Introduction
Several electrocardiogram (ECG) abnormalities are
frequently found in patients without heart disease who experience
an ischemic stroke or subarachnoid hemorrhage (SAH). However,
knowledge about the ECG changes in patients with intracranial
hemorrhage (ICH) is currently limited, including subdural
hemorrhage and intraparenchymal hemorrhage (IPH), which are the
most frequent and second most frequent ICHs, respectively (1,2). Among
the most common ICH ECG abnormalities are ST segment depression,
left ventricular hypertrophy, prolongation of the corrected QT
interval and inversion of the T wave (3). These nonspecific ECG findings may
confuse medical diagnoses and, consequently, lead to erroneous
therapeutic decisions.
ECG variations are usual in supratentorial
hemorrhagic stroke, predominantly in basal ganglia and thalamic
localization (4,5). These changes are frequently present in
patients with ventricular effraction and include mainly QTc
prolongation, followed by brady/tachycardia and subsequently ST
segment modification (5,6). Certain investigations substantiate the
assumption that a cardiac cortical rhythm control site is possibly
inside the middle cerebral artery context or in the frontal
cingulate cortex (4,6). Vascular injury to this part may be
followed by cardiac arrhythmias linked to a disinhibition of the
right insular cortex with subsequent augmented sympathetic tone.
Ischemic commitment of the right hemisphere stimulates a greater
risk for cardiac arrhythmia incidence than that of the left
hemisphere (4-6).
Imbalances of the autonomic nervous system role are important for
these disruptions of rate, rhythm and conduction. Tachycardia and
pressor replies are more usual following stimulus of the right
insular cortex and after experimental stimulus of the left vagus,
which innervates the atrioventricular node and the cardiac
transference arrangement. Bradycardia appears to be more usual
following stimulation of the left insular cortex or the right vagus
nerve, which innervates the sinoatrial node, or it may be a source
of the Cushing outcome (4-6).
ECG fluctuations appear in 49-100% of patients after
SAH (7). The most usual ECG changes
after SAH and IPH include repolarization irregularities, such as QT
interval extension, ST-segment and T-wave modifications (8). Atrioventricular block, atrial flutter
and ventricular arrhythmia are the most usual modifications related
to cardiac arrhythmias; however, the mechanisms remain to be
completely elucidated (7,8).
It was reported that ECG fluctuations were existent
in >90% of unselected patients with ischemic stroke and
intracerebral hemorrhage, but the occurrence was inferior regarding
the omission of patients with preexistent heart sickness. Those
patients with subarachnoid hemorrhage, repolarization and
ischemic-like ECG alterations are related to direct effects of the
cerebral disorder (9).
Furthermore, the nontraumatic IPH group comprises
IPH and SAH. Acute SAH is noticed on the tomography as hyperdensity
(blood clot density) in the cerebrospinal fluid spaces surrounding
the brain. While the main source of SAH is a disrupted aneurysm, it
may additionally be caused by intracranial dissection, trauma,
vasculitis, dural AVM or cervical fistulas (10).
The scientific literature has reported various ECG
abnormalities, particularly in SAH (11-13),
but no case reports of ECG abnormalities in patients with IPH have
been published, to the best of our knowledge. The present study
reported on the ECG findings and the therapeutic management of a
patient with IPH who was initially diagnosed with acute myocardial
infarction.
Case report
A 78-year-old male patient was transferred from a
lower-complexity to a high-complexity hospital in August 2021
because imaging and hemodynamic services were unavailable in the
former setting. At the lower-complexity hospital, the patient
presented with weakness and decreased muscle strength in the left
half of the body with 2 h of evolution, which was associated with
dysarthria and deviation of the right labial commissure. The
patient was admitted with elevated blood pressure (220/110 mmHg),
for which 20 mg of labetalol was administered intravenously. An ECG
was immediately performed, which revealed elevation of the ST
segment, a situation that was managed pharmacologically as a
myocardial infarction by administering 80 mg of atorvastatin and
300 mg of clopidogrel. Subsequently, the patient was transferred to
a higher-complexity hospital, where it was observed that the
patient neither had a history of angina or dyspnea, nor symptoms of
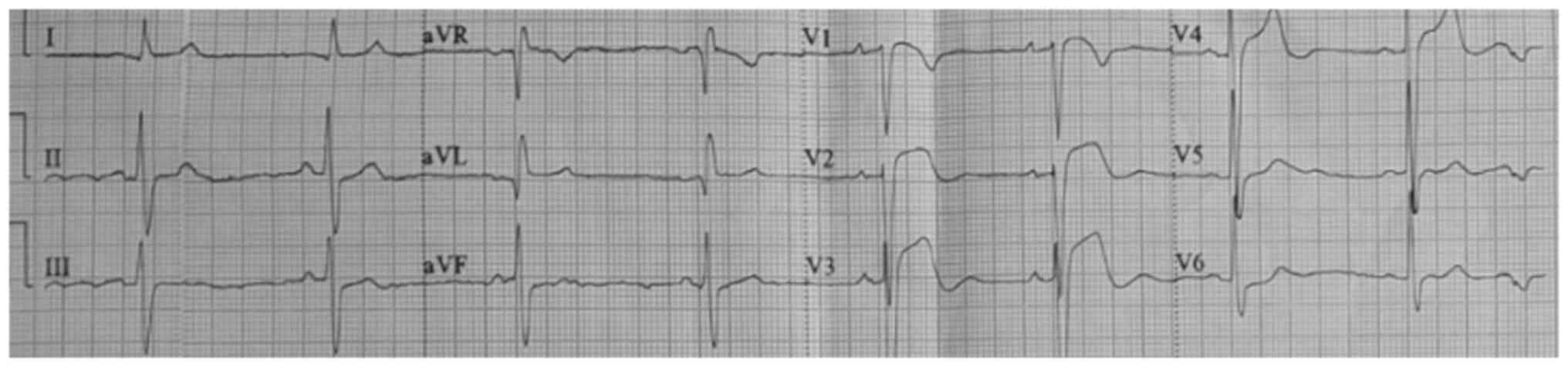
a coronary syndrome. Fig. 1
presents ST-segment elevation in V1, V2 and V3 (acquired at the
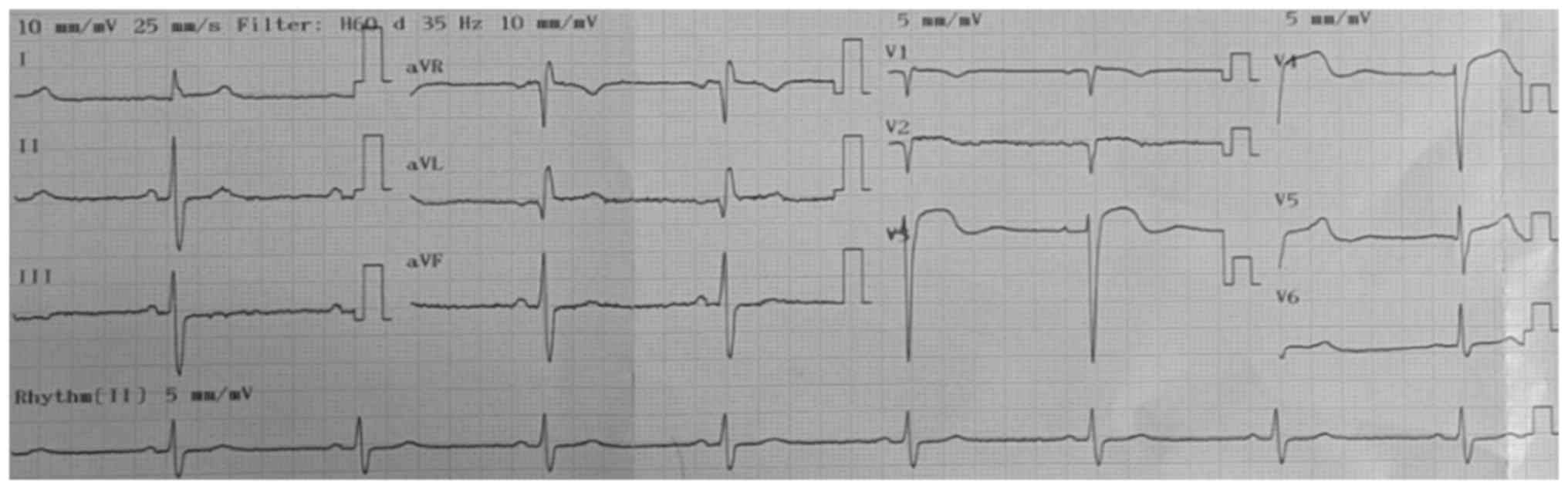
low-complexity hospital). Fig. 2
depicts ST-segment elevation in V3 and V4 (acquired at the
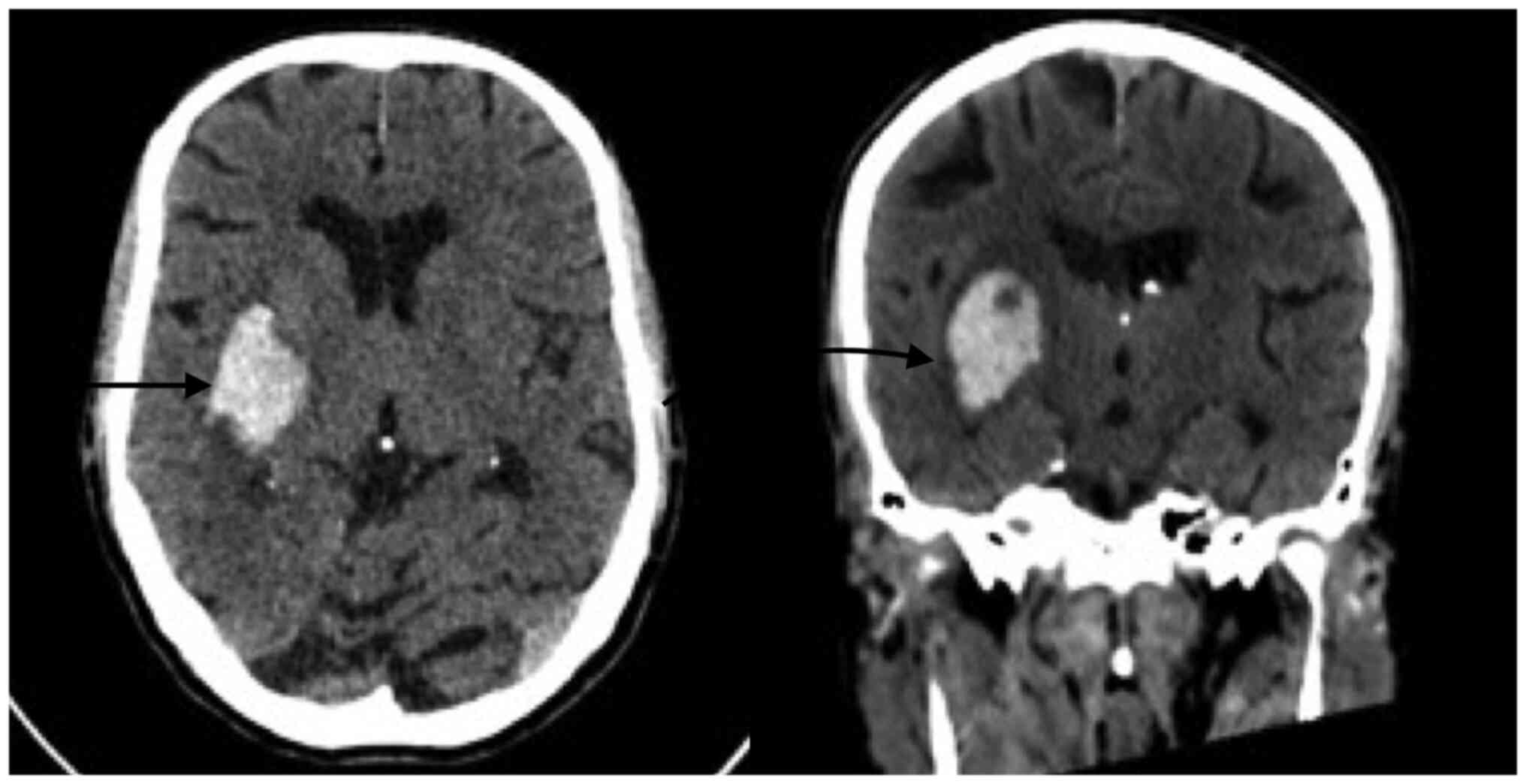
high-complexity hospital) and Fig.
3 presents right temporal intraparenchymal hematoma with
vasogenic edema and ventricular involvement (acquired at the
high-complexity hospital).
After 6 h, the patient was admitted to Hospital San
Vicente Fundación (Rionegro, Colombia), a highly complex hospital
(a hospital with the infrastructure, technology and specialists
that allow it to provide the population with a health service that
treats the most complex diseases), where the following vital signs
were recorded: Blood pressure, 184/83 mmHg (hypertensive); heart
rate, 52 beats per minute (bradycardia); respiratory rate, 22
breaths per minute tachypneic; and oxygen saturation, 97% (normal
value). On physical examination, the patient was drowsy and
oriented in three spheres. Muscular strength was 5/5 in the right
half of the body and 4/5 in the left upper limb without alteration
in sensitivity, aphasia or dysarthria. The patient denied symptoms
including chest pain, angina, dyspnea, limitation in functional
class, palpitations and previous cardio and neuromuscular events.
Acute neurovascular syndrome was suspected, for which a simple
skull tomography was performed, revealing spontaneous IPH of the
right basal ganglion in the context of an acute cerebrovascular
accident of hypertensive origin without any criteria for
neurosurgical surgical intervention; furthermore, the patient did
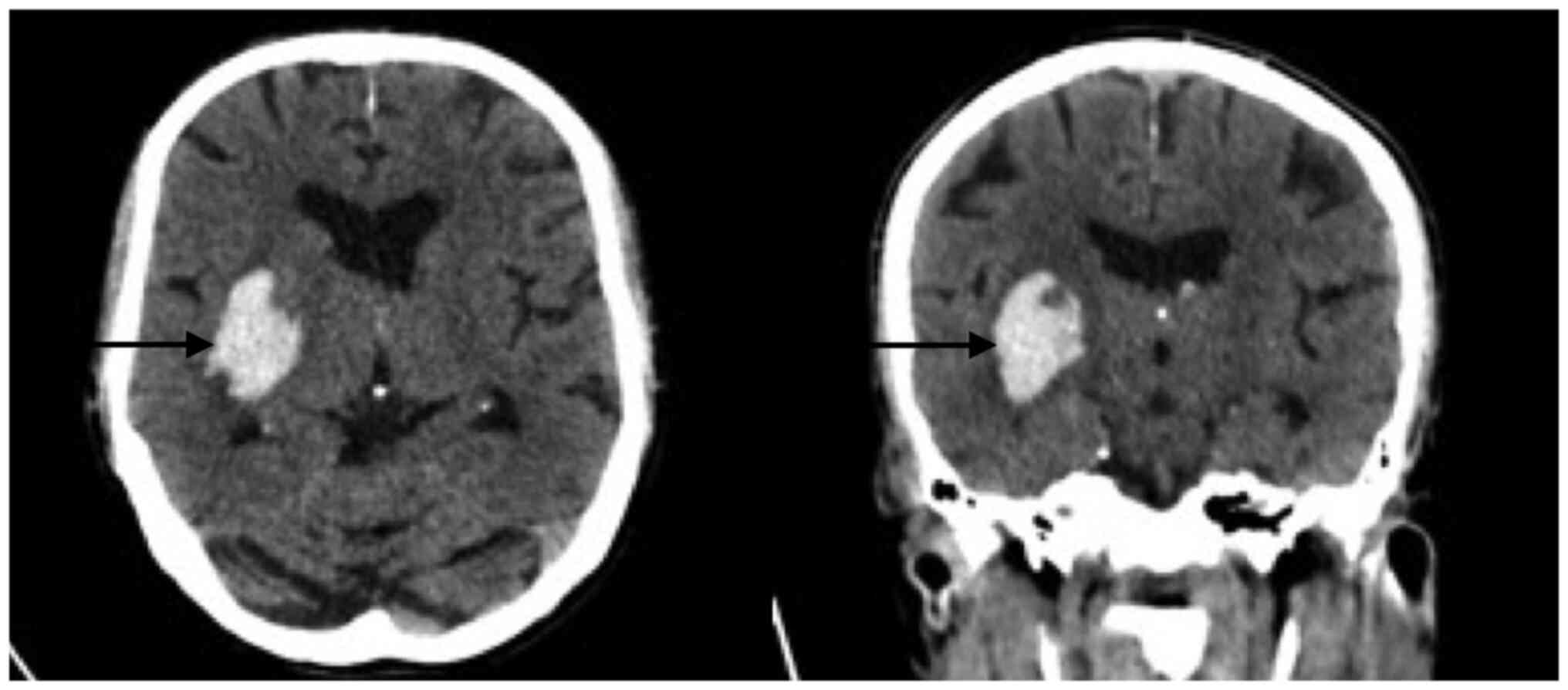
not require reversal of the administered antiplatelets. Fig. 4 reveals a right temporal
intraparenchymal hematoma with vasogenic edema and ventricular
involvement without any changes compared with the previous one.
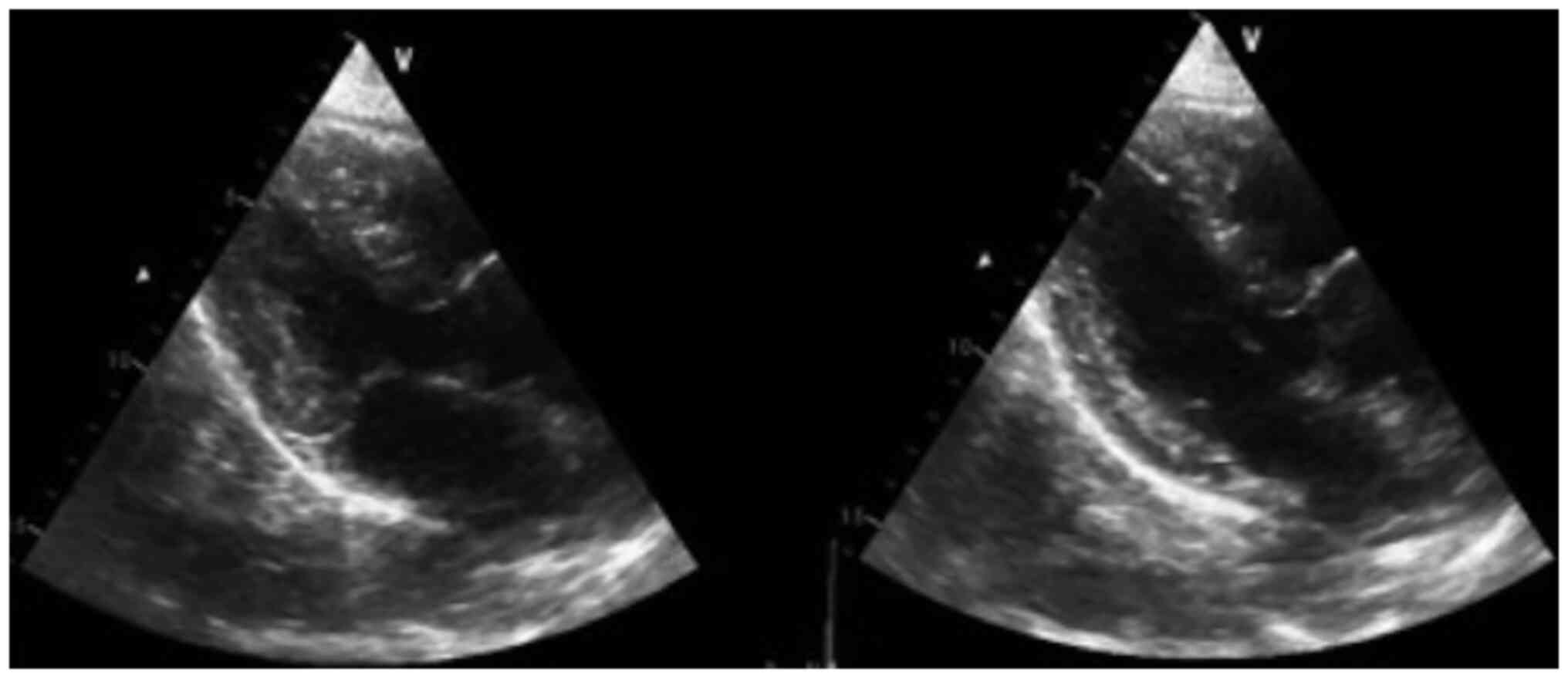
Fig. 5 suggests no akinesia or
hypokinesia, but diastolic dysfunction and relaxation disorders are
present.
It is important to note that the ECG changes were
transient. At the time of establishing medical management for the
patient's pathology (neurovascular syndrome), the ECG changes began
to normalize.
The patient was hospitalized in the intensive care
unit for a hypertensive brain emergency and required a vasodilator
for 2 days (nitroglycerin and IV labetalol). A transthoracic ECG
was performed, revealing an ejection fraction of 65% (calculated
using Simpson's method) with type I diastolic dysfunction due to
relaxation disorders and without signs of ischemia, intracavitary
masses or thrombi (Fig. 5).
The patient subsequently required transfer to the
hospitalization room where the antihypertensive management was
adjusted. The patient was discharged on the ninth day after
admission with left hemiparesis and without spasticity. Follow-up
and outpatient rehabilitation were planned.
Discussion
Several cases of ST segment deviation, inversion of
the T wave and prolongation of the corrected QT interval in
patients with SAH have been reported (11-14).
As showcased in these previous SAH studies and the present case,
clinicians still face challenges in differentiating the ECG changes
of ICH or SAH from those of acute coronary syndrome. In the present
study, ST-segment elevation was evidenced in a patient with IPH who
was initially treated as a myocardial infarction. The most common
ECG alterations after IPH include repolarization abnormalities,
such as QT interval prolongation and ST-segment and T-wave changes
(15), of which QT interval
prolongation is the most frequent (2,3).
As observed in the present case, nontraumatic IPH
results from small artery bleeding, commonly due to hypertension
(15,16), and it generally occurs in patients
older than 60 years of age (16).
Previously, Yaghmoor et al (2) reported an IPH prevalence of 23% among
all ICHs.
Contrary to what occurred in the present case,
previous research reported that death in patients with IPH was more
common in cases in which changes in the ST segment were not
observed (2).
It has been suggested that, to decrease or predict
the occurrence of unfavorable results after ICH, cardiac monitoring
should be performed in addition to the ECG to detect arrhythmias
during the first 2 to 3 days after hospitalization (2).
The most important aspects that must be considered
to differentiate the ECG changes of ICH or SAH from those of acute
coronary syndrome are the clinical manifestations. Not every
patient with IPH should have an ECG. If the clinical manifestations
indicate chest pain, discomfort, dyspnea and other suggestive
symptoms, it is more likely that it is a coronary syndrome. In the
patient described in the present study, the main reason for
consultation was weakness and decreased muscle strength in the left
side of the body, associated with dysarthria, as well as labial
commissure deviation, suggesting a neurovascular syndrome (probable
ischemic stroke or IPH).
It is important to note that, in addition to the
presence of nonspecific ECG findings, clinicians should consider
immediate brain computed tomography to confirm ICH.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
MAGD, MZG, DGA and CMA contributed to the conception
and design of the study. CMA wrote the manuscript. DGA and CMA
searched the literature. MAGD, MZG and DGA provided clinical
assistance to the patient and were responsible for the treatments.
DGA and CMA revised the manuscript. MZG and CMA confirm the
authenticity of all the raw data. All authors have read and
approved the final manuscript.
Ethics approval and consent to
participate
The Bioethics Committee of San Vicente Fundación
Hospital (Rionegro, Colombia) approved the publication of this
case.
Patient consent for publication
Written informed consent for the publication of
clinical details and images was obtained from the patient.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Junttila E, Vaara M, Koskenkari J, Ohtonen
P, Karttunen A, Raatikainen P and Ala-Kokko T: Repolarization
abnormalities in patients with subarachnoid and intracerebral
hemorrhage: Predisposing factors and association with outcome.
Anesth Analg. 116:190–197. 2013.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Yaghmoor BE, Alotaibi SM, Enani MZ,
AlQudsi HS, Aljehani MA, Althomali MH, Hisan FM, Sindi GJ,
Alshoaibi NA and Sabbagh AJ: Electrocardiographic changes following
intracranial haemorrhage: A retrospective cohort study.
Neurosciences (Riyadh). 25:104–111. 2020.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Takeuchi S, Nagatani K, Otani N, Wada K
and Mori K: Electrocardiograph abnormalities in intracerebral
hemorrhage. J Clin Neurosci. 22:1959–1962. 2015.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Daniele O, Caravaglios G, Fierro B and
Natalè E: Stroke and cardiac arrhythmias. J Stroke Cerebrovasc Dis.
11:28–33. 2002.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Critchley HD, Mathias CJ, Josephs O,
O'Doherty J, Zanini S, Dewar BK, Cipolotti L, Shallice T and Dolan
RJ: Human cingulate cortex and autonomic control: Converging
neuroimaging and clinical evidence. Brain. 126:2139–2152.
2003.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Keller C and Williams A: Cardiac
dysrhythmias associated with central nervous system dysfunction. J
Neurosci Nurs. 25:349–355. 1993.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Bhattacharya IS, Sandeman D, Dweck M,
McKie S and Francis M: Electrocardiographic abnormalities in a
patient with subarachnoid haemorrhage. BMJ Case Rep.
2011(bcr0820103253)2011.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Sakr YL, Ghosn I and Vincent JL: Cardiac
manifestations after subarachnoid hemorrhage: A systematic review
of the literature. Prog Cardiovasc Dis. 45:67–80. 2002.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Gölbaşi Z, Selçoki Y, Eraslan T, Kaya D
and Aydoğdu S: QT dispersion. Is it an independent risk factor for
in-hospital mortality in patients with intracerebral hemorrhage?
Jpn Heart J. 40:405–411. 1999.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Delgado Almandoz JE, Schaefer PW, Forero
NP, Falla JR, Gonzalez RG and Romero JM: Diagnostic accuracy and
yield of multidetector CT angiography in the evaluation of
spontaneous intraparenchymal cerebral hemorrhage. AJNR Am J
Neuroradiol. 30:1213–1221. 2009.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Cima K, Grams A and Metzler B:
Subarachnoid haemorrhage mimicking a STEMI. Eur Heart J Acute
Cardiovasc Care. 6:736–737. 2017.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Laundon RK and Littmann L: Spiked helmet
pattern ST elevation in subarachnoid hemorrhage. J Electrocardiol.
52:96–98. 2019.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Park I, Kim YJ, Ahn S, Sohn CH, Seo DW and
Kim WY: Subarachnoid hemorrhage mimicking ST-segment elevation
myocardial infarction after return of spontaneous circulation. Clin
Exp Emerg Med. 2:260–263. 2015.PubMed/NCBI View Article : Google Scholar
|
|
14
|
SPRINT Research Group. Wright JT Jr,
Williamson JD, Whelton PK, Snyder JK, Sink KM, Rocco MV, Reboussin
DM, Rahman M, Oparil S, et al: A randomized trial of intensive
versus standard blood-pressure control. N Engl J Med.
377:2103–2116. 2015.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Qaqa AY, Suleiman A, Alsumrain M, Debari
VA, Kirmani J and Shamoon FE: Electrocardiographic abnormalities in
patients presenting with intracranial parenchymal haemorrhage. Acta
Cardiol. 67:635–639. 2012.PubMed/NCBI View Article : Google Scholar
|
|
16
|
An SJ, Kim TJ and Yoon BW: Epidemiology,
risk factors, and clinical features of intracerebral hemorrhage: An
update. J Stroke. 19:3–10. 2017.PubMed/NCBI View Article : Google Scholar
|