated to be significantly decreased on day 14
compared with that in the DM + Vehicle group (P<0.05). A
negative correlation was identified between MDA level and %WC on
day 4 (r=-0.889; P=0.001; Fig. 1B)
and day 7 (r=-0.556; P=0.025; Fig.
1C), but not on day 14 (r=-0.451; P=0.122; Fig. 1D).
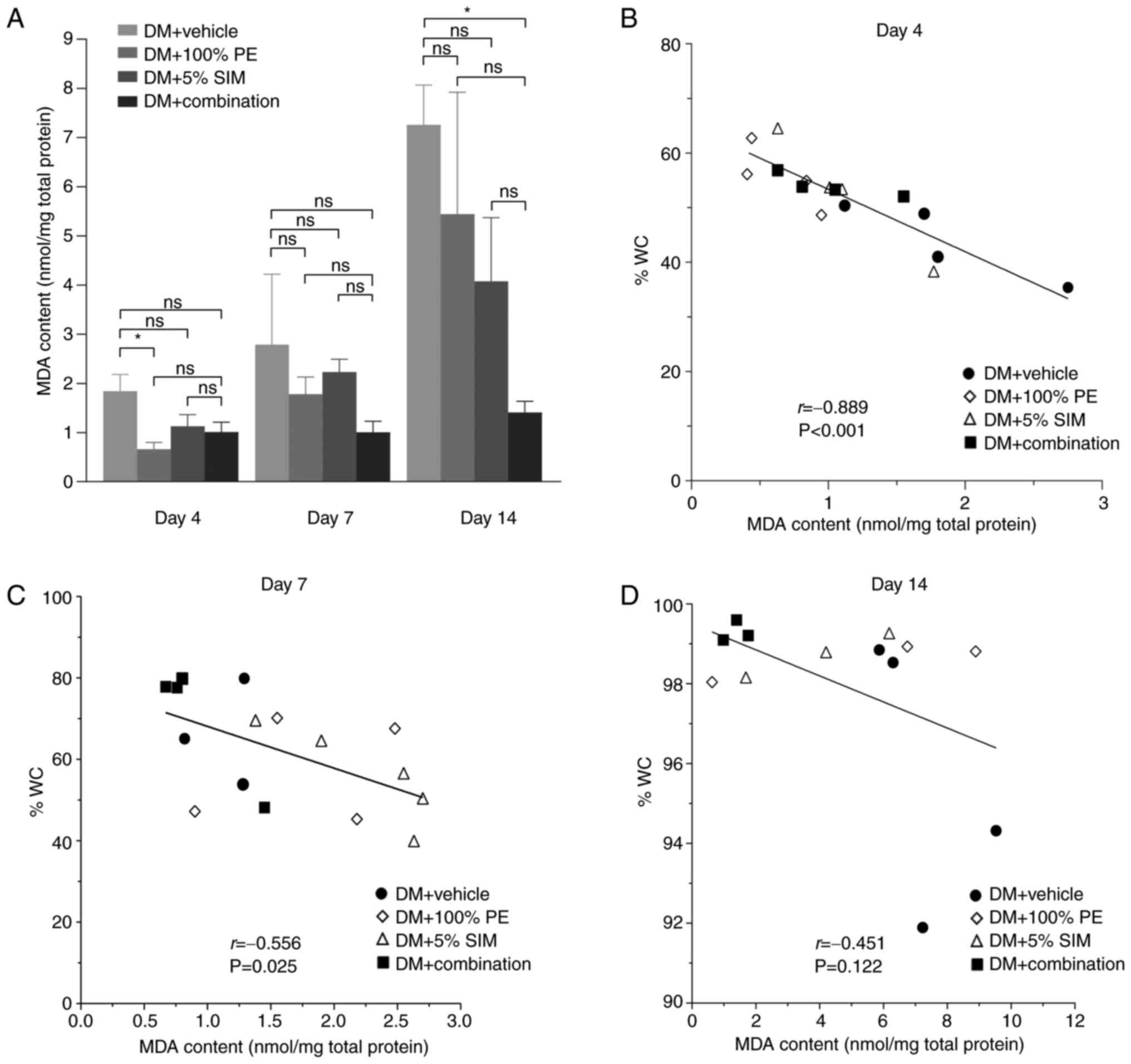 | Figure 1MDA content and correlation analysis
between MDA and %WC. (A) Tissue MDA levels in the various groups at
day 4, 7 and 14. Data are presented as the mean ± SEM. Pearson's
correlation analyses between MDA contents (nmol/mg total protein)
and %WC on day (B) 4, (C) 7 and (D) 14. Number of animals in each
group, 4-5; number of wounds in each group, 3-5.
*P<0.05. %WC, percentage wound closure; DM, diabetes
mellitus; MD, malondialdehyde; ns, not significant; PE,
Phyllanthus emblica Linn.; SIM, simvastatin. |
Effects of topical application of
combined PE and SIM on wound neutrophil infiltration
Images of neutrophil infiltration at the wound site
(H&E staining; x400 magnification) on days 4, 7 and 14
post-wounding are shown in Fig. 2A.
The number of infiltrated neutrophils detected during the
inflammation and proliferation phases of the wound (days 4 and 7)
were significantly downregulated in the DM + Combination group
compared with those in the DM + Vehicle group (P<0.05; Fig. 2B).
Effects of topical application of
combined PE and SIM on wound IL-6 protein levels
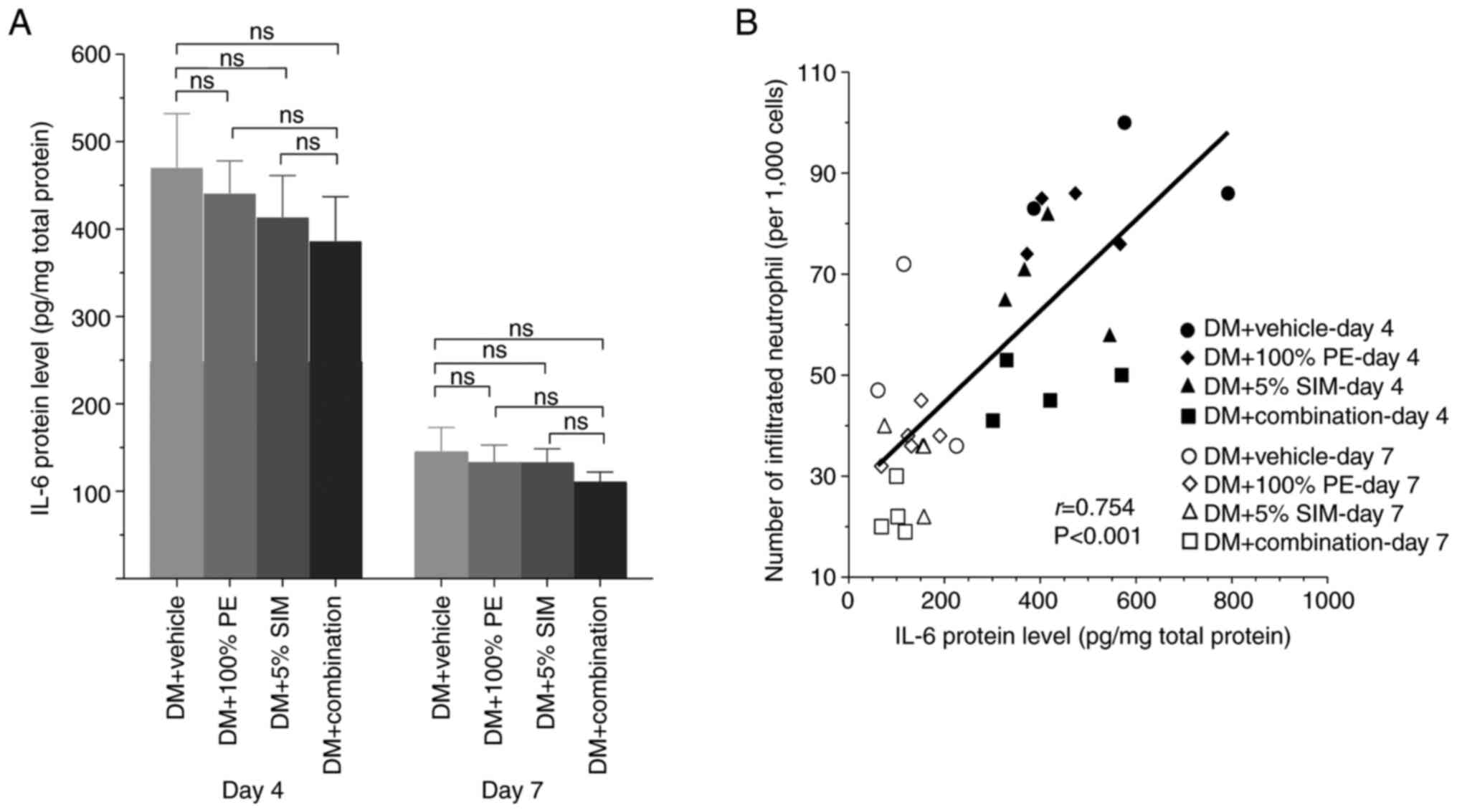
The levels of IL-6 (pg/mg total protein) in the
wound tissue on days 4 and 7 post-wounding are shown in Fig. 3A. The results showed that the IL-6
protein levels in all groups seemed to increase during the wound
inflammation phase on day 4 and appeared to be downregulate on day
7, the proliferation phase of wound healing. However, there was no
statistically significant difference among all groups in each phase
(P<0.05). Notably, a strong positive correlation was detected
between IL-6 protein levels and neutrophil infiltration (r=0.754;
P=0.001; Fig. 3B).
Effect of topical application of
combined PE and SIM on wound VEGF protein levels and
angiogenesis
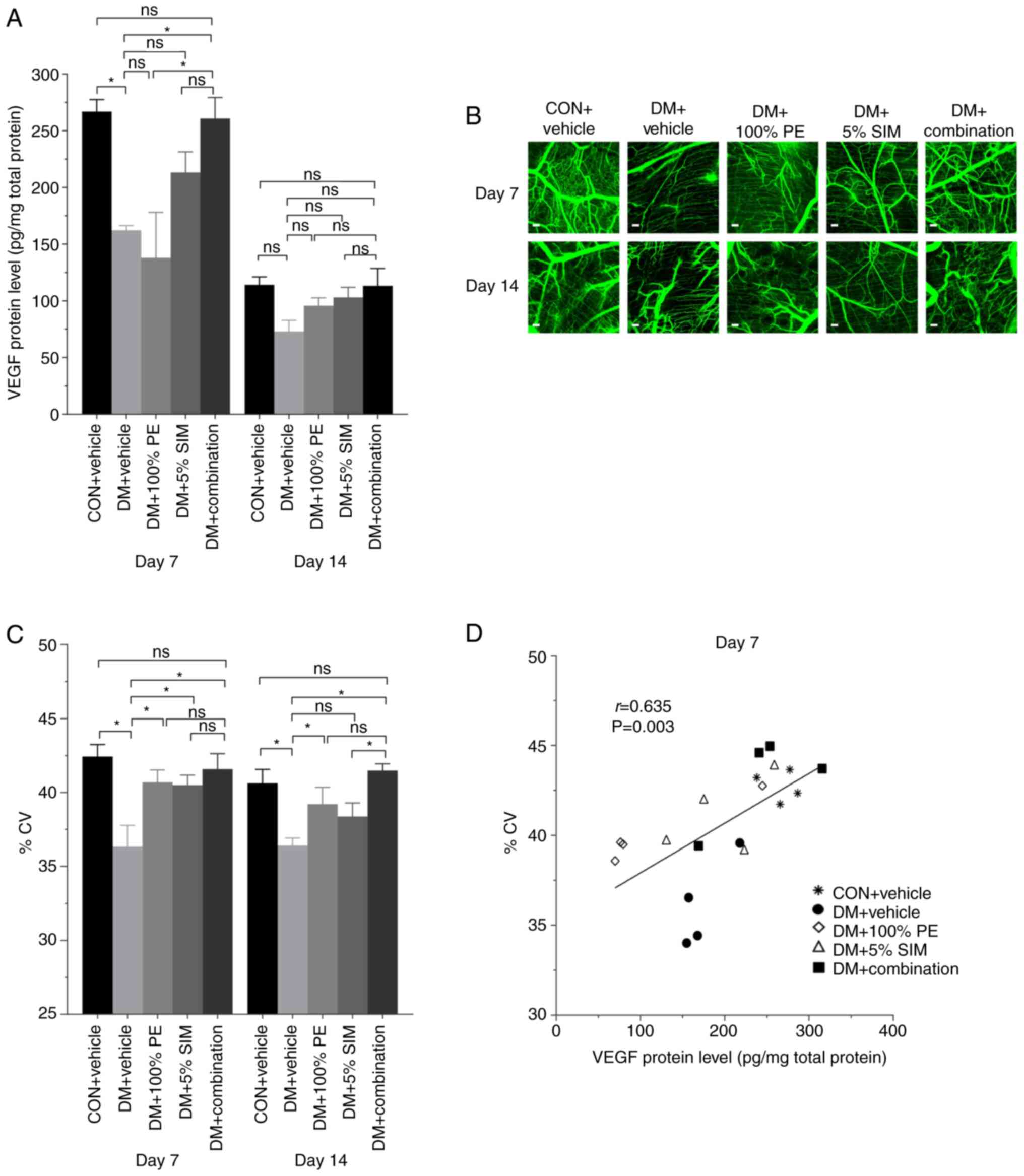
The VEGF protein levels (pg/mg total protein) in all
groups on days 7 and 14 post-wounding are shown in Fig. 4A. The results showed that VEGF
protein levels were significantly upregulated in the DM +
Combination group compared with those in the DM + Vehicle group on
days 7; however, there was no significant difference among groups
on day 14 post-wounding. Microscopic images of CV near the wound
area are shown in Fig. 4B on days 7
and 14 post-wounding. Notably, %CV was significantly increased in
the DM + Combination group compared with those in the DM + Vehicle
group on days 7 and 14 post-wounding (P<0.05; Fig. 4C); however, there was no significant
difference in the DM + Combination group when compared with the CON
+ Vehicle group. These results indicated that the combination cream
could enhance wound healing in diabetic mice as good as the
spontaneous wound healing observed in non-diabetic control mice. As
shown in Fig. 4D, on day 7
post-wounding, there was a moderate positive correlation (r=0.635;
P=0.003) between VEGF protein levels and %CV in the mouse
wounds.
Effect of topical application of
combined PE and SIM on wound RE
Images of RE at the wound site (H&E staining;
x400 magnification) on days 4, 7 and 14 post-wounding are shown in
Fig. 5A. The results showed that
%RE at the wound site in the DM + 5% SIM and DM + Combination
groups were slightly upregulated compared with that in the DM +
Vehicle group on day 4; however, by day 14 post-wounding, all
groups had completed 100% RE except for the DM + Vehicle group
(Fig. 5B).
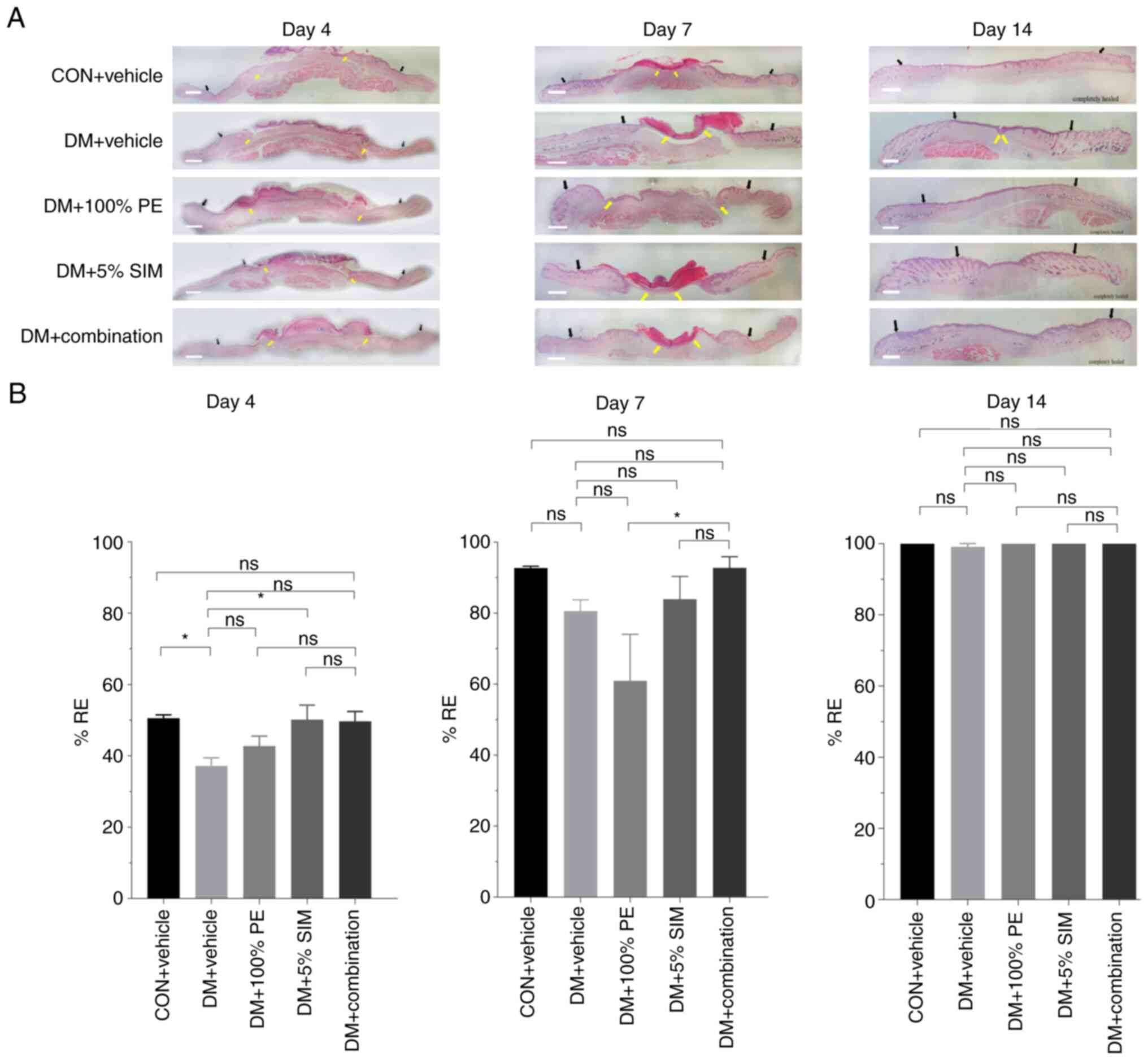 | Figure 5Effects of topical application of
combined PE and SIM cream on %RE. (A) RE on days 4, 7 and 14. Black
arrows indicate the wound edge with features of increased wound
thickness and proliferated epidermis; yellow arrows indicate the
tip of the endothelial cell in the wound area (hematoxylin and
eosin staining; x400 magnification; scale bar, 1 mm). (B) %RE in
diabetic groups on days 4, 7 and 14. Data are presented as the mean
± SEM. Number of animals in each group, 4-5; number of wounds in
each group, 4-5. *P<0.05. %RE, percentage of
re-epithelialization; CON, control; DM, diabetes mellitus; ns, not
significant; PE, Phyllanthus emblica Linn.; SIM,
simvastatin. |
Effect of topical application of
combined PE and SIM on WC
Images of WC in all groups on days 7 and 14 are
shown in Fig. 6A. Regarding %WC,
none of the treatment groups had significant differences when
compared with the DM + Vehicle group on day 7, except the DM +
Combination group. However, on day 14 all of the treatment groups
exhibited increased %WC compared with the DM + Vehicle group
(Fig. 6B). Furthermore, %WC in the
DM + Combination group was not significantly different from that in
the CON + Vehicle group. As shown in Fig. 6C, there were positive correlations
between %CV and %WC on days 7 (r=0.736; P=0.0003) and 14 (r=0.457;
P=0.006).
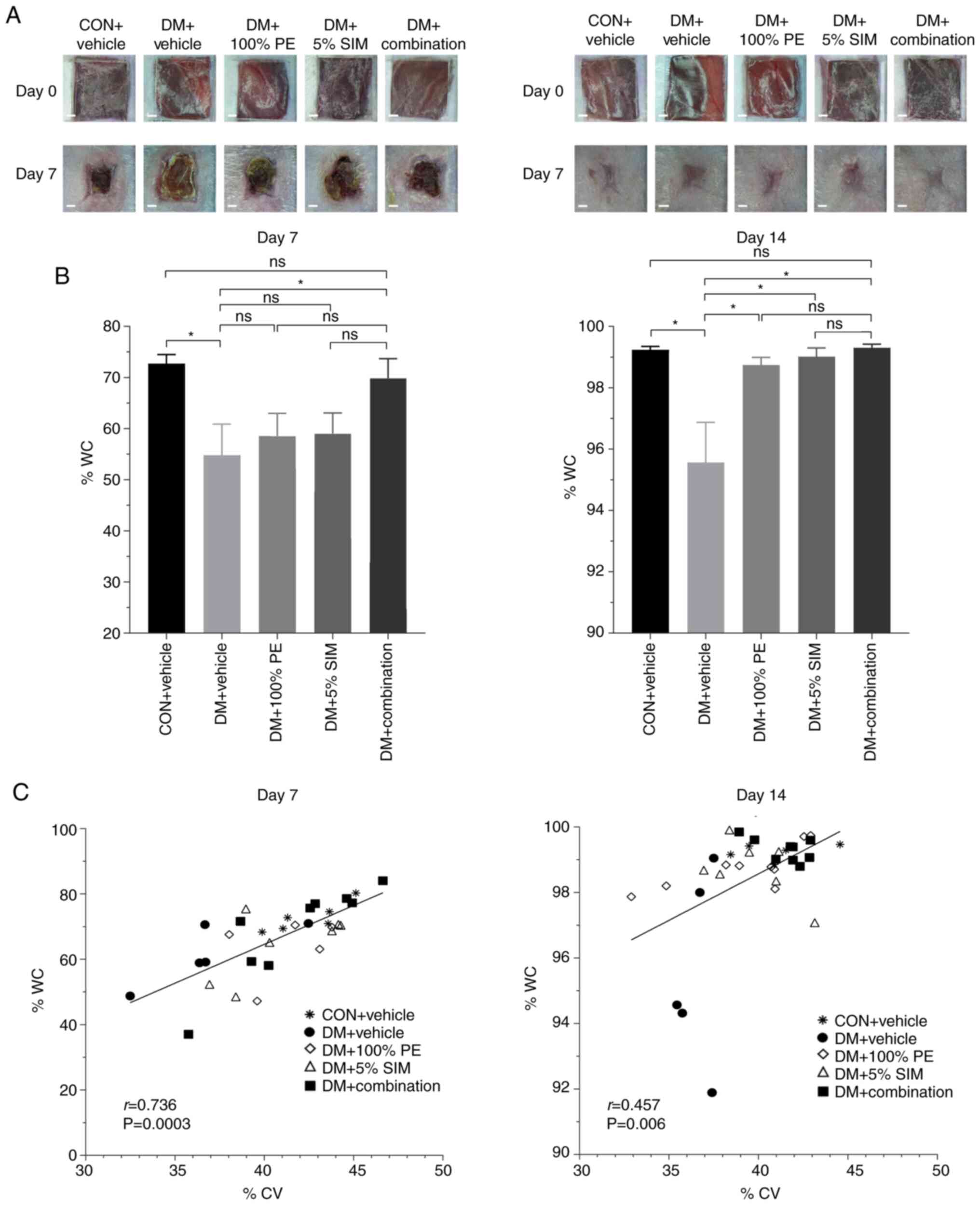 | Figure 6Effects of topical application of
combined PE and SIM cream on %WC. (A) Images of wound area (scale
bar, 1 mm). (B) %WC in the groups on days 7 and 14 post-wounding.
Data are presented as the mean ± SEM. *P<0.05. (C)
Pearson's correlation analysis between %CV and %WC in the five
groups on days 7 and 14. Number of animals in each group, 4-5;
number of wounds in each group, 4-9. %CV, percentage of capillary
vascularity; %WC, percentage of wound closure; CON, control; DM,
diabetes mellitus; ns, not significant; PE, Phyllanthus
emblica Linn.; SIM, simvastatin. |
Discussion
In diabetes, chronic or poor control of
hyperglycemia can result in the production of reactive oxygen
species (ROS) via both enzymatic and nonenzymatic pathways
(18). NADPH oxidase, mitochondrial
electron transport chain pathways and other sources of ROS, such as
advanced glycation end products and uncoupled nitric oxide synthase
(NOS), are the prominent sources of ROS generation in diabetic
wounds (18,19). Under this circumstance, excessive
ROS serve a critical role in promoting pathophysiological events,
prolonging inflammation and preventing diabetic wound healing
(19,20). In the present study, the results of
a Pearson's correlation analysis between MDA levels and %WC on days
4 and 7 indicated a reduction in oxidative stress at the wound site
may markedly increase wound closure. In the DM + Combination group,
a significant downregulation in MDA content was observed when
compared with DM + Vehicle on day 14, thus confirming the
antioxidant effect of combined PE and SIM treatment. Previous
studies on animals and patients with diabetes have confirmed the
effects of SIM and PE on decreasing MDA levels. Both SIM and PE
have been reported for their antioxidant properties, including the
restoration of endogenous antioxidant enzymes and scavenging free
radicals (13,17,21-25).
Therefore, combined PE and SIM cream could improve the healing
process in chronic diabetic wounds via its beneficial effects on
antioxidant-targeting pathways.
The present study also detected a reduction in the
number of infiltrated neutrophils in the DM + Combination group.
This may contribute to a reduction in oxidative stress since
neutrophil infiltration is recognized as a rich source of ROS
during the wound inflammatory phase (18).
Both PE and SIM have been reported to exert
anti-inflammatory effects. Nain et al (25) proposed that the active components,
fisetin and gallic acid, in PE extract may be responsible for
reducing IL-6 protein levels in a mouse macrophage model.
Furthermore, SIM-induced downregulation of IL-6 protein expression
has been observed in both animal models and clinical patients
(26,27). In the present study, combined
treatment with PE and SIM exhibited a better effect on
downregulating IL-6 protein levels in wound tissue compared with
single treatment with PE or SIM on days 4 and 7; however, this was
not significant. Moreover, in the present study, on days 4 and 7
post-wounding, there was a strong positive correlation (r=0.754;
P=0.001) between the IL-6 protein levels and the number of
infiltrating neutrophils.
In the present study, the results showed that
topical application of combined PE and SIM significantly enhanced
VEGF protein levels on day 7. In the DM + Combination group, %CV on
days 7 and 14 were significantly improved compared with those in
the DM + Vehicle group. In addition, there was a positive
correlation (r=0.635; P=0.003) detected between VEGF levels and %CV
on day 7. In the wound-healing process, PE has been reported to
increase wound healing by increasing angiogenesis and VEGF protein
levels in an in vitro model and in mouse gastric ulcers
(12,28). Furthermore, the gallic acid-enriched
fraction of PE has been shown to improve indomethacin-induced
gastric ulcer healing via an arginine catabolism-related
endothelial NOS (eNOS)-dependent pathway while promoting VEGF
expression (28). SIM can also
enhance wound healing through angiogenesis and VEGF mRNA levels in
rats and diabetic mice (5-6,29). In
addition, SIM has been reported to enhance eNOS mRNA expression and
protein levels in cultured endothelial cells preconditioned with a
steady laminar flow (30). The
upregulation of VEGF protein levels may occur through the eNOS/NO
pathway; therefore, modulating eNOS activity may enhance
angiogenesis in diabetic wound healing.
In the present study, the DM + 5% SIM and DM +
Combination groups exhibited a slight promoting effect on RE on day
4; however, there was no significant difference in %RE when
compared with the DM + Vehicle group on days 7 and 14
post-wounding. The possible pleiotropic effects of SIM on wound
healing may explain the increased RE (5-8).
RE is one of the major features that indicates wound healing is
successful and the migration of keratinocytes at the wound edge
across the wound gap starts the RE process (31). In the present study, %RE was used to
determine the degree of new epithelial tissue growing from the edge
of the wound.
The present study revealed positive correlations
between %CV and %WC on days 7 and 14. These findings indicated that
improving angiogenesis may be beneficial to diabetic wound healing.
The present findings demonstrated that the combination cream
successfully improved diabetic wound healing associated with
increased angiogenesis. Moreover, the results of %CV also confirm
that the combination cream could enhance wound angiogenesis in
diabetic mice comparatively to the spontaneous wound healing
process in the control non-diabetic mice.
Notably, SIM has been reported to exert
lipid-lowering- independent effects and a pleiotropic effect on
wound healing. The pleiotropic effects of SIM are explained well
through effective reduction of inflammation, and upregulation of
VEGF production, angiogenesis and RE in wound healing (5-8).
Based on the present findings, both PE and SIM had
wound healing promoting effects, possibly mediated through their
antioxidant and anti-inflammatory properties. PE extract contains
polyphenolic compounds, such as gallic acid and ellagic acid, which
have been reported to exert antioxidant, anti-inflammatory and
wound-healing effects (17,32-35).
In previous studies, the wound-healing property of PE extract was
revealed to be most likely due to the free radical-scavenging
ability of its polyphenols (32-35).
Ascorbic acid, emblicanin A, emblicanin B and low molecular weight
tannins from PE have been shown to exhibit antioxidant activity at
the wound site in dermal wound healing by restoring tissue ascorbic
acid, α-tocopherol, SOD, CAT and GPx activities (13,14).
The antioxidant and anti-inflammatory properties of statins, which
are independent of their lipid-lowering effects, could be partly
explained by modulation of the Nrf2/HO-1 pathway. Nrf2 and other
proteins involved in the Nrf2/HO-1 signaling pathway have a crucial
role in cellular responses to oxidative stress (23). To the best of our knowledge, there
is no research addressing the direct effect of PE on the ERK1/2
pathway in wound inflammation. However, the Nrf2 pathway-activating
mechanisms of PE have been reported to be associated with the roles
of ERK and p38MAPK in directly phosphorylating Nrf2 and promoting
Nrf2 nuclear import to increase its nuclear accumulation (24).
The promising effects of the combination of the
multi-chemical-containing plant PE and the single structure
chemical SIM on diabetic wound healing may offer targets for
compounds that effect amelioration of oxidative stress, prolonged
inflammation and other impaired healing processes.
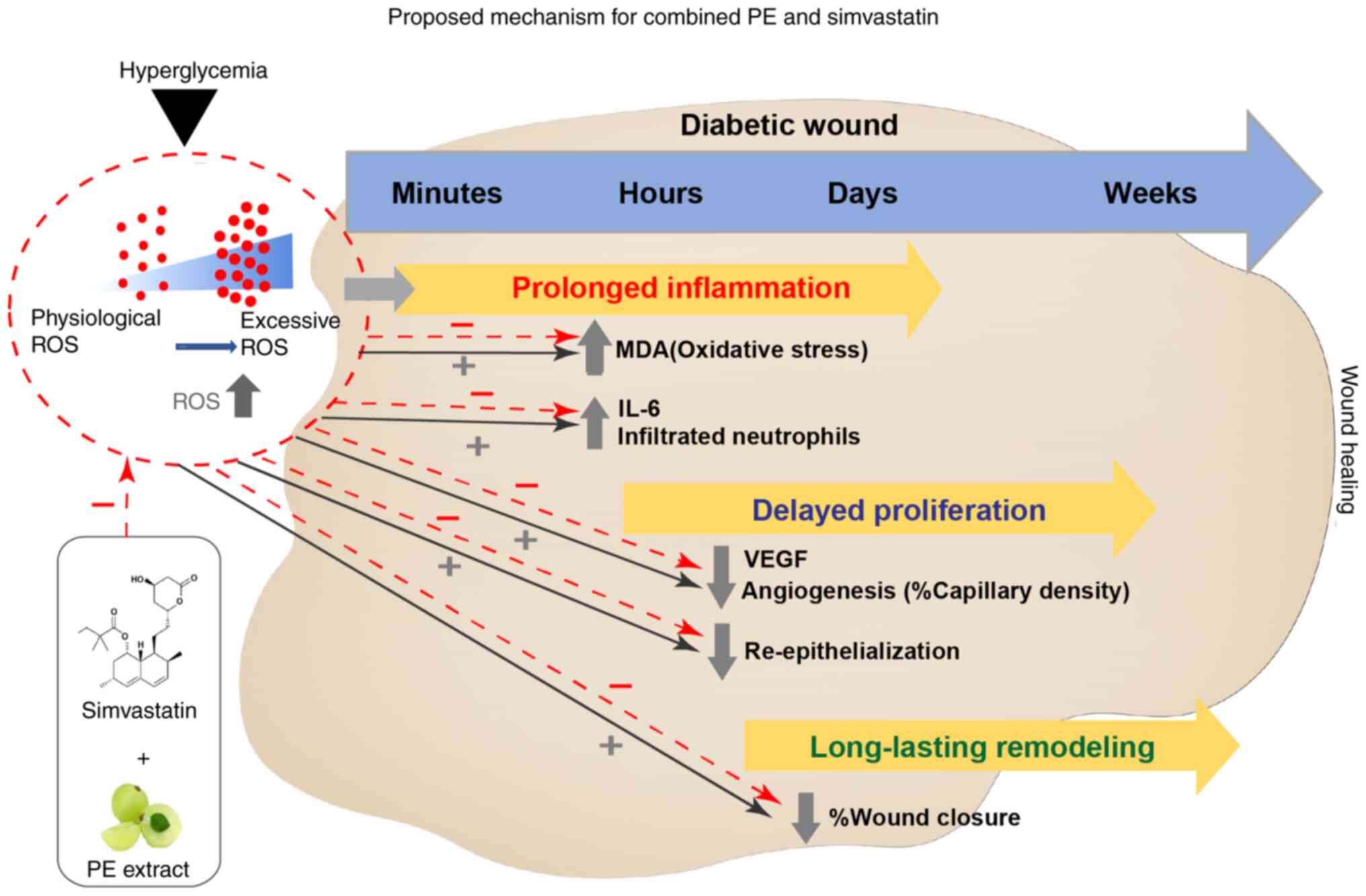
Notably, wound healing is a complex process that
includes three overlapping phases, as shown in Fig. 7 however, there are a number of
transcription factors and signalling pathways associated with
cutaneous wound healing (24,27-31).
A limitation of the present study is the lack of investigation of
all signaling parameters involved in the potential mechanisms;
these require further study and verification in the future.
Based on the present findings, the proposed
mechanism of topical application of PE and SIM is shown in Fig. 7. The combined effect of PE and SIM
on wound healing in diabetic mice may be associated with a
reduction in oxidative stress and inflammation, thus leading to
improved angiogenesis and wound closure.
In conclusion, to the best of our knowledge, the
present study is the first in vivo evidence showing that
topical application of combined PE and SIM may have beneficial
effects on diabetic wound healing through a reduction in excessive
oxidative stress and inflammation, and increased angiogenesis. The
present study revealed the highly promising topical use of combined
PE and SIM for the future treatment of diabetic wound healing.
Acknowledgements
Not applicable.
Funding
Funding: This study was supported by the 100th Anniversary
Chulalongkorn University for Doctoral Scholarship, The Graduate
School at Chulalongkorn University, Thailand.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
CC and SP contributed to the conception and design
of the study. TTL helped with the acquisition, analysis and
interpretation of the data. SS helped in the data analysis and
checking the statistical analysis. TTL performed the drafting and
writing of the manuscript. TTL and SP confirm the authenticity of
all the raw data. CC and SP gave final approval of the article. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
The experimental procedures and daily care were
approved by the Animal Care and Use Ethics Committee, Faculty of
Medicine, Chulalongkorn University, Thailand (IRB no.
007/2562).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Alexiadou K and Doupis J: Management of
diabetic foot ulcers. Diabetes Ther. 3(4)2012.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Johnson JB, Broszczak DA, Mani JS, Anesi J
and Naiker M: A cut above the rest: Oxidative stress in chronic
wounds and the potential role of polyphenols as therapeutics. J
Pharm Pharmacol. 74:485–502. 2022.PubMed/NCBI View Article : Google Scholar
|
|
3
|
González AC, Costa TF, Andrade ZA and
Medrado AR: Wound healing-A literature review. An Bras Dermatol.
91:614–620. 2016.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Bitto A, Minutoli D, Altavilla D, Polito
F, Fiumara T, Marini H, Galeano M, Calò M, Cascio PL, Bonaiuto M,
et al: Simvastatin enhances VEGF production and ameliorates
impaired wound healing in experimental diabetes. Pharmacol Res.
57:159–169. 2008.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Sukpat S, Israsena N and Patumraj S:
Pleiotropic effects of simvastatin on wound healing in diabetic
mice. J Med Assoc Thai. 99:213–219. 2016.PubMed/NCBI
|
|
6
|
Rego AC, Araújo Filho IA, Damasceno BP,
Egito ES, Silveira IA, Brandão-Neto J and Medeiros AC: Simvastatin
improves the healing of infected skin wounds of rats. Acta Cir
Bras. 22 (Suppl 1):S57–S63. 2007.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Asai J, Takenaka H, Hirakawa S, Sakabe J,
Hagura A, Kishimoto S, Maruyama K, Kajiya K, Kinoshita S, Tokura Y
and Katoh N: Topical simvastatin accelerates wound healing in
diabetes by enhancing angiogenesis and lymphangiogenesis. Am J
Pathol. 181:2217–2224. 2012.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Wang CC, Yang PW, Yang SF, Hsieh KP, Tseng
SP and Lin YC: Topical simvastatin promotes healing of
Staphylococcus aureus-contaminated cutaneous wounds. Int Wound J.
13:1150–1157. 2016.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Yadav SS, Singh MK, Singh PK and Kumar V:
Traditional knowledge to clinical trials: A review on therapeutic
actions of emblica officinalis. Biomed Pharmacother. 93:1292–1302.
2017.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Chansriniyom C, Bunwatcharaphansakun P,
Eaknai W, Nalinratana N, Ratanawong A, Khongkow M and
Luechapudiporn R: A synergistic combination of Phyllanthus emblica
and Alpinia galanga against H2O2-induced
oxidative stress and lipid peroxidation in human ECV304 cells. J
Funct Foods. 43:44–54. 2018.
|
|
11
|
Ansari A, Shahriar MS, Hassan MM, Das SR,
Rokeya B, Haque MA, Haque ME, Biswas N and Sarkar T: Emblica
officinalis improves glycemic status and oxidative stress in STZ
induced type 2 diabetic model rats. Asian Pac J Trop Med. 7:21–25.
2014.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Usharani P, Fatima N and Muralidhar N:
Effects of Phyllanthus emblica extract on endothelial dysfunction
and biomarkers of oxidative stress in patients with type 2 diabetes
mellitus: A randomized, double-blind, controlled study. Diabetes
Metab Syndr Obes. 6:275–284. 2013.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Muthuraman A, Sood S and Singla SK: The
antiinflammatory potential of phenolic compounds from Emblica
officinalis L. in rat. Inflammopharmacology. 19:327–334.
2011.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Golechha M, Sarangal V, Ojha S, Bhatia J
and Arya DS: Anti-inflammatory effect of Emblica officinalis in
rodent models of acute and chronic inflammation: Involvement of
possible mechanisms. Int J Inflam. 2014(178408)2014.PubMed/NCBI View Article : Google Scholar
|
|
15
|
National Research Council of Thailand
(NRCT), The Ethical Principles for the Use of Animal for Scientific
Purposes (2015).
|
|
16
|
Sukpat S, Isarasena N, Wongphoom J and
Patumraj S: Vasculoprotective effects of combined endothelial
progenitor cells and mesenchymal stem cells in diabetic wound care:
Their potential role in decreasing wound-oxidative stress. BioMed
Res Int Jan. 2013(459196)2013.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Liao TT, Sukpat S, Chansriniyom C and
Patumraj S: Dose-dependent effect of local treatment of
Phyllanthus emblica L. cream on diabetic wound. Chula Med J.
66:129–136. 2022.
|
|
18
|
Cano Sanchez M, Lancel S, Boulanger E and
Neviere R: Targeting oxidative stress and mitochondrial dysfunction
in the treatment of impaired wound healing: A systematic review.
Antioxidants (Basel). 7(98)2018.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Fakhruddin S, Alanazi W and Jackson KE:
Diabetes-induced reactive oxygen species: Mechanism of their
generation and role in renal injury. J Diabetes Res.
2017(8379327)2017.PubMed/NCBI View Article : Google Scholar
|
|
20
|
El-Benna J, Hurtado-Nedelec M, Marzaioli
V, Marie JC, Gougerot-Pocidalo MA and Dang PM: Priming of the
neutrophil respiratory burst: Role in host defense and
inflammation. Immunol Rev. 273:180–193. 2016.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Zinellu A, Paliogiannis P, Usai MF, Carru
C and Mangoni AA: Effect of statin treatment on circulating
malondialdehyde concentrations: A systematic review and
meta-analysis. Ther Adv Chronic Dis.
10(2040622319862714)2019.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Cowled PA, Khanna A, Laws PE, Field JB,
Varelias A and Fitridge RA: Statins inhibit neutrophil infiltration
in skeletal muscle reperfusion injury. J Surg Res. 141:267–276.
2007.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Zhang Y, Rong S, Feng Y, Zhao L, Hong J,
Wang R and Yuan W: Simvastatin attenuates renal
ischemia/reperfusion injury from oxidative stress via targeting
Nrf2/HO-1 pathway. Exp Ther Med. 14:4460–4466. 2017.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Wang F and Wang H: Phyllanthus emblica L.
extract activates Nrf2 signalling pathway in HepG2 cells. Biomed
Res. 28:3383–3386. 2017.
|
|
25
|
Nain P, Saini V, Sharma S and Nain J:
Antidiabetic and antioxidant potential of Emblica officinalis
Gaertn. leaves extract in streptozotocin-induced type-2 diabetes
mellitus (T2DM) rats. J Ethnopharmacol. 142:65–71. 2012.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Rezaie-Majd A, Maca T, Bucek RA, Valent P,
Müller MR, Husslein P, Kashanipour A, Minar E and Baghestanian M:
Simvastatin reduces expression of cytokines interleukin-6,
interleukin-8, and monocyte chemoattractant protein-1 in
circulating monocytes from hypercholesterolemic patients.
Arterioscler Thromb Vasc Biol. 22:1194–1199. 2002.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Paumelle R, Blanquart C, Briand O, Barbier
O, Duhem C, Woerly G, Percevault F, Fruchart JC, Dombrowicz D,
Glineur C and Staels B: Acute anti-inflammatory properties of
statins involve peroxisome proliferator–activated receptor-α via
inhibition of the protein kinase C signaling pathway. Circ Res.
98:361–369. 2006.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Chatterjee A, Chatterjee S, Biswas A,
Bhattacharya S, Chattopadhyay S and Bandyopadhyay SK: Gallic acid
enriched fraction of Phyllanthus emblica potentiates
indomethacin-induced gastric ulcer healing via e-NOS-dependent
pathway. Evid Based Complement Alternat Med.
2012(487380)2012.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Khoshneviszadeh M, Ashkani-Esfahani S,
Namazi MR, Noorafshan A, Geramizadeh B and Miri R: Topical
simvastatin enhances tissue regeneration in full-thickness skin
wounds in rat models. Iran J Pharm Sci. 13:263–269. 2014.PubMed/NCBI
|
|
30
|
Rossi J, Rouleau L, Tardif JC and Leask
RL: Effect of simvastatin on Kruppel-like factor2, endothelial
nitric oxide synthase and thrombomodulin expression in endothelial
cells under shear stress. Life Sci. 87:92–99. 2010.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Braiman-Wiksman L, Solomonik I, Spira R
and Tennenbaum T: Novel insights into wound healing sequence of
events. Toxicol Pathol. 35:767–779. 2007.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Poltanov EA, Shikov AN, Dorman HD,
Pozharitskaya ON, Makarov VG, Tikhonov VP and Hiltunen R: Chemical
and antioxidant evaluation of Indian gooseberry (Emblica
officinalis Gaertn., syn. Phyllanthus emblica L.) supplements.
Phytother Res. 23:1309–1315. 2009.PubMed/NCBI View
Article : Google Scholar
|
|
33
|
Pozharitskaya ON, Ivanova SA, Shikov AN
and Makarov VG: Separation and evaluation of free
radical-scavenging activity of phenol components of Emblica
officinalis extract by using an HPTLC–DPPH* method. J Sep Sci.
30:1250–1254. 2007.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Scartezzini P, Antognoni F, Raggi MA, Poli
F and Sabbioni C: Vitamin C content and antioxidant activity of the
fruit and of the Ayurvedic preparation of Emblica officinalis
Gaertn. J Ethnopharmacol. 104:113–118. 2006.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Fatima N, Pingali U and Muralidhar N:
Study of pharmacodynamic interaction of Phyllanthus emblica extract
with clopidogrel and ecosprin in patients with type II diabetes
mellitus. Phytomedicine. 21:579–585. 2014.PubMed/NCBI View Article : Google Scholar
|