Introduction
Immunoglobulin A nephropathy (IgAN) is the most
prevalent type of primary glomerulonephritis and the main cause of
end stage renal disease (ESRD) in the world (1,2). In
India, IgAN presents as nephrotic syndrome with rapid progression
to ESRD, requiring renal replacement therapy (3). Experimental models suggest that
surplus production of aberrantly-glycosylated IgA in mucosal-allied
lymphoid tissue in the gut leads to IgA nephropathy (4,5). The
four important steps in the pathogenesis of IgAN are i) mucosal IgA
production by plasma cells, ii) production of under-glycosylated
IgA, iii) production of immune complexes and iv) deposition of
these nephritogenic immune complexes in the kidney leading to IgA
nephropathy. The heterogeneous clinical features of IgAN range from
asymptomatic microscopic haematuria to a rapidly progressive form
of glomerulonephritis (6,7). Renal biopsy is the primary method for
IgAN diagnosis and disease assessment. However, it is difficult to
perform due to its invasiveness (8). Hence, there is a need to investigate
the underlying mechanisms of disease development for the
identification of new disease biomarkers/target genes for IgAN
diagnosis that may provide an alternative to invasive methods.
MicroRNAs (miRNAs/miRs) are endogenous, small
non-coding RNAs of ~18-22 nucleotides in length, which have a major
role in mRNA degradation or translation repression, thereby
regulating the expression of their target proteins. Several
biological and molecular processes are mediated by miRNAs. Certain
miRNAs regulate the target genes involved in different cellular
processes, such as proliferation, differentiation, migration,
signaling and apoptosis (9). The
expression profiles of miRNAs are specific and vary in different
cancer types, such as lung (10),
liver (11), breast (12), colon (13), pancreatic (14) and renal cancer (15,16).
In addition, the dysregulation of miRNAs is associated with several
pathological conditions, including IgAN (17-19).
Recently, a small number of miRNAs that are differentially
expressed in the blood, urine and renal tissue were identified to
have a direct connection with IgAN (18,20,21).
Identifying miRNAs in tissues responsible for the pathway leading
to aberrant galactosylation of IgA helps to plan oral therapy that
selectively inhibits the responsible miRNAs locally in the
intestine without systemic serious adverse events. Studies have
indicated that miRNAs are associated with a spectrum of clinical
aspects in kidney disease, including fibrosis and inflammation
(22,23). The association of miRNAs with IgAN
progression in tissues and their role in disease pathogenesis
remain largely elusive. In the present study, new miRNAs related to
IgAN were discovered by sequencing miRNAs from renal biopsies.
Furthermore, bioinformatics analysis was used to look into the
probable functions of differentially expressed miRNAs (DEMs). A
total of 25 miRNAs that were differentially expressed were found
and the relationship of the majority of these miRNAs with IgAN
suggests that they may act as targets or biomarkers for IgAN in the
Indian population. The objectives of the present study were to
assess miRNAs as potential disease markers and further explore
miRNA-based targeted therapeutic strategies for IgAN.
Materials and methods
Clinical sample collection
The present study was a single-centre hospital-based
case-control, retrospective study, which was conducted at AIG (Mayo
Clinic Care Network) Hospital (Hyderabad, India) from February 2021
to April 2022, that included the kidney biopsy blocks of patients
with IgAN (n=6) and control tissue (n=6) from patients with renal
cell carcinoma (‘healthy’ tissue). The samples were confirmed by
in-house pathologists. After obtaining informed consent from the
patients, biopsy samples were collected from the surgical wards.
The inclusion criteria for patients with IgAN were as follows: i)
IgAN was confirmed by renal biopsy; ii) normal renal function
(blood urea nitrogen, blood creatinine); iii) age ≥25 years; iv) no
previous hormone, immunosuppressant or kidney transplantation
treatments; and v) the patients did not have any secondary IgAN,
such as lupus nephritis, purpura nephritis or hepatitis B-related
nephritis. The exclusion criteria for healthy participants were
patients with i) chronic diseases, such as coronary heart disease,
hypertension, acute and chronic cerebrovascular disease or
diabetes; ii) infectious diseases and fever; iii) mental illnesses;
and iv) any metabolic syndromes. The research study was approved by
the Institutional Research and Ethics Committee of AIG Hospital
(AHF/AIGH-IRB: 02/47/2021) and written informed consent was
obtained from all patients included in the present study. The study
protocol conformed to the ethical guidelines of the 1975
Declaration of Helsinki. The clinical features of patients with
IgAN after diagnosis by experienced pathologists are provided in
Table I.
 | Table IDemographic and baseline clinical
data of the healthy participants and patients with IgAN. |
Table I
Demographic and baseline clinical
data of the healthy participants and patients with IgAN.
| Characteristic | IgAN (n=6) | Healthy controls
(n=6) |
|---|
| Sex,
male/female | 5:1 | 5:1 |
| Age, years | 51.2±7.49 | 59±5.91 |
Tissue collection and miRNA
isolation
Formalin-fixed paraffin-embedded (FFPE) blocks of
the biopsy specimens were collected. Sections (3-5 mm) were cut
from each block and a single section from each block was mounted on
previously prepared poly-L-lysine-coated slides. The sections were
then subjected to haematoxylin and eosin staining according to a
standard protocol. Images of the stained slides were acquired using
a bright-field microscope (DM 2000; Leica Microsystems). The
pathological analysis of samples was performed by evaluating both
macroscopic and microscopic features of kidney tissues. The slides
were reviewed by experienced in-house pathologists in a blinded
manner.
Frozen kidney biopsy specimens from six patients
with IgAN and six healthy control samples were selected for miR
transcriptomic profiling by next generation sequencing (NGS). miRNA
was extracted and purified using the miRNeasy FFPE Kit (cat. no.
217504; Qiagen GmbH) following the manufacturer's protocol.
Isolated miRNAs were quantified with the Qubit microRNA Assay Kit
(cat. no. Q32880; Qiagen GmbH) and were included in the further
analysis.
Library preparation for small RNA
sequencing (RNA-seq)
Small RNA library preparation was performed using
the CleanTag™ Small RNA Library Preparation Kit (cat.
no. L-3206; TriLink Biotechnologies) according to the
manufacturer's protocol. In brief, isolated miRNA molecules were
ligated with a 3' RNA adapter, followed by ligation with a 5'
adapter. The cDNA was prepared at 50˚C for 1 h and amplified. PCR
was performed using the following thermocycling conditions: Initial
denaturation at 98˚C for 30 sec; followed by 18 cycles of 98˚C for
10 sec, 60˚C for 30 sec and 72˚C for 15 sec; followed by a final
extension at 72˚C for 10 min. Purified library products were
evaluated using the High Sensitivity DNA Chip for Agilent 2100 Tape
Station (Agilent Technologies, Inc.) and the samples were again
quantified by using the Qubit microRNA Assay Kit, followed by NGS
on the ION S5 Torrent (Thermo Fisher Scientific, Inc.) at 1x50 base
pairs.
miRNA expression analyses. Quality
control and reads mapping to the reference genome
BAM files of raw reads were processed and
high-quality reads were obtained. The upstream and downstream
analyses were mainly based on the clean and high-quality reads. The
sequences of miRNA reads were mapped to the reference Human genome
(hg38) by Bowtie2 (v2.2) using Partek Flow Genomic Analysis
Software (https://www.partek.com/partek-flow/).
Known miRNA alignment and novel miRNA
prediction. miRBase version 22 (https://www.mirbase.org/) was used as a reference. The
Partek Flow Genomic Analysis Software was used to compare the miRNA
expression between healthy participants and patients with IgAN and
to calculate the P-value. These softwares were used to predict
novel miRNA as well as helped to identify novel miR counts, base
bias on either the first position or on each position of all novel
miRs.
Quantification of miRNA. The miR read counts
were normalized as transcript per million (TPM) based on the
following formula: Normalized expression=mapped read count/total
reads x106.
Differential expression of miRNA. The
uniquely mapped reads were then subjected to differential gene
expression (DGE) analysis using DeSeq2 in Partek Flow Genomic
Analysis software. The Benjamini and Hochberg's false discovery
rate (FDR) was used to adjust the P-values. In the expression
profile, statistically significant differences of genes were
considered with a fold change (FC) ≥±2 and P<0.05. The list of
differentially expressed genes is provided in Table II for the respective comparison.
P<0.05 was considered the significant threshold for the signal
transduction and disease pathways. Furthermore, the variance of
gene expression patterns between the test and healthy samples was
assessed using principal component analysis (PCA). Specifically, a
three-component PCA analysis and visualization were conducted with
default parameters using Partek Flow Genomic Analysis Software.
 | Table IIDifferentially expressed miRNAs and
their respective associated upregulated and downregulated
genes. |
Table II
Differentially expressed miRNAs and
their respective associated upregulated and downregulated
genes.
| A, Significant
differentially expressed genes summary (Test vs. Control) |
|---|
| MiRNA ID | FDR step up | Ratio | Fold change | P-value |
|---|
| hsa-miR-21-5p | 0.0008 | 6.894 | 6.89 | 0.00002 |
| hsa-miR-10a-5p | 0.4835 | 0.650 | -1.54 | 0.2805 |
|
hsa-miR-146b-5p | 0.0346 | 3.494 | 3.49 | 0.0037 |
| hsa-miR-29c-3p | 0.8341 | 0.810 | -1.23 | 0.7148 |
| hsa-miR-192-5p | 0.6392 | 1.255 | 1.26 | 0.5015 |
| hsa-miR-204-3p | 0.0530 | 0.269 | -3.71 | 0.0114 |
| hsa-miR-92a-3p | 0.1016 | 1.992 | 1.99 | 0.0297 |
| hsa-miR-328-3p | 0.0530 | 0.303 | -3.30 | 0.0113 |
|
hsa-miR-146a-5p | 0.4055 | 1.827 | 1.83 | 0.2059 |
| hsa-miR-155-5p | 0.0501 | 2.786 | 2.79 | 0.0092 |
| hsa-let-7g-5p | 0.2391 | 2.791 | 2.79 | 0.1067 |
| hsa-miR-184 | 0.1211 | 0.241 | -4.15 | 0.0404 |
| hsa-miR-28-3p | 0.1211 | 1.803 | 1.80 | 0.0410 |
| hsa-miR-320c | 0.0601 | 0.217 | -4.62 | 0.0148 |
| hsa-miR-486-5p | 0.0189 | 0.150 | -6.65 | 0.0017 |
| hsa-miR-139-3p | 0.0110 | 0.416 | -2.40 | 0.0006 |
| hsa-miR-99a-5p | 0.0110 | 0.329 | -3.04 | 0.0008 |
|
hsa-miR-1307-3p | 0.8103 | 0.854 | -1.17 | 0.6732 |
|
hsa-miR-30c-2-3p | 0.0413 | 0.433 | -2.31 | 0.0057 |
| hsa-miR-30c-5p | 0.1130 | 2.470 | 2.47 | 0.0348 |
| hsa-miR-100-5p | 0.0815 | 0.457 | -2.19 | 0.0213 |
| hsa-miR-320b | 0.0501 | 0.272 | -3.67 | 0.0085 |
|
hsa-miR-181a-5p | 0.1390 | 1.750 | 1.75 | 0.0513 |
|
hsa-miR-151a-3p | 0.1003 | 0.505 | -1.98 | 0.0278 |
| hsa-miR-127-3p | 0.0110 | 0.159 | -6.29 | 0.0007 |
| B, Top 10
upregulated miRNAs in small RNA sequencing analysis |
| MiRNA ID | FDR step up | Ratio | Fold change | P-value |
| hsa-miR-21-5p | 0.0008 | 6.894 | 6.89 | 0.00002 |
|
hsa-miR-146b-5p | 0.0346 | 3.494 | 3.49 | 0.0037 |
| hsa-miR-192-5p | 0.6392 | 1.255 | 1.26 | 0.5015 |
|
hsa-miR-181a-5p | 0.1390 | 1.750 | 1.75 | 0.0513 |
| hsa-miR-92a-3p | 0.1016 | 1.992 | 1.99 | 0.0297 |
|
hsa-miR-146a-5p | 0.4055 | 1.827 | 1.83 | 0.2059 |
| hsa-miR-155-5p | 0.0501 | 2.786 | 2.79 | 0.0092 |
| hsa-let-7g-5p | 0.2391 | 2.791 | 2.79 | 0.1067 |
| hsa-miR-28-3p | 0.1211 | 1.803 | 1.80 | 0.0410 |
| hsa-miR-30c-5p | 0.1130 | 2.470 | 2.47 | 0.0348 |
| C, Top 15
downregulated miRNAs in small RNA sequencing analysis |
| MiRNA ID | FDR step up | Ratio | Fold change | P-value |
| hsa-miR-10a-5p | 0.4835 | 0.650 | -1.54 | 0.2805 |
| hsa-miR-320c | 0.0601 | 0.217 | -4.62 | 0.0148 |
| hsa-miR-486-5p | 0.0189 | 0.150 | -6.65 | 0.0017 |
| hsa-miR-139-3p | 0.0110 | 0.416 | -2.40 | 0.0006 |
| hsa-miR-99a-5p | 0.0110 | 0.329 | -3.04 | 0.0008 |
| hsa-miR-328-3p | 0.0530 | 0.303 | -3.30 | 0.0113 |
|
hsa-miR-1307-3p | 0.8103 | 0.854 | -1.17 | 0.6732 |
|
hsa-miR-30c-2-3p | 0.0413 | 0.433 | -2.31 | 0.0057 |
| hsa-miR-100-5p | 0.0815 | 0.457 | -2.19 | 0.0213 |
| hsa-miR-320b | 0.0501 | 0.272 | -3.67 | 0.0085 |
| hsa-miR-29c-3p | 0.8341 | 0.810 | -1.23 | 0.7148 |
| hsa-miR-184 | 0.1211 | 0.241 | -4.15 | 0.0404 |
|
hsa-miR-151a-3p | 0.1003 | 0.505 | -1.98 | 0.0278 |
| hsa-miR-204-3p | 0.0530 | 0.269 | -3.71 | 0.0114 |
| hsa-miR-127-3p | 0.0110 | 0.159 | -6.29 | 0.0007 |
Gene Ontology (GO) and Kyoto
Encyclopedia of Genes and Genomes (KEGG) enrichment analysis of
genes encoding the DEMs
The most overrepresented GO terms and KEGG pathways
that were closely linked to the DEMs were highlighted using KEGG
and GO functional annotations. The KEGG Database served as the
basis for the KEGG Pathways study (KEGG enrichment pathway
database; http://www.genome.jp/.). The Target Scan
(https://www.targetscan.org/vert_80)
and miRDB (http://www.mirdb.org/) tools were used
to acquire the signal transduction and disease pathway annotation
data for the potential target genes. A Fisher's exact test was used
to determine the P-value and a significant threshold of P<0.05
was used to evaluate the statistical significance of the signal
transduction and disease pathways to the background. Cluster
Profiler (Bioconductor) was used for the GO functional analysis to
offer molecular function (MF), biological process (BP) and cellular
component (CC) annotation for potential target genes (https://bioconductor.org/packages/release/bioc/html/clusterProfiler.html.).
A statistically significant difference in the level of GO
annotation enrichment was defined as P<0.05.
Regulatory network construction of
miRNA-target genes
To recognize the function of miRs in the context of
regulatory interactions between miRNAs and their target genes, the
regulatory network of miRNA-target genes was constructed using
CyTransFinder (Cytoscape3.x plugin) (24). The combined scores >0.4 were
selected to construct the network and Cytoscape (version 3.6.1)
software was used to further analyse the interactive miRNA-target
gene network.
Statistical and bioinformatics
analysis
Partek Flow Genomics Analysis Software (Build
version 9.0.20.0417) was used for the quantification and
statistical analysis. DGE analysis was performed using one-way
ANOVA followed by with Dunnett's post-hoc test. P≤0.05 and Log2FC
≥2 was considered to indicate differentially-expressed genes. Data
were further processed for GO enrichment analysis with Cluster
Profiler and KEGG pathway analysis with the KEGG database. The
scatter plots for gene expression and heat map were constructed
using Partek Flow Analysis Software.
Results
miRNA sequencing and analysis in
healthy participants and patients with IgAN
To study the aberrant expression of miRNAs in the
tissues of patients with IgAN, miRNAs were sequenced and the data
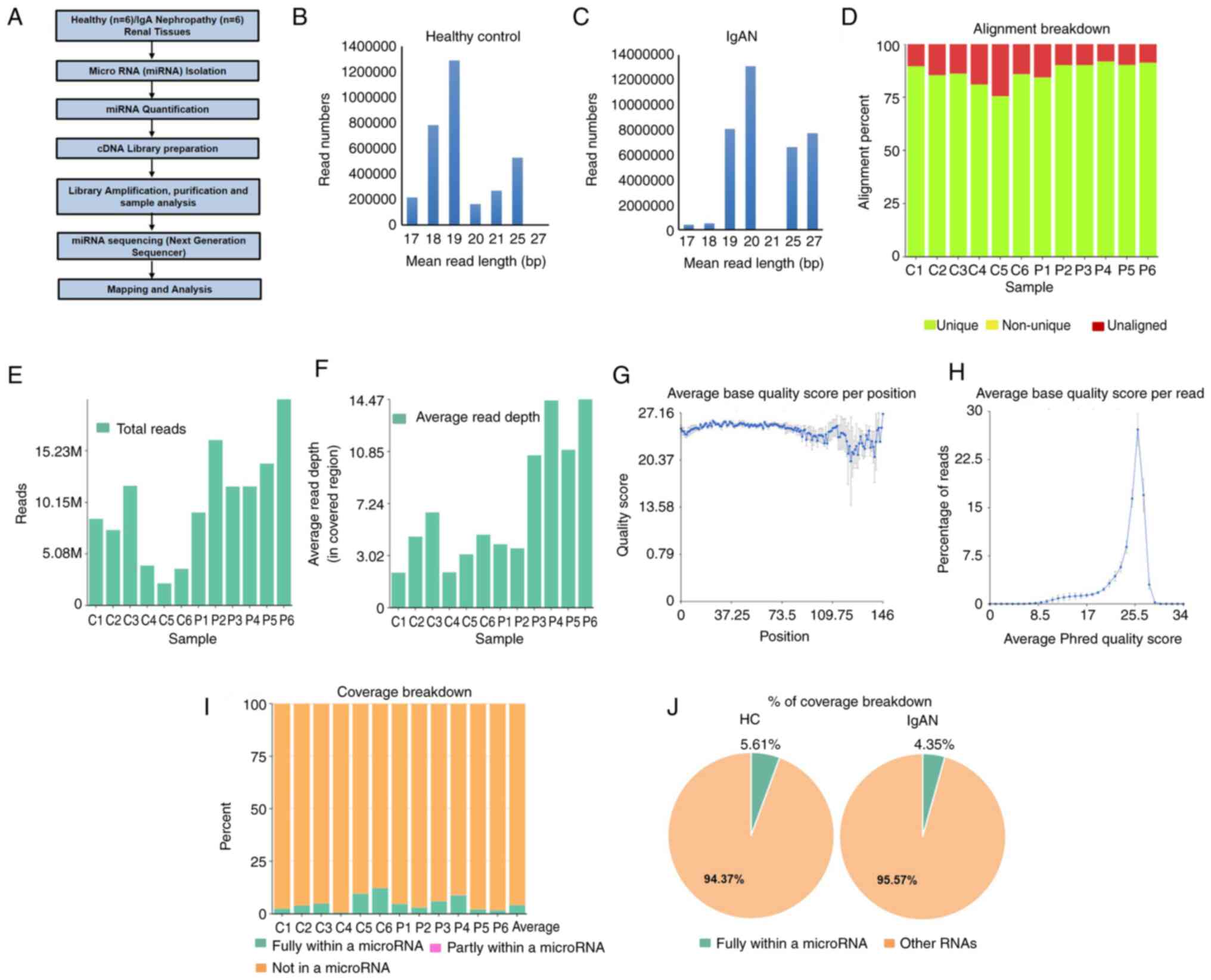
were analyzed following the workflow presented in Fig. 1A. miRNAs isolated from the tissues
of healthy participants and patients with IgAN were mainly composed
of ~18-27-bp sequences (Fig. 1B and
C). 19 and 20 bp nucleotides were
most abundant in cells, indicating that mature miRNAs were the most
common class of small RNAs in tissues. The miRNA sequences were
mapped to databases containing human miRNAs and the quality of the
transcriptome data is presented in Fig.
1D-H. Fig. 1D displays the
alignment percentage for each sample. Sample alignment to hg38 was
in a good range i.e. 75-92%. The average base quality score
indicated that all reads in each sample had an average quality
scoring from the 5'-3' end and distribution of how many reads have
a particular base quality score on average (Fig. 1E-H). Fig. 1I and J indicate the quantification of the
coverage breakdown of miRNAs; the percentage of fully within miRNAs
in healthy participants was 5.61% of total RNAs and that in
patients with IgAN was 4.35% of total RNAs in this study.
Significant DEMs in tissue samples of
patients with IgAN
Gene expression signatures occur due to cellular
perturbations, such as drug treatments, gene knockdown or diseases,
and may be quantified using DGE methods comparing the gene
expression between two groups of samples to identify significantly
expressed genes altered in the perturbation. To identify miRNAs
associated with the development of IgAN, miRNAs that were
significantly differentially expressed were assessed (P<0.05 and
FC ≥±2). Furthermore, to identify the similarity of biological
samples in RNA-seq datasets, gene expression values were
transformed into principal components (PCs), a set of linearly
uncorrelated features representing the most relevant sources of
variance in the data. The PCs scatter plot of the data for given
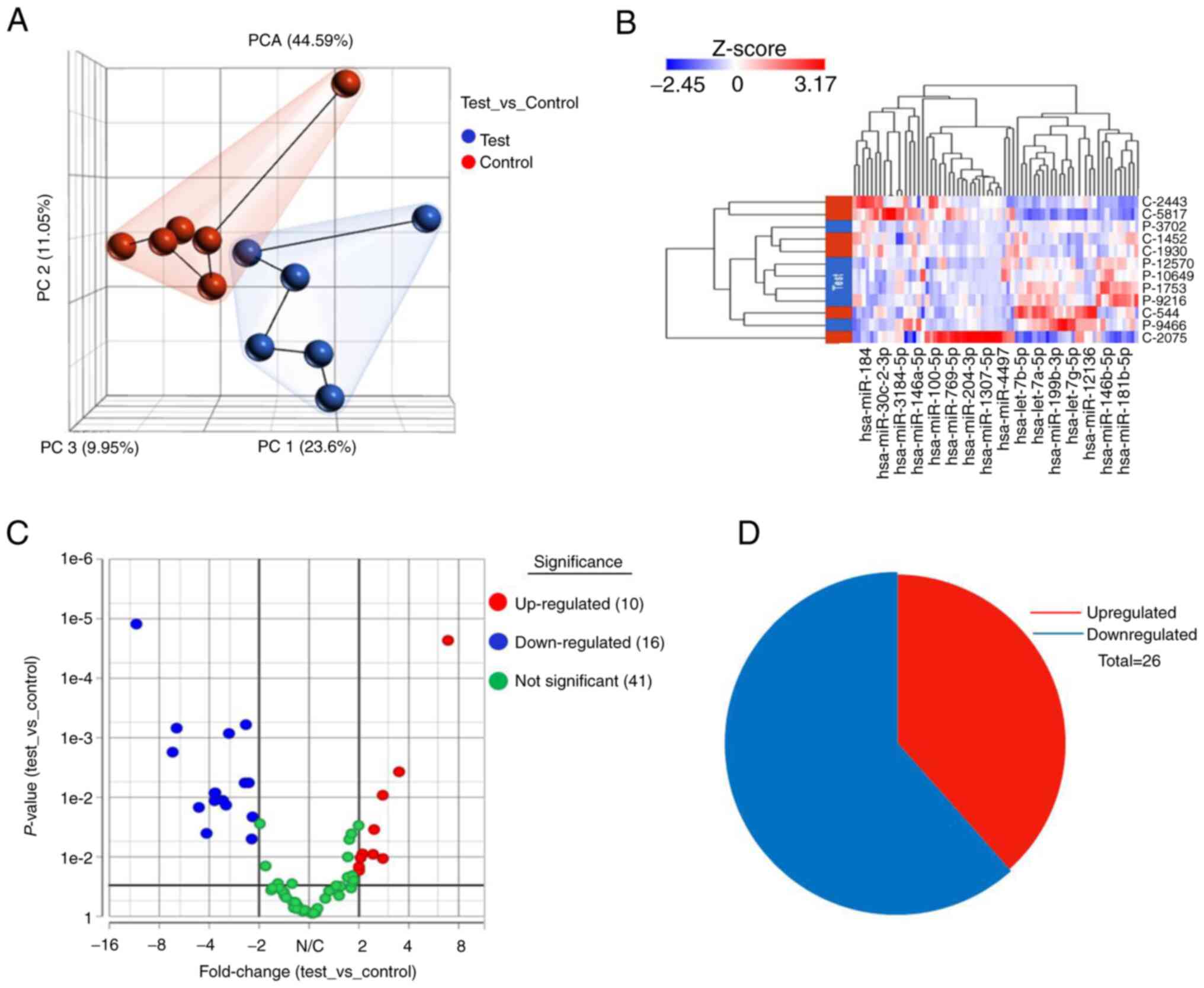
samples is presented in Fig. 2A.
Each point represents each sample and samples with similar gene
expression profiles came closer in the three-dimensional space.
Fig. 2B presents the hierarchical
clustering data of differentially expressed genes in the analysis.
Differential expression analysis of the set revealed that a total
of 67 miRNAs were identified. Of the 67 miRNAs, 25 were
significantly dysregulated: 10 were upregulated and 15 miRNAs were
downregulated with a significant P-value of ≤0.05 and Log2FC ≥±2 in
tissues from patients with IgAN as compared with healthy
participants (Fig. 2C). A list of
significantly dysregulated miRNAs, comprising upregulated and
downregulated miRNAs, with the FC and P-values, is provided in
Table II. A pie chart indicating
distributions of significantly differentially expressed up- and
downregulated miRNAs in patients with IgAN as compared with healthy
participants is displayed in Fig.
2D.
Functional prediction for upregulated
genes
The top 10 upregulated miRNAs were first compiled in
order to clarify the probable functions of DEMs in IgAN. With
regard to these, hsa-miR-21-5p was significantly upregulated in
patients with IgAN, whereas hsa-miR-146b-5p, hsa-miR-155-5p and
hsa-let-7g-5p displayed substantial abundance in both healthy and
test samples (IgAN) (Table IIB).
When miRNAs target the 3'-UTRs of mRNAs, the stability of the mRNAs
is decreased or translation efficiency is impeded. A total of 990
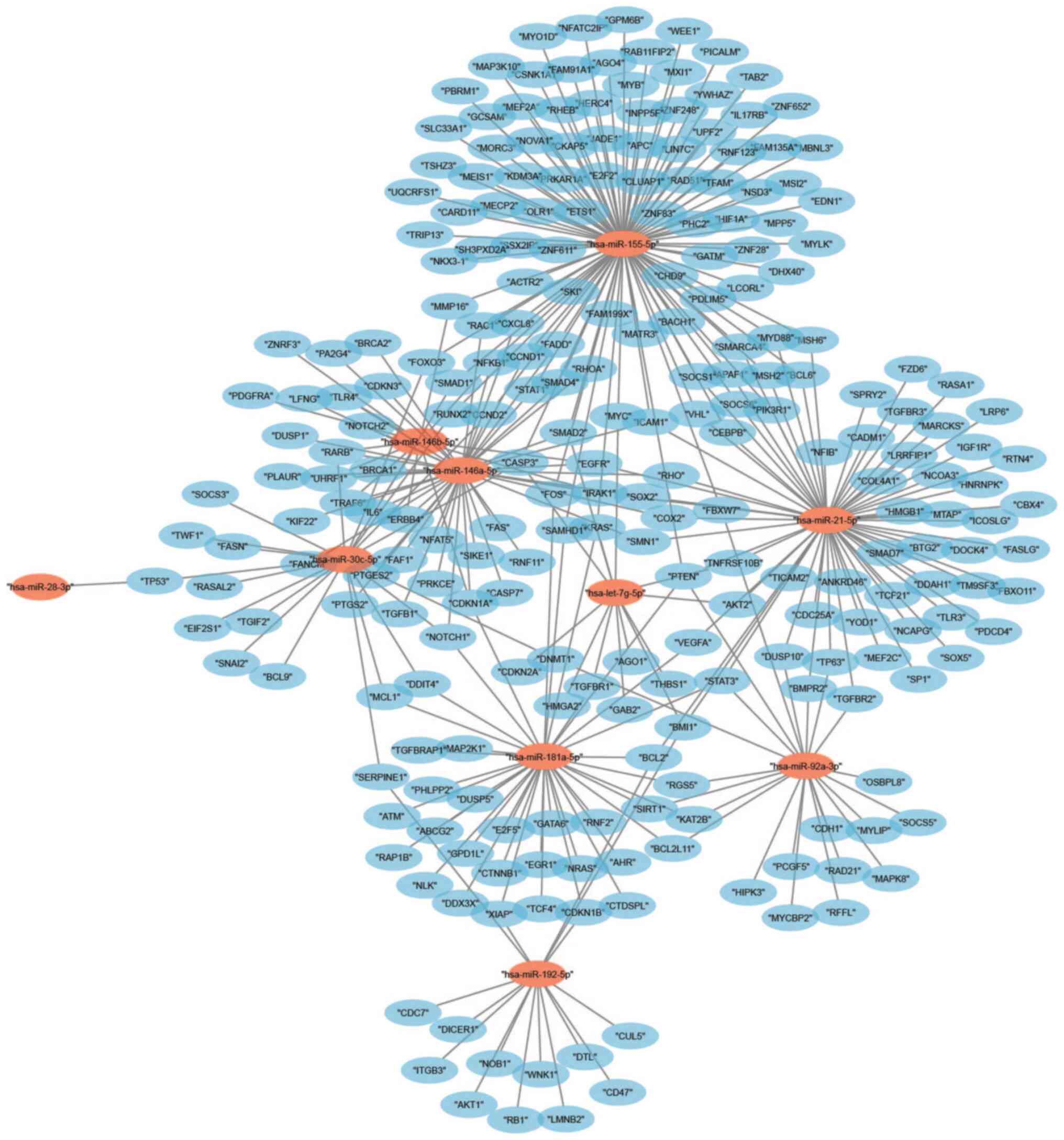
potential target genes were identified from the top 10 upregulated
miRNAs and furthermore, an interaction network for miRNAs-target
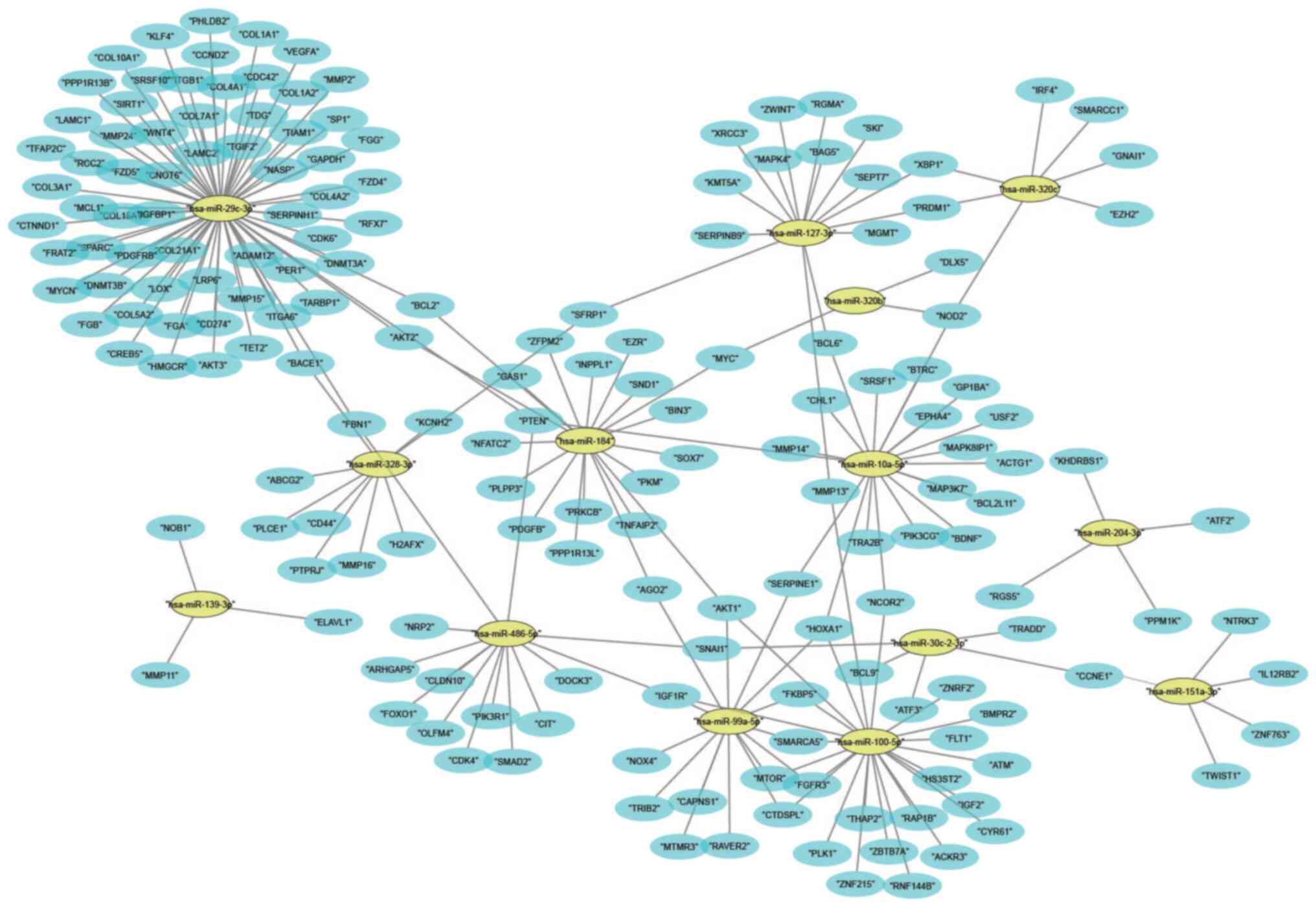
genes was created (Fig. 3). The
largest subnetwork was found for miR-155-5p, which included 375
potential target genes.
The gene functions were examined by performing KEGG
analysis and GO enrichment in terms of BP, CC and MF, as well as
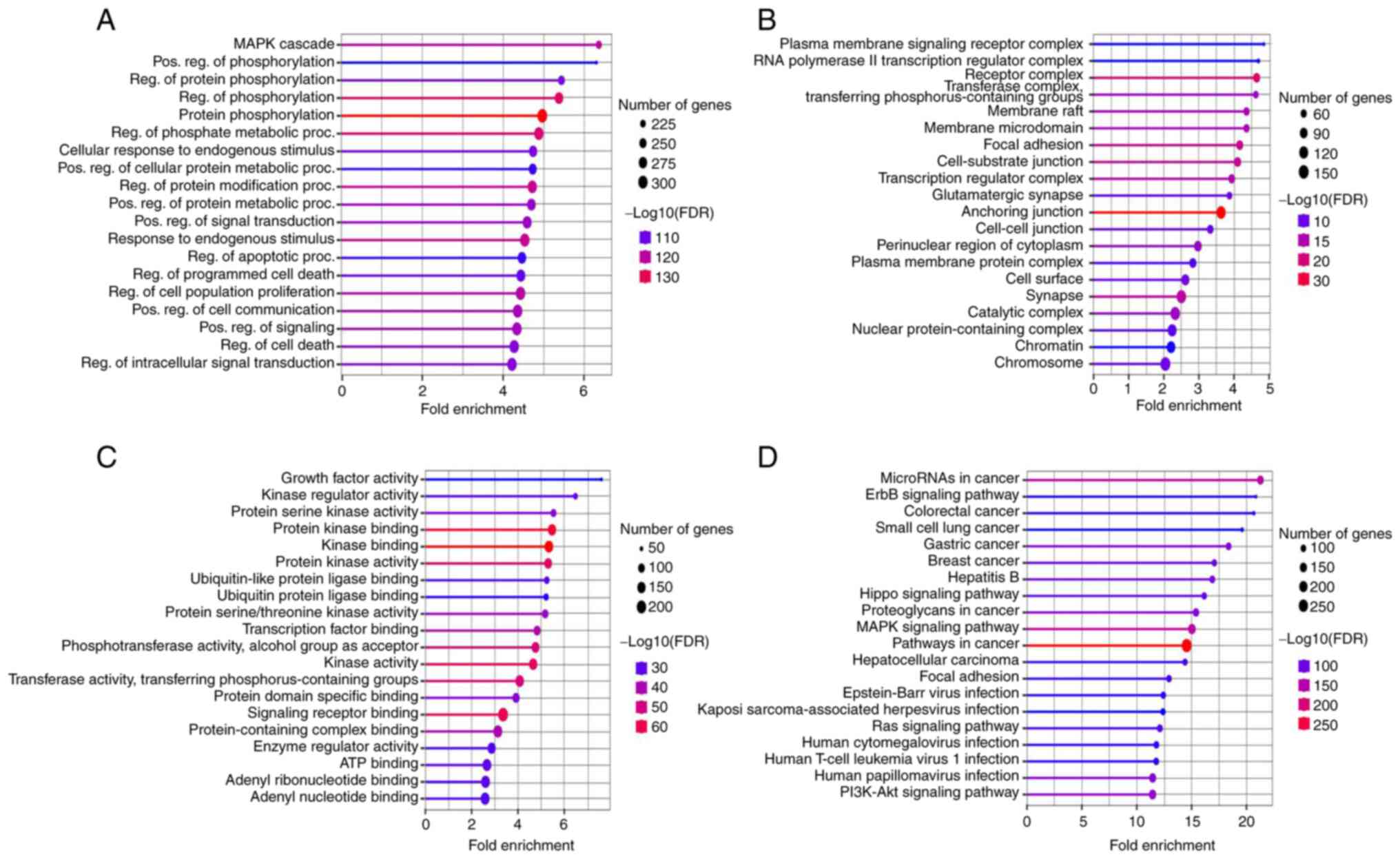
their associated pathways. The FDR cut-off was set at 0.05 and the
top 20 significantly enriched terms were displayed for BP, CC and
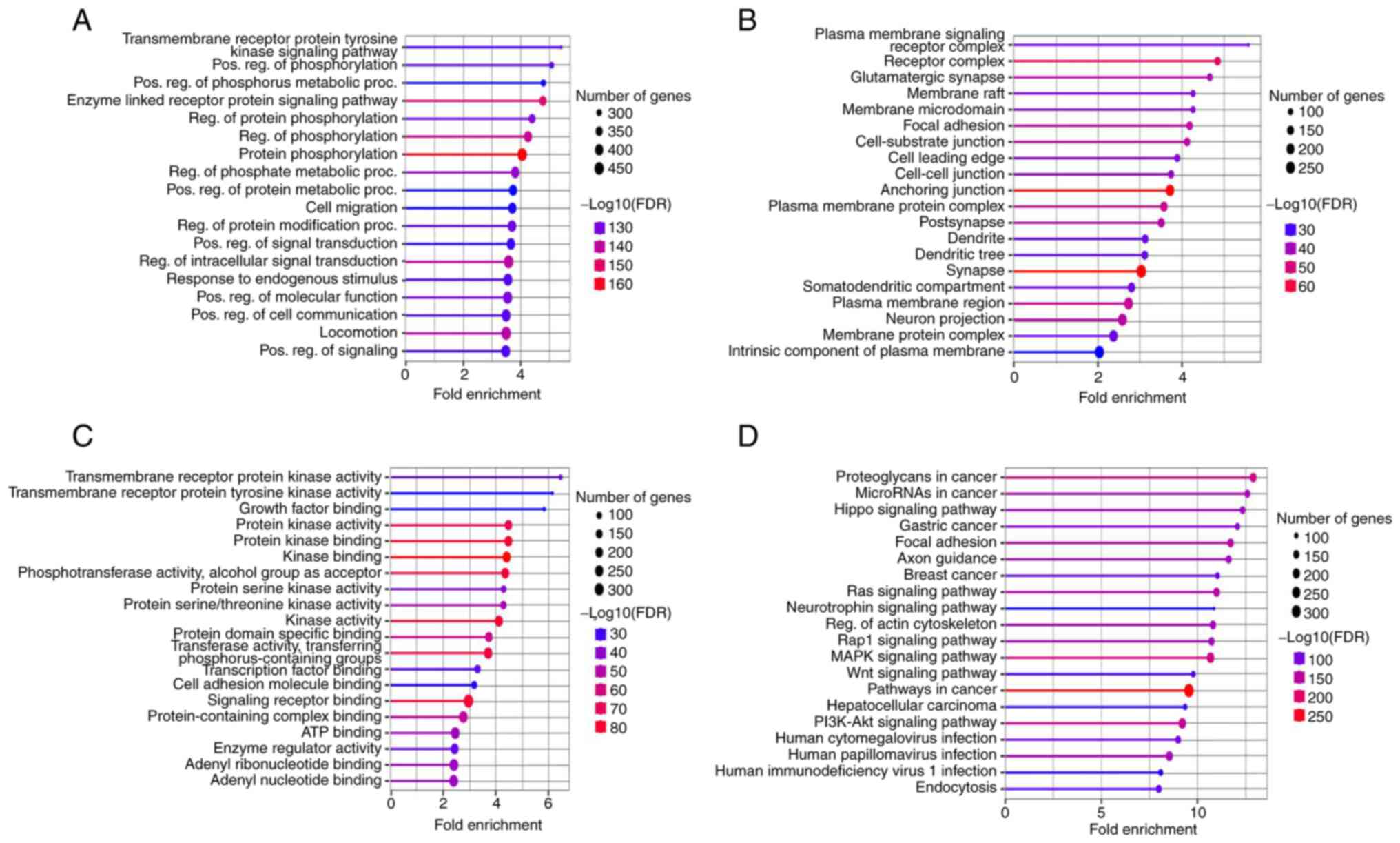
MF (Fig. 4). Most of the genes in
the category BP were active and related to responses to the MAPK
pathway, positive regulation of phosphorylation, protein
phosphorylation and regulation (Fig.
4A). In the category CC, the majority of the genes were
associated with ‘Plasma membrane signaling receptor complex’ and
‘RNA polymerase II transcription regulator complex and receptor
complex’ (Fig. 4B). The terms with
the maximum number of genes involved were ‘Growth factor activity’,
‘Kinase regulator activity’ and ‘Protein serine kinase activity’ in
the MF category (Fig. 4C).
Furthermore, KEEG pathways analysis revealed that the upregulated
miRNA target genes were predominantly involved in ‘Cancer
pathways’, ‘ErbB signaling cascade’ and in ‘Colorectal cancer
pathways’ (Fig. 4D).
Functional prediction for
downregulated genes
The top 15 miRNAs that were downregulated are
presented in Table IIC; miR-127-3p
had the highest abundance and miR-486-5p had the most significant
downregulation in patients with IgAN. The 15 downregulated miRNAs
have >1,600 target genes and the connections of these genes were
presented in a network including the interactions between them
(Fig. 5). The regulatory network
revealed that hsa-miR-29c-3p had the largest subnetwork, which
contained 245 potential target genes, while hsa-miR-1307-3p did not
have any regulatory network.
The gene functions for the downregulated miRNAs were
examined by performing KEGG analysis and GO enrichment in the
categories BP, CC and MF, as well as their associated pathways.
Most of the genes in the BP group were active and related to
responses to the transmembrane receptor ‘Protein tyrosine kinase
signaling pathway’ and ‘Positive regulation of phosphorylation and
phosphorus metabolic process’ (Fig.
6A). However, in the category CC, most of the genes were
involved in ‘Plasma membrane signaling receptor complex’,
‘Glutamatergic synapse’ and ‘Membrane raft’ (Fig. 6B). In the category MF, the genes
mostly covered ‘Transmembrane receptor protein kinase activity’,
‘Tyrosine kinase’ and ‘Growth factor binding’ functions (Fig. 6C). Furthermore, KEGG pathway
analysis revealed that ‘Proteoglycans in cancer’, ‘miRNA in cancer’
and ‘Hippo signaling pathway’ are the key pathways associated with
the majority of genes (Fig.
6D).
Discussion
IgAN is the most commonly occurring type of
glomerulonephritis worldwide in adults, with an estimated frequency
of 2.5 per 100,000 individuals per year (25). Although IgAN is characterized by the
aberrant glycosylation and deposition of IgA in mesangial areas,
the exact pathogenesis remains elusive. Renal biopsy is the
standard and most accurate method for IgAN diagnosis and disease
assessment. However, it is difficult to perform due to haematuria
and invasiveness. It has been indicated that miRNAs provide the
possibility of non-invasive diagnosis of IgAN, as they have
important roles in the occurrence and development of IgAN (25). miRNAs are also of certain value in
predicting the disease prognosis. In 2010, Wang et al
(26) described the relation of
urinary sediment miR-192 in IgAN with the severity of renal injury
and IgAN prognosis, which provided novel ideas for the study of
miRNAs in IgAN. The deregulation of miR-148b was a key factor in
the pathogenesis of IgAN, which may result in galactose-deficient
IgA1 in IgAN, as reported by Serino et al (27) in 2012. Since then, studies of miRNAs
have mainly focused on the mechanisms by which miRNAs influence the
pathogenesis of IgAN, the association between miRNAs and the
severity of IgAN and the relationship between miRNAs and the
prognosis of IgAN (27).
miRNAs have a vital role in regulating various
cellular processes, such as cell migration, proliferation and
apoptosis. miRNAs are stable, ubiquitous and abundant in body
fluids and cells; hence, miRNAs may be considered good biomarkers
as well as drug targets (28,29).
According to previous studies, miR-148b, let7b, miR-146a, miR-155,
miR-21, miR-200 and miR-429 miRNAs may be involved in IgAN
development (30). At the time of
writing, 2,885 published articles were identified using the search
terms ‘kidney’ and ‘microRNA’ in PubMed [US National Library of
Medicine (NLM) at the National Center for Biotechnology Information
(NCBI); https://pubmed.ncbi.nlm.nih.gov] in the last 5 years,
but the exact role of miRNA expression in the pathogenesis of IgAN
has not been well explored. In the present study, small RNA-seq was
performed for the identification of additional functional miRNAs
and the results demonstrated that nearly 75% of miRNAs were present
in renal tissues from both healthy individuals and patients with
IgAN. Among the 25 significant DEMs, miR-21-5p (31,32)
and miR-155-5p (22,28) were previously reported to be
associated with IgAN. However, the other three miRNAs, miR-146a,
miR-192 and miR-146b, were newly identified in patients with IgAN.
The immune regulator miR-146a is known to be upregulated in
patients with IgAN (22,28) and is significantly related to
inflammatory cell infiltration and interstitial lesions. In
addition, miR-192 is also associated with interstitial fibrosis and
epithelial-to-mesenchymal transition (28,33).
In the present study, amongst the top 10 upregulated miRNAs,
miR-21-5p had the maximum FC, followed by miR-146b-5p and
miR-155-5p. miR-181a-5p exhibited the most abundant expression in
patients with IgAN as well as in healthy individuals, followed by
miR-30c-5p. Amongst the top downregulated miRNAs, miR-486-5p had
the maximum FC, followed by miR-127-3p and miR-320c, and miR-184
exhibited the most abundant expression followed by miR-100-5b and
miR-320c in both healthy individuals as well as patients with IgAN.
The important factor for the progression of IgAN is inflammation).
miR-146a was previously reported to inhibit inflammation by
targeting tumor necrosis factor receptor-associated factor 6
(TRAF6), TLR4 and IL-1 receptor-associated kinase 1 and suppressed
NF-κB signaling (34). In addition,
the expression of miR-146a is also inhibited in TGF-β1-Smad
signaling and TRAF6-NF-κB signaling pathways (35,36).
In the present study, the expression of miR-146a-5p and miR-146b-5p
was also identified in renal tissues of patients with IgAN and the
upregulation of these two miRNAs was also found in tissue samples
targeting most of the genes along with TRL4, TRAF6, TGF-β1, SMAD
and NF-κB. miR-146a and miR-146b may decrease renal fibrosis by
inhibiting profibrotic and inflammatory signaling pathways and may
be used as another therapeutic target for renal fibrosis treatment;
these findings are in line with the previously published work by
Ichii et al (34) and Lin
et al (35). The present
study reported, for the first time, that the 5 upregulated miRNAs,
miR-181a-5p, miR-28-3p, let-7g-5p, miR-30c-5p, miR-92a-3p are
interconnected with IgAN disease. Numerous studies have indicated
that the overexpressed miRNAs may deregulate phosphatase and tensin
homolog (PTEN) and silencing PTEN at the post-transcriptional
level, considering another form of epigenetic modification
(37). Zhang et al (38) reported that miR-21 directly bound to
the 3'-UTR of PTEN and promoted the proliferation, migration and
invasion of gastric cancerous cells. Furthermore, the expression of
PTEN is increased in the PI3K/AKT/mTOR signaling pathway by the
antisense oligoneucleotides against PTEN, leading to reverse
effects on the cellular microenvironment of gastric cancerous cells
(38). In the present study, PTEN
was found to be the target gene of miR-21-5p, miR-155-5p,
miR-92a-3p and let-7g-5p miRNAs in the tissue samples of patients
with IgAN; however, the association of let-7g-5p and PTEN in
patients with IgAN remains to be further investigated. The
dysregulation of miR-92a-3p is known to have a role in tumorigenic
processes; therefore, it is associated with tumor progression and
prognosis. In 2018, Zhu et al (39) demonstrated that miR-92a-3p blocks
the progression of Wilms' tumor by targeting notch receptor 1
(NOTCH1). In the present study, NOTCH1 was also found as a targeted
gene of miR-92a-3p, suggesting NOTCH1 is the main target of
miR-92a-3p for the inhibition of the proliferation, migration and
invasion of tumor cells.
KEGG pathway and gene enrichment analysis identified
that the MAPK pathway, Cancer pathways, the ErbB signaling cascade,
Colorectal cancer pathway, Protein tyrosine kinase signaling
pathway, miRNAs in cancer, Proteoglycans in cancer and Hippo
signaling pathways were the most significantly enriched key
pathways associated with the majority of genes (Figs. 4D and 6D) and also involved in regulating cell
proliferation or IgAN development. These findings suggest that
miRNAs that are differentially expressed may be involved in the
progression and deposition of IgAN through their target genes
directly or by regulating various signaling pathways. Studies have
indicated that renal ischemia/reperfusion injury (I/R) frequently
occurs in kidney transplantations and acute kidney injuries and the
upregulation of miR-30c-5p has a reno-protective effect against
renal I/R by reducing inflammation (40). Schneider et al (41) reported that miR-28-3p is known to
control the proliferation of cells and is downregulated in B-cell
lymphomas and acts as a tumor suppressor. It is already reported
that tumor suppressor p53 has a major role in preventing tumors and
the activity or function of p53 may be regulated by miRNAs through
the direct suppression of p53 or its regulators in cells (42). In the present study, p53 was found
to be the only target of miR-28-3p, suggesting miR-28-3p may be
involved in the prevention of tumor progression in patients with
IgAN. Wu et al (43) also
demonstrated that the downregulation of miR-127-3p has an
inhibitory effect on IFN-1 signalling and may also inhibit the
induction of interferon-stimulated response element and
gamma-activated sequence-mediated gene expression in the renal
tissues of patients with lupus nephritis. In the present study,
miR-127-3p expression was significantly downregulated, which is in
agreement with the previously published reports. On the other hand,
miR-127-3p has been indicated to target BCL6, a key transcriptional
factor for the differentiation of follicular T-helper cells, and is
involved in immune disorders of various autoimmune diseases
(44-46).
This further supports that miR-127-3p may serve as a therapeutic
target for autoimmune diseases. However, to identify the unique
biomarkers for IgAN, further research is required to compare the
variations in the expression of these miRNAs with other types of
kidney disorders.
Over the last few years, the potential value of
miRNAs as biomarkers in IgAN has increasingly developed.
Identification of aberrant signaling pathways in IgAN may help to
uncover the fundamental molecular mechanisms behind those signaling
pathways linked to IgAN and to identify more promising molecular
candidates with effective diagnostic and prognostic value. These
findings further elucidate the pathogenesis of IgAN and help to
develop personalized treatments for IgAN. Reports have indicated
that circulating miRNA-mediated gene regulation is dynamic and
exhibits a bio-fluids-specific profile in relation to a
pathophysiological state. Besides, the attractiveness of
circulating miRNAs as biomarkers is associated with the
tissue-specific nature of miRNA expression. This gives rise to a
specific mechanism for intercellular communication and an
application of miRNAs as diagnostic/prognostic biomarkers (47,48).
Integrated bioinformatics analysis revealed that the pathways
identified in the present study may have important roles in the
pathogenesis of IgAN. The present study is a pilot study to
determine whether miRNAs are viable therapeutic targets for IgAN in
the Indian population, and the findings will serve as a springboard
for data collection in a larger sample. However, a restriction is
the limited sample size. Therefore, additional research using
larger sample sizes is necessary to confirm the biomarkers and look
into the molecular mechanisms underlying the aberrant signalling
pathways that led to the development of IgAN.
In conclusion, the current investigation discovered
25 miRNAs that were differentially expressed and linked to IgAN.
Many of these connections are novel and may have a significant role
in the pathogenesis of IgAN. In addition, the DEMs miR-181a-5p,
miR-92a-3p, let-7g-5p, miR-28-3p and miR-30c-5p may govern the
development of IgAN by controlling the behaviour of tissues or IgA
deposition via targeting the signaling pathways. It is of great
interest to the area of nephrology that these miRNA panels or
individual miRNAs are employed as circulating miRNA biomarkers or
in conjunction with other biomarkers and therapeutic targets for
IgAN diagnosis and therapy to increase the diagnostic value.
Acknowledgements
The authors would like to acknowledge Dr D.
Nageshwar Reddy, Chairman of AIG (Mayo Clinic Care Network)
Hospitals (Hyderabad, India) and Dr M. Sasikala, Director,
Translational Research Center, Asian Healthcare Foundation, AIG
Hospitals (Hyderabad, India) for their guidance in the execution of
the study.
Funding
Funding: This work was supported by the Granules India Project
Research Grant (grant no. GIG/AHF/2020-04).
Availability of data and materials
The datasets generated, used and/or analyzed during
the present study are available from the corresponding author upon
reasonable request. The NGS data are available from the Mendeley
Data repository [http://dx.doi.org/10.17632/j7bz9v33w8.1].
Authors' contributions
AT designed the study, performed all the experiments
and data analysis and prepared the manuscript. PCY and RVV
performed the bioinformatics analysis, participated in its
interpretation and revised the manuscript. AS performed the
histopathological analysis. SKR supervised and was involved in the
clinical diagnosis, surgical resection and providing the patients'
samples. AT and PCY verified the authenticity of the raw data. All
authors have read and approved the final version of the
manuscript.
Ethics approval and consent to
participate
The research study was approved by the Institutional
Research and Ethics Committee of AIG Hospital (Hyderabad, India;
AHF/AIGH-IRB: 02/47/2021) and written informed consent was obtained
from all patients included in the study. The study protocol
conformed to the ethical guidelines of the 1975 Declaration of
Helsinki.
Patient consent for publication
Not applicable.
Competing interests
All the authors declare that they have no competing
interests.
References
|
1
|
Pawluczyk I, Nicholson M, Barbour S, Er L,
Selvaskandan H, Bhachu JS and Barratt J: A pilot study to predict
risk of IgA nephropathy progression based on miR-204 expression.
Kidney Int Rep. 6:2179–2188. 2021.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Das U, Dakshinamurty KV, Prayaga A and
Uppin M: Spectrum of IgA nephropathy in a single center. Saudi J
Kidney Dis Transpl. 26:1057–1063. 2015.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Selvaskandan H, Pawluczyk I and Barratt J:
MicroRNAs: A new avenue to understand, investigate and treat
immunoglobulin A nephropathy? Clin Kidney J. 11:29–37.
2018.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Molyneux K, Wimbury D, Pawluczyk I, Muto
M, Bhachu J, Mertens PR, Feehally J and Barratt J:
β1,4-galactosyltransferase 1 is a novel receptor for IgA in human
mesangial cells. Kidney Int. 92:1458–1468. 2017.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Currie EG, Coburn B, Porfilio EA, Lam P,
Rojas OL, Novak J, Yang S, Chowdhury RB, Ward LA, Wang PW, et al:
Immunoglobulin A nephropathy is characterized by anticommensal
humoral immune responses. JCI Insight. 7(e141289)2022.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Suzuki H, Kiryluk K, Novak J, Moldoveanu
Z, Herr AB, Renfrow MB, Wyatt RJ, Scolari F, Mestecky J, Gharavi AG
and Julian BA: The pathophysiology of IgA nephropathy. J Am Soc
Nephrol. 22:1795–1803. 2011.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Lv J, Wong MG, Hladunewich MA, Jha V, Hooi
LS, Monaghan H, Zhao M, Barbour S, Jardine MJ, Reich HN, et al:
Effect of oral methylprednisolone on decline in kidney function or
kidney failure in patients with IgA nephropathy: The TESTING
randomized clinical trial. JAMA. 327:1888–1898. 2022.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Lai KN, Tang SC, Schena FP, Novak J,
Tomino Y, Fogo AB and Glassock RJ: IgA nephropathy. Nat Rev Dis
Primers. 2(16001)2016.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Chuammitri P, Vannamahaxay S, Sornpet B,
Pringproa K and Patchanee P: Detection and characterization of
microRNA expression profiling and its target genes in response to
canine parvovirus in crandell reese feline kidney cells. PeerJ.
8(e8522)2020.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Yanaihara N, Caplen N, Bowman E, Seike M,
Kumamoto K, Yi M, Stephens RM, Okamoto A, Yokota J, Tanaka T, et
al: Unique microRNA molecular profiles in lung cancer diagnosis and
prognosis. Cancer Cell. 9:189–198. 2006.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Huang YS, Dai Y, Yu XF, Bao SY, Yin YB,
Tang M and Hu CX: Microarray analysis of microRNA expression in
hepatocellular carcinoma and non-tumorous tissues without viral
hepatitis. J Gastroenterol Hepatol. 23:87–94. 2008.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Iorio MV, Ferracin M, Liu CG, Veronese A,
Spizzo R, Sabbioni S, Magri E, Pedriali M, Fabbri M, Campiglio M,
et al: MicroRNA gene expression deregulation in human breast
cancer. Cancer Res. 65:7065–7070. 2005.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Schetter AJ, Leung SY, Sohn JJ, Zanetti
KA, Bowman ED, Yanaihara N, Yuen ST, Chan TL, Kwong DL, Au GK, et
al: MicroRNA expression profiles associated with prognosis and
therapeutic outcome in colon adenocarcinoma. JAMA. 299:425–236.
2008.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Bloomston M, Frankel WL, Petrocca F,
Volinia S, Alder H, Hagan JP, Liu CG, Bhatt D, Taccioli C and Croce
CM: MicroRNA expression patterns to differentiate pancreatic
adenocarcinoma from normal pancreas and chronic pancreatitis. JAMA.
297:1901–1908. 2007.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Schultz NA, Werner J, Willenbrock H,
Roslind A, Giese N, Horn T, Wøjdemann M and Johansen JS: MicroRNA
expression profiles associated with pancreatic adenocarcinoma and
ampullary adenocarcinoma. Mod Pathol. 25:1609–1622. 2012.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Rozenblum E, Schutte M, Goggins M, Hahn
SA, Panzer S, Zahurak M, Goodman SN, Sohn TA, Hruban RH, Yeo CJ and
Kern SE: Tumor-suppressive pathways in pancreatic carcinoma. Cancer
Res. 57:1731–1734. 1997.PubMed/NCBI
|
|
17
|
Jansson MD and Lund AH: MicroRNAs and
cancer. Mol Oncol. 6:590–610. 2012.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Vegter EL, van der Meer, de Windt LJ,
Pinto MY and Voors AA: MicroRNAs in kidney physiology and disease.
Eur J Heart Fail. 18:457–468. 2016.
|
|
19
|
Trionfini P, Benigni A and Remuzzi G:
MicroRNAs in kidney physiology and disease. Nat Rev Nephrol.
11:23–33. 2015.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Liu L, Duan A, Guo Q, Sun G, Cui W, Lu X,
Yu H and Luo P: Detection of microRNA-33a-5p in serum, urine and
renal tissue of patients with IgA nephropathy. Exp Ther Med.
21(205)2021.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Wang G, Kwan BC, Lai FM, Choi PC, Chow KM,
Li PK and Szeto CC: Intrarenal expression of microRNAs in patients
with IgA nephropathy. Lab Invest. 90:98–103. 2010.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Wang G, Kwan BC, Lai FM, Chow KM, Li PK
and Szeto CC: Elevated levels of miR-146a and miR-155 in kidney
biopsy and urine from patients with IgA nephropathy. Dis Markers.
30:171–179. 2011.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Qin W, Chung AC, Huang XR, Meng XM, Hui
DS, Yu CM, Sung JJ and Lan HY: TGF-β/Smad3 signaling promotes renal
fibrosis by inhibiting miR-29. J Am Soc Nephrol. 22:1462–1474.
2011.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Politano G, Orso F, Raimo M, Benso A,
Savino A, Taverna D and Di Carlo S: CyTRANSFINDER: A Cytoscape 3.3
plugin for three-component (TF, gene, miRNA) signal transduction
pathway construction. BMC Bioinformatics. 17(157)2016.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Scionti K, Molyneux K, Selvaskandan H,
Barratt J and Cheung CK: New insights into the pathogenesis and
treatment strategies in IgA nephropathy. Glomerular Dis. 2:15–29.
2021.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Wang G, Kwan BC, Lai FM, Chow KM, Kam-Tao
Li P and Szeto CC: Expression of microRNAs in the urinary sediment
of patients with IgA nephropathy. Dis Markers. 28:79–86.
2010.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Serino G, Sallustio F, Cox SN, Pesce F and
Schena FP: Abnormal miR-148b expression promotes aberrant
glycosylation of IgA1 in IgA nephropathy. J Am Soc Nephrol.
23:814–824. 2012.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Yao X, Zhai Y, An H, Gao J, Chen Y, Zhang
W and Zhao Z: MicroRNAs in IgA nephropathy. Ren Fail. 43:1298–1310.
2021.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Liang H, Gong F, Zhang S, Zhang CY, Zen K
and Chen X: The origin, function, and diagnostic potential of
extracellular microRNAs in human body fluids. Wiley Interdiscip Rev
RNA. 5:285–300. 2014.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Wang J, Chen J and Sen S: MicroRNA as
biomarkers and diagnostics. J Cell Physiol. 231:25–30.
2016.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Xu BY, Meng SJ, Shi SF, Liu LJ, Lv JC, Zhu
L and Zhang H: MicroRNA-21-5p participates in IgA nephropathy by
driving T helper cell polarization. J Nephrol. 33:551–560.
2020.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Rodriguez A, Vigorito E, Clare S, Warren
MV, Couttet P, Soond DR, van Dongen S, Grocock RJ, Das PP, Miska
EA, et al: Requirement of bic/microRNA-155 for normal immune
function. Science. 316:608–611. 2007.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Fan Q, Lu R, Zhu M, Yan Y, Guo X, Qian Y,
Zhang L, Dai H, Ni Z and Gu L: Serum miR-192 is related to
tubulointerstitial lesion and short-term disease progression in IgA
nephropathy. Nephron. 142:195–207. 2019.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Ichii O, Otsuka S, Sasaki N, Namiki Y,
Hashimoto Y and Kon Y: Altered expression of microRNA miR-146a
correlates with the development of chronic renal inflammation.
Kidney Int. 81:280–292. 2012.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Lin TJ, Yang SS, Hua KF, Tsai YL, Lin SH
and Ka SM: SPAK plays a pathogenic role in IgA nephropathy through
the activation of NF-κB/MAPKs signaling pathway. Free Radic Biol
Med. 99:214–224. 2016.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Wang Z, Liao Y, Wang L, Lin Y, Ye Z, Zeng
X, Liu X, Wei F and Yang N: Small RNA deep sequencing reveals novel
miRNAs in peripheral blood mononuclear cells from patients with IgA
nephropathy. Mol Med Rep. 22:3378–3386. 2020.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Hu M, Zhu S, Xiong S, Xue X and Zhou X:
MicroRNAs and the PTEN/PI3K/Akt pathway in gastric cancer (review).
Oncol Rep. 41:1439–1454. 2019.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Zhang BG, Li JF, Yu BQ, Zhu ZG, Liu BY and
Yan M: microRNA-21 promotes tumor proliferation and invasion in
gastric cancer by targeting PTEN. Oncol Rep. 27:1019–1026.
2012.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Zhu S, Zhang L, Zhao Z, Fu W, Fu K, Liu G
and Jia W: MicroRNA-92a-3p inhibits the cell proliferation,
migration and invasion of Wilms tumor by targeting NOTCH1. Oncol
Rep. 40:571–578. 2018.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Zhang C, Yu S, Zheng B, Liu D, Wan F, Ma
Y, Wang J, Gao Z and Shan Z: miR-30c-5p reduces renal
ischemia-reperfusion involving macrophage. Med Sci Monit.
25:4362–4369. 2019.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Schneider C, Setty M, Holmes AB, Maute RL,
Leslie CS, Mussolin L, Rosolen A, Dalla-Favera R and Basso K:
MicroRNA 28 controls cell proliferation and is down-regulated in
B-cell lymphomas. Proc Natl Acad Sci USA. 111:8185–8190.
2014.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Feng Z, Zhang C, Wu R and Hu W: Tumor
suppressor p53 meets microRNAs. J Mol Cell Biol. 3:44–50.
2011.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Wu L, Han X, Jiang X, Ding H, Qi C, Yin Z,
Xiao J, Xiong L, Guo Q, Ye Z, et al: Downregulation of renal
Hsa-miR-127-3p contributes to the overactivation of type I
interferon signaling pathway in the kidney of lupus nephritis.
Front Immunol. 12(747616)2021.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Saito Y, Liang G, Egger G, Friedman JM,
Chuang JC, Coetzee GA and Jones PA: Specific activation of
microRNA-127 with downregulation of the proto-oncogene BCL6 by
chromatin-modifying drugs in human cancer cells. Cancer Cell.
9:435–443. 2006.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Craft JE: Follicular helper T cells in
immunity and systemic autoimmunity. Nat Rev Rheumatol. 8:337–347.
2012.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Lv W, Fan F, Wang Y, Gonzalez-Fernandez E,
Wang C, Yang L, Booz GW and Roman RJ: Therapeutic potential of
microRNAs for the treatment of renal fibrosis and CKD. Physiol
Genomics. 50:20–34. 2018.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Li JY, Yong TY, Michael MZ and Gleadle JM:
Review: The role of microRNAs in kidney disease. Nephrology
(Carlton). 15:599–608. 2010.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Miguel V: The extracellular miRNA
fingerprint of kidney disease: A narrative review. ExRNA.
4(12)2022.
|