1. Introduction
Hypertension is one of the most common chronic
diseases in the world. It may lead to target organ damage, which is
a major cause of increased morbidity and mortality from
cardiovascular disease. The disease burden attributable to arterial
hypertension is substantial, accounting for 62% of all strokes and
49% of all cases of heart disease. Furthermore, it culminates in an
estimated 7.1 million deaths a year, equivalent to 13% of total
worldwide deaths. In addition, those individuals classified as
having resistant hypertension (RH) have been observed to have
increased target organ damage and cardiovascular risk. Compared to
those hypertensive patients with superior pharmacological
treatment, the damage and above-mentioned risk are still
increasing, in spite of them being treated with anti-hypertensive
medications (typically an angiotensin-converting enzyme inhibitor
or angiotensin II receptor blocker with a calcium channel blocker
and a thiazide/thiazide-type diuretic). The prevalence of RH varies
by definition from study to study, with a reported prevalence range
of 5-30% in patients with treated hypertension. However, compared
with the general hypertensive population, the population with RH is
older and contains a high-risk sub-population with more
cardiovascular complications (1).
Thus, patients with RH are encouraged to adhere to intensive
lifestyle-based therapy with salt restriction, aerobic exercise,
smoking cessation and weight reduction in conjunction with
pharmacological blood pressure (BP) control. However, medication
side effects, poor adherence to treatment and difficulty in
adhering to lifestyle changes limit BP control to target values in
patients with RH. Due to the target organ damage and the autonomic
imbalance, RH has become common and costly (2). In recent years, there has been an
increasing emphasis on complementary and alternative treatments
without medication. Furthermore, growing evidence has been provided
for bio-electric techniques in the treatment of hypertension, with
results seen in the fields of renal sympathetic denervation (RDN),
carotid baroreflex activation therapy (CBAT) and Chinese medicine
electroacupuncture for the lowering of BP. However, only a small
number of studies on transcutaneous electrical nerve stimulation
(TENS) applied to hypertension are currently available. This
technique is gradually gaining attention due to its low side
effects and high acceptability, and the application prospect is
promising. In the present review, the progress, advantages and
disadvantages of common bio-electric techniques for the treatment
of RH were discussed in order to deepen the knowledge in this
field.
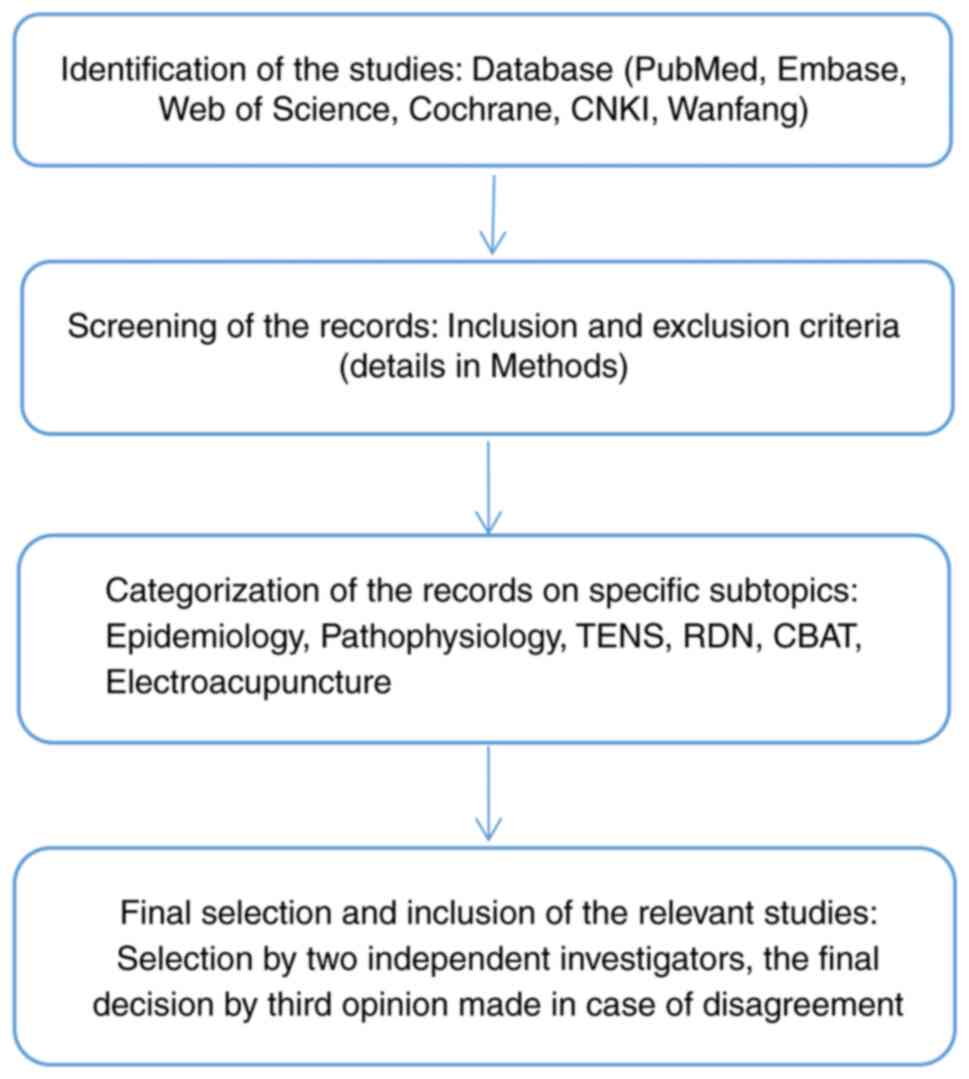
2. Methodology
Search strategy and study
selection
The literature search was conducted covering six
important online databases (PubMed, Embase, Web of Science,
Cochrane, Chinese National Knowledge Infrastructure and Wanfang)
using relevant keywords for the present study on the topic of
interest. For the epidemiological and pathophysiology information,
the following terms were used: ‘resistant hypertension’,
‘hypertension’, ‘blood pressure’, ‘epidemiology’, ‘pathophysiology’
and ‘mechanism’. Only research conducted on humans (double-blind,
single-blind and unblinded trials) published after the year 2,000
was included. Abstract-only articles, letters to editors and
studies on animal or cell models were excluded. When referring to
investigations on bioelectric techniques and therapeutic options,
medical subject headings terms, such as ‘transcutaneous electric
nerve stimulation’, ‘renal sympathetic denervation’, ‘carotid
baroreflex activation therapy’ and ‘electroacupuncture’ were used.
The same study inclusion criteria as those stated above were
applied (Fig. 1).
Diagnostic criteria
Optimal doses (or best-tolerated doses) of an
appropriate therapeutic strategy, which should include a diuretic
(typically an angiotensin-converting enzyme inhibitor or an
angiotensin II receptor blocker with a calcium channel blocker and
a thiazide/thiazide-type diuretic), fail to lower the clinical
systolic BP (SBP) and diastolic BP (DBP) values to <140 and/or
<90 mmHg, respectively. Inadequate control of the BP has been
confirmed by ambulatory BP monitoring (ABPM) or home BP monitoring
(HBPM), and after the exclusion of various causes of pseudo-RH
(particularly poor medication adherence) and secondary hypertension
(3). The diagnosis of RH is
becoming more clear after several international guidelines for
hypertension unified their definitions, but the details require to
be further explored. According to the latest 2018 European Society
of Cardiology/European Society of Hypertension guidelines for the
management of arterial hypertension (4), the diagnosis of RH requires detailed
information regarding the following items: i) The patient's
history, including lifestyle characteristics, alcohol and dietary
sodium intake, interfering drugs or substances and sleep history;
ii) the nature and dosing of the antihypertensive treatment; iii) a
physical examination, with a particular focus on determining the
presence of hypertension-mediated organ damage (HMOD) and signs of
secondary hypertension; iv) confirmation of treatment resistance by
out-of-office BP measurements (i.e. ABPM or HBPM); v) laboratory
tests to detect electrolyte abnormalities (hypokalaemia),
associated risk factors (diabetes), organ damage (advanced renal
dysfunction) and secondary hypertension; vi) confirmation of
adherence to BP-lowering therapy.
3. High prevalence and treatment bottleneck
with RH
Due to the complexity of the diagnosis of RH, it has
rarely been included in large experimental studies. In addition,
definitive epidemiological data are not available internationally.
However, the importance of RH cannot be ignored. Over the past
decade, RH has appeared to account for a larger proportion of all
hypertensive patients receiving treatment. The findings of a
meta-analysis indicated that the prevalence of RH among patients
with treated hypertension ranged between 13.72 and 16.32% (5). This data suggests that the prevalence
of RH is significant. Furthermore, the ReHOT study recruited
patients with hypertension stage 2 (never treated or under previous
antihypertensive treatment) at 26 sites in Brazil. A more precise
assessment indicated that the prevalence of RH using a 12-week
open-label forced-titration regimen of 3 antihypertensive drugs was
11.7%. Patients with true RH were then randomized into 2 treatment
groups comparing the 2 drugs spironolactone vs. clonidine as a
fourth-drug therapy (fourth drug to be added to the commonly
prescribed triple antihypertension regimen) for RH. The results
indicated that clonidine was not superior to spironolactone as a
fourth-drug therapy in patients with RH (6). No ideal medication or treatment
adherence technique is available. Furthermore, the 2017 American
College of Cardiology/American Heart Association hypertension
guidelines suggest that the treatment goal for RH should be a BP
<130/80 mmHg (7). A more simple
definition of RH and more aggressive anti-hypertensive goals would
lead to a substantial increase in the prevalence of RH among those
who have already received medication. RH is likely to be <10% of
treated patients, even if the optimal drug treatment of RH has been
poorly studied. However, patients with RH are at higher risk of
HMOD, chronic kidney disease and premature cardiovascular events.
Diabetes mellitus or BP ≥180/100 mmHg at study entry was
independently associated with an RH diagnosis (6). The increased prevalence and the
magnitude of the risk of its coexisting conditions have led to a
further focus on the importance of RH in the field of hypertension.
In the case of resistance to existing drugs, it is worth paying
attention to the improvement of the treatment of RH through the
pathophysiological mechanisms of RH, which are complex. In the
study of new anti-hypertensive drugs that may treat hypertension in
different ways, whether they are able to cooperate with other
auxiliary methods to treat RH or whether they may increase the
medical compliance of patients or increase the antihypertensive
efficacy requires to be investigated.
4. Pathophysiology by which mechanisms
promote RH
Multifactorial stimulation
The pathogenesis of RH is the persistence of
multiple factors that influence the abnormal activation of the
central integration system. Studies have indicated that
continuously activated sympathetic as well as
renin-angiotensin-aldosterone system (RAAS) activity are among the
important pathogenic mechanisms of RH (8). RH tends to have sustained stimulation
by longer-term risk factors. Specifically, there may be four major
pathways, including sodium overload, sympathetic nervous system
activation, endothelial dysfunction and atherosclerosis. These
co-stimulations cause vascular remodeling and volume expansion,
leading to the development of RH. On the basis of multiple risk
factors, excessive activation of the RAAS in vivo increases
sympathetic activity in central or local tissues and activates
inflammatory factors. The resulting state of inflammation initiates
oxidative stress processes and promotes the progression of arterial
calcification and atherosclerosis, exacerbating abnormalities in
vascular structure and function and causing systemic vascular
remodeling. Thus, the series of changes make the BP, which had
already reached beyond the limit, more difficult to control. Under
pathological conditions, reactive oxygen species production may
cause oxidative stress, leading to endoplasmic reticulum stress and
autophagy, resulting in vascular endothelial dysfunction, vascular
remodeling and reduced compliance (9). The renal sympathetic nerve is composed
of afferent and efferent fibers. Excessive activation of renal
sympathetic afferent fibers enhances central sympathetic nervous
system activity, resulting in systemic sympathetic hyperactivity
and increased adrenaline release, which causes structural and
functional changes in target organs such as the kidney, heart and
blood vessels. Eventually, the maintenance and progression of
hypertension are established. Excessive excitation of renal
sympathetic efferent fibers leads to the production and excessive
secretion of norepinephrine. Thereby, renal vasoconstriction and
decreased renal blood flow occur, activating the renal and systemic
RAAS systems; norepinephrine also causes the small inlet arteries
to constrict more than the small outlet arteries, resulting in a
decreased glomerular filtration rate and increased sodium
reabsorption; at the same time, the stimulated granulosa cells
release renin, which also further activates the RAAS. The above
pathophysiological processes exacerbate the elevated BP levels and
participate in the maintenance and progression of RH (10-12).
Central integration
The role of central integration has received
increasing attention in the study of pathogenesis in hypertension.
The central nervous system (CNS) controls peripheral vasodilation
and vasoconstriction mainly through the vegetative nerves. The
regulatory centers are mainly in the medulla oblongata, pons,
hypothalamus and other parts of certain nerve clusters. In recent
years, rostral ventrolateral medulla (RVLM) has been considered to
be closely related to the occurrence and development of
hypertension. The RVLM is the final pathway of the CNS in the
regulation of cardiovascular activity and is indispensable in
maintaining BP homeostasis (13).
The nucleus tractus solitarius (NTS) is located in the RVLM. The
NTS is the main central relay station that integrates multiple
inhibitory and excitatory sensory inputs from the viscera, as well
as downstream cortical inputs. Carotid pressure receptors send
axons via the glossopharyngeal and vagus nerves. Aortic pressure
receptors send axons via the aortic inhibitory nerves to the cell
bodies in nodal ganglia. Then both ways assemble in the NTS
(14). Over-regulation by the
center after receiving abnormally activated peripheral signals is
significantly associated with RH. If the body is subjected to a
sustained increase in arterial pressure, the pressure reflex adapts
to the new ‘normal’ and the receptors are reset to respond to the
higher pressure with reduced sensitivity. The increase in
intravascular volume leads to activation of stretch-sensitive nerve
fibers in pressure receptors located in the carotid sinus, aortic
arch and thoracic great vessels. As arterial pressure increases,
the rate of firing through the glossopharyngeal and vagus nerves
into the medullary dorsal solitary bundle nuclei also increases.
The brain interprets the increased signal as an increase in BP and
attempts to counteract the perceived increase by transmitting the
signal to various end organs (15).
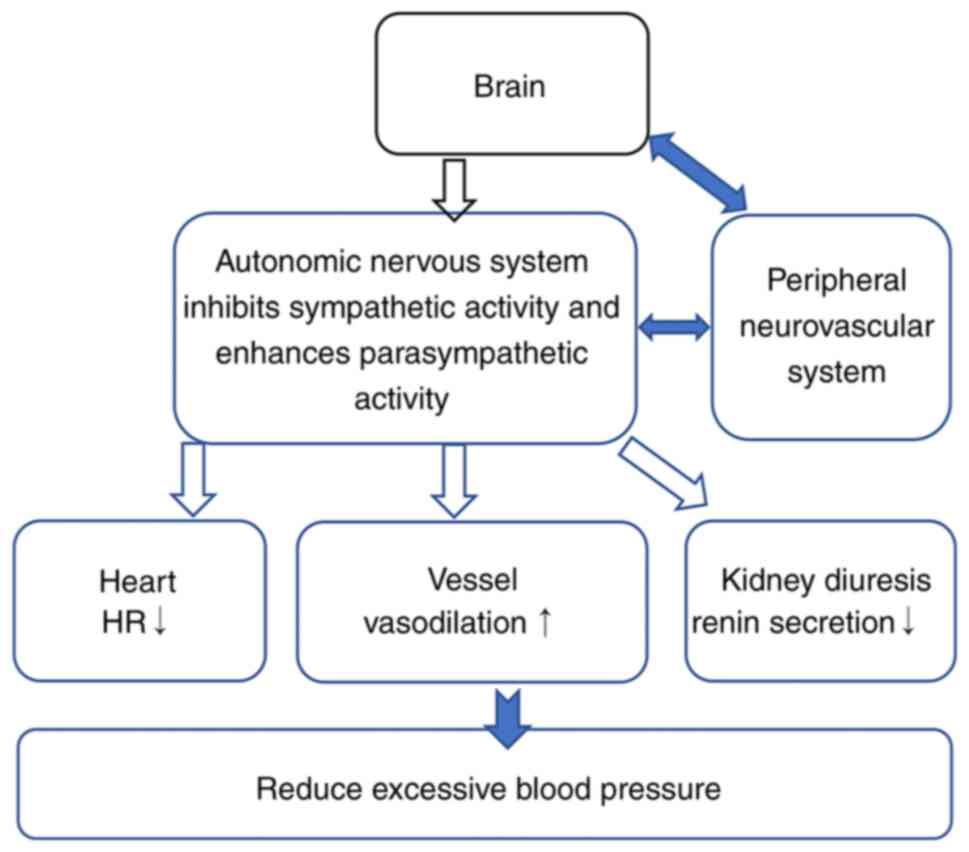
The lateral ventral lateral medullary region and the medullary
questioning nucleus result in decreased sympathetic outflow
(inhibitory effect) and increased parasympathetic outflow
(excitatory effect). The practical effect is a decrease in heart
rate, myocardial contractility and vascular tone, sodium benefit
and hypotension (Fig. 2).
5. What is bioelectricity technology?
Bioelectricity is a technical method for diagnosis
and treatment of human diseases using electrical stimulation and
biofeedback that collects electrical signals from living organisms
(human body). Bioelectric technology may be divided into
electrophysiological examination and electrophysiological
treatment. Common electrophysiological examinations include
electrocardiogram and electromyogram, and treatments include
electroshock defibrillation, cardiac pacing, pulsed radiofrequency
techniques and other clinically common modalities. What is being
described in the present study is mainly the electrical stimulation
therapy in electrophysiological treatment, i.e., the application of
electric current without producing significant heat consists of
electrical stimulation of nerves or muscles, the passage of
electric currents into the body or the use of low-intensity
interrupted currents to raise the skin's threshold for pain
detection. Electrical stimulation therapies include artificial
pacemakers (cardiac resynchronization therapy), deep brain
stimulation, electroshock, electroacupuncture, pulsed
radiofrequency therapy, spinal cord stimulation, intracranial
direct current stimulation, TENS and vagus nerve stimulation.
Electrical stimulation therapy is now widely used in fields such as
sports rehabilitation and postoperative analgesia. It may indicate
a normal or abnormal muscle activity state through biofeedback and
guide patients to carry out correct muscle rehabilitation training.
It is used to stimulate nerve reflexes or neuromuscles by means of
electrical pulses of different frequencies, which may awaken the
proprioceptors, make the muscles stretch passively, have an
analgesic effect and promote blood circulation.
Electrical stimulation in rehabilitation therapy can
not only improve motor deficits at individual sites, but also
connect systems to create functional multi-joint movements. TENS,
originally designed as an electrophysiological technique to
overcome painful impulses, is a form of low-frequency stimulation
that acts on smaller afferent sensory fibres. Low-frequency TENS
does not cause site muscle contraction because it only acts on
sensory nerve fibers and does not activate motor fibers (16). Low-frequency high-intensity TENS
enhances ongoing alpha oscillations in the primary sensorimotor
cortex and increasing functional connectivity between the brains,
leading to long-term changes in ongoing brain activity. This
TENS-induced regulation of sustained brain states allows for a
wider range of analgesic effects of low-frequency and
high-intensity TENS (17). There is
moderate-quality evidence that TENS reduces pain and has no serious
adverse events compared to placebo (18). TENS may also be applied in the
treatment of neurological disorders. TENS may improve cognition and
behavior in patients with early and mid-stage Alzheimer's disease.
This is evidenced by improvements in long-term and short-term
memory, verbal fluency, circadian rest activity rhythms, as well as
physical, social and emotional functioning of patients (16). A study indicated that proprioceptive
neuromuscular facilitation (PNF) stretch combined with TENS on the
triceps gastrocnemius muscle triggered muscle contraction during
the muscle contraction phase of PNF stretch, significantly improved
ankle function in subjects after ankle sprain and was a good
adjunctive rehabilitation technique (19). In most countries, TENS devices and
accessories are available without prescription, and the running
costs and follow-up clinical support for TENS are not expensive.
These conditions allow for self-administration of the treatment
without risk of harm, thus potentially relieving patients' symptoms
throughout the day (Fig. 3).
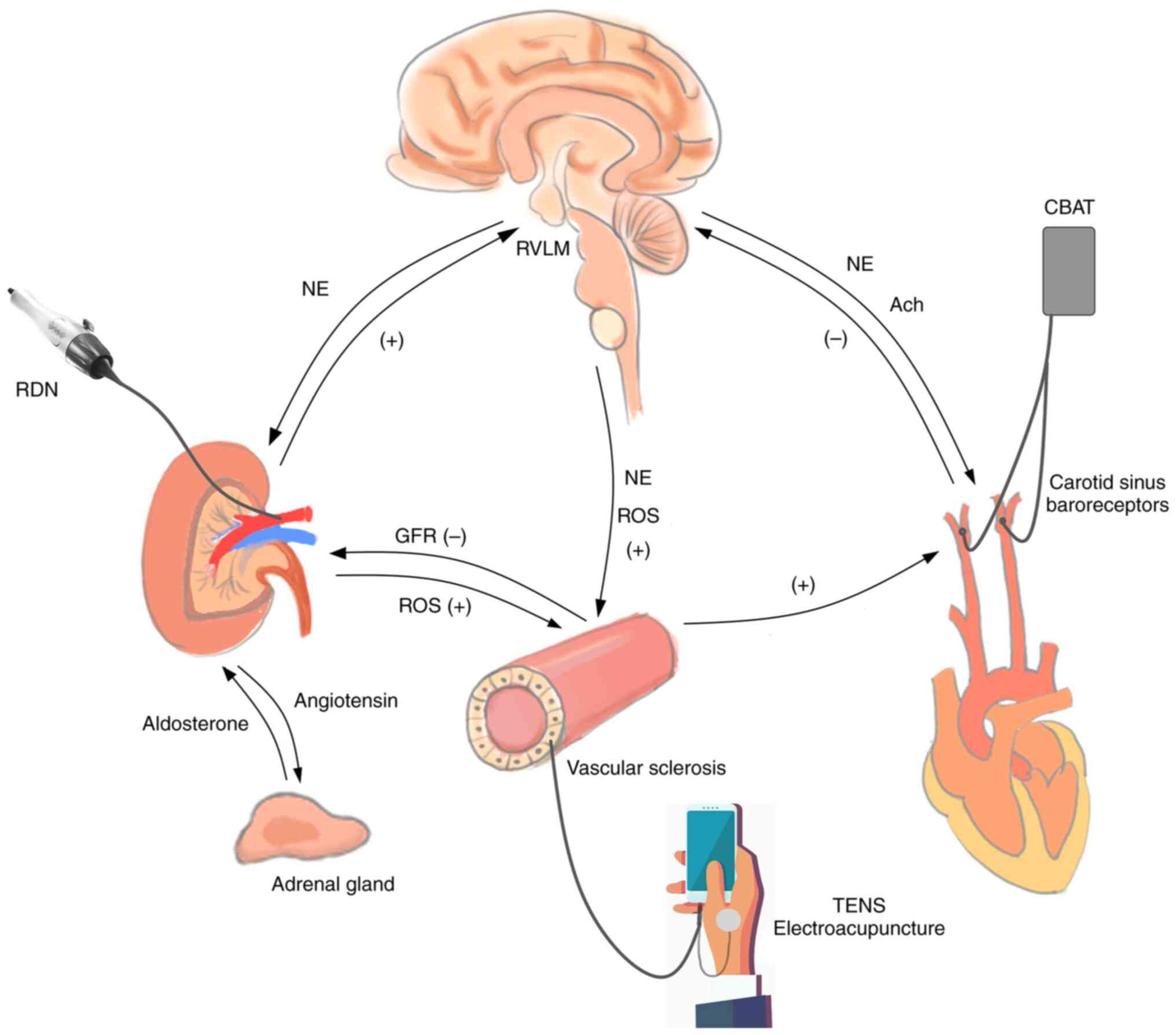 | Figure 3Mechanism of central integration and
bioelectric technology. RDN, renal sympathetic denervation; CBAT,
carotid baroreflex activation therapy; TENS, transcutaneous
electrical nerve stimulation; RVLM, rostral ventrolateral medulla;
NE, noradrenaline; Ach, acetylcholine; ROS, reactive oxygen
species; GFR, glomerular filtration rate; (+), promotion; (-),
restraint. |
6. Role of bioelectric technology in RH
Renal sympathetic denervation for
RH
RDN mainly includes radiofrequency, ultrasound,
chemical ablation and cryoablation. The persistent increase in the
activity of the renal sympathetic-associated RAAS system is one of
the important pathogenic mechanisms of RH, which has been confirmed
by numerous basic experiments (20). The emergence of RDN based on these
mechanisms is considered to be a superior means of lowering BP in
the future, in addition to the possibility of renal protection
(21). The widely studied and
effective first-generation radiofrequency RDN catheter has problems
such as being affected by respiration, difficult operation and
inadequate ablation, but related trials have also obtained
significant antihypertensive efficacy with the advantage of
sustained pressure reduction (22-24).
The second-generation catheter currently used is able to better
achieve combined ablation of the renal aorta and branches than the
first-generation catheter, which overcomes certain technical
difficulties of the first-generation catheter and improves the
ablation effect (25,26). SPYRAL HTN-OFF MED is an
international multicenter randomized single-blind sham-operated
controlled study that included patients with mild to moderate
hypertension (non-RH), with up to 1 antihypertensive drug applied.
The patients were randomized into either the RDN group or the
sham-operated group. The study used a second-generation standard
spiral spherical catheter, which may reach branch arteries of 3-8
mm in diameter, to perform a combined ablation of the distal renal
artery and branch vessels in the RDN group, and found that the 24-h
ABP decreased better in the RDN group than in the sham-operated
group at 3 months after the procedure, and there were no adverse
effects in either group (26,27).
The RADIANCE-HTN SOLO study indicated that intravascular ultrasound
RDN was significantly more effective in lowering BP than
intracatheter ablation of RDN (28); however, further evidence is required
to confirm this. Numerous existing studies have demonstrated good
results of RDN application in mild to moderate hypertension
(22,28,29).
However, based on the obvious correlation between the mechanism of
RDN in lowering BP and the pathophysiological mechanism of RH, the
application of RDN in RH still cannot be ignored. The effect of RDN
treatment on RH is also significant. The SPYRAL HTN-ON MED study of
patients with RH based on ABPM and evaluation at 6 months indicated
reduced RDN in the main renal arteries and branches compared to
sham controls without any major safety event (25). The current clinical trials of RDN
for RH have problems of patient population selection and a lesser
amount of data, and a short follow-up time. One study found a more
pronounced decrease in BP in patients with RH with high renin after
RDN therapy (30). In addition,
young and middle-aged patients with high renin activity and high
DBP and SBP are more suitable for RDN (30,31).
There are also a small number of trials demonstrating the safety of
RDN for RH, but the current paucity of evidence also poses
limitations for the use of RDN. Thus, regarding RDN treatment for
RH, further exploration of the efficacy and safety, further
improvement of the procedure and devices, selection of the patients
who are most likely to benefit from the treatment and more accurate
detection methods are required.
CBAT for RH
CBAT is one of the bioelectrical applications based
on the carotid baroreflex and is central integration-related.
Animal studies have indicated that stimulation of the carotid
baroreceptors in dogs results in a significant decrease in BP and
prolonged activation of the pressure reflex, leading to a sustained
increase in arterial pressure and sustained sympathetic excitation
(32). A US company,
CVRx®, has developed a carotid pressure receptor
electrical stimulation device and the second generations of the
current device have been tested in clinical trials. The
first-generation Rheos™ system consists of a pulse generator and
two carotid sinus electrodes. Clinical trials related to the system
have confirmed certain hypotensive efficacy in lowering BP, but
safety appears to be lacking. In the Rheos pivotal trial, 86,265
patients with RH with a mean baseline BP 169/101 mmHg received
bilateral implantation of the Rheos™ device. All patients received
pressure receptor modulation for an additional 6 months for various
periods, with 50% achieving intra-month systolic BP control at 12
months, but 9% experienced transient or permanent facial nerve
injury (33). It was indicated that
after 6 months of CBAT, the greatest reduction in systolic BP was
achieved with unilateral right carotid stimulation, intermediate
values of pressure reduction with bilateral stimulation, and the
smallest reduction in systolic BP with unilateral left stimulation,
which is consistent with animal studies demonstrating the
superiority of the right carotid pressure reflex (34,35).
The second-generation device, Barostim neo™, based on the Rheos™,
requires only unilateral carotid sinus electrode implantation, is
smaller and lighter, has a longer battery life, improved surgical
implantation procedures and safety, and has no clear difference in
efficacy from the first generation. In a phase I and IIa trial
involving 30 patients with RH in Europe and Canada, no major
adverse events were observed 6 months after implantation of the
Barostim neo™ device. Of note, no temporary or permanent facial
nerve palsy were observed in the patients. Furthermore, the
antihypertensive efficacy of this trial was similar to that of the
phase II and III trials of the Rheos™ system (14,36).
CVRx received Food and Drug Administration approval for Barostim
neo™ implantation in US patients considered to be responders in the
Rheos™ trial. In addition, the advantage of CBAT is that it not
only reduces BP by counteracting sympathetic activation through the
carotid reflex, but also serves to improve cardiac function, renal
function and insulin sensitivity through sympathetic axis-related
pathways (37). However, there are
also issues that cannot be ignored, including the invasiveness of
the implantation and the definition of the applicable population of
CBAT. It is required to further optimize the device efficacy and
trial design to obtain better antihypertensive efficacy on the
basis of safe use and to select a more suitable population of
patients for CBAT.
Electroacupuncture for RH
Electroacupuncture is a form of acupuncture that
applies electrical impulses to stimulate nerve tissue through
needles, which may be used for analgesia, anesthesia,
rehabilitation and treatment of diseases. Acupuncture has been
widely accepted in China as a traditional modality for the
treatment of diseases. However, its international promotion is
limited by the fact that its mechanism is more complex and not yet
well-defined. It is currently thought that the antihypertensive
mechanism of acupuncture is related to RAAS system inhibition,
regulation of oxidative stress, modulation of peripheral nerves and
inhibition of central sympathetic excitation (38-42).
Tjen-A-Looi et al (41)
indicated that acupuncture may cause a decrease in sympathetic
excitability in the RVLM region by modulating the projections from
the paraventricular nucleus to the RVLM and the expression of
opioid receptors and γ-aminobutyric acid receptors, which in turn
causes a decrease in BP, and a significant decrease in renal
vascular resistance was found by Doppler ultrasound measurement of
renal artery vascular resistance after at least three sessions of
moxibustion treatment at bilateral dorsal acupoints. The efficacy
of acupuncture in the treatment of hypertension has been initially
recognized, and a randomized controlled clinical trial has
indicated that acupuncture may reduce the BP by 3-5 mmHg in
patients with hypertension (43).
It also reduces the risk of cardiovascular disease and mortality
(44). A systematic review compared
several sham acupuncture-controlled trials of acupuncture to lower
the BP, revealing that the acupuncture group was effective in
lowering both SBP and DBP. A systematic review comparing
acupuncture-controlled trials of RAAS inhibitors suggested that
acupuncture was more beneficial than RAAS inhibitors for lowering
BP (45). Electroacupuncture also
has good antihypertensive efficacy, but superiority over
non-current acupuncture for lowering BP has not been demonstrated.
Certain systematic analyses have indicated that acupuncture
combined with ACEI therapy and acupuncture combined therapy are
more effective than acupuncture alone in the treatment of
hypertension (46). In conclusion,
electroacupuncture has good prospects as an adjunctive treatment
for hypertension in the future, and on the basis of further
refinement of pathophysiological mechanisms, future clinical
studies may be considered in terms of adjunctive treatment of
RH.
TENS for RH
TENS uses small, specially placed electrodes to
deliver electrical impulses across the skin. Its use generally
involves less anesthesia. TENS is now widely used in pain and
rehabilitation and its safety has been validated by more advanced
evidence. TENS has a similar mechanism of action to
electroacupuncture for RH, with joint hypotension lowering by
afferent nerves and their central regulation through electrical
pulse stimulation of acupuncture points or ganglion areas.
Low-frequency TENS is able to modulate the autonomic nervous system
by releasing endogenous opioids. TENS lacks the invasiveness of
traditional acupuncture therapy and has a certified safety profile.
It leads to higher patient acceptance and helps to improve BP
compliance. However, there is less evidence to analyze both in
terms of pathophysiological mechanisms and clinical studies. The
lack of clinical evidence is the main problem with TENS for RH. In
a trial of non-invasive vagus nerve stimulation to prevent cerebral
vascular rupture and improve intracranial aneurysm models in mice,
TENS was indicated to rapidly reduce BP in mice by activating the
vagus nerve mechanism (47). The
study suggested that TENS rapidly reduced BP in mice by activating
the vagus nerve. Human trials have also indicated that TENS is
effective in reducing sympathetic nerve activity in healthy
subjects and patients with cardiovascular disease (48). The effective stimulation point
remains elusive. The effective stimulation sites remain to be
clearly defined, but the commonly used electrode placement sites
are on the thumb abductor muscle between the first and second
metacarpal bones (Hegu point), between the palmaris longus tendon
and the radial flexor tendon (Neiguan point), part of the
sympathetic ganglion area (paravertebral ganglion) and the stellate
ganglion. The control group exhibited a significant reduction in
SBP. The available research reports have generated information
regarding the effectiveness of TENS treatment. Jacobsson et
al (49) administered 28 days
of TENS to 12 hypertensive patients and observed a significant
reduction in office BP after treatment. Silverdal et al
(50) applied different frequencies
of TENS to treat hypertensive patients taking ≤1 antihypertensive
drug and demonstrated a significant reduction in BP in the
low-frequency TENS compared to the sham stimulation group. However,
neither of these studies was blinded. the effectiveness of TENS
remains to be standardized. Do Amaral Sartori et al
(51) designed a double-blinded
trial, indicating that low-frequency TENS decreased sympathetic
nervous system activity and increased parasympathetic nervous
system activity compared to the placebo group, but there was no
change in BP. The available evidence for the use of TENS in the
treatment of RH indicates that the approach is not promising. It is
related to the imperfect clinical design and small amount of data
(Table I), but more and more
patients with RH are in urgent need of a safer adjunctive therapy
to increase BP lowering. More attention needs to be paid to the
study of TENS in RH. A more comprehensive clinical trial design has
been registered with the US Clinical Trials Center (52). Future research and development are
needed to evaluate the mechanism and effectiveness of TENS in the
treatment of RH.
 | Table IStudies examining transcutaneous
electrical nerve stimulation vs. sham denervation. |
Table I
Studies examining transcutaneous
electrical nerve stimulation vs. sham denervation.
| First author,
year | Type of study | Duration of single
stimulation and total course | Types of enrolled
patients | Electrode
position | Cases (intervention
vs. sham) | Type of BP measured
at study endpoint (ABPM/office BP) and the difference between
groups (post-vs. pre-treatment: mean ± standard deviation) | P-value | Lowering of BP | (Refs.) |
|---|
| Do Amaral Sartori,
2018 | Double blind | 30 min, single
test | Patients with
hypertension | Bilateral
paravertebral region (from T1 to L2) | 18 (8/10) | Office BP, ∆SBP:
-2.68 (129.37±15.48 vs. 126.69±15.21) | <0.490 | Not
significant | (51) |
| Silverdal,
2012 | Single blind | 30 min a.m., 30 min
p.m., daily for 28±4 d | Patients with
hypertension taking ≥1 antihypertensive medication | On the hand over
musculus abductor pollicis between the first and second metacarpal
bone and over musculus extensor carpiradialis two finger breadths
distal to the radial part of the bend of the arm | 32 (16/16) | Office BP, ∆SBP:
-4.7 (152.7±8.9 vs. 148.0±12.1) | <0.01 | Significant | (50) |
| Jacobsson,
2000 | Single blind | 30 min a.m., 30 min
p.m., daily for 28 d | Patients with
hypertension taking ≥1 antihypertensive medication | Dorsal web between
the first and second metacarpal bones and at the proximal portion
of the brachioradial muscle bilaterally | 56 (28/28) | ABPM, ∆SBP: -6.3
(161.9±22.1 vs. 155.6±19.9) | <0.05 | Significant | (49) |
| Chen, 2022 | Single blind | 65 min, single
test | Patients with
controlled hypertension | Hegu and Neiguan
acupoints | 91 (45/45) | Office BP, ∆SBP:
-2.6 (105.2±8.9 vs. 102.6±9.0) | 0.172 | Not
significant | (53) |
7. Bioelectricity technology has great
potential for RH
In recent years, increasing attention has been paid
to bioelectric technology. For RDN or CBAT, improved device safety
and better clinical trials are needed. With the help of TENS, the
treatment and rehabilitation of a variety of neurological, skeletal
and muscle-related diseases have been markedly enhanced. However,
whether it may be better used to assist in the treatment of
resistant hypertension still requires to be further explored. In
the current review, relevant articles have been analyzed and the
effectiveness and application prospects of TENS for RH have been
demonstrated. However, there is only a small number of trials of
TENS performed to date and the randomized clinical trials with
available results have the disadvantages of lack of blinded design,
inability to avoid the generation of bias and short duration of
certain comparisons. Due to these weaknesses, the durability of its
antihypertensive efficacy cannot be proven. A future systematic
review and meta-analysis on the efficacy of TENS on hypertension is
encouraged. More attention should be paid to the following in
future scientific studies: i) Further improvement of the
anti-hypertensive mechanisms of TENS; ii) overcoming the
shortcomings of the design of existing clinical trials, increase of
the sample size, design of sham stimulation control groups, use of
superior stimulation sites, observation of long-term
antihypertensive efficacy and selection of high-quality monitoring
indicators; iii) development of a sensitive and responsive
closed-loop feedback system based on the existing TENS instrument,
which calculates data on BP and heart rate to automatically adjust
the amount of transcutaneous current stimulation within a safe
range (Table II).
 | Table IIInfluencing factors and evaluation
indexes of clinical trials in resistant hypertension. |
Table II
Influencing factors and evaluation
indexes of clinical trials in resistant hypertension.
| Factors to be
excluded | Evaluation indexes
to be considered |
|---|
| • Patients with
recent diabetes mellitus, congestive heart failure, myocardial
infarction, cardiac pacemaker, obesity, ECG changes, cigarette
smoking and beta receptor blocker treatment | • Systolic and
diastolic blood pressure in multiple different patient positions
(sitting or standing) |
| • Congenital renal
vascular malformation and chronic kidney disease | • Heart rate
variability |
| • Abnormal skin
conditions at the stimulation site | • Body mass index
and abdominal circumference |
| • Pregnancy and
lactation in peri-test period | • Inflammatory
factors, natriuretic peptide |
| | • Renal vascular
resistance, glomerular filtration rate |
| | • Plasma
norepinephrine, epinephrine, renin activity, plasma aldosterone or
cortisol concentration |
| | • Vascular
endothelial function and volume load |
8. Conclusions
The epidemiological survey (54-56)
of cardiovascular disease and hypertension in recent years
indicated that the level of awareness and control of hypertension
are low, and the overall prevention and treatment of hypertension
still require further improvement. The public awareness of
hypertension vigilance, treatment and control requires to be
enhanced. Therefore, patients are encouraged to focus on BP control
and to discover more scientific and suitable treatments to manage
hypertension internationally. In particular, attention should be
paid to complementary alternative treatment options. The present
review aimed to provide the current knowledge on the role of
bioelectric techniques for the treatment of RH. The results from
epidemiological studies, although with significant heterogeneity,
demonstrate a clear increase in the prevalence of RH. Both RDN and
CABT have significant anti-hypertensive efficacy and are currently
supported by sufficient clinical evidence. The continuous
improvement of the design of subsequent clinical trials, which
allows them to improve safety in the update, allows for them to be
gradually applied in the clinic. The use of electroacupuncture is
associated with a certain degree of pain, which may make it
difficult to accept for certain patients with RH. However, its
long-term efficacy and domestic popularity make it more widely used
in China.
To overcome this, a gentle stimulation treatment
called TENS has been implemented. It is applied for treating the
corresponding disease by placing double electrodes on the surface
of the respective part of the body and feeding specific low-voltage
and low-frequency pulsed currents into the body. In TENS, the
afferent nerve electrodes are stimulated by the low-voltage
electric pulse delivered by the skin electrodes. This method has
few side effects and offers the possibility of patient
self-treatment and full treatment at home, which may markedly
reduce the impact of poor medical compliance to the treatment. To
date, TENS has been in use in the fields of pain relief and
rehabilitation. It also has the advantages of non-invasiveness, low
cost, high safety and ease of use. However, compared with
RDN/CABT/electroacupuncture, the mechanism of TENS for RH is not
perfect. Existing studies indicated that its antihypertensive
efficacy fluctuates in a small range and it may be considered
separately for the treatment of mild to moderate hypertension
alone. Furthermore, the treatment of RH requires to be combined
with other techniques to achieve the BP target. Therefore, TENS is
considered a better adjunctive treatment for RH in the future.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author upon reasonable
request.
Authors' contributions
GQ conceived and designed the direction of the
manuscript. CW and PW performed the literature search and GQ made
the final decision regarding study inclusion. CW prepared the
original manuscript, as well as figures and tables. CW and PW
provided help and advice regarding the manuscript. CW and GQ
reviewed and proofread the manuscript. All authors contributed to
editorial changes in the manuscript. All authors have read and
approved the final manuscript. Data authentication is not
applicable.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Myat A, Redwood SR, Qureshi AC, Spertus JA
and Williams B: Resistant hypertension. BMJ.
345(e7473)2012.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Vilela-Martin JF, Giollo-Junior LT,
Chiappa GR, Cipriano-Junior G, Vieira PJ, dos Santos Ricardi F,
Paz-Landim MI, de Andrade DO, Cestário Edo E, Cosenso-Martin LN, et
al: Effects of transcutaneous electrical nerve stimulation (TENS)
on arterial stiffness and blood pressure in resistant hypertensive
individuals: Study protocol for a randomized controlled trial.
Trials. 17(168)2016.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Rimoldi SF, Messerli FH, Bangalore S and
Scherrer U: Resistant hypertension: What the cardiologist needs to
know. Eur Heart J. 36:2686–2695. 2015.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Williams B, Mancia G, Spiering W, Agabiti
Rosei E, Azizi M, Burnier M, Clement DL, Coca A, de Simone G,
Dominiczak A, et al: 2018 ESC/ESH Guidelines for the management of
arterial hypertension. Eur Heart J. 39:3021–3104. 2019.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Achelrod D, Wenzel U and Frey S:
Systematic review and meta-analysis of the prevalence of resistant
hypertension in treated hypertensive populations. Am J Hypertens.
28:355–361. 2015.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Krieger EM, Drager LF, Giorgi DMA, Pereira
AC, Barreto-Filho JAS, Nogueira AR, Mill JG, Lotufo PA, Amodeo C,
Batista MC, et al: Spironolactone versus clonidine as a fourth-drug
therapy for resistant hypertension: The ReHOT randomized Study
(Resistant Hypertension Optimal Treatment). Hypertension.
71:681–690. 2018.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Whelton PK, Carey RM, Aronow WS, Casey DE
Jr, Collins KJ, Dennison Himmelfarb C, DePalma SM, Gidding S,
Jamerson KA, Jones DW, et al: 2017
ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the
prevention, detection, evaluation, and management of high blood
pressure in adults: A Report of the American College of
Cardiology/American Heart Association Task Force on Clinical
Practice Guidelines. J Am Coll Cardiol. 71:e127–e248.
2018.PubMed/NCBI View Article : Google Scholar
|
|
8
|
de Jager RL and Blankestijn PJ:
Pathophysiology I: The kidney and the sympathetic nervous system.
Eurointervention. 9:R42–R47. 2013.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Fan H, Tian ZX, Ma SM, Yang NN and Liu CZ:
Research progress of mechanism of oxidase stress-endoplasmic
reticulum stress-autophagy of acupuncture in rostral ventrolateral
medulla of hypertension. CJTCMP. 36:4128–4131. 2021.
|
|
10
|
Cicconetti P, Di Berardino A, Tortorelli
D'Ambrosio M and Cacciafesta M: Resistant hypertension in the
elderly. Recenti Prog Med. 108:316–323. 2017.PubMed/NCBI View Article : Google Scholar : (In Italian).
|
|
11
|
Hering D, Mahfoud F, Walton AS, Krum H,
Lambert GW, Lambert EA, Sobotka PA, Böhm M, Cremers B, Esler MD and
Schlaich MP: Renal denervation in moderate to severe CKD. J Am Soc
Nephrol. 23:1250–1257. 2012.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Laurent S, Schlaich M and Esler M: New
drugs, procedures, and devices for hypertension. Lancet.
380:591–600. 2012.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Li HB, Lu Y and Zheng TZ: Rostral
ventrolateral medulla and primary hypertension. Chin J Hypertens.
19:917–919. 2011.
|
|
14
|
Victor RG: Carotid baroreflex activation
therapy for resistant hypertension. Nat Rev Cardiol. 12:451–463.
2015.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Zar T and Peixoto AJ: Paroxysmal
hypertension due to baroreflex failure. Kidney Int. 74:126–131.
2008.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Doucet BM, Lam A and Griffin L:
Neuromuscular electrical stimulation for skeletal muscle function.
Yale J Biol Med. 85:201–215. 2012.PubMed/NCBI
|
|
17
|
Peng WW, Tang ZY, Zhang FR, Li H, Kong YZ,
Iannetti GD and Hu L: Neurobiological mechanisms of TENS-induced
analgesia. Neuroimage. 195:396–408. 2019.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Johnson MI, Paley CA, Jones G, Mulvey MR
and Wittkopf PG: Efficacy and safety of transcutaneous electrical
nerve stimulation (TENS) for acute and chronic pain in adults: A
systematic review and meta-analysis of 381 studies (the meta-TENS
study). BMJ Open. 12(e051073)2022.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Alahmari KA, Silvian P, Ahmad I, Reddy RS,
Tedla JS, Kakaraparthi VN and Rengaramanujam K: Effectiveness of
low-frequency stimulation in proprioceptive neuromuscular
facilitation techniques for post ankle sprain balance and
proprioception in adults: A randomized controlled trial. Biomed Res
Int. 2020(9012930)2020.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Hering D, Trzebski A and Narkiewicz K:
Recent advances in the pathophysiology of arterial hypertension:
Potential implications for clinical practice. Pol Arch Intern Med.
127:195–204. 2017.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Ott C, Janka R, Schmid A, Titze S, Ditting
T, Sobotka PA, Veelken R, Uder M and Schmieder RE: Vascular and
renal hemodynamic changes after renal denervation. Clin J Am Soc
Nephrol. 8:1195–1201. 2013.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Esler MD, Böhm M, Sievert H, Rump CL,
Schmieder RE, Krum H, Mahfoud F and Schlaich MP: Catheter-based
renal denervation for treatment of patients with
treatment-resistant hypertension: 36 month results from the
SYMPLICITY HTN-2 randomized clinical trial. Eur Heart J.
35:1752–1759. 2014.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Esler MD, Krum H, Schlaich M, Schmieder
RE, Böhm M and Sobotka PA: Renal sympathetic denervation for
treatment of drug-resistant hypertension: One-year results from the
Symplicity HTN-2 randomized, controlled trial. Circulation.
126:2976–2982. 2012.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Esler MD, Krum H, Sobotka PA, Schlaich MP,
Schmieder RE and Böhm M: Renal sympathetic denervation in patients
with treatment-resistant hypertension (The Symplicity HTN-2 Trial):
A randomised controlled trial. Lancet. 376:1903–1909.
2010.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Kandzari DE, Böhm M, Mahfoud F, Townsend
RR, Weber MA, Pocock S, Tsioufis K, Tousoulis D, Choi JW, East C,
et al: Effect of renal denervation on blood pressure in the
presence of antihypertensive drugs: 6-month efficacy and safety
results from the SPYRAL HTN-ON MED proof-of-concept randomised
trial. Lancet. 391:2346–2355. 2018.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Townsend RR, Mahfoud F, Kandzari DE, Kario
K, Pocock S, Weber MA, Ewen S, Tsioufis K, Tousoulis D, Sharp ASP,
et al: Catheter-based renal denervation in patients with
uncontrolled hypertension in the absence of antihypertensive
medications (SPYRAL HTN-OFF MED): A randomised, sham-controlled,
proof-of-concept trial. Lancet. 390:2160–2170. 2017.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Yang XX and Zhao D: Study on the
antihypertensive effect of renal denervation. Chin J Hypertens.
29:1190–1195. 2021.
|
|
28
|
Azizi M, Schmieder RE, Mahfoud F, Weber
MA, Daemen J, Davies J, Basile J, Kirtane AJ, Wang Y, Lobo MD, et
al: Endovascular ultrasound renal denervation to treat hypertension
(RADIANCE-HTN SOLO): A multicentre, international, single-blind,
randomised, sham-controlled trial. Lancet. 391:2335–2345.
2018.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Chen S, Kiuchi MG, Schmidt B, Hoye NA,
Acou WJ, Liu S, Chun KRJ and Pürerfellner H: Renal denervation for
mild-moderate treatment-resistant hypertension : A timely
intervention? Herz. 44:412–418. 2019.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Wang L, Lu CZ, Zhang X, Zhang F, Xia DS,
Chen X, Zhao XD, Guo SZ, Yu X and Jian L: Efficacy of renal
sympathetic denervation in resistent hypertension with high renin
levels. Chin J Hypertens. 22:1084–1086. 2014.
|
|
31
|
Mahfoud F, Bakris G, Bhatt DL, Esler M,
Ewen S, Fahy M, Kandzari D, Kario K, Mancia G, Weber M, et al:
Reduced blood pressure-lowering effect of catheter-based renal
denervation in patients with isolated systolic hypertension: Data
from SYMPLICITY HTN-3 and the Global SYMPLICITY Registry. Eur Heart
J. 38:93–100. 2017.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Lohmeier TE, Irwin ED, Rossing MA, Serdar
DJ and Kieval RS: Prolonged activation of the baroreflex produces
sustained hypotension. Hypertension. 43:306–311. 2004.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Bisognano JD, Bakris G, Nadim MK, Sanchez
L, Kroon AA, Schafer J, de Leeuw PW and Sica DA: Baroreflex
activation therapy lowers blood pressure in patients with resistant
hypertension: Results from the double-blind, randomized,
placebo-controlled rheos pivotal trial. J Am Coll Cardiol.
58:765–773. 2011.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Campón-Checkroun AM, Luceño-Mardones A,
Riquelme I, Oliva-Pascual-Vaca J, Ricard F and Oliva-Pascual-Vaca
Á: Effects of the right carotid sinus compression technique on
blood pressure and heart rate in medicated patients with
hypertension. J Altern Complement Med. 24:1108–1112.
2018.PubMed/NCBI View Article : Google Scholar
|
|
35
|
de Leeuw PW, Alnima T, Lovett E, Sica D,
Bisognano J, Haller H and Kroon AA: Bilateral or unilateral
stimulation for baroreflex activation therapy. Hypertension.
65:187–192. 2015.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Hoppe UC, Brandt MC, Wachter R, Beige J,
Rump LC, Kroon AA, Cates AW, Lovett EG and Haller H: Minimally
invasive system for baroreflex activation therapy chronically
lowers blood pressure with pacemaker-like safety profile: Results
from the Barostim neo trial. J Am Soc Hypertens. 6:270–276.
2012.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Bakris GL, Nadim MK, Haller H, Lovett EG,
Schafer JE and Bisognano JD: Baroreflex activation therapy provides
durable benefit in patients with resistant hypertension: Results of
long-term follow-up in the Rheos Pivotal Trial. J Am Soc Hypertens.
6:152–158. 2012.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Chang Lee SN, Ho TJ, Shibu MA, Day CH,
Viswanadha VP, Lai CH, Chen YL, Hsieh DJ, Chen YS and Huang CY:
Protective effects of electroacupuncture at LR3 on cardiac
hypertrophy and apoptosis in hypertensive rats. Acupunct Med.
34:201–208. 2016.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Guo ZL, Fu LW, Su HF, Tjen ALSC and
Longhurst JC: Role of TRPV1 in acupuncture modulation of reflex
excitatory cardiovascular responses. Am J Physiol Regul Integr Comp
Physiol. 314:R655–R66. 2018.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Li P, Tjen-A-Looi SC, Cheng L, Liu D,
Painovich J, Vinjamury S and Longhurst JC: Long-lasting reduction
of blood pressure by electroacupuncture in patients with
hypertension: Randomized controlled trial. Med Acupunct.
27:253–266. 2015.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Tjen ALSC, Guo ZL, Fu LW and Longhurst JC:
Paraventricular nucleus modulates excitatory cardiovascular
reflexes during Electroacupuncture. Sci Rep.
6(25910)2016.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Tjen ALSC, Li P and Longhurst JC:
Prolonged inhibition of rostral ventral lateral medullary premotor
sympathetic neurons by electroacupuncture in cats. Auton Neurosci.
106:119–1131. 2003.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Liu Y, Park JE, Shin KM, Lee M, Jung HJ,
Kim AR, Jung SY, Yoo HR, Sang KO and Choi SM: Acupuncture lowers
blood pressure in mild hypertension patients: A randomized,
controlled, assessor-blinded pilot trial. Complement Ther Med.
23:658–665. 2015.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Feldstein CA: Lowering blood pressure to
prevent stroke recurrence: A systematic review of long-term
randomized trials. J Am Soc Hypertens. 8:503–513. 2014.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Yang J, Chen J, Yang M, Yu S, Ying L, Liu
GJ, Ren YL, Wright JM and Liang FR: Acupuncture for hypertension.
Cochrane Database Syst Rev. 11(CD008821)2018.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Tan X, Pan Y, Su W, Gong S, Zhu H, Chen H
and Lu S: Acupuncture therapy for essential hypertension: A network
meta-analysis. Ann Transl Med. 7(266)2019.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Wang Y, Shi GX, Tian ZX, Liu JH, Qi YS, Tu
JF, Yang JW, Wang LQ and Liu CZ: Transcutaneous electrical acupoint
stimulation for high-normal blood pressure: Study protocol for a
randomized controlled pilot trial. Trials. 22(140)2021.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Stein C, Dal Lago P, Ferreira JB, Casali
KR and Plentz RD: Transcutaneous electrical nerve stimulation at
different frequencies on heart rate variability in healthy
subjects. Auton Neurosci. 165:205–208. 2011.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Jacobsson F, Himmelmann A, Bergbrant A,
Svensson A and Mannheimer C: The effect of transcutaneous electric
nerve stimulation in patients with therapy-resistant hypertension.
J Hum Hypertens. 14:795–798. 2000.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Silverdal J, Mourtzinis G, Stener-Victorin
E, Mannheimer C and Manhem K: Antihypertensive effect of
low-frequency transcutaneous electrical nerve stimulation (TENS) in
comparison with drug treatment. Blood Press. 21:306–310.
2012.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Do Amaral Sartori S, Stein C, Coronel CC,
Macagnan FE and Plentz RDM: Effects of transcutaneous electrical
nerve stimulation in autonomic nervous system of hypertensive
patients: A randomized controlled trial. Curr Hypertens Rev.
14:66–71. 2018.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Vilela-Martin JF, Giollo-Junior LT,
Chiappa GR, Cipriano-Junior G, Vieira PJ, dos Santos Ricardi F,
Paz-Landim MI, de Andrade DO, Cestário Edo E, Cosenso-Martin LN, et
al: Effects of transcutaneous electrical nerve stimulation (TENS)
on arterial stiffness and blood pressure in resistant hypertensive
individuals: Study protocol for a randomized controlled trial.
Trials. 17(168)2016.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Chen L, Shen Y, Liu S and Cao Y:
Transcutaneous electrical acupoint stimulation improved
preoperative blood pressure in gynecological malignant tumor
patients with hypertension: A randomized, controlled trial. Front
Oncol. 12(906528)2022.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Mills KT, Stefanescu A and He J: The
global epidemiology of hypertension. Nat Rev Nephrol. 16:223–237.
2020.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Lee JH, Kim KI and Cho MC: Current status
and therapeutic considerations of hypertension in the elderly.
Korean J Intern Med. 34:687–695. 2019.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Flynn JT: Hypertension in the young:
Epidemiology, sequelae and therapy. Nephrol Dial Transplant.
24:370–375. 2009.PubMed/NCBI View Article : Google Scholar
|