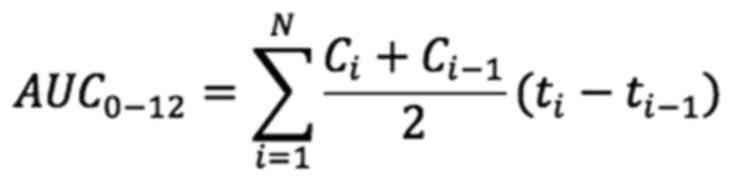Introduction
Antimicrobial resistance has become a global public
health concern, endangering both human health and quality of life.
The issue of antimicrobial resistance in China is becoming
increasingly severe (1-3).
The results of the China Bacterial Surveillance Network in 2021
showed that carbapenem-resistant Klebsiella pneumoniae is
only sensitive to certain antibiotics, such as tigecycline (92.7%),
colistin (94.7%), polymyxin (94.1%), ceftazidime and avibactam
(89.9%) (4). Carbapenem-resistant
Acinetobacter baumannii also shows sensitivity to
tigecycline (97.5%), colistin (98.4%) and polymyxin (99.3%). The
resistance rates of A. baumannii to imipenem and meropenem
are as high as 72.3 and 71.5%, respectively (4). The detection of multidrug-resistant
and pan-drug-resistant A. baumannii and carbapenem-resistant
Enterobacter is one of the main reasons for the significant
increase in the use of tigecycline in recent years (5-7).
Tigecycline was the first new-generation,
broad-spectrum, glycyl tetracycline antibiotic approved by the US
Food and Drug administration in 2005, which exerted good
antibacterial activity against common pathogenic bacteria and
multidrug-resistant bacteria, such as multidrug-resistant
Acinetobacter baumannii, carbapenem-resistant Klebsiella
pneumoniae and carbapenem-resistant Acinetobacter
baumannii (8,9). At present, it is mainly used
clinically for the treatment of complicated intra-abdominal
infections, severe community-acquired pneumonia,
multidrug-resistant A. baumannii infections and infections
caused by carbapenem-resistant Enterobacteriaceae (8-11).
With the wide application of tigecycline in clinical practice, its
irrational use has attracted the attention of the Chinese Health
Commission. It is required that medical institutions document the
prescription of tigecycline in a special file, invite experts on
infectious diseases for consultation before prescription, and
promptly review, analyse and summarise prescriptions (12). Feedback on numerous problems
(inappropriate drug selection, inappropriate dosage and usage, lack
of consultation record and lack of dynamic laboratory tests to
evaluate efficacy) to the clinical frontline is also required to
improve clinical efficacy, and reduce adverse drug reactions and
drug resistance.
The present study intended to investigate the
rational use of tigecycline in the intensive care unit (ICU) of The
First Affiliated Hospital of Bengbu Medical College (Bengbu, China)
through prescription review. Currently, there are two regimens used
by clinicians for maintenance administration of tigecycline to
treat pulmonary infection caused by carbapenem-resistant A.
baumannii: i) 50 mg twice daily (every 12 h); and ii) 100 mg
every 12 h. The blood drug concentrations of the two doses were
monitored and the association with clinical prognosis was analysed.
This provided a basis for the empirical treatment of
carbapenem-resistant A. baumannii pulmonary infection. The
present study may provide a reference for the rational use and
management of tigecycline in hospitals.
Patients and methods
Patients. The inclusion criterion for blood
concentration monitoring consisted of pulmonary infection cases
caused by multidrug-resistant and pan-drug-resistant A.
baumannii between September 2019 and March 2022 from The First
Affiliated Hospital of Bengbu Medical College (Bengbu, China). The
patients were divided into two groups according to the ‘Evaluation
Criteria for Clinical Application of Tigecycline’ (Table I) formulated by Chinese experts and
previous reports (8,13-15):
i) The low-dose group (first loading dose of 100 mg or no loading
dose, followed by 50 mg of maintenance dose), 9 patients (8 male
and 1 female; age range, 42-84 years); and ii) the high-dose group
(first loading dose of 200 mg, followed by 100 mg of maintenance
dose), 9 patients (5 male and 4 female; age range, 31-71 years).
The exclusion criterion was the use of tigecycline for <72
h.
 | Table IEvaluation criteria for the clinical
application of tigecycline. |
Table I
Evaluation criteria for the clinical
application of tigecycline.
| Category | Parameters |
|---|
| Indication | i) Severe cases of
complicated intra-abdominal infection, complicated skin and soft
tissue infection, and community-acquired pneumonia. |
| | ii)
Multidrug-resistant A. baumannii infections (excluding CNS
and UT infections). |
| | iii)
Carbapenem-resistant Enterobacteriaceae infections (excluding CNS
and UT infections). For multidrug or carbapenem resistance, the use
is considered rational even if the infection site is not specified,
provided that it is not a CNS or UT infection. |
| Regimen | i) Monotherapy is not
appropriate for treating extensively drug-resistant gram-negative
bacterial infections. |
| | ii) The first loading
dose is 100 mg, and the maintenance dose is 50 mg every 12 h; for
children aged 8-11 years old, 1.2 mg/kg every 12 h, and the maximum
dose is 50 mg every 12 h; for children aged 12-17 years, 50 mg
every 12 h. |
| | iii) Hepatic
insufficiency: No dose adjustment is required for patients with
mild to moderate hepatic insufficiency (Child Pugh class A and B);
for patients with severe hepatic impairment (Child Pugh class C),
the first dose should be adjusted to 100 mg, followed by 25 mg
every 12 h. |
| | iv) When treating
hospital-acquired pneumonia or ventilator-associated pneumonia, the
dose can be increased. The maintenance dose can be up to 100 mg
every 12 h; for severe infections caused by carbapenem-resistant
Enterobacterales or carbapenem-resistant A. baumannii, the
dose can be doubled. |
| Aetiology and
efficacy evaluation | i) Aetiological
testing should be applied before the use of antibacterial drugs,
such as bacterial culture, including effective aetiological
evidence from other hospitals. |
| | ii) During treatment,
dynamic laboratory tests should be performed to evaluate the
efficacy, such as routine blood tests, procalcitonin level tests
and bacterial culture. |
| Prescription and
consultation of the ‘special use class’ of antibiotics | i) Prescription is
issued by a senior physician and supported by the information
management system. |
| | ii) Timely
consultation with in-hospital or out-of-hospital experts
specialized in the ‘special use class’ of antibacterial drugs, with
appropriate consultation records. |
| | iii) Prescribing
bypassing a senior consultant is limited to within 24 h after
tigecycline is administered, and there is a record of the
corresponding disease course. |
| | iv) Special file
registration is carried out in accordance with the ‘National Health
Office (2017) no. 10’ document. |
| | v) Physicians who are
authorised to prescribe and consult on the ‘special use class’ of
antibiotics require regular training and assessment and should have
the corresponding records. |
The inclusion criterion for the tigecycline
prescription review consisted of 40 patients (24 male and 16
female; age range, 29-85 years) treated with tigecycline at the ICU
of The First Affiliated Hospital of Bengbu Medical College (Bengbu,
China) between September 2019 and March 2022. Prescriptions were
randomly selected from 100 prescriptions for review. The exclusion
criterion was the use of tigecycline for <72 h. The present
study was approved by the Ethics Committee of The First Affiliated
Hospital of Bengbu Medical College (Bengbu, China) and written
informed consent was obtained from each patient.
Observation indicators
The observation indicators were as follows: i)
Observation indicators of tigecycline blood concentration
monitoring: Use of tigecycline, white blood cell count, neutrophil
count, C-reactive protein (CRP) level, and body temperature before
and after treatment; and ii) observation indicators of prescription
review: Patient's admission number, sex, age, diagnosis, doctor's
advice regarding the administration of tigecycline alone or a
combination therapy, and bacterial culture results before and after
medication. The indications, dosing schedule, aetiology and
efficacy were analysed according to the ‘Evaluation Criteria for
Clinical Application of Tigecycline’ (Table I), previous publications (15-18)
and the instruction manual for tigecycline. Prescription reviews
were conducted based on four factors: i) Indication; ii) dosing
schedule; iii) aetiology and efficacy evaluation; and iv)
prescription and consultation on the special use grade of
antibiotics.
Blood drug concentration
monitoring
The blood concentrations of tigecycline were
monitored at five time-points: 0 h at the 7th administration, and
30 min, 1, 6 and 12 h after the 7th administration. From each
patient, 1-2 ml blood was collected, and following centrifugation
(22,000 x g, 5 min, 25˚C), the supernatants were subjected to
high-performance liquid chromatography. An Agilent 1200
high-performance liquid chromatography instrument (Agilent
Technologies Co., Ltd.) with a Kromasil C18 chromatographic column
(4.6x150 mm, 5 µm; Agilent Technologies Co., Ltd.) was used to
check the blood concentrations of tigecycline. The mobile phase was
acetonitrile -0.023 mmol/l phosphate buffer (24:76,
v/v, pH=3.0), the flow velocity was 1.0 ml/min, the
temperature of the column was 25˚C and the injection volume was 50
µl. Tigecycline standard (Beijing Bei Ao Lai Bo Technology Co.,
Ltd.) was diluted with distilled water to 1.00 mg/ml stock
solution, then the above stock solution was diluted successively
with PBS (pH=3.0, 0.1 mmol/l) to a series of standard solutions
with concentrations of 25,000, 10,000, 5,000, 2,500, 1,250 and
0.500 µg/ml and the quality control solutions were 15,000, 5,000,
0,125 µg/ml. The internal standard solution of minocycline (1.00
mg/ml; Sigma-Aldrich; Merck KGaA) was prepared in distilled water,
then diluted in phosphate buffer (pH=3.0, 0.1 mmol/l) to obtain a
100 µg/ml concentration. Phoenix WinNonlin software 6.4 (Pharsight
Corporation) was used for non-compartmental analysis and for
fitting pharmacokinetic parameters. The area under the curve
(AUC)0-12 h was calculated using the statistical moment
method.
(i=0, 0.5, 1, 6, 12)
Evaluation of the clinical efficacy of
tigecycline
Efficacy evaluation was performed based on the
‘Technical Guidelines for Clinical Trials of Antibacterial Drugs’
published by Chinese experts (12).
Evaluation parameters included: i) Clinical symptoms (cough,
expectoration, dyspnoea, chest distress and elevated body
temperature); ii) signs (lung auscultation with thickened
respiratory sounds and rales); iii) laboratory test results
(hematology and CRP); and iv) bacteriological test results. The
definitions of efficacy were as follows: i) ‘Cure’ was defined when
the patients showed absence or negative results for all four
aforementioned parameters; ii) ‘significant improvement’ was
defined as the patient having a positive result for three of the
four parameters; iii) ‘effective’ was defined as a positive result
for two of the aforementioned parameters; and iv) ‘ineffective’ was
defined as the patient's condition worsening or no obvious
improvement after 72 h of treatment. The overall effective rate was
calculated as: Overall effective rate (%)=(cure + significant
improvement + effective cases)/total number of patients x100%.
Statistical analysis
SPSS 22.0 (IBM Corp.) was used for statistical
analysis. Continuous data were presented as the mean ± SD. A paired
or an independent sample Student's t-test was used for inter-group
comparisons of data that conformed to a normal distribution,
whereas the Mann-Whitney U-test or the Wilcoxon signed-rank test
was used for non-normally distributed data. Count data are
presented as n (%) and Fisher's exact test was used for inter-group
comparisons. P<0.05 was considered to indicate a statistically
significant difference.
Results
Pharmacodynamic profile and clinical
efficacy of tigecycline
A total of 18 ICU patients with pulmonary infection
caused by multidrug-resistant or pan-drug-resistant A.
baumannii were included in the blood concentration monitoring
study. The patients were divided into two groups: i) The low-dose
group; and ii) the high-dose group, with 9 cases in each group.
There were 7 cases of carbapenem-resistant A. baumannii
pulmonary infection in each of the two groups. Blood drug
concentrations at five different time points (0 h, 30 min, 1, 6 and
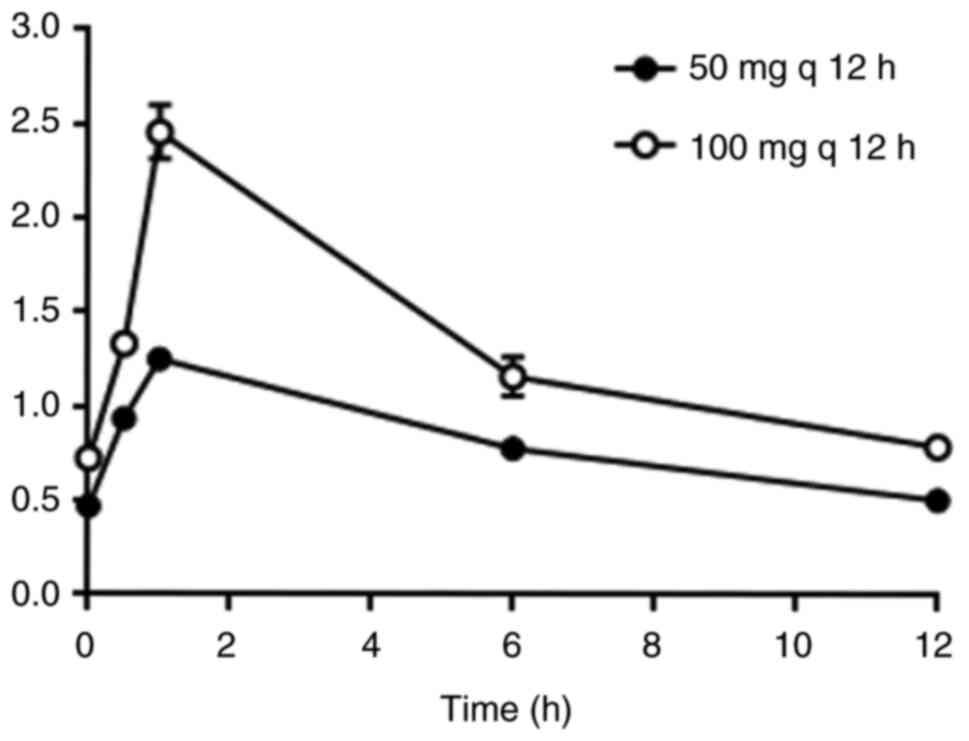
12 h after the 7th administration) were detected. The results
showed that the blood drug concentrations in the high-dose group
were significantly higher compared with those in the low-dose group
(Fig. 1). The maximum concentration
(Cmax) in the high-dose group was 2.46±0.43 µg/ml, which
was significantly higher compared with the Cmax of
1.25±0.16 µg/ml in the low-dose group (P<0.001). AUC0-12
h was 16.35±3.09 h·µg/ml in the high-dose group and only
9.83±1.23 h·µg/ml in the low-dose group (Table II), and there was a significant
difference between the two groups (P<0.001). The overall rates
of efficacy in the high-dose and low-dose groups were 77.78 and
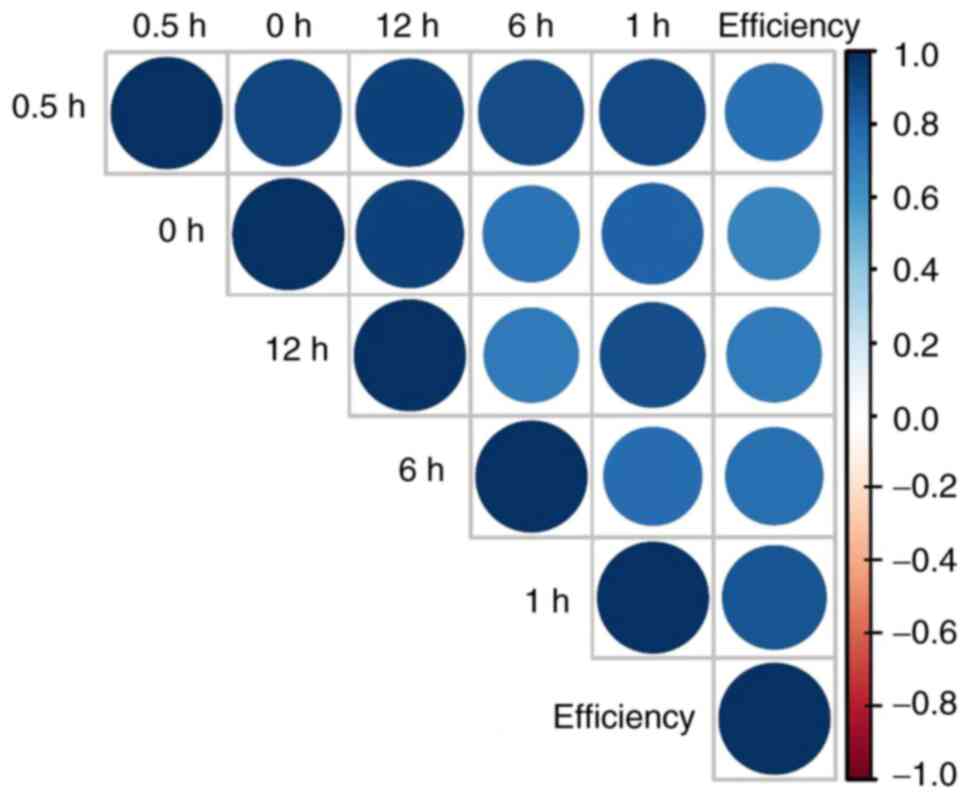
55.56%, respectively. In addition, the concentration of tigecycline
was positively associated with the efficacy (Fig. 2). These results suggested that for
multidrug-resistant or pan-drug-resistant A. baumannii,
especially carbapenem-resistant A. baumannii pulmonary
infection, the regimen of 200 mg for the first loading dose plus
100 mg every 12 h for the maintenance dose is the most
effective.
 | Table IIComparison of the pharmacokinetic
parameters and efficacy between the two groups. |
Table II
Comparison of the pharmacokinetic
parameters and efficacy between the two groups.
| Parameter | Low-dose group
(n=9) | High-dose group
(n=9) | P-value |
|---|
| Cmax,
µg/ml | 1.25±0.16 | 2.46±0.43 | 0.001 |
| AUC0-12 h,
h·µg/ml | 9.83±1.23 | 16.35±3.09 | 0.001 |
| Effective rate, n
(%) | 5 (55.56) | 7 (77.78) | 0.317 |
Inflammatory factors before and after
tigecycline treatment
After tigecycline treatment, the body temperature,
white blood cell and neutrophil counts, and CRP level in both the
low-dose and high-dose groups were lower than the corresponding
pre-treatment values. The body temperature and CRP level were
significantly decreased (P<0.05) compared with the pre-treatment
values in the high-dose group, whereas only body temperature showed
a significant decrease (P<0.05) compared with pre-treatment in
the low-dose group, the white blood cell and neutrophil counts, and
the CRP level did not show a significant difference in the low-dose
group (Table III).
 | Table IIIChanges in inflammatory factors in the
two groups before and after treatment. |
Table III
Changes in inflammatory factors in the
two groups before and after treatment.
| Group | Time-point | Temperature, ˚C | White blood cell
count, x109/l | Neutrophil count,
x109/l | CRP, mg/l |
|---|
| Low-dose | Pre-treatment | 38.31±0.89 | 12.02±4.73 | 10.41±4.21 | 81.71±57.89 |
| | Post-treatment |
36.93±0.91a | 10.47±5.49 | 8.49±5.40 | 72.64±68.52 |
| High-dose | Pre-treatment | 38.19±1.24 | 15.18±5.28 | 13.35±4.69 | 113.37±80.07 |
| | Post-treatment |
36.61±0.59a | 12.76±5.70 | 10.52±5.98 |
44.10±28.81a |
Infection sites and infecting
pathogens for tigecycline prescriptions
A total of 40 tigecycline prescriptions were
analysed. Pulmonary infections accounted for the largest proportion
(n=29, 72.5%), followed by multi-site infections (n=8, 20%) and
abdominal infections (n=3, 7.5%) (Table IV). All 40 cases (100%) received an
aetiological test before tigecycline treatment. In total, 42
pathogen strains were isolated from the 40 cases, with A.
baumannii accounting for the largest proportion (n=28, 66.67%),
followed by Klebsiella pneumoniae (n=4, 9.52%) (Table V).
 | Table IVInfection site distribution among the
40 cases. |
Table IV
Infection site distribution among the
40 cases.
| Site | Cases |
|---|
| Lung | 29 (72.5) |
| Abdominal
cavity | 3 (7.5) |
| Multiple sites | 8 (20.0) |
 | Table VDistribution of the 42 pathogenic
strains isolated from the 40 patients. |
Table V
Distribution of the 42 pathogenic
strains isolated from the 40 patients.
| Pathogen | Strain cases |
|---|
| Acinetobacter
baumannii | 28 (66.67) |
| Staphylococcus
aureus | 3 (7.14) |
| Stenotrophomonas
maltophilia | 1 (2.38) |
| Enterococcus
faecium | 2 (4.76) |
| Klebsiella
pneumoniae | 4 (9.52) |
| Pseudomonas
aeruginosa | 2 (4.76) |
| Escherichia
coli | 2 (4.76) |
Tigecycline prescription review
A total of 40 prescriptions of tigecycline were
reviewed following the predefined standards. In total, 11
prescriptions were considered rational and the remaining 29
prescriptions were considered irrational. In both rational and
irrational groups, tigecycline was used with other antibiotics,
especially carbapenems, which accounted for the largest proportion
(Table VI). The infections in the
rational and irrational groups are shown in Table VII. Both groups had severe
infection (90.9% in the rational and 79.3% in the irrational
group), including carbapenem-resistant A. baumannii
(rational group) and carbapenem-resistant Klebsiella
pneumoniae (irrational group), which caused lung and other
serious infections, such as abdominal infection, septic shock and
sepsis (P=0.65; Table VII). No
significant differences were observed in the body temperature,
white blood cell count, neutrophil count and CRP between both
groups before treatment with tigecycline (Table VIII).
 | Table VITigecycline combined with various
antimicrobials in the rational use and irrational use groups. |
Table VI
Tigecycline combined with various
antimicrobials in the rational use and irrational use groups.
| Antimicrobial | Rational use group
(n=15) | Irrational use
group (n=37) |
|---|
| Carbapenems | 10 (66.7) | 19 (51.4) |
| β-Lactamase | 1 (6.7) | 11 (29.7) |
| Quinolones | 1 (6.7) | 2 (5.4) |
|
Aminoglycosides | 1 (6.7) | 1 (2.7) |
| Polypeptides | 1 (6.7) | 3 (8.1) |
| Polyphosphates | 1 (6.7) | 0 (0.0) |
|
Nitroimidazoles | 0 (0.0) | 1 (2.7) |
 | Table VIIInfections in the rational/irrational
groups. |
Table VII
Infections in the rational/irrational
groups.
| Variable | Rational use
group | Irrational use
group | P-value |
|---|
| Infections caused
by carbapenem-resistant bacteria or other infections (abdominal
infection, septic shock, sepsis) | 10 (90.9) | 23 (79.3) | 0.65 |
| Infections caused
by multidrug-resistant or pan-drug-resistant bacteria | 1 (9.1) | 6 (20.7) | |
 | Table VIIIInflammatory factors in the two
groups. |
Table VIII
Inflammatory factors in the two
groups.
| Group | Temperature,
˚C | White blood cell
count, x109/l | Neutrophil count,
x109/l | CRP, mg/l |
|---|
| Rational use | 38.17±0.93 | 12.58±5.55 | 10.98±5.05 | 79.35±52.98 |
| Irrational use | 37.91±0.98 | 14.16±10.48 | 11.93±10.12 | 83.61±55.24 |
| P-value | 0.448 | 0.716 | 0.868 | 0.891 |
In the irrational group with 29 cases, 20
prescriptions (20/40, 50%) lacked consultation records. The first
doses stated on 17 prescriptions were not the recommended loading
administration, thereby resulting in inappropriate usage and dosage
of tigecycline. Drug selection was inappropriate in two
prescriptions, and four prescriptions lacked dynamic laboratory
tests for the evaluation of efficacy (Table IX).
 | Table IXIrrational use of tigecycline
(n=40). |
Table IX
Irrational use of tigecycline
(n=40).
| Type | Cases |
|---|
| Inappropriate
dosage and usage | 17(42) |
| Inappropriate drug
selection | 2(5) |
| Lack of
consultation record | 20(50) |
| Lack of dynamic
laboratory tests to | 4(10) |
| evaluate
efficacy | |
Discussion
By conducting a prescription review, the present
study showed that the major problems in tigecycline usage in the
ICU of The First Affiliated Hospital of Bengbu Medical College
included: i) Inappropriate usage and dosage (the first dose was not
the recommended loading administration); ii) inappropriate drug
selection; iii) lack of consultation before administration; and iv)
lack of dynamic laboratory tests to assess treatment efficacy.
Blood drug concentration monitoring of 18 patients showed that the
blood drug concentration in the high-dose group was significantly
higher compared with that in the low-dose group, and the rate of
clinical efficacy in the high-dose group was also higher compared
with that in the low-dose group.
Based on blood concentration monitoring and
prescription review analysis, the results of the present study
suggested that, for the rational use of tigecycline, the first dose
should be administered with loading (double dose), so that the
steady-state trough concentration can be reached quicker (19). This approach may improve efficacy
and reduce the chance of drug resistance. Tigecycline is not
recommended for severe infections caused by Pseudomonas
aeruginosa, since tigecycline shows low antibacterial activity
against this pathogen (20,21). Hospitals should strengthen the
management and consultation systems for tigecycline usage. The
present study highlighted that for severe pulmonary infections
caused by carbapenem-resistant A. baumannii, 200 mg of the
first loading dose and 100 mg of the maintenance dose would be
recommended for initial treatment. The recommendation of the
present study is in line with previous studies indicating that high
doses of tigecycline should be used to treat infections involving
carbapenem-resistant A. baumannii (5,22,23).
The current study confirms that the recommended regimen has a
higher AUC0-12 h and higher efficacy compared with the
regimen using 50 mg of the maintenance dose.
Certain measures can be taken into consideration to
improve the rational use of tigecycline in The First Affiliated
Hospital of Bengbu Medical College. Firstly, the hospital
management can organise a hospital prescription review team to
conduct special prescription reviewing for tigecycline in the ICU.
Secondly, based on the review results, the hospital should aim to
correct major problems and formulate rewards and penalties, to
ensure the rational use of tigecycline in the ICU. Thirdly, the
drug susceptibility test kit can be updated. The currently used kit
cannot show the minimum inhibitory concentration (MIC) values of
gram-negative bacteria. It has been reported that the dosage of
tigecycline can be empirically prescribed based on the MIC
(24,25). At MIC <0.5, the regimen of 100 mg
of the loading dose and 50 mg every 12 h of the maintenance dose
should be used; however, at MIC >1.0, the regimen of 200 mg of
the loading dose and 100 mg of the maintenance dose is recommended.
The aforementioned dosage schedule is also suitable for
hospital-acquired pneumonia, ventilator-associated pneumonia or
carbapenem-resistant Enterobacterales, as well as other severe
infections caused by carbapenem-resistant A. baumannii
(21,22). The current kit does not provide the
MIC of tigecycline, but rather a positive/negative result;
therefore, it is difficult for clinicians to formulate an initial
treatment plan. The drug susceptibility test kit should therefore
be replaced. Fourthly, the role of clinical pharmacists can be
enhanced. Clinical pharmacists may give feedback to medical staff
in the form of lectures based on the results of prescription
reviews. The latest research on tigecycline can be summarized and
taught to improve awareness of the rational use of tigecycline
among medical staff. In addition, clinical pharmacists can conduct
large sample pharmacokinetics/pharmacodynamics studies to guide
clinical medication. Lastly, hospitals may formulate measures to
regularly conduct random inspections of the use of tigecycline,
carbapenems and colistin, to increase their rational use and ensure
pre-administration consultation.
In summary, irrational use of tigecycline exists in
the ICU of The First Affiliated Hospital of Bengbu Medical College
where the present study was conducted. The major problems include
irrational usage and dosage, inappropriate drug selection, lack of
consultation and lack of dynamic laboratory tests to evaluate the
efficacy of treatment. The dose of 50 mg q12 h tigecycline used to
treat pulmonary infections caused by A. baumannii is
relatively low in the hospital. Hospitals may improve the rational
use of tigecycline through special prescription reviews, regular
spot checks, training and relevant research.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by the 512 Talent
Cultivation Plan Foundation of Bengbu Medical College (grant no.
by51201320).
Availability of data and materials
The datasets used and/or analysed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
MY and XD designed the study and interpreted the
data. MY drafted the manuscript. BQ and XW evaluated the cases
treated with tigecyclin and collected the blood samples. SW
analysed the data and constructed the graphs. XD revised the
manuscript. All authors have read and approved the final
manuscript. MY and XD confirm the authenticity of all the raw
data.
Ethics approval and consent to
participate
Ethics approval (approval no. 2022KY012) was
obtained from the Ethics Committee of The First Affiliated Hospital
of Bengbu Medical College (Bengbu, China) and written informed
consent was obtained from each patient.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Tan J, Wang Y, Gong X, Li J, Zhong W, Shan
L, Lei X, Zhang Q, Zhou Q, Zhao Y, et al: Antibiotic resistance in
neonates in China 2012-2019: A multicenter study. J Microbiol
Immunol Infect. 55:454–462. 2022.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Wang Q, Jin L, Sun S, Yin Y, Wang R, Chen
F, Wang X, Zhang Y, Hou J, Zhang Y, et al: Occurrence of high
levels of cefiderocol resistance in carbapenem-resistant
escherichia coli before its approval in China: A report from China
CRE-network. Microbiol Spectr. 10(e0267021)2022.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Jiang Y, Yang S, Deng S, Lu W, Huang Q and
Xia Y: Epidemiology and resistance mechanisms of tigecycline- and
carbapenem-resistant Enterobacter cloacae in Southwest
China: A 5-year retrospective study. J Glob Antimicrob Resist.
28:161–167. 2022.PubMed/NCBI View Article : Google Scholar
|
|
4
|
http://www.carss.cn/Report/Details?aId=862.
|
|
5
|
Bartal C, Rolston KVI and Nesher L:
Carbapenem-resistant Acinetobacter baumannii: Colonization,
infection and current treatment options. Infect Dis Ther.
11:683–694. 2022.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Chen Y, Ji J, Ying C, Liu Z, Yang Q, Kong
H and Xiao Y: Blood Bacterial Resistant Investigation Collaborative
System (BRICS) Study Group. Blood bacterial resistant investigation
collaborative system (BRICS) report: A national surveillance in
China from 2014 to 2019. Antimicrob Resist Infect Control.
11(17)2022.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Jing N, Yan W, Zhang Q, Yuan Y, Wei X,
Zhao W, Guo S, Guo L, Gao Y, Zhao L, et al: Epidemiology and
genotypic characteristics of carbapenem resistant Enterobacterales
in Henan, China: A multicentre study. J Glob Antimicrob Resist.
29:68–73. 2022.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Dimopoulos G, Almyroudi MP, Kapralos I,
Apostolopoulou O, Flevari A, Nicolau DP and Dokoumetzidis A:
Intrapulmonary pharmacokinetics of high doses of tigecycline in
patients with ventilator-associated pneumonia. Int J Antimicrob
Agents. 59(106487)2022.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Yang T, Mei H, Wang J and Cai Y:
Therapeutic drug monitoring of tigecycline in 67 infected patients
and a population pharmacokinetics/microbiological evaluation of
A. baumannii study. Front Microbiol.
12(678165)2021.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Chang K, Wang H, Zhao J, Yang X, Wu B, Sun
W, Huang M, Cheng Z, Chen H, Song Y, et al: Polymyxin B/tigecycline
combination vs polymyxin B or tigecycline alone for the treatment
of hospital-acquired pneumonia caused by carbapenem-resistant
enterobacteriaceae or carbapenem-resistant Acinetobacter
baumannii. Front Med (Lausanne). 9(772372)2022.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Yaghoubi S, Zekiy AO, Krutova M, Gholami
M, Kouhsari E, Sholeh M, Ghafouri Z and Maleki F: Tigecycline
antibacterial activity, clinical effectiveness, and mechanisms and
epidemiology of resistance: narrative review. Eur J Clin Microbiol
Infect Dis. 41:1003–1022. 2022.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Zhou CY, Zhang L, Zhu N, Cai H, Chen G and
Luo Z: Dept.of Pharmacy, Anhui Provincial Children's Hospital,
Dept.of Pharmacy, the Fourth Affiliated Hospital of Anhui Medical
University. Specialized management and rationality analysis of
carbapenems and tigecycline in one hospital. Eval Anal Drug-Use
Hosp China. 20:989–995. 2020.
|
|
13
|
Bai XR, Jiang DC and Yan SY: High-dose
tigecycline in elderly patients with pneumonia due to
multidrug-resistant Acinetobacter baumannii in intensive
care unit. Infect Drug Resist. 13:1447–1454. 2020.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Zha L, Pan L, Guo J, French N, Villanueva
EV and Tefsen B: Effectiveness and safety of high dose tigecycline
for the treatment of severe infections: A systematic review and
meta-analysis. Adv Ther. 37:1049–1064. 2020.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Xia G and Jiang R: Clinical study on the
safety and efficacy of high-dose tigecycline in the elderly
patients with multidrug-resistant bacterial infections: A
retrospective analysis. Medicine (Baltimore).
99(e19466)2020.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Chuang YC, Cheng CY, Sheng WH, Sun HY,
Wang JT, Chen YC and Chang SC: Effectiveness of tigecycline-based
versus colistin-based therapy for treatment of pneumonia caused by
multidrug-resistant Acinetobacter baumannii in a critical
setting: A matched cohort analysis. BMC Infect Dis.
14(102)2014.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Zhou Y, Chen X, Xu P, Zhu Y, Wang K, Xiang
D, Wang F and Banh HL: Clinical experience with tigecycline in the
treatment of hospital-acquired pneumonia caused by multidrug
resistant Acinetobacter baumannii. BMC Pharmacol Toxicol.
20(19)2019.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Liu B, Li S, Li HT, Wang X, Tan HY, Liu S,
Pan PH, Li XG and Li XM: Outcomes and prognostic factors of
tigecycline treatment for hospital-acquired pneumonia involving
multidrug-resistant Acinetobacter baumannii. J Int Med Res.
48(300060520910917)2020.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Huang F, Cao WX, Yan YY, Mao TT, Wang XW,
Huang D, Qiu YS, Lu WJ, Li DJ and Zhuang YG: Influence of
continuous renal replacement therapy on the plasma concentration of
tigecycline in patients with septic shock: A prospective
observational study. Front Pharmacol. 14(1118788)2023.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Doi Y: Treatment options for
carbapenem-resistant gram-negative bacterial infections. Clin
Infect Dis. 69 (Suppl 7):S565–S575. 2019.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Bouza E: The role of new carbapenem
combinations in the treatment of multidrug-resistant gram-negative
infections. J Antimicrob Chemother. 76 (Suppl 4):iv38–iv45.
2021.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Sheu CC, Chang YT, Lin SY, Chen YH and
Hsueh PR: Infections caused by carbapenem-resistant
enterobacteriaceae: An update on therapeutic options. Front
Microbiol. 10(80)2019.PubMed/NCBI View Article : Google Scholar
|
|
23
|
De Pascale G, Lisi L, Ciotti GMP,
Vallecoccia MS, Cutuli SL, Cascarano L, Gelormini C, Bello G,
Montini L, Carelli S, et al: Pharmacokinetics of high-dose
tigecycline in critically ill patients with severe infections. Ann
Intensive Care. 10(94)2020.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Xie J, Roberts JA, Alobaid AS, Roger C,
Wang Y, Yang Q, Sun J, Dong H, Wang X, Xing J, et al: Population
pharmacokinetics of tigecycline in critically ill patients with
severe infections. Antimicrob Agents Chemother. 61:e00345–17.
2017.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Xie J, Wang T, Sun J, Chen S, Cai J, Zhang
W, Dong H, Hu S, Zhang D, Wang X and Dong Y: Optimal tigecycline
dosage regimen is urgently needed: Results from a
pharmacokinetic/pharmacodynamic analysis of tigecycline by Monte
Carlo simulation. Int J Infect Dis. 18:62–67. 2014.PubMed/NCBI View Article : Google Scholar
|